Differential Distribution of the wlaN and cgtB Genes, Associated with Guillain-Barré Syndrome, in Campylobacter jejuni Isolates from Humans, Broiler Chickens, and Wild Birds
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. PCR Amplification
2.3. Analysis of LOS
2.4. Invasion Assay
3. Results
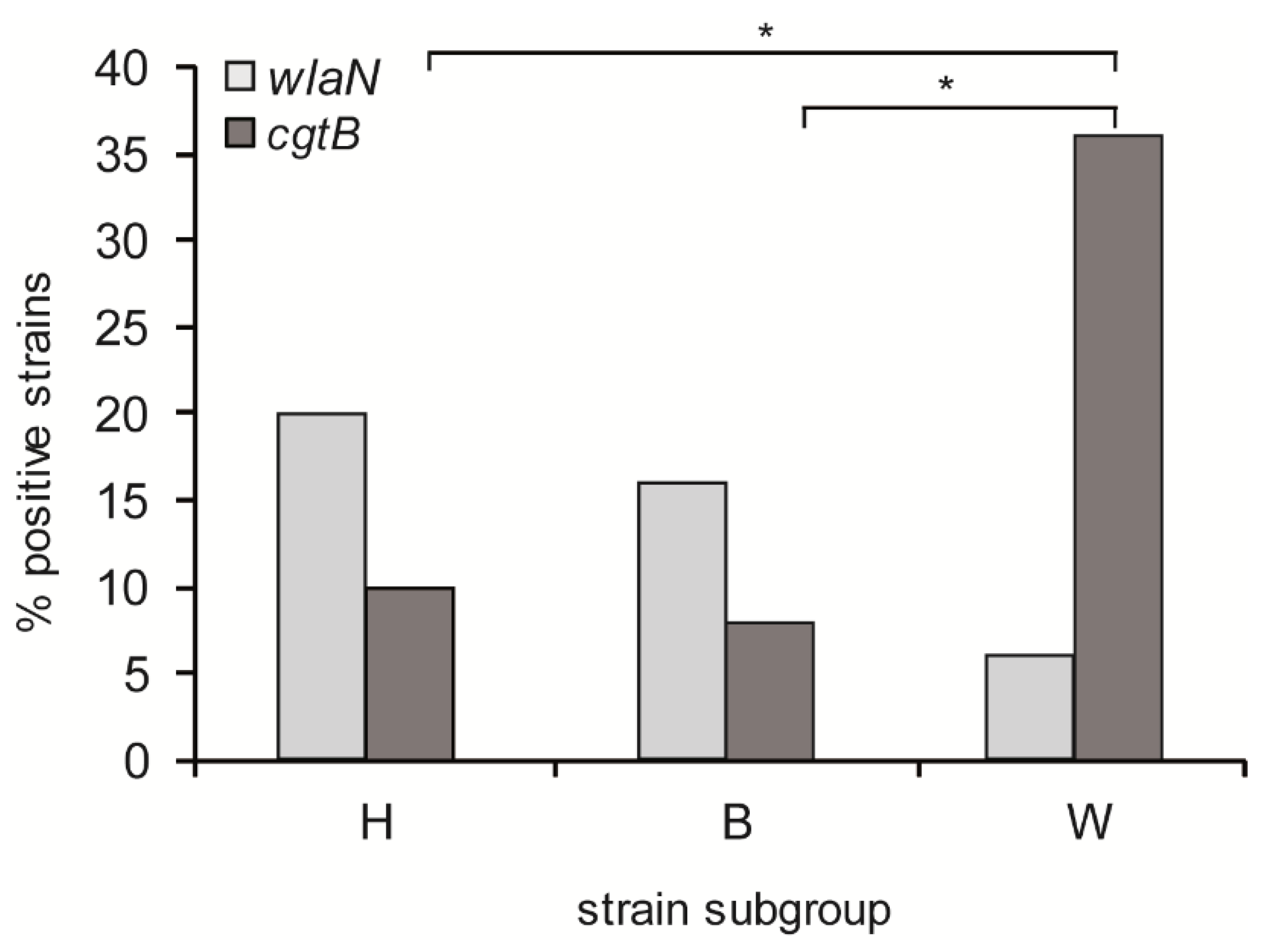
3.1. Differential Prevalence of wlaN and cgtB Genes in C. jejuni Isolates from Human Patients, Broiler Chickens, and Wild Birds
3.2. The Presence of the cgtB and wlaN Genes is Associated with Certain MLST Clonal Complexes
3.3. Homopolymeric G-tract variants in wlaN and cgtB genes
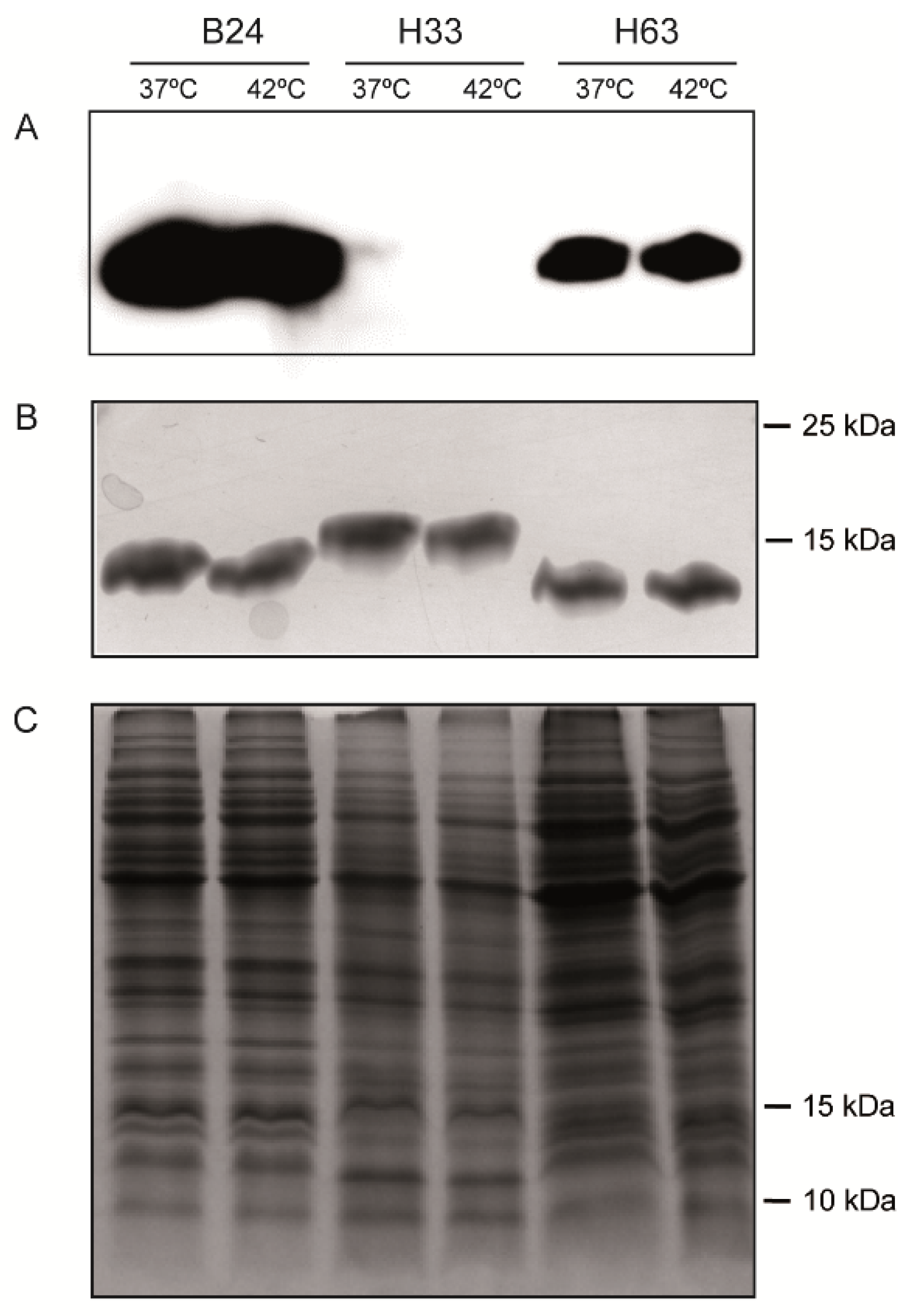
3.4. The production of LOSSIAL is Not Affected by Temperature
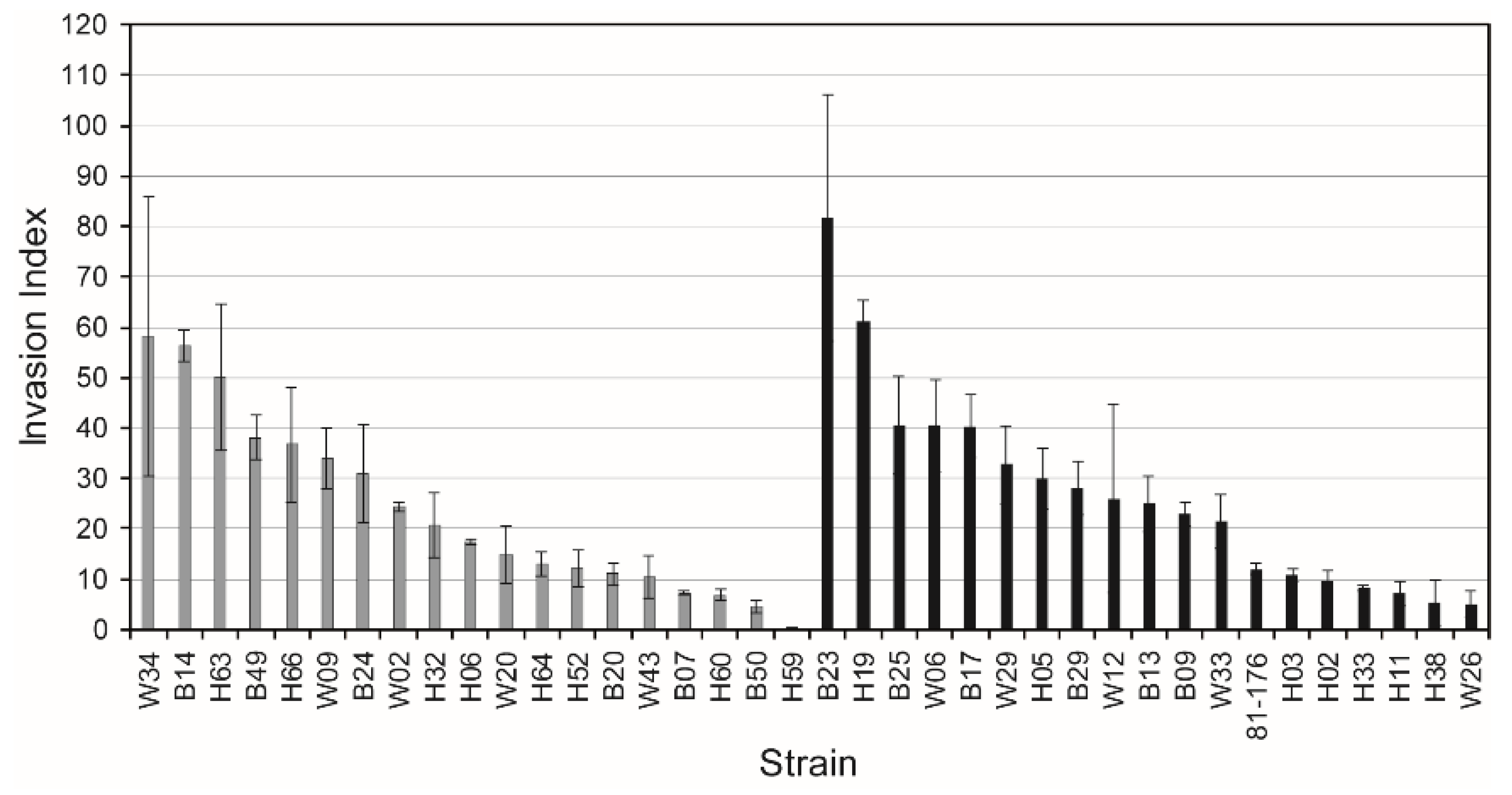
3.5. Sialylation is Not Needed for Invasiveness
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Blaser, M.J.; Engberg, J. Clinical and Epidemiological Aspects of Campylobacter Infections. In Campylobacter, 3rd ed.; Nachamkin, I., Szymanski, C., Eds.; ASM Press: Washington, DC, USA, 2008; pp. 99–121. [Google Scholar]
- EFSA. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017, 15, e05077. [Google Scholar]
- Pires, S.M.; Vigre, H.; Makela, P.; Hald, T. Using outbreak data for source attribution of human salmonellosis and sampylobacteriosis in Europe. Foodborne Pathog. Dis. 2010, 7, 1351–1361. [Google Scholar] [CrossRef]
- French, N.P.; Midwinter, A.; Holland, B.; Collins-Emerson, J.; Pattison, R.; Colles, F.; Carter, P. Molecular epidemiology of Campylobacter jejuni isolates from wild-bird fecal material in children’s playgrounds. Appl. Environ. Microbiol. 2009, 75, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.E.; Corcoran, D.; Dooley, J.S.G.; Fanning, S.; Lucey, B.; Matsuda, M.; Mcdowell, D.A.; Mégraud, F.; Millar, B.C.; O’mahony, R.; et al. Campylobacter. Vet. Res. 2005, 36, 351–382. [Google Scholar] [CrossRef] [PubMed]
