Evolutionarily-Related Helicobacter pylori Genotypes and Gastric Intraepithelial Neoplasia in a High-Risk Area of Northern Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Cases and Histopathological Evaluation
2.2. DNA Extraction, Polymerase Chain Reaction (PCR) and Sequence Analyses
2.3. Data Analysis
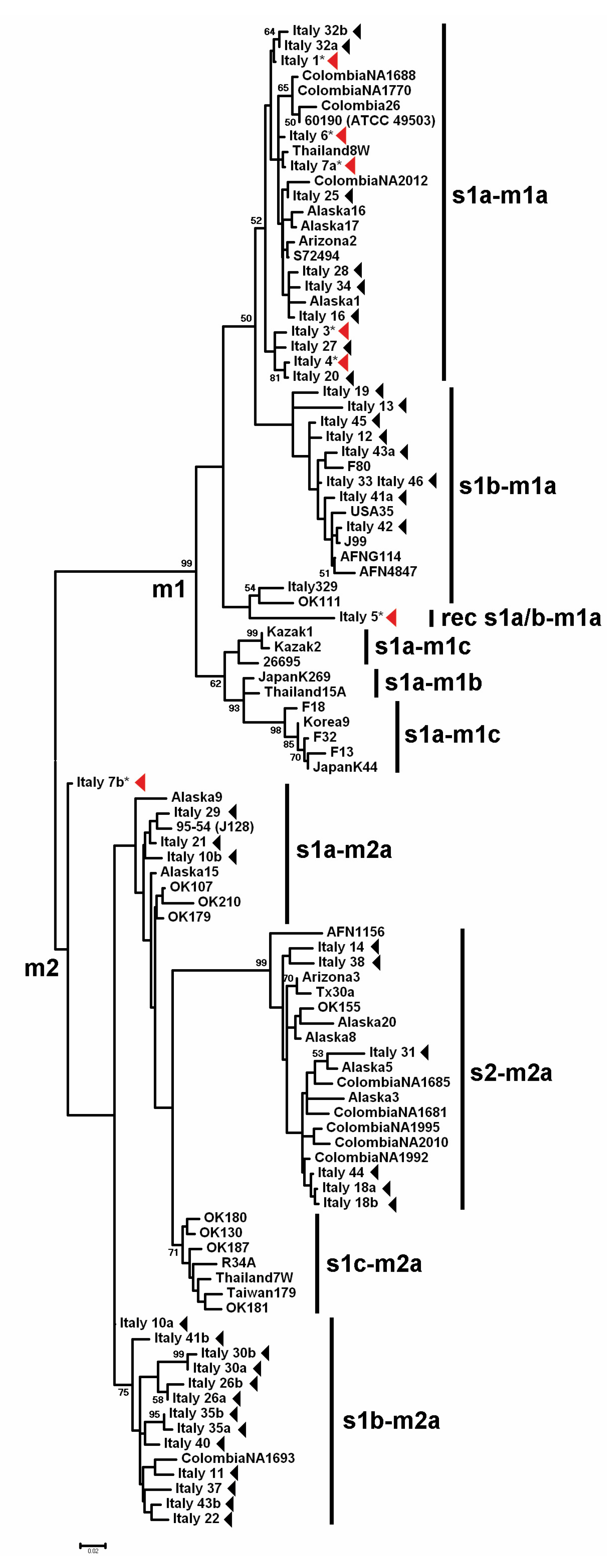
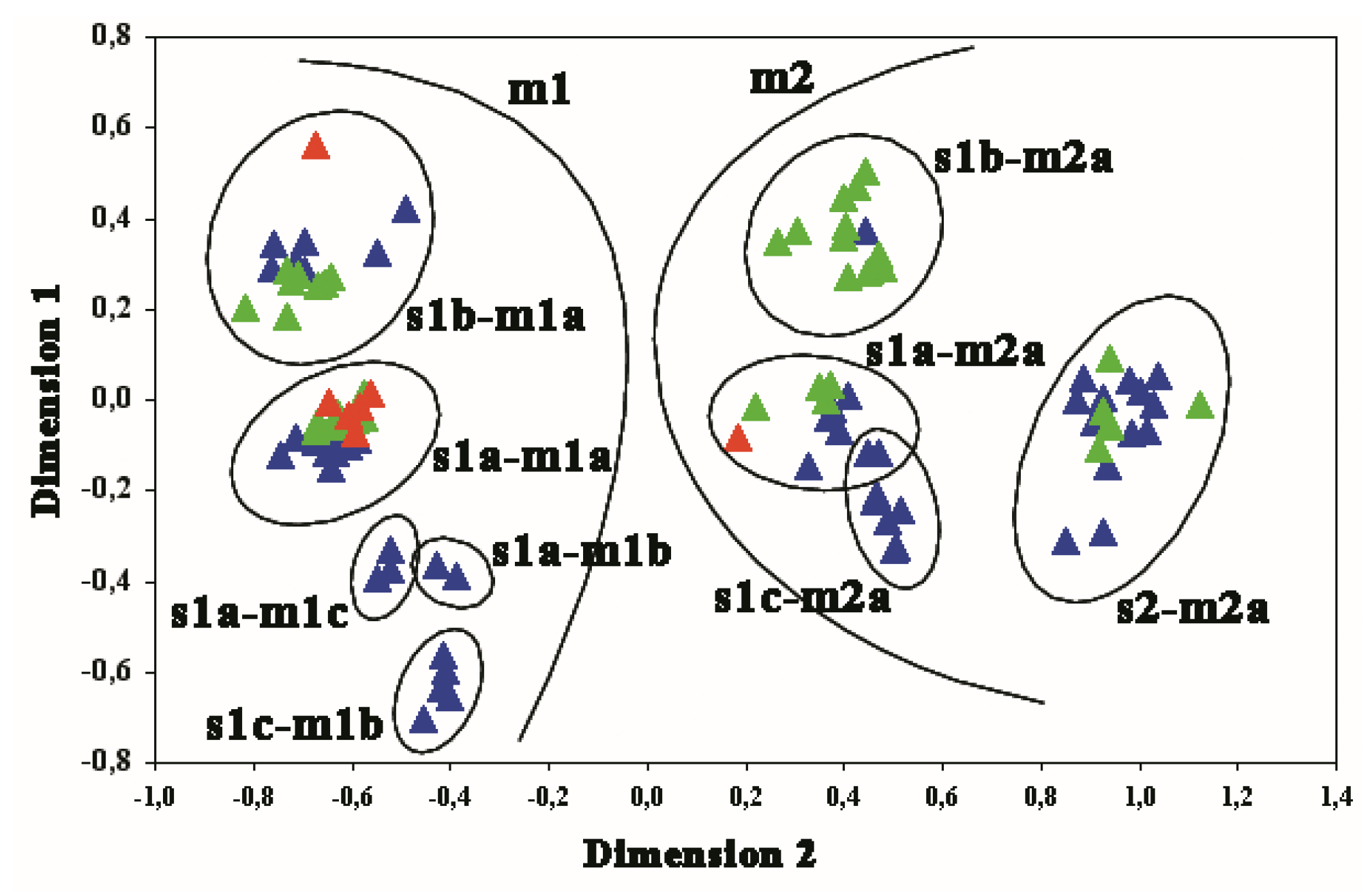
3. Results
3.1. GIN-Associated Hp Genotypes
3.2. NNGDL-Associated Hp Genotypes
3.3. Comparison between the Hp Genotypes of GINs and NNGDLs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Polk, D.B.; Peek, R.M., Jr. Helicobacter pylori: Gastric cancer and beyond. Nat. Rev. Cancer 2010, 10, 403–414. [Google Scholar] [CrossRef]
- Parsonnet, J.; Friedman, G.D.; Vandersteen, D.P.; Chang, Y.; Vogelman, J.H.; Orentreich, N.; Sibley, R.K. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 1991, 325, 1127–1131. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological Agents. A Review of Human Carcinogens; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100, pp. 1–441. [Google Scholar]
- Lyons, K.; Le, L.C.; Pham, Y.T.; Borron, C.; Park, J.Y.; Tran, C.T.D.; Tran, T.V.; Tran, H.T.; Vu, K.T.; Do, C.D.; et al. Gastric cancer: Epidemiology, biology, and prevention: A mini review. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. 2019, 28, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Cover, T.L. Helicobacter pylori diversity and gastric cancer risk. MBio 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Censini, S.; Lange, C.; Xiang, Z.; Crabtree, J.E.; Ghiara, P.; Borodovsky, M.; Rappuoli, R.; Covacci, A. Cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 1996, 93, 14648–14653. [Google Scholar] [CrossRef] [PubMed]
- Covacci, A.; Censini, S.; Bugnoli, M.; Petracca, R.; Burroni, D.; Macchia, G.; Massone, A.; Papini, E.; Xiang, Z.; Figura, N.; et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc. Natl. Acad. Sci. USA 1993, 90, 5791–5795. [Google Scholar] [CrossRef] [PubMed]
- Kuck, D.; Kolmerer, B.; Iking-Konert, C.; Krammer, P.H.; Stremmel, W.; Rudi, J. Vacuolating cytotoxin of Helicobacter pylori induces apoptosis in the human gastric epithelial cell line AGS. Infect. Immun. 2001, 69, 5080–5087. [Google Scholar] [CrossRef] [PubMed]
