Effects of Light Pollution on the Early Life Stages of the Most Abundant Northern Red Sea Coral
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Planulae Collection
2.3. Experimental Setup
2.4. Settlement, Survivorship, and Growth Rate
2.5. Photosynthesis vs. Light Energy (PE) and PSII Efficiency
2.5.1. PE Curves
2.5.2. Imaging-PAM
2.6. Calcification Rate
2.7. Statistical Analyses
3. Results
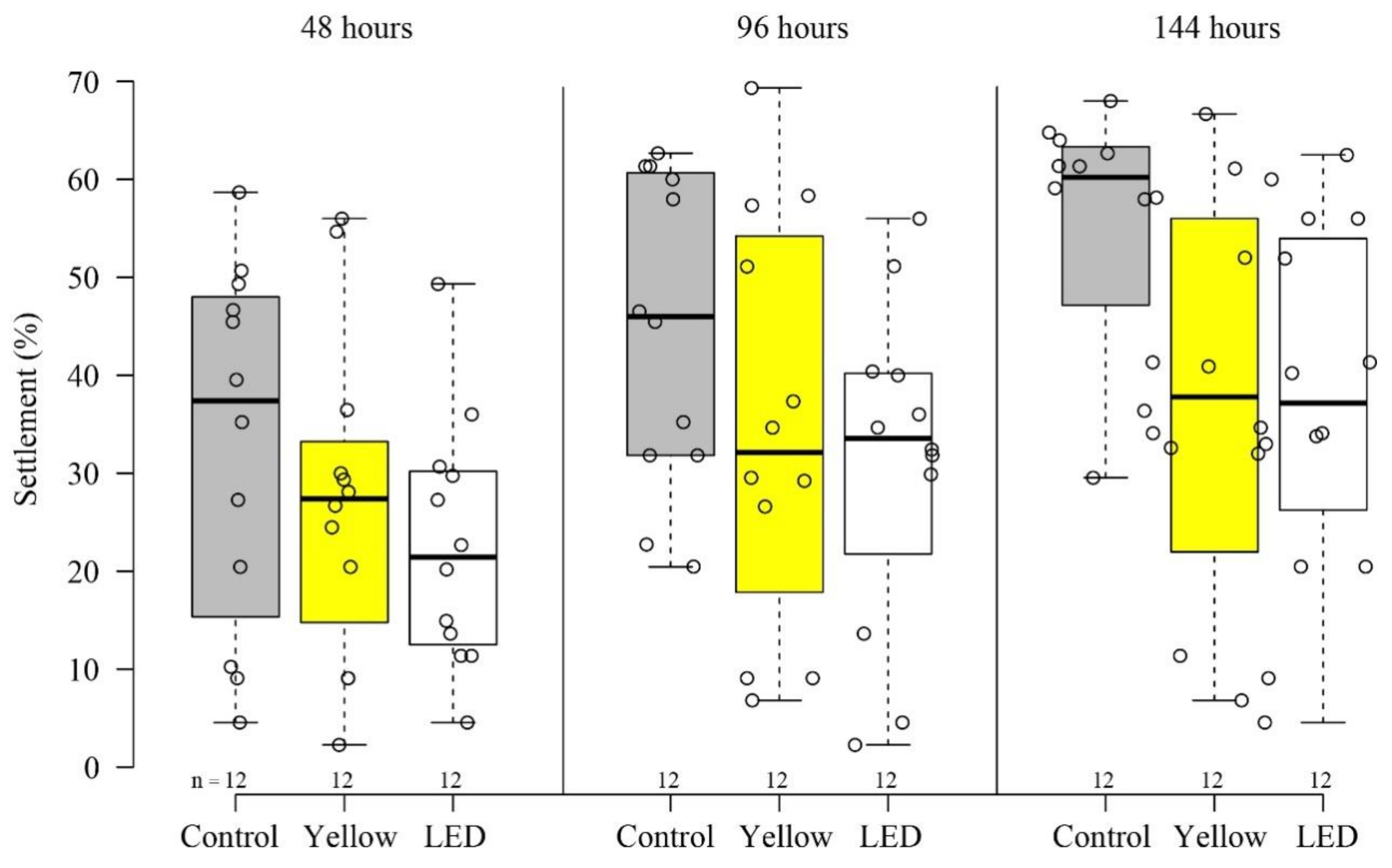
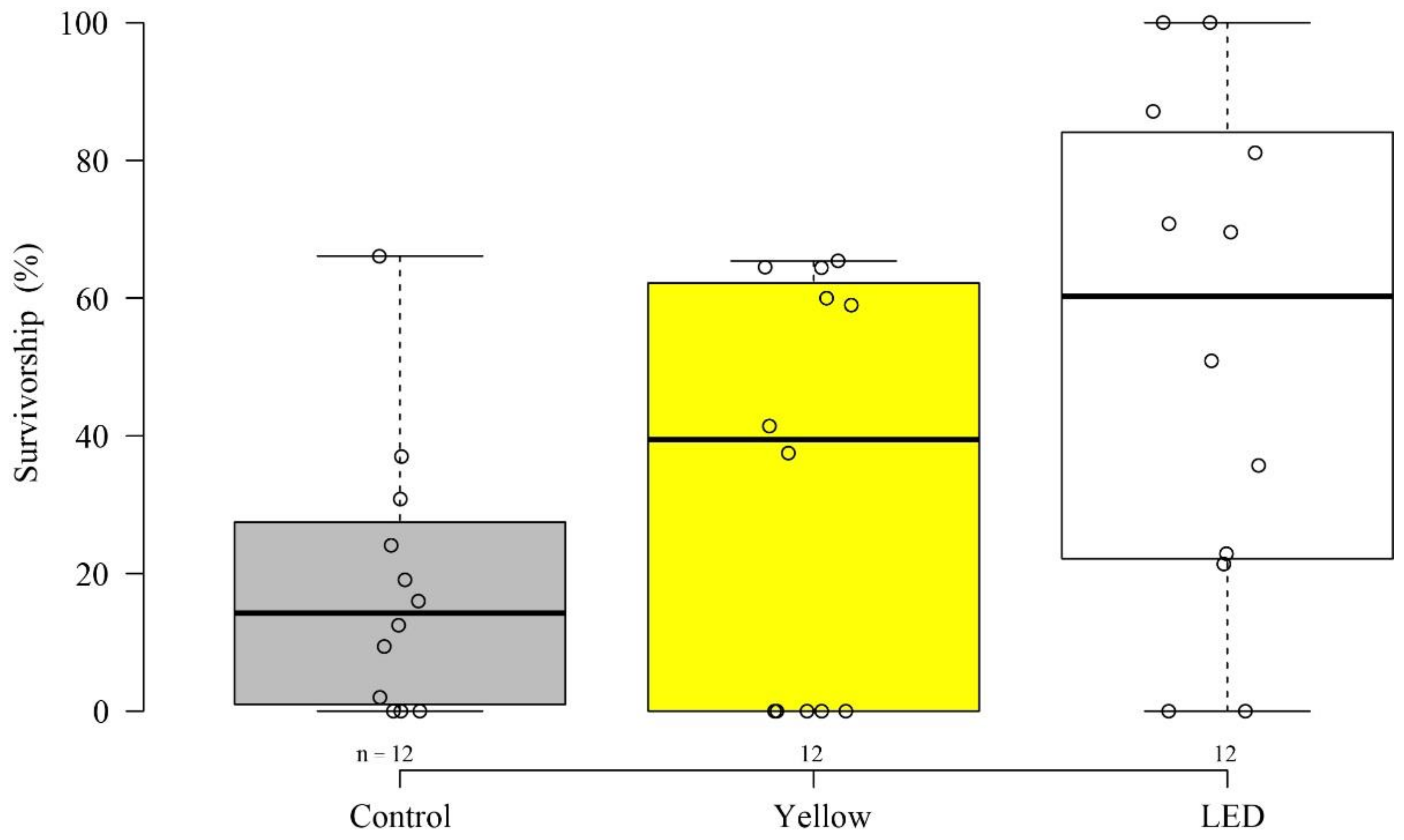
3.1. Settlement and Survivorship
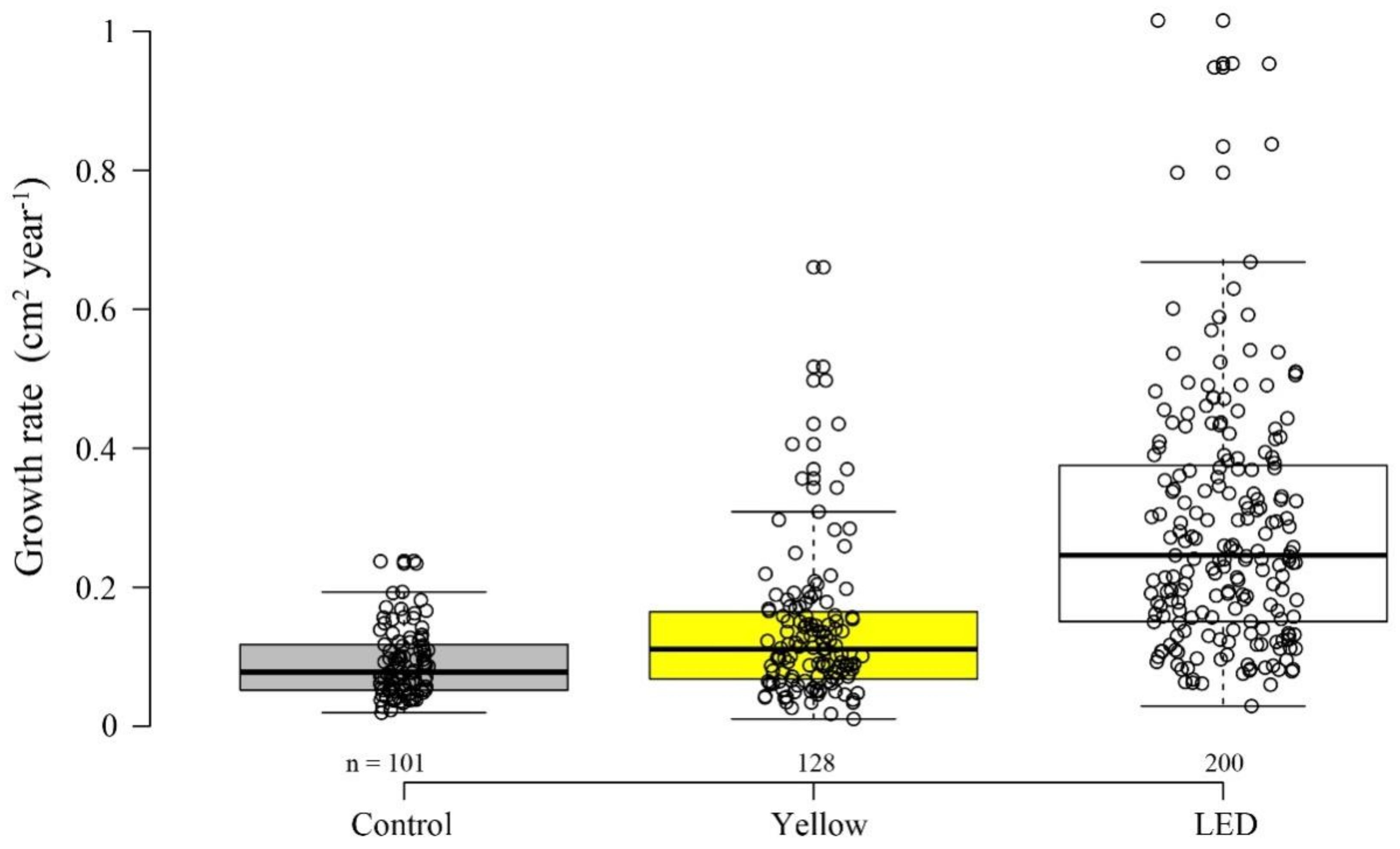
3.2. Growth Rate
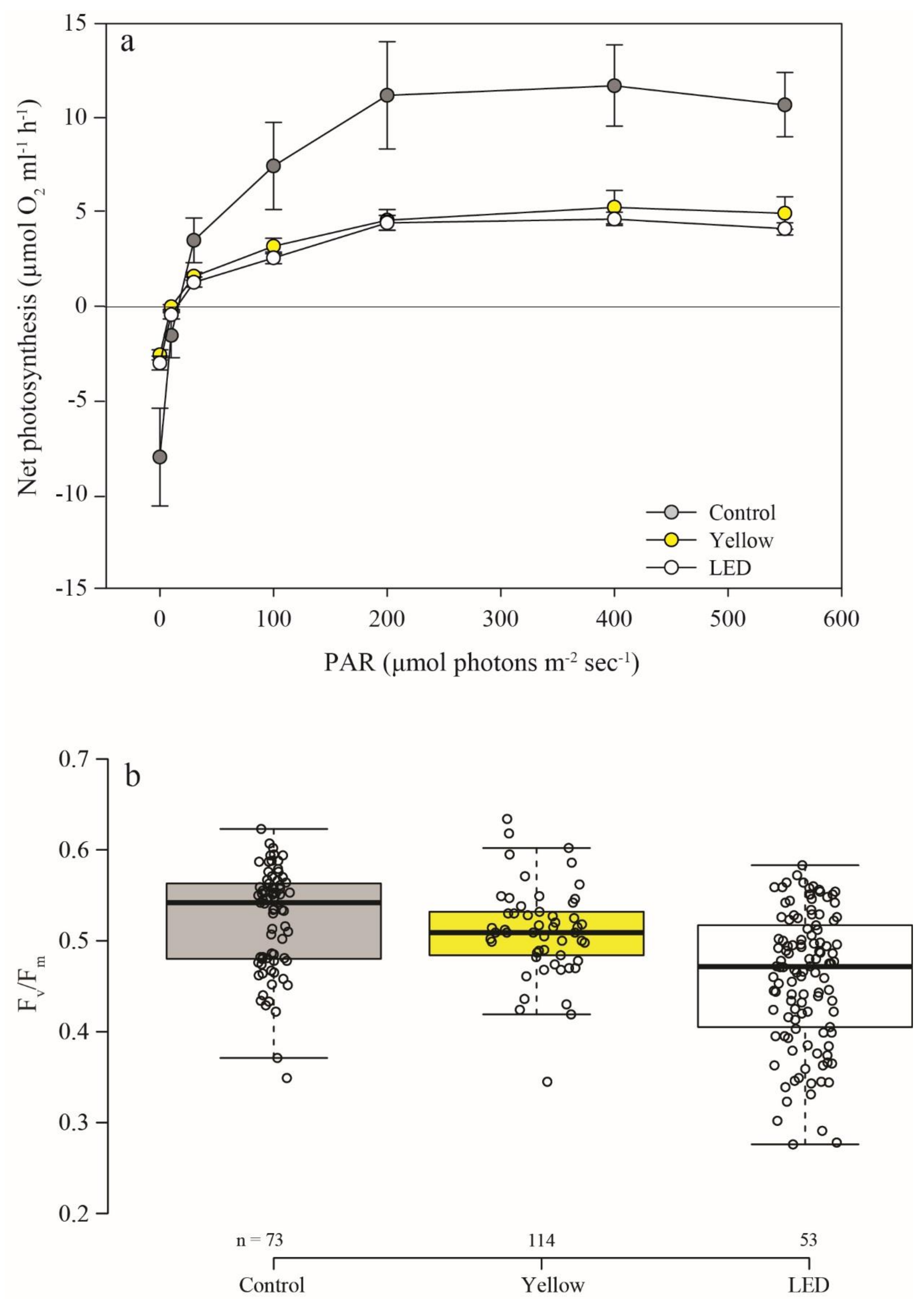
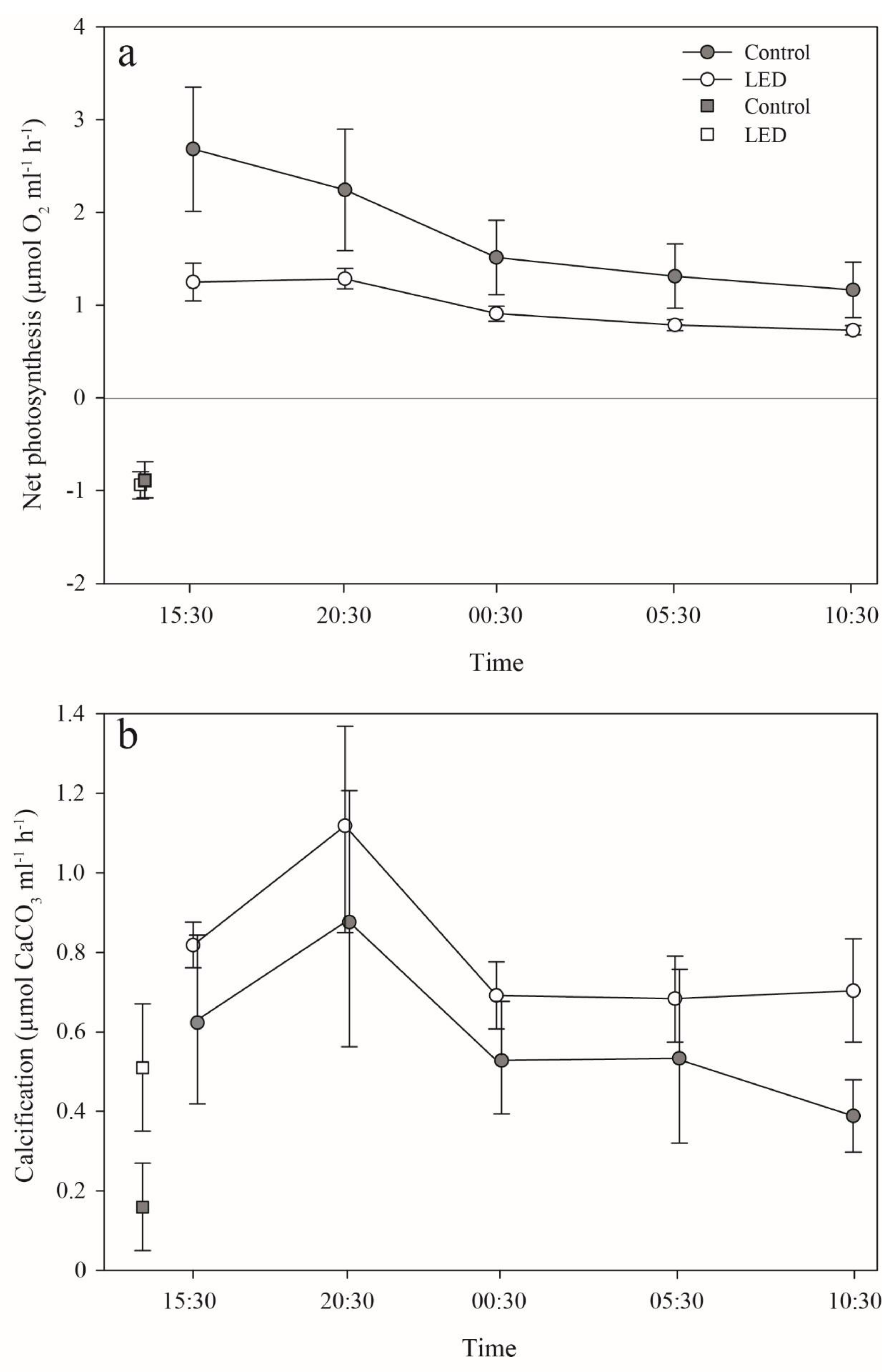
3.3. Photosynthesis and Calcification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loya, Y. The coral reefs of Eilat—past, present and future: Three decades of coral community structure studies. In Coral Health and Disease; Springer: Berlin/Heidelberg, Germany, 2004; pp. 1–34. [Google Scholar]
- Anthony, K.R.; Kline, D.I.; Diaz-Pulido, G.; Dove, S.; Ove, H.-G.O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. USA 2008, 105, 17442–17446. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Aanderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Baird, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Vermeij, M.; Bak, R. How are coral populations structured by light? Marine light regimes and the distribution of Madracis. Mar. Ecol. Prog. Ser. 2002, 233, 105–116. [Google Scholar] [CrossRef]
- Strader, M.E.; Davies, S.W.; Matz, M.V. Differential responses of coral larvae to the colour of ambient light guide them to suitable settlement microhabitat. R. Soc. Open Sci. 2015, 2, 150358. [Google Scholar] [CrossRef]
- Schlacher, T.A.; Stark, J.; Fischer, A.B. Evaluation of artificial light regimes and substrate types for aquaria propagation of the staghorn coral Acropora solitaryensis. Aquaculture 2007, 269, 278–289. [Google Scholar] [CrossRef]
- Gleason, D.F.; Edmunds, P.J.; Gates, R.D. Ultraviolet radiation effects on the behavior and recruitment of larvae from the reef coral Porites astreoides. Mar. Biol. 2006, 148, 503–512. [Google Scholar]
- Mundy, C.; Babcock, R. Role of light intensity and spectral quality in coral settlement: Implications for depth-dependent settlement? J. Exp. Mar. Biol. Ecol. 1998, 223, 235–255. [Google Scholar] [CrossRef]
- Kaniewska, P.; Alon, S.; Karako-Lampert, S.; Hoegh-Guldberg, O.; Levy, O. Signaling cascades and the importance of moonlight in coral broadcast mass spawning. Elife 2015, 4, e09991. [Google Scholar] [CrossRef]
- Brady, A.K.; Willis, B.L.; Harder, L.D.; Vize, P.D. Lunar phase modulates circadian gene expression cycles in the broadcast spawning coral Acropora millepora. Biol. Bull. 2016, 230, 130–142. [Google Scholar] [CrossRef]
- Boch, C.A.; Ananthasubramaniam, B.; Sweeney, A.M.; Doyle Iii, F.J.; Morse, D.E. Effects of light dynamics on coral spawning synchrony. Biol. Bull. 2011, 220, 161–173. [Google Scholar] [CrossRef]
- Levy, O.; Appelbaum, L.; Leggat, W.; Gothlif, Y.; Hayward, D.C.; Miller, D.J.; Hoegh-Guldberg, O. Light-responsive cryptochromes from a simple multicellular animal, the coral Acropora millepora. Science 2007, 318, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Tamir, R.; Lerner, A.; Haspel, C.; Dubinsky, Z.; Iluz, D. The spectral and spatial distribution of light pollution in the waters of the northern Gulf of Aqaba (Eilat). Sci. Rep. 2017, 7, 42329. [Google Scholar] [CrossRef] [PubMed]