- Koga, M.; Gilbert, M.; Takahashi, M.; Li, J.; Koike, S.; Hirata, K.; Yuki, N. Comprehensive analysis of bacterial risk factors for the development of Guillain-Barré syndrome after Campylobacter jejuni enteritis. J. Infect. Dis. 2006, 193, 547–555. [Google Scholar] [CrossRef]
- Ang, C.W.; De Klerk, M.A.; Endtz, H.P.; Jacobs, B.C.; Laman, J.D.; van der Meche, F.G.A.; van Doorn, P.A. Guillain-Barre syndrome- and Miller Fisher syndrome-associated Campylobacter jejuni lipopolysaccharides induce anti-GM1 and anti-GQ1b antibodies in rabbits. Infect. Immun. 2001, 69, 2462–2469. [Google Scholar] [CrossRef] [PubMed]
- Yuki, N. Infectious origins of, and molecular mimicry in, Guillain-Barré and Fisher syndromes. Lancet Infect. Dis. 2001, 1, 29–37. [Google Scholar] [CrossRef]
- Parker, C.T.; Gilbert, M.; Yuki, N.; Endtz, H.P.; Mandrell, R.E. Characterization of lipooligosaccharide-biosynthetic loci of Campylobacter jejuni reveals new lipooligosaccharide classes: Evidence of mosaic organizations. J. Bacteriol. 2008, 190, 5681–5689. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.T.; Horn, S.T.; Gilbert, M.; Miller, W.G.; Woodward, D.L.; Mandrell, R.E. Comparison of Campylobacter jejuni lipooligosaccharide biosynthesis loci from a variety of sources. J. Clin. Microbiol. 2005, 43, 2771–2781. [Google Scholar] [CrossRef]
- Gilbert, M.; Karwaski, M.-F.; Bernatchez, S.; Young, N.M.; Taboada, E.; Michniewicz, J.; Cunningham, A.-M.; Wakarchuk, W.W. The genetic bases for the variation in the lipo-oligosaccharide of the mucosal pathogen, Campylobacter jejuni biosynthesis of sialylated ganglioside mimics in the core oligosaccharide. J. Biol. Chem. 2002, 277, 327–337. [Google Scholar] [CrossRef]
- Linton, D.; Gilbert, M.; Hitchen, P.G.; Dell, A.; Morris, H.R.; Wakarchuk, W.W.; Gregson, N.A.; Wren, B.W. Phase variation of a beta-1,3 galactosyltransferase involved in generation of the ganglioside GM1-like lipo-oligosaccharide of Campylobacter jejuni. Mol. Microbiol. 2000, 37, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Parkhill, J.; Wren, B.W.; Mungall, K.; Ketley, J.M.; Churcher, C.; Basham, D.; Chillingworth, T.; Davies, R.M.; Feltwell, T.; Holroyd, S.; et al. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 2000, 403, 665–668. [Google Scholar] [CrossRef]