- Galmiche, A.; Rassow, J.; Doye, A.; Cagnol, S.; Chambard, J.C.; Contamin, S.; de Thillot, V.; Just, I.; Ricci, V.; Solcia, E.; et al. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 2000, 19, 6361–6370. [Google Scholar] [CrossRef] [PubMed]
- Atherton, J.C.; Cao, P.; Peek, R.M., Jr.; Tummuru, M.K.; Blaser, M.J.; Cover, T.L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 1995, 270, 17771–17777. [Google Scholar] [CrossRef]
- Atherton, J.C.; Peek, R.M., Jr.; Tham, K.T.; Cover, T.L.; Blaser, M.J. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology 1997, 112, 92–99. [Google Scholar] [CrossRef]
- Palli, D.; Vaira, D.; Menegatti, M.; Saieva, C. A serologic survey of Helicobacter pylori infection in 3281 Italian patients endoscoped for upper gastrointestinal symptoms. The Italian Helicobacter Pylori Study Group. Aliment. Pharmacol. Ther. 1997, 11, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Gasbarrini, A.; Anti, M.; Franceschi, F.; Armuzzi, A.; Cotichini, R.; Ojetti, V.; Candelli, M.; Lippi, M.E.; Paolucci, M.; Cicconi, V.; et al. Prevalence of and risk factors for Helicobacter pylori infection among healthcare workers at a teaching hospital in Rome: The Catholic University Epidemiological Study. Eur. J. Gastroenterol. Hepatol. 2001, 13, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.H.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M.H. Systematic review with meta-analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Toracchio, S.; Marzio, L. Primary and secondary antibiotic resistance of Helicobacter pylori strains isolated in central Italy during the years 1998-2002. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2003, 35, 541–545. [Google Scholar] [CrossRef]
- Capocaccia, R.; De Angelis, R.; Frova, L.; Sant, M.; Buiatti, E.; Gatta, G.; Micheli, A.; Berrino, F.; Barchielli, A.; Conti, E.; et al. Estimation and projections of stomach cancer trends in Italy. Cancer Causes Control CCC 1995, 6, 339–346. [Google Scholar] [CrossRef]
