Processing of Biltong (Dried Beef) to Achieve USDA-FSIS 5-log Reduction of Salmonella without a Heat Lethality Step
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Beef Handling and Inoculation
2.3. Biltong Processing: Antimicrobials
2.4. Biltong Processing: Marination and Drying
2.4.1. Short-Term Biltong Marination Process
2.4.2. Extended Overnight Biltong Marination Process
2.4.3. Biltong Drying Process
2.5. Water Activity and Moisture-Loss Determination
2.6. Microbial Sampling and Enumeration of Beef
2.7. Statistical Analysis
3. Results
3.1. Short-Term Biltong Marination Process
3.2. Long-Term Biltong Marination Process
3.3. Temperature and Relative Humidity Measurements
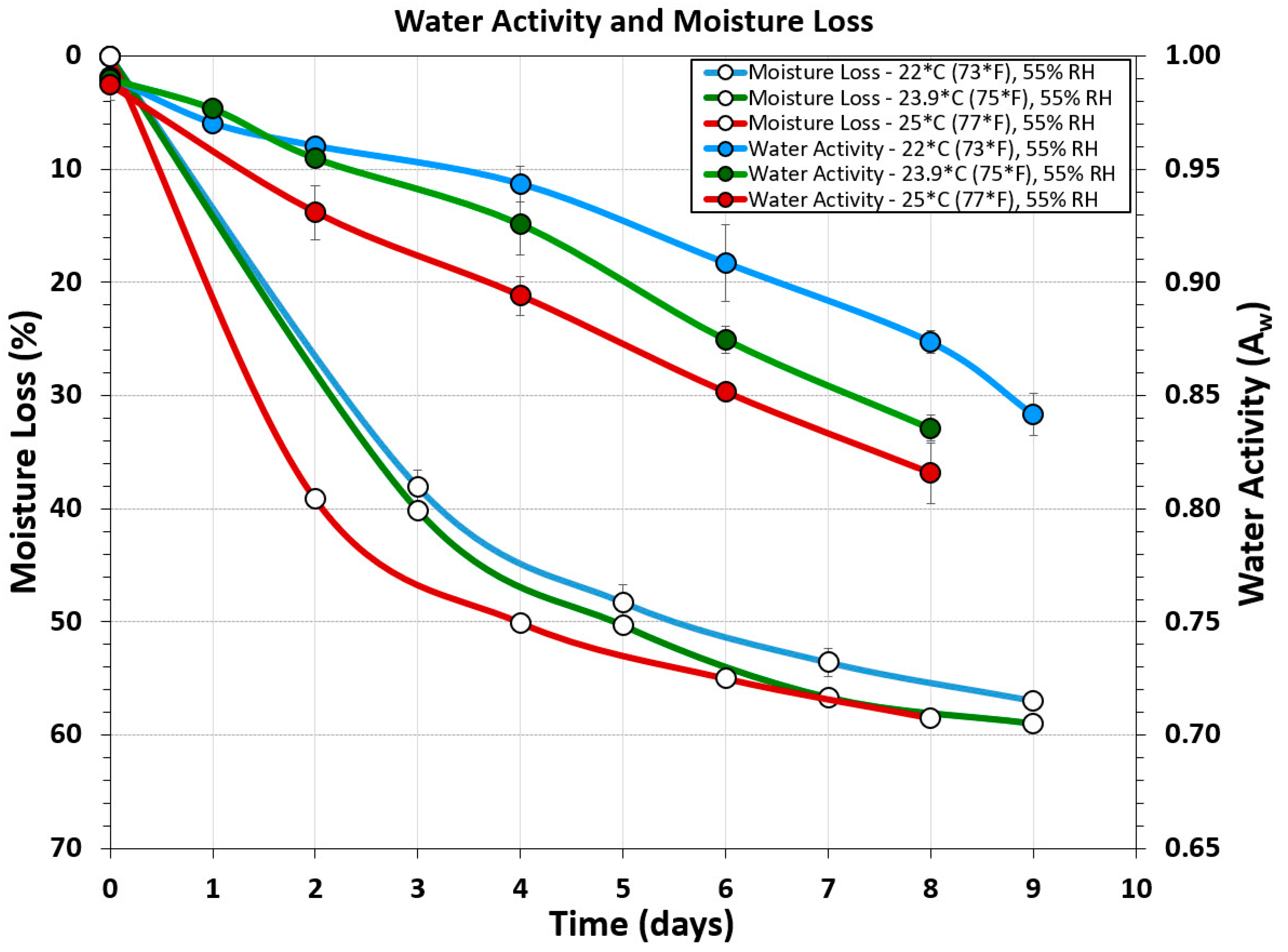
3.4. Water Activity and Moisture-Loss Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taormina, P.J.; Sofos, J.N. Low-water activity meat products. In The Microbiological Safety of Low Water Activity Foods and Spices; Gurtler, J.B., Doyle, M.P., Kornacki, J.L., Eds.; Springer: New York, NY, USA, 2014; pp. 127–164. [Google Scholar]
- Wentworth, E.N. Dried meat: Early man’s travel ration. Agric. Hist. 1956, 30, 2–10. [Google Scholar]
- Jones, M.; Arnaud, E.; Gouws, P.; Hoffman, L.C. Processing of South African biltong—A review. South Afr. J. Anim. Sci. 2017, 47, 743. [Google Scholar] [CrossRef]
- Petit, T.; Caro, Y.; Petit, A.-S.; Santchurn, S.J.; Collignan, A. Physicochemical and microbiological characteristics of biltong, a traditional salted dried meat of South Africa. Meat Sci. 2014, 96, 1313–1317. [Google Scholar] [CrossRef]
- Jones, M.; Arnaud, E.; Gouws, P.; Hoffman, L.C. Effects of the addition of vinegar, weight loss and packaging method on the physicochemical properties and microbiological profile of biltong. Meat Sci. 2019, 156, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.; Lindsay, D. Survival of Listeria monocytogenes, and enterotoxin-producing Staphylococcus aureus and Staphylococcus pasteuri, during two types of biltong-manufacturing processes. Food Control 2010, 21, 1042–1050. [Google Scholar] [CrossRef]
- Naidoo, K.; Lindsay, D. Pathogens associated with biltong product and their in vitro survival of hurdles used during production. Food Prot. Trends 2010, 30, 532–538. [Google Scholar]
- Buege, D.R.; Searls, G.; Ingham, S. Lethality of Commercial Whole-Muscle Beef Jerky Manufacturing Processes against Salmonella Serovars and Escherichia coli O157:H7. J. Food Prot. 2006, 69, 2091–2099. [Google Scholar] [CrossRef] [PubMed][Green Version]
- USDA-FSIS. Verification Procedures for Lethality and Stabilization; USDA-FSIS: Washington, DC, USA, 2017; Volume 7111.1, pp. 1–21.