- Baird, A.; Marshall, P. Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2002, 237, 133–141. [Google Scholar] [CrossRef]
- Edmunds, P.; Gates, R.; Gleason, D. The biology of larvae from the reef coral Poritesastreoides, and their response to temperature disturbances. Mar. Biol. 2001, 139, 981–989. [Google Scholar] [CrossRef]
- Muscatine, L.; Porter, J.W. Reef corals: Mutualistic symbioses adapted to nutrient-poor environments. Bioscience 1977, 27, 454–460. [Google Scholar] [CrossRef]
- LaJeunesse, T.C.; Parkinson, J.E.; Gabrielson, P.W.; Jeong, H.J.; Reimer, J.D.; Voolstra, C.R.; Santos, S.R. Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr. Biol. 2018, 28, 2570–2580. [Google Scholar] [CrossRef]
- Frade, P.R.; De Jongh, F.; Vermeulen, F.; Van Bleijswijk, J.; Bak, R.P.M. Variation in symbiont distribution between closely related coral species over large depth ranges. Mol. Ecol. 2008, 17, 691–703. [Google Scholar] [CrossRef]
- Byler, K.A.; Carmi-Veal, M.; Fine, M.; Goulet, T.L. Multiple symbiont acquisition strategies as an adaptive mechanism in the coral Stylophora pistillata. PLoS ONE 2013, 8, e5959. [Google Scholar] [CrossRef]