- Müller, J.; Meyer, B.; Hänel, I.; Hotzel, H. Comparison of lipooligosaccharide biosynthesis genes of Campylobacter jejuni strains with varying abilities to colonize the chicken gut and to invade Caco-2 cells. J. Med. Microbiol. 2007, 56, 1589–1594. [Google Scholar] [CrossRef][Green Version]
- Iglesias-Torrens, Y.; Miró, E.; Guirado, P.; Llovet, T.; Muñoz, C.; Cerdà-Cuéllar, M.; Madrid, C.; Balsalobre, C.; Navarro, F. Population structure, antimicrobial resistance, and virulence-associated genes in Campylobacter jejuni isolated from three ecological niches: Gastroenteritis patients, broilers, and wild birds. Front. Microbiol. 2018, 9, 1676. [Google Scholar] [CrossRef] [PubMed]
- Koolman, L.; Whyte, P.; Burgess, C.; Bolton, D. Distribution of virulence-associated genes in a selection of Campylobacter isolates. Foodborne Pathog. Dis. 2015, 12, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.W.; Dung, T.T.N.; Siddiqui, F.; Korbrisate, S.; Bukhari, H.; Tra, M.P.V.; Hoang, N.V.M.; Carrique-Mas, J.; Bryant, J.; Campbell, J.I.; et al. Identification of possible virulence marker from Campylobacter jejuni isolates. Emerg. Infect. Dis. 2014, 20, 1026–1029. [Google Scholar] [CrossRef]
- Tsai, C.M.; Frasch, C.E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 1982, 119, 115–119. [Google Scholar] [CrossRef]
- Hänel, I.; Müller, J.; Müller, W.; Schulze, F. Correlation between invasion of Caco-2 eukaryotic cells and colonization ability in the chick gut in Campylobacter jejuni. Vet. Microbiol. 2004, 101, 75–82. [Google Scholar] [CrossRef]
- Taboada, E.N.; Acedillo, R.R.; Carrillo, C.D.; Findlay, W.A.; Medeiros, D.T.; Mykytczuk, O.L.; Roberts, M.J.; Valencia, C.A.; Farber, J.M.; Nash, J.H.E. Large-scale comparative genomics meta-analysis of Campylobacter jejuni isolates reveals low level of genome plasticity. J. Clin. Microbiol. 2004, 42, 4566–4576. [Google Scholar] [CrossRef]