- USDA-FSIS. Fsis Compliance Guideline for Meat and Poultry Jerky Produced by Small and Very Small Establishments; USDA-FSIS: Washington, DC, USA, 2014; pp. 1–54.
- Carr, M.; Miller, M.; Daniel, D.; Yarbrough, C.; Petrosky, J.; Thompson, L. Evaluation Of The Physical, Chemical And Sensory Properties Of Jerky Processed From Emu, Beef, And Turkey. J. Food Qual. 1997, 20, 419–425. [Google Scholar] [CrossRef]
- Laufer, A.S.; Grass, J.; Holt, K.; Whichard, J.M.; Griffin, P.M.; Gould, L.H. Outbreaks of Salmonella infections attributed to beef –United States, 1973–2011. Epidemiol. Infect. 2014, 143, 2003–2013. [Google Scholar] [CrossRef]
- Outbreak of Salmonellosis Associated with Beef Jerky—New Mexico, 1995. JAMA 1995, 274, 1669. [CrossRef]
- Eidson, M.; Swell, C.M.; Graves, G.; Olson, R. Beef jerky gastroenteritis outbreaks. J. Environ. Health 2000, 62, 9. [Google Scholar]
- Nickelson, R.; Luchansky, J.B.; Kaspar, C.; Johnson, E. Dry fermented sausage and escherichia coli o157:H7 validation research. In An Executive Summary Prepared by the Blue Ribbon Task Force of the National Cattlemen’s Beef Association; Research report no. 11-316; National Cattlemen’s Asssociation: Chicago, IL, USA, 1996. [Google Scholar]
- Burnham, G.M.; Hanson, D.J.; Koshick, C.M.; Ingham, S. Death of Salmonella Serovars, Escherichia Coli O157: H7, Staphylococcus Aureus And Listeria Monocytogenes During The Drying Of Meat: A Case Study Using Biltong And Droëwors. J. Food Saf. 2008, 28, 198–209. [Google Scholar] [CrossRef]
- Juneja, V.K.; Hwang, C.A.; Friedman, M. Thermal inactivation and postthermal treatment growth during storage of multiple salmonella serotypes in ground beef as affected by sodium lactate and oregano oil. J. Food Sci. 2010, 75, M1-6. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.E.; Smith, J.; Broadbent, J. Efficacy of washing meat surfaces with 2% levulinic, acetic, or lactic acid for pathogen decontamination and residual growth inhibition. Meat Sci. 2011, 88, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Juneja, V.K. Modeling non-linear survival curves to calculate thermal inactivation of Salmonella in poultry of different fat levels. Int. J. Food Microbiol. 2001, 70, 37–51. [Google Scholar] [CrossRef]
- Juneja, V.K.; Yadav, A.S.; Hwang, C.-A.; Sheen, S.; Mukhopadhyay, S.; Friedman, M. Kinetics of Thermal Destruction of Salmonella in Ground Chicken Containing trans-Cinnamaldehyde and Carvacrol†. J. Food Prot. 2012, 75, 289–296. [Google Scholar] [CrossRef]
- Karolenko, C.E.; Bhusal, A.; Gautam, D.; Muriana, P.M. Selenite Cystine Agar for Enumeration of Inoculated Salmonella Serovars Recovered from Stressful Conditions during Antimicrobial Validation Studies. Microorganisms 2020, 8, 338. [Google Scholar] [CrossRef]