- Ferlay, J.; Autier, P.; Boniol, M.; Heanue, M.; Colombet, M.; Boyle, P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann. Oncol. Off. J. Eur. Soc. Med Oncol. 2007, 18, 581–592. [Google Scholar] [CrossRef]
- Forman, D.B.F.; Brewster, D.H.; Gombe Mbalawa, C.; Kohler, B.; Piñeros, M.; Steliarova-Foucher, E.; Swaminathan, R.; Ferlay, J. (Eds.) Cancer Incidence in Five Continents. In Cancer Incidence in Five Continents, 164th ed.; International Agency for Research on Cancer: Lyon, France, 2014; Volume X, pp. 714–779. [Google Scholar]
- Stroffolini, T.; Rosmini, F.; Ferrigno, L.; Fortini, M.; D’Amelio, R.; Matricardi, P.M. Prevalence of Helicobacter pylori infection in a cohort of Italian military students. Epidemiol. Infect. 1998, 120, 151–155. [Google Scholar] [CrossRef]
- Gallo, N.; Zambon, C.F.; Navaglia, F.; Basso, D.; Guariso, G.; Grazia Piva, M.; Greco, E.; Mazza, S.; Fogar, P.; Rugge, M.; et al. Helicobacter pylori infection in children and adults: A single pathogen but a different pathology. Helicobacter 2003, 8, 21–28. [Google Scholar] [CrossRef]
- Russo, F.; Berloco, P.; Cuomo, R.; Caruso, M.L.; Di Matteo, G.; Giorgio, P.; De Francesco, V.; Di Leo, A.; Ierardi, E. Helicobacter pylori strains and histologically-related lesions affect the outcome of triple eradication therapy: A study from southern Italy. Aliment. Pharmacol. Ther. 2003, 17, 421–428. [Google Scholar] [CrossRef]
- Zambon, C.F.; Navaglia, F.; Basso, D.; Rugge, M.; Plebani, M. Helicobacter pylori babA2, cagA, and s1 vacA genes work synergistically in causing intestinal metaplasia. J. Clin. Pathol. 2003, 56, 287–291. [Google Scholar] [CrossRef]
- Figura, N.; Valassina, M.; Roviello, F.; Pinto, F.; Lenzi, C.; Giannace, R.; Marrelli, D.; Valentini, M.; Valensin, P.E. Helicobacter pylori cagA and vacA types and gastric carcinoma. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2000, 32, S182–S183. [Google Scholar] [CrossRef]