- Gilbert, M.; Brisson, J.R.; Karwaski, M.F.; Michniewicz, J.; Cunningham, A.M.; Wu, Y.; Young, N.M.; Wakarchuk, W.W. Biosynthesis of ganglioside mimics in Campylobacter jejuni OH4384. Identification of the glycosyltransferase genes, enzymatic synthesis of model compounds, and characterization of nanomole amounts by 600-mhz (1)h and (13)c NMR analysis. J. Biol. Chem. 2000, 275, 3896–3906. [Google Scholar] [CrossRef]
- Naito, M.; Frirdich, E.; Fields, J.A.; Pryjma, M.; Li, J.; Cameron, A.; Gilbert, M.; Thompson, S.A.; Gaynor, E.C. Effects of sequential 81-176 lipooligosaccharide core truncations on biofilm formation, stress survival, and pathogenesis. J. Bacteriol. 2010, 192, 2182–2192. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Habib, I.; Louwen, R.; Uyttendaele, M.; Houf, K.; Vandenberg, O.; Nieuwenhuis, E.E.; Miller, W.G.; van Belkum, A.; De Zutter, L. Correlation between genotypic diversity, lipooligosaccharide gene locus class variation, and Caco-2 cell invasion potential of Campylobacter jejuni isolates from chicken meat and humans: Contribution to virulotyping. Appl. Environ. Microbiol. 2009, 75, 4277–4288. [Google Scholar] [CrossRef] [PubMed]
- Ellström, P.; Feodoroff, B.; Hänninen, M.-L.; Rautelin, H. Characterization of clinical Campylobacter jejuni isolates with special emphasis on lipooligosaccharide locus class, putative virulence factors and host response. Int. J. Med. Microbiol. 2013, 303, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H.; Brü Ggemann, H.; Sheppard, S.K.; Ekawa, T.; Meyer, T.F.; Yamamoto, S.; Igimi, S. Molecular Evidence for the thriving of Campylobacter jejuni ST-4526 in Japan. PLoS ONE 2012, 7, e48394. [Google Scholar] [CrossRef]
- Hald, B.; Skov, M.N.; Nielsen, E.M.; Rahbek, C.; Madsen, J.J.; Wainø, M.; Chriél, M.; Nordentoft, S.; Baggesen, D.L.; Madsen, M. Campylobacter jejuni and Campylobacter coli in wild birds on Danish livestock farms. Acta Vet. Scand. 2015, 58, 11. [Google Scholar] [CrossRef]