- Foster, J.W. Salmonella acid shock proteins are required for the adaptive acid tolerance response. J. Bacteriol. 1991, 173, 6896–6902. [Google Scholar] [CrossRef]
- Leyer, G.J.; Johnson, E.A. Acid adaptation promotes survival of salmonella spp. Cheese. Appl. Environ. Microbiol. 1992, 58, 2075–2080. [Google Scholar] [CrossRef]
- Wilde, S.; Jørgensen, F.; Campbell, A.; Rowbury, R.; Humphrey, T. Growth of salmonella enterica serovar enteritidis pt4 in media containing glucose results in enhanced rpos-independent heat and acid tolerance but does not affect the ability to survive air-drying on surfaces. Food Microbiol. 2000, 17, 679–686. [Google Scholar] [CrossRef]
- The National Advisory Committee on Microbiological Criteria for Foods. J. Food Prot. 1994, 57, 1101–1121. [CrossRef] [PubMed][Green Version]
- USDA-AMS. Institutional Meat Purchase Specifications; Fresh Beef Series 100; Agricultural Marketing Service: Washington, DC, USA, 2014; pp. 1–71.
- USDA-FSIS. Safe and Suitable Ingredients Used in the Production of Meat and Poultry, and Egg Products; USDA-FSIS: Washington, DC, USA, 2018.
- USDA-FSIS. Fsis Compliance Guideline Haccp Systems Validation (April 2015); USDA-FSIS: Washington, DC, USA, 2015; pp. 1–68.
- Muriana, P.M.; Eager, J.; Wellings, B.; Morgan, B.; Nelson, J.; Kushwaha, K. Evaluation of Antimicrobial Interventions against E. coli O157:H7 on the Surface of Raw Beef to Reduce Bacterial Translocation during Blade Tenderization. Foods 2019, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- USDA-FSIS. Salmonella Compliance Guidelines for Small and Very Small Meat and Poultry Establishments that Produce Ready-to-Eat (rte) Products and Revised Appendix A, June 2017; U.S. Food Safety and Inspection Service: Washington, DC, USA, 2017; pp. 1–37.
- Allen, K.; Cornforth, D.; Whittier, D.; Vasavada, M.; Nummer, B. Evaluation of High Humidity and Wet Marinade Methods for Pasteurization of Jerky. J. Food Sci. 2007, 72, C351–C355. [Google Scholar] [CrossRef] [PubMed]
- Porto-Fett, A.C.S.; Call, J.E.; Luchansky, J.B. Validation of a Commercial Process for Inactivation of Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on the Surface of Whole Muscle Beef Jerky†. J. Food Prot. 2008, 71, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Human Services, F.D.A. Voluntary sodium reduction goals: Target mean and upper bound concentrations for sodium in commercially processed, packaged, and prepared foods; draft guidance for industry; availability. Fed. Regist. 2016, 81, 35363–35367. [Google Scholar]
- United States Food and Drug Administration, U.-F. Cpg Sec 525.825 Vinegar, Definitions-Adulteration with Vinegar Eels; Office of Foods and Veterinary Medicine: Washington, DC, USA, 1995.
