Candida albicans Antifungal Resistance and Tolerance in Bloodstream Infections: The Triad Yeast-Host-Antifungal
Abstract
1. Introduction
2. Antifungal Drugs: Mechanisms of Action and Resistance
2.1. Polyenes
2.2. Pyrimidine Analogues
2.3. Triazoles
2.4. Echinocandins
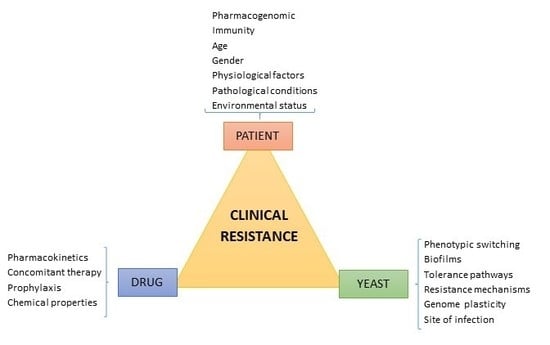
3. Factors Contributing to Candida albicans Clinical Resistance
3.1. The Tolerance Pathways
3.2. Cell Plasticity
3.3. Effect of Concomitant Therapy
4. Strategies to Overcome Antifungal Resistance and Tolerance
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Calderone, R. (Ed.) Candida and Candidiasis; ASM Press: Washington, DC, USA, 2002. [Google Scholar]
- Cannon, R.D.; Holmes, A.R.; Mason, A.B.; Monk, B.C. Oral Candida: Clearance, colonization, or candidiasis? J. Dent. Res. 1995, 74, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Pirofski, L.A. Accidental virulence, cryptic pathogenesis, martians, lost hosts, and the pathogenicity of environmental microbes. Eukaryot. Cell 2007, 6, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- Segal, B.H.; Almyroudis, N.G.; Battiwalla, M.; Herbrecht, R.; Perfect, J.R.; Walsh, T.J.; Wingard, J.R. Prevention and early treatment of invasive fungal infection in patients with cancer and neutropenia and in stem cell transplant recipients in the era of newer broad-spectrum antifungal agents and diagnostic adjuncts. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2007, 44, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Costa-de-Oliveira, S.; Pina-Vaz, C.; Mendonca, D.; Goncalves Rodrigues, A. A first Portuguese epidemiological survey of fungaemia in a university hospital. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2008, 27, 365–374. [Google Scholar] [CrossRef]
- Montagna, M.T.; Lovero, G.; Borghi, E.; Amato, G.; Andreoni, S.; Campion, L.; Lo Cascio, G.; Lombardi, G.; Luzzaro, F.; Manso, E.; et al. Candidemia in intensive care unit: A nationwide prospective observational survey (GISIA-3 study) and review of the European literature from 2000 through 2013. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 661–674. [Google Scholar]
- Quindos, G. Epidemiology of candidaemia and invasive candidiasis. A changing face. Rev. Iberoam. Micol. 2014, 31, 42–48. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Karabinis, A.; Samonis, G.; Falagas, M.E. Candidemia in immunocompromised and immunocompetent critically ill patients: A prospective comparative study. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2007, 26, 377–384. [Google Scholar] [CrossRef]
- Blumberg, H.M.; Jarvis, W.R.; Soucie, J.M.; Edwards, J.E.; Patterson, J.E.; Pfaller, M.A.; Rangel-Frausto, M.S.; Rinaldi, M.G.; Saiman, L.; Wiblin, R.T.; et al. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: The NEMIS prospective multicenter study. The National Epidemiology of Mycosis Survey. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2001, 33, 177–186. [Google Scholar] [CrossRef]
- Nolla-Salas, J.; Sitges-Serra, A.; Leon-Gil, C.; Martinez-Gonzalez, J.; Leon-Regidor, M.A.; Ibanez-Lucia, P.; Torres-Rodriguez, J.M. Candidemia in non-neutropenic critically ill patients: Analysis of prognostic factors and assessment of systemic antifungal therapy. Study Group of Fungal Infection in the ICU. Intensive Care Med. 1997, 23, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Garey, K.W.; Rege, M.; Pai, M.P.; Mingo, D.E.; Suda, K.J.; Turpin, R.S.; Bearden, D.T. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: A multi-institutional study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2006, 43, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.; Benjamin, D.K., Jr.; Calandra, T.F.; Edwards, J.E., Jr.; Filler, S.G.; Fisher, J.F.; Kullberg, B.J.; Ostrosky-Zeichner, L.; et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2009, 48, 503–535. [Google Scholar] [CrossRef]
- Park, S.; Kelly, R.; Kahn, J.N.; Robles, J.; Hsu, M.J.; Register, E.; Li, W.; Vyas, V.; Fan, H.; Abruzzo, G.; et al. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 2005, 49, 3264–3273. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Lewis, R.E. The echinocandin antifungals: An overview of the pharmacology, spectrum and clinical efficacy. Expert Opin. Investig. Drugs 2003, 12, 1313–1333. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Hospenthal, D.R.; Murray, C.K.; Rinaldi, M.G. The role of antifungal susceptibility testing in the therapy of candidiasis. Diagn. Microbiol. Infect. Dis. 2004, 48, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Costa-de-Oliveira, S.; Araujo, R.; Silva-Dias, A.; Pina-Vaz, C.; Rodrigues, A.G. Propofol lipidic infusion promotes resistance to antifungals by reducing drug input into the fungal cell. BMC Microbiol. 2008, 8, 9. [Google Scholar] [CrossRef]
- Rodrigues, A.G.; Araujo, R.; Pina-Vaz, C. Human albumin promotes germination, hyphal growth and antifungal resistance by Aspergillus fumigatus. Med. Mycol. 2005, 43, 711–717. [Google Scholar] [CrossRef]
- Pedrosa, A.F.; Rodrigues, A.G. Candidemia in burn patients: Figures and facts. J. Trauma Acute Care Surg. 2011, 70, 498–506. [Google Scholar] [CrossRef]
- Brion, L.P.; Uko, S.E.; Goldman, D.L. Risk of resistance associated with fluconazole prophylaxis: Systematic review. J. Infect. 2007, 54, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Kanafani, Z.A.; Perfect, J.R. Antimicrobial resistance: Resistance to antifungal agents: Mechanisms and clinical impact. Clin. Infect. Dis. 2008, 46, 120–128. [Google Scholar] [CrossRef]
- Peman, J.; Canton, E.; Espinel-Ingroff, A. Antifungal drug resistance mechanisms. Expert Rev. Anti-Infect. Ther