- Kwan, P.S.L.; Xavier, C.; Santovenia, M.; Pruckler, J.; Stroika, S.; Joyce, K.; Gardner, T.; Fields, P.I.; McLaughlin, J.; Tauxe, R.V.; et al. Multilocus sequence typing confirms wild birds as the source of a Campylobacter outbreak associated with the consumption of raw peas. Appl. Environ. Microbiol. 2014, 80, 4540–4546. [Google Scholar] [CrossRef]
- Datta, S.; Niwa, H.; Itoh, K. Prevalence of 11 pathogenic genes of Campylobacter jejuni by PCR in strains isolated from humans, poultry meat and broiler and bovine faeces. J. Med. Microbiol. 2003, 52, 345–348. [Google Scholar] [CrossRef]
- Ellström, P.; Hansson, I.; Nilsson, A.; Rautelin, H.; Olsson Engvall, E. Lipooligosaccharide locus classes and putative virulence genes among chicken and human Campylobacter jejuni isolates. BMC Microbiol. 2016, 16, 1–6. [Google Scholar] [CrossRef]
- Khoshbakht, R.; Tabatabaei, M.; Hosseinzadeh, S.; Shekarforoush, S.S.; Aski, H.S. Distribution of nine virulence-associated genes in Campylobacter jejuni and C. coli isolated from broiler feces in Shiraz, Southern Iran. Foodborne Pathog. Dis. 2013, 10, 764–770. [Google Scholar] [CrossRef]
- Kordinas, V.; Nicolaou, C.; Ioannidis, A.; Papavasileiou, E.; John Legakis, N.; Chatzipanagiotou, S. Prevalence of four virulence genes in Campylobacter jejuni determined by PCR and sequence analysis. Mol. Diagn. 2005, 9, 211–215. [Google Scholar] [CrossRef]
- Mortensen, N.P.; Kuijf, M.L.; Ang, C.W.; Schiellerup, P.; Krogfelt, K.A.; Jacobs, B.C.; van Belkum, A.; Endtz, H.P.; Bergman, M.P. Sialylation of Campylobacter jejuni lipo-oligosaccharides is associated with severe gastro-enteritis and reactive arthritis. Microbes Infect. 2009, 11, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Kang, M.; Jang, H.-K. Genetic characterization and epidemiological implications of Campylobacter isolates from wild birds in South Korea. Transbound. Emerg. Dis. 2018, 66, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, A.; Sensale, M.; Marzocco, L.; Fioretti, A.; Menna, L.F.; Dipineto, L. Campylobacter jejuni, Campylobacter coli, and cytolethal distending toxin (CDT) genes in common teals (Anas crecca). Vet. Microbiol. 