- Rosenberg, Y.; Doniger, T.; Levy, O. Sustainability of coral reefs are affected by ecological light pollution in the Gulf of Aqaba/Eilat. Commun. Biol. 2019, 2, 1–9. [Google Scholar] [CrossRef]
- Aubrecht, C.; Elvidge, C.D.; Longcore, T.; Rich, C.; Safran, J.; Strong, A.E.; Eakin, C.M.; Baugh, K.E.; Tuttle, B.T.; Howard, A.T.; et al. A global inventory of coral reef stressors based on satellite observed nighttime lights. Geocarto Int. 2008, 23, 467–479. [Google Scholar] [CrossRef]
- Davies, T.W.; Duffy, J.P.; Bennie, J.; Gaston, K.J. Stemming the tide of light pollution encroaching into marine protected areas. Conserv. Lett. 2016, 9, 164–171. [Google Scholar] [CrossRef]
- Gattuso, J.-P.; Gentili, B.; Duarte, C.M.; Kleypas, J.A.; Middelburg, J.J.; Antoine, D. Light availability in the coastal ocean: Impact on the distribution of benthic photosynthetic organisms and contribution to primary production. Biogeosciences Discuss. 2006, 3, 895–959. [Google Scholar] [CrossRef]
- Davies, T.W.; Smyth, T. Why artificial light at night should be a focus for global change research in the 21st century. Glob. Chang. Biol. 2018, 24, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Gaston, K.J.; Bennie, J.; Davies, T.W.; Hopkins, J. The