- Cellini, L.; Grande, R.; Di Campli, E.; Di Bartolomeo, S.; Capodicasa, S.; Marzio, L. Analysis of genetic variability, antimicrobial susceptibility and virulence markers in Helicobacter pylori identified in Central Italy. Scand. J. Gastroenterol. 2006, 41, 280–287. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, V.; Margiotta, M.; Zullo, A.; Hassan, C.; Valle, N.D.; Burattini, O.; D’Angelo, R.; Stoppino, G.; Cea, U.; Giorgio, F.; et al. Claritromycin resistance and Helicobacter pylori genotypes in Italy. J. Microbiol. 2006, 44, 660–664. [Google Scholar] [PubMed]
- Basso, D.; Zambon, C.F.; Letley, D.P.; Stranges, A.; Marchet, A.; Rhead, J.L.; Schiavon, S.; Guariso, G.; Ceroti, M.; Nitti, D.; et al. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology 2008, 135, 91–99. [Google Scholar] [CrossRef]
- Grande, R.; Di Campli, E.; Di Bartolomeo, S.; Verginelli, F.; Di Giulio, M.; Baffoni, M.; Bessa, L.J.; Cellini, L. Helicobacter pylori biofilm: A protective environment for bacterial recombination. J. Appl. Microbiol. 2012, 113, 669–676. [Google Scholar] [CrossRef]
- Donida, B.M.; Tomasello, G.; Ghidini, M.; Buffoli, F.; Grassi, M.; Liguigli, W.; Maglietta, G.; Pergola, L.; Ratti, M.; Sabadini, G.; et al. Epidemiological, clinical and pathological characteristics of gastric neoplasms in the province of Cremona: The experience of the first population-based specialized gastric cancer registry in Italy. BMC Cancer 2019, 19, 212. [Google Scholar] [CrossRef]
- McColl, K.E.; el-Omar, E.; Gillen, D. Helicobacter pylori gastritis and gastric physiology. Gastroenterol. Clin. North Am. 2000, 29, 687–703. [Google Scholar] [CrossRef]
- Schlemper, R.J.; Riddell, R.H.; Kato, Y.; Borchard, F.; Cooper, H.S.; Dawsey, S.M.; Dixon, M.F.; Fenoglio-Preiser, C.M.; Flejou, J.F.; Geboes, K.; et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000, 47, 251–255. [Google Scholar] [CrossRef]
- Kato, M. Diagnosis and therapies for gastric non-invasive neoplasia. World J. Gastroenterol. 2015, 21, 12513–12518. [Google Scholar] [CrossRef]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef]
- Linee Guida. Tracciabilità, Raccolta, Trasporto, Conservazione e Archiviazione Di Cellule e Tessuti Per Indagini Diagnostiche Di Anatomia Patologica. Consiglio Superiore Di Sanità, Sezione I, Ministero Della Salute, Rome, Italy; 2015. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2369_allegato.pdf (accessed on 6 January 2020).