2011, 150, 401–404. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Primer | Sequence 5’–3’ | PCR product (bp) | Reference |
|---|---|---|---|
| cgtB-F | TTAAGAGCAAGATATGAAGGT | 740 | [12] |
| cgtB-R | GCACATAGAGAACGCTACAA | ||
| wlaN-F | TGCTGGGTATACAAAGGTTGTG | 561 | [16] |
| wlaN-R | AGGTCCATTACCGCATACCA | ||
| gltA-F | GCCCAAAGCCCATCAAGCGGA | 141 | [17] |
| gltA-R | GCGCTTTGGGGTCATGCACA |
| Strain | cgtB | wlaN | ST-CC (ST) | Strain | cgtB | wlaN | ST-CC (ST) | Strain | cgtB | wlaN | ST-CC (ST) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| H01 | S (441) | H62 | ST-206 (572) | B45 | ST-354 (354) | ||||||
| H72 | S (441) | H68 | ST-21 (19) | W28 | ST-354 (354) | ||||||
| B44 | S (441) | H06 | 9 | ST-21 (19) | W54 | ST-354 (354) | |||||
| H53 | S (531) | H11 | 9 | ST-21 (21) | W55 | ST-354 (354) | |||||
| B16 | S (531) | H32 | 9 | ST-21 (21) | W56 | ST-354 (354) | |||||
| W02 | 5 | S (996) | H52 | 8 | ST-21 (21) | B37 | ST-354 (8498) | ||||
| W11 | S (1261) | H59 | 8 | ST-21 (21) | H61 | 5 | ST-42 (459) | ||||
| W13 | S (1343) | B20 | 9 | ST-21 (21) | H58 | 6 | ST-42 (4016) | ||||
| W23 | S (1343) | B36 | 8 | ST-21 (21) | H46 | ST-443 (51) | |||||
| H07 | S (1710) | B46 | 8 | ST-21 (21) | H19 | ST-443 (5799) | |||||
| B10 | S (1710) | H12 | ST-21 (50) | W12 | ST-446 (3552) | ||||||
| B26 | S (1710) | H34 | 9 | ST-21 (50) | H48 | ST-45 (45) | |||||
| B35 | S (1710) | H35 | ST-21 (50) | B27 | ST-45 (45) | ||||||
| B02 | S (2331) | H66 | 8 | ST-21 (50) | B31 | ST-45 (45) | |||||
| W17 | 5 | S (2351) | H73 | 9 | ST-21 (50) | W30 | ST-45 (45) | ||||
| W06 | S (4355) | B07 | 8 | ST-21 (50) | W32 | 5 | ST-45 (45) | ||||
| W07 | S (4355) | B14 | 8 | ST-21 (50) | W36 | 5 | ST-45 (45) | ||||
| W08 | S (4355) | B50 | 10/11 | ST-21 (50) | W37 | 5 | ST-45 (45) | ||||
| B39 | S (7114) | H37 | 5 | ST-21 (883) | W40 | 5 | ST-45 (45) | ||||