- USDA-FSIS. Compliance Guide on the Determination of Processing Aids; USDA-FSIS: Washington, DC, USA, 2008; pp. 1–2.
- Post, R.; Budak, C.; Canavan, J.; Duncan-Harrington, T.; Jones, B.J.; Kegley, M. A Guide to Federal Food Labeling Requirements for Meat and Poultry Products; US Department of Agriculture: Washington, DC, USA, 2007.
- United States Food and Drug Administration, U.F. Title 21—food and drugs; chapter I—Food and drug administration, department of health and human services; subchapter b—Food for human consumption; part 101—Food labeling; subpart g—Exemptions from food labeling requirements. In Code of Federal Regulations; Government Publishing Office: Washington, DC, USA, 2016; Volume 2. [Google Scholar]






| Category | Substance | Intended Use of Product | Amount | Label Key a |
|---|---|---|---|---|
Antimicrobials
| Lactic acid (5%). | Beef and pork sub-primals and trimmings. | 2% to 5 % solution of lactic acid, not to exceed 55 °C. | None under the accepted conditions of use (1) |
Antimicrobials
| An aqueous solution of sulfuric acid and sodium sulfate. | In the form of a spray, wash, or dip on the surface of meat (beef and pork) and poultry products processing. | Solution of sulfuric acid and sodium sulfate at concentrations sufficient to achieve a targeted pH range of 1.0–2.2 on the surface of meat and poultry. | None under the accepted conditions of use (1) |
Antimicrobials
| An aqueous solution of acidic calcium sulfate and lactic acid. | Applied as a continuous spray or a dip on raw poultry carcasses, parts, giblets, and ground poultry. | Acidic calcium sulfate sufficient for purpose; lactic acid not to exceed 5.0 % and 55 °C. | None under the accepted conditions of use (1) |
Antimicrobials
| A solution of water, acidic calcium sulfate, lactic acid, and sodium phosphate (solution with a pH range of 1.45 to 1.55). | Raw whole muscle beef cuts and cooked roast beef and similar cooked beef products (e.g., corned beef, pastrami, etc.). | A solution of water, acidic calcium sulfate, lactic acid, and sodium phosphate (solution with a pH range of 1.45 to 1.55) spray applied for up to 30 s of continual application *sodium phosphate on finished product must not exceed 5000 ppm. | Listed by common name in ingredients statement of multi-ingredient products. Single ingredient raw whole muscle beef cuts must be descriptively labeled (2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karolenko, C.E.; Bhusal, A.; Nelson, J.L.; Muriana, P.M. Processing of Biltong (Dried Beef) to Achieve USDA-FSIS 5-log Reduction of Salmonella without a Heat Lethality Step. Microorganisms 2020, 8, 791. https://doi.org/10.3390/microorganisms8050791
Karolenko CE, Bhusal A, Nelson JL, Muriana PM. Processing of Biltong (Dried Beef) to Achieve USDA-FSIS 5-log Reduction of Salmonella without a Heat Lethality Step. Microorganisms. 2020; 8(5):791. https://doi.org/10.3390/microorganisms8050791
Chicago/Turabian StyleKarolenko, Caitlin E., Arjun Bhusal, Jacob L. Nelson, and Peter M. Muriana. 2020. "Processing of Biltong (Dried Beef) to Achieve USDA-FSIS 5-log Reduction of Salmonella without a Heat Lethality Step" Microorganisms 8, no. 5: 791. https://doi.org/10.3390/microorganisms8050791
APA StyleKarolenko, C. E., Bhusal, A., Nelson, J. L., & Muriana, P. M. (2020). Processing of Biltong (Dried Beef) to Achieve USDA-FSIS 5-log Reduction of Salmonella without a Heat Lethality Step. Microorganisms, 8(5), 791. https://doi.org/10.3390/microorganisms8050791