ecological impacts of nighttime light pollution: A mechanistic appraisal. Biol. Rev. 2013, 88, 912–927. [Google Scholar] [CrossRef] [PubMed]
- Loya, Y. The Red Sea coral Stylophora pistillata is an r strategist. Nature 1976, 259, 478. [Google Scholar] [CrossRef]
- Kramer, N.; Eyal, G.; Tamir, R.; Loya, Y. Upper mesophotic depths in the coral reefs of Eilat, Red Sea, offer suitable refuge grounds for coral settlement. Sci. Rep. 2019, 9, 2263. [Google Scholar] [CrossRef]
- Winkler, L. Die Bestimmung des im Wasser gelösten Sauerstoffes. Ber. Dtsch. Chem. Ges. 1888, 21, 2843–2854. [Google Scholar] [CrossRef]
- Chalker, B. Simulating light-saturation curves for photosynthesis and calcification by reef-building corals. Mar. Biol. 1981, 63, 135–141. [Google Scholar] [CrossRef]
- Edmond, J.M. High precision determination of titration alkalinity and total carbon dioxide content of sea water by potentiometric titration. Deep Sea Res. Oceanogr. Abstracts. Elsevier 1970, 17, 737–750. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2018. Available online: https://www.R-project.org/ (accessed on 15 December 2019).