- Rigoli, L.; Di Bella, C.; Verginelli, F.; Falchetti, M.; Bersiga, A.; Rocco, A.; Nardone, G.; Mariani-Costantini, R.; Caruso, R.A. Histological heterogeneity and somatic mtDNA mutations in gastric intraepithelial neoplasia. Mod. Pathol. Off. J. USA Can. Acad. Pathol. 2008, 21, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Rocco, A.; Caruso, R.; Toracchio, S.; Rigoli, L.; Verginelli, F.; Catalano, T.; Neri, M.; Curia, M.C.; Ottini, L.; Agnese, V.; et al. Gastric adenomas: Relationship between clinicopathological findings, Helicobacter pylori infection, APC mutations and COX-2 expression. Ann. Oncol. Off. J. Eur. Soc. Med Oncol. 2006, 17, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Wright, S. The genetical structure of populations. Ann. Eugen. 1951, 15, 323–354. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Forster, P.; Rohl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Fu, Y.X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 1997, 147, 915–925. [Google Scholar]
- Polzin, T.D.; Daneshmand, S.V. On Steiner trees and minimum spanning trees in hypergraphs. Oper. Res. Lett. 2003, 12–20. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evol. Int. J. Org. Evol. 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, M. Helicobacter pylori CagA and gastric cancer: A paradigm for hit-and-run carcinogenesis. Cell Host Microbe 2014, 15, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y.; Orito, E.; Mizokami, M.; Gutierrez, O.; Saitou, N.; Kodama, T.; Osato, M.S.; Kim, J.G.; Ramirez, F.C.; Mahachai, V.; et al. Helicobacter pylori in North and South America before Columbus. FEBS Lett. 2002, 517, 180–184. [Google Scholar] [CrossRef]
- Covacci, A.; Telford, J.L.; Del Giudice, G.; Parsonnet, J.; Rappuoli, R. Helicobacter pylori virulence and genetic geography. Science 1999, 284, 1328–1333. [Google Scholar] [CrossRef]
- Kersulyte, D.; Mukhopadhyay, A.K.; Velapatino, B.; Su, W.; Pan, Z.; Garcia, C.; Hernandez, V.; Valdez, Y.; Mistry, R.S.; Gilman, R.H.; et al. Differences in genotypes of Helicobacter pylori from different human populations. J. Bacteriol. 2000, 182, 3210–3218. [Google Scholar] [CrossRef]
- Van Doorn, L.J.; Figueiredo, C.; Megraud, F.; Pena, S.; Midolo, P.; Queiroz, D.M.; Carneiro, F.; Vanderborght, B.; Pegado, M.D.; Sanna, R.; et al. Geographic distribution of vacA allelic types of Helicobacter pylori. Gastroenterology 1999, 116, 823–830. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Kodama, T.; Gutierrez, O.; Kim, J.G.; Kashima, K.; Graham, D.Y. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: Studies in four different countries. J. Clin. Microbiol. 1999, 37, 2274–2279. [Google Scholar] [CrossRef]
- Naumann, M.; Crabtree, J.E. Helicobacter pylori-induced epithelial cell signalling in gastric carcinogenesis. Trends Microbiol. 2004, 12, 29–36. [Google Scholar] [CrossRef]
- van Doorn, L.J.; Figueiredo, C.; Sanna, R.; Plaisier, A.; Schneeberger, P.; de Boer, W.; Quint, W. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology 1998, 115, 58–66. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toracchio, S.; Caruso, R.A.; Perconti, S.; Rigoli, L.; Betri, E.; Neri, M.; Verginelli, F.; Mariani-Costantini, R. Evolutionarily-Related Helicobacter pylori Genotypes and Gastric Intraepithelial Neoplasia in a High-Risk Area of Northern Italy. Microorganisms 2020, 8, 324. https://doi.org/10.3390/microorganisms8030324
Toracchio S, Caruso RA, Perconti S, Rigoli L, Betri E, Neri M, Verginelli F, Mariani-Costantini R. Evolutionarily-Related Helicobacter pylori Genotypes and Gastric Intraepithelial Neoplasia in a High-Risk Area of Northern Italy. Microorganisms. 2020; 8(3):324. https://doi.org/10.3390/microorganisms8030324
Chicago/Turabian StyleToracchio, Sonia, Rosario Alberto Caruso, Silvia Perconti, Luciana Rigoli, Enrico Betri, Matteo Neri, Fabio Verginelli, and Renato Mariani-Costantini. 2020. "Evolutionarily-Related Helicobacter pylori Genotypes and Gastric Intraepithelial Neoplasia in a High-Risk Area of Northern Italy" Microorganisms 8, no. 3: 324. https://doi.org/10.3390/microorganisms8030324
APA StyleToracchio, S., Caruso, R. A., Perconti, S., Rigoli, L., Betri, E., Neri, M., Verginelli, F., & Mariani-Costantini, R. (2020). Evolutionarily-Related Helicobacter pylori Genotypes and Gastric Intraepithelial Neoplasia in a High-Risk Area of Northern Italy. Microorganisms, 8(3), 324. https://doi.org/10.3390/microorganisms8030324