| H33 | S (8479) | H60 | 5 | ST-21 (883) | W43 | 5 | ST-45 (45) | ||||
| W53 | S (8514) | B06 | 5 | ST-21 (883) | W44 | 5 | ST-45 (45) | ||||
| W05 | ST-1034 (4001) | B17 | ST-21 (883) | B42 | ST-45 (137) | ||||||
| W24 | ST-1275 (637) | H54 | ST-21 (1214) | B22 | ST-45 (652) | ||||||
| W27 | 5 | ST-1275 (637) | H08 | ST-21 (3769) | W47 | 5 | ST-45 (8512) | ||||
| W03 | ST-1275 (1223) | H02 | ST-21 (4664) | H18 | ST-464 (464) | ||||||
| W10 | 5 | ST-1275 (1223) | H03 | ST-257 (257) | B08 | ST-464 (464) | |||||
| W15 | 5 | ST-1275 (1223) | H50 | ST-257 (257) | B15 | ST-464 (464) | |||||
| W18 | 5 | ST-1275 (1223) | H56 | ST-257 (257) | H38 | ST-48 (48) | |||||
| W21 | ST-1275 (1223) | B52 | ST-257 (367) | B09 | ST-48 (48) | ||||||
| W22 | ST-1275 (1268) | B53 | ST-257 (367) | W29 | ST-48 (48) | ||||||
| W04 | ST-1275 (1275) | B54 | ST-257 (367) | H71 | ST-49 (49) | ||||||
| W09 | 5 | ST-1275 (1275) | B55 | ST-257 (367) | H51 | ST-52 (52) | |||||
| W20 | 5 | ST-1275 (1275) | B56 | ST-257 (367) | B21 | ST-574 (305) | |||||
| W14 | ST-1275 (1292) | B57 | ST-257 (367) | B25 | ST-574 (305) | ||||||
| W16 | ST-1275 (1292) | H67 | ST-257 (2254) | B04 | 5 | ST-607 (607) | |||||
| W26 | ST-1275 (3049) | H69 | ST-257 (2254) | B41 | 5 | ST-607 (607) | |||||
| W19 | ST-1275 (3629) | B13 | ST-257 (2254) | H04 | ST-607 (904) | ||||||
| W25 | 5 | ST-1275 (8511) | B33 | ST-283 (267) | B38 | ST-607 (904) | |||||
| W33 | ST-179 (179) | B28 | ST-353 (5) | B47 | ST-607 (904) | ||||||
| W41 | ST-179 (220) | B29 | ST-353 (5) | B24 | 8 | ST-607 (1707) | |||||
| W34 | 5 | ST-179 (2209) | H63 | 9 | ST-353 (353) | B19 | ST-607 (7110) | ||||
| W39 | 5 | ST-179 (2209) | B48 | ST-353 (356) | B51 | ST-607 (7110) | |||||
| W46 | 5 | ST-179 (2209) | H36 | ST-353 (400) | H05 | ST-61 (61) | |||||
| W48 | ST-179 (2209) | B05 | ST-353 (400) | H40 | ST-61 (61) | ||||||
| W49 | 5 | ST-179 (2209) | B18 | ST-353 (400) | H57 | ST-61 (61) | |||||
| B49 | 9 | ST-206 (46) | B30 | ST-353 (400) | H65 | ST-61 (61) | |||||