- Hervé, M. RVAideMemoire: Testing and Plotting Procedures for Biostatistics. R Package Version 0.9–70. Available online: https://cran.r-project.org/web/packages/RVAideMemoire/RVAideMemoire.pdf (accessed on 15 December 2019).
- Dongwen, L.; Ganesh, S.; Koolaard, J. Predictmeans: Calculate Predicted Means for Linear Models. R Package Version 0.99. 2018. Available online: https://CRAN.R-project.org/package=predictmeans (accessed on 15 December 2019).
- Falchi, F.; Cinzano, P.; Duriscoe, D.; Kyba, C.C.M.; Elvidge, C.D.; Baugh, K.; Portnov, B.A.; Rybnikova, N.A.; Furgoni, R. The new world atlas of artificial night sky brightness. Sci. Adv. 2016, 2, e1600377. [Google Scholar] [CrossRef]
- Bolton, D.; Mayer-Pinto, M.; Clark, G.F.; Dafforn, K.A.; Brassil, W.A.; Becker, A.; Johnston, E.L. Coastal urban lighting has ecological consequences for multiple trophic levels under the sea. Sci. Total Environ. 2017, 576, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Pulido, G.; Harii, S.; McCook, L.J.; Hoegh-Guldberg, O. The impact of benthic algae on the settlement of a reef-building coral. Coral Reefs 2010, 29, 203–208. [Google Scholar] [CrossRef]
- Doherty, P.P. Coral reef fishes: Recruitment-limited assemblages? The Reef and Man. In Proceedings of the 4th International Coral Reef Symposium, Manila, Philippines, 18–22 May 1981; pp. 465–470. [Google Scholar]
- Gaines, S.; Roughgarden, J. Larval settlement rate: A leading determinant of structure in an ecological community of the marine intertidal zone. Proc. Natl. Acad. Sci. USA 1985, 82, 3707–3711. [Google Scholar] [CrossRef] [PubMed]
- Underwood, A.; Fairweather, P. Supply-side ecology and benthic marine assemblages. Trends Ecol. Evol. 1989, 4, 16–20. [Google Scholar] [CrossRef]
- Westneat, M.W.; Resing, J.M. Predation on coral spawn by planktivorous fish. Coral Reefs 1988, 7, 89–92. [Google Scholar] [CrossRef]
- Fisk, D.; Harriott, V. Spatial and temporal variation in coral recruitment on the Great Barrier Reef: Implications for dispersal hypotheses. Mar. Biol. 1990, 107, 485–490. [Google Scholar] [CrossRef]
- Jokiel, P.L.; Ito, R.Y.; Liu, P.M. Night irradiance and synchronization of lunar release of planula larvae in the reef coral Pocillopora damicornis. Mar. Biol. 1985, 88, 167–174. [Google Scholar] [CrossRef]
- Knowlton, N.; Maté, J.L.; Guzmán, H.M.; Rowan, R.; Jara, J. Direct evidence for reproductive isolation among the three species of the Montastraea annularis complex in Central America (Panamá and Honduras). Mar. Biol. 1997, 127, 705–711. [Google Scholar] [CrossRef]
- Davies, T.W.; Duffy, J.P.; Bennie, J.; Gaston, K.J. The nature, extent, and ecological implications of marine light pollution. Front. Ecol. Environ. 2014, 12, 347–355. [Google Scholar] [CrossRef]
- Shlesinger, T.; Loya, Y. Breakdown in spawning synchrony: A silent threat to coral persistence. Science 2019, 365, 1002–1007. [Google Scholar] [CrossRef]
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. B: Biol. Sci. 2014, 369, 20130243. [Google Scholar] [CrossRef] [PubMed]
- Massa, G.D.; Kim, H.-H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to LED lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Parry, M.A.; Keys, A.J.; Madgwick, P.J.; Carmo-Silva, A.E.; Andralojc, P.J. Rubisco regulation: A role for inhibitors. J. Exp. Bot. 2008, 59, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Poulin, C.; Bruyant, F.; Laprise, M.-H.; Cockshutt, A.M.; Vandenhecke, J.M.-R.; Huot, Y. The impact of light pollution on diel changes in the photophysiology of Microcystis aeruginosa. J. Plankton Res. 2013, 36, 286–291. [Google Scholar] [CrossRef]