| H64 | 8 | ST-206 (227) | B40 | ST-353 (400) | H70 | ST-61 (61) | |||||
| H09 | ST-206 (572) | H49 | ST-354 (354) | W50 | ST-952 (8513) | ||||||
| H13 | ST-206 (572) | H74 | ST-354 (354) | W51 | ST-952 (8513) | ||||||
| H14 | ST-206 (572) | B23 | ST-354 (354) | W52 | ST-952 (8513) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guirado, P.; Paytubi, S.; Miró, E.; Iglesias-Torrens, Y.; Navarro, F.; Cerdà-Cuéllar, M.; Stephan-Otto Attolini, C.; Balsalobre, C.; Madrid, C. Differential Distribution of the wlaN and cgtB Genes, Associated with Guillain-Barré Syndrome, in Campylobacter jejuni Isolates from Humans, Broiler Chickens, and Wild Birds. Microorganisms 2020, 8, 325. https://doi.org/10.3390/microorganisms8030325
Guirado P, Paytubi S, Miró E, Iglesias-Torrens Y, Navarro F, Cerdà-Cuéllar M, Stephan-Otto Attolini C, Balsalobre C, Madrid C. Differential Distribution of the wlaN and cgtB Genes, Associated with Guillain-Barré Syndrome, in Campylobacter jejuni Isolates from Humans, Broiler Chickens, and Wild Birds. Microorganisms. 2020; 8(3):325. https://doi.org/10.3390/microorganisms8030325
Chicago/Turabian StyleGuirado, Pedro, Sonia Paytubi, Elisenda Miró, Yaidelis Iglesias-Torrens, Ferran Navarro, Marta Cerdà-Cuéllar, Camille Stephan-Otto Attolini, Carlos Balsalobre, and Cristina Madrid. 2020. "Differential Distribution of the wlaN and cgtB Genes, Associated with Guillain-Barré Syndrome, in Campylobacter jejuni Isolates from Humans, Broiler Chickens, and Wild Birds" Microorganisms 8, no. 3: 325. https://doi.org/10.3390/microorganisms8030325
APA StyleGuirado, P., Paytubi, S., Miró, E., Iglesias-Torrens, Y., Navarro, F., Cerdà-Cuéllar, M., Stephan-Otto Attolini, C., Balsalobre, C., & Madrid, C. (2020). Differential Distribution of the wlaN and cgtB Genes, Associated with Guillain-Barré Syndrome, in Campylobacter jejuni Isolates from Humans, Broiler Chickens, and Wild Birds. Microorganisms, 8(3), 325. https://doi.org/10.3390/microorganisms8030325