- Ayalon, I.; De Barros Marangoni, L.F.; Benichou, J.I.; Avisar, D.; Levy, O. Red Sea corals under Artificial Light Pollution at Night (ALAN) undergo oxidative stress and photosynthetic impairment. Glob. Chang. Biol. 2019, 25, 4194–4207. [Google Scholar] [CrossRef]
- Goreau, T.F. The physiology of skeleton formation in corals. I. A method for measuring the rate of calcium deposition by corals under different conditions. Biol. Bull. 1959, 116, 59–75. [Google Scholar] [CrossRef]
- Cohen, I.; Dubinsky, Z.; Erez, J. Light enhanced calcification in hermatypic corals: New insights from light spectral responses. Front. Mar. Sci. 2016, 2, 122. [Google Scholar] [CrossRef]
- Eyal, G.; Cohen, I.; Shaham-Eyal, L.; Ben-Zvi, O.; Tikochinski, Y.; Loya, Y. Photoacclimation and induction of light-enhanced calcification in the mesophotic coral Euphyllia paradivisa. R. Soc. Open Sci. 2019, 6, 180527. [Google Scholar] [CrossRef]
- Gorbunov, M.Y.; Falkowski, P.G. Photoreceptors in the cnidarian hosts allow symbiotic corals to sense blue moonlight. Limnol. Oceanogr. 2002, 47, 309–315. [Google Scholar] [CrossRef]
- Rocha, R.J.; Pimentel, T.; Serôdio, J.; Rosa, R.; Calado, R. Comparative performance of light emitting plasma (LEP) and light emitting diode (LED) in ex situ aquaculture of scleractinian corals. Aquaculture 2013, 402, 38–45. [Google Scholar] [CrossRef]
- Wijgerde, T.; Henkemans, P.; Osinga, R. Effects of irradiance and light spectrum on growth of the scleractinian coral Galaxea fascicularis—Applicability of LEP and LED lighting to coral aquaculture. Aquaculture 2012, 344, 188–193. [Google Scholar] [CrossRef]
- Wijgerde, T.; Van Melis, A.; Silva, C.I.F.; Leal, M.C.; Vogels, L.; Mutter, C.; Osinga, R. Red light represses the photophysiology of the scleractinian coral Stylophora pistillata. PLoS ONE 2014, 9, e92781. [Google Scholar] [CrossRef] [PubMed]
- Bennie, J.; Davies, T.W.; Cruse, D.; Gaston, K.J. Ecological effects of artificial light at night on wild plants. J. Ecol. 2016, 104, 611–620. [Google Scholar] [CrossRef]
- Connell, J.H.; Hughes, T.P.; Wallace, C.C.; Tanner, J.E.; Harms, K.E.; Kerr, A.M. A long-term study of competition and diversity of corals. Ecol. Monogr. 2004, 74, 179–210. [Google Scholar] [CrossRef]
| Treatment | R2 | Slope (α) | SE | Ec | SE | Pmax | SE | Ek | SE |
|---|---|---|---|---|---|---|---|---|---|
| Control | 0.94 | 0.49 | 0.19 | 22.57 | 6.53 | 18.77 | 4.59 | 58.80 | 23.89 |
| Yellow | 0.96 | 0.22 | 0.07 | 13.00 | 0.61 | 8.10 | 0.80 | 38.46 | 7.77 |
| LED | 0.93 | 0.19 | 0.04 | 19.39 | 2.07 | 7.01 | 0.62 | 44.50 | 6.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamir, R.; Eyal, G.; Cohen, I.; Loya, Y. Effects of Light Pollution on the Early Life Stages of the Most Abundant Northern Red Sea Coral. Microorganisms 2020, 8, 193. https://doi.org/10.3390/microorganisms8020193
Tamir R, Eyal G, Cohen I, Loya Y. Effects of Light Pollution on the Early Life Stages of the Most Abundant Northern Red Sea Coral. Microorganisms. 2020; 8(2):193. https://doi.org/10.3390/microorganisms8020193
Chicago/Turabian StyleTamir, Raz, Gal Eyal, Itay Cohen, and Yossi Loya. 2020. "Effects of Light Pollution on the Early Life Stages of the Most Abundant Northern Red Sea Coral" Microorganisms 8, no. 2: 193. https://doi.org/10.3390/microorganisms8020193
APA StyleTamir, R., Eyal, G., Cohen, I., & Loya, Y. (2020). Effects of Light Pollution on the Early Life Stages of the Most Abundant Northern Red Sea Coral. Microorganisms, 8(2), 193. https://doi.org/10.3390/microorganisms8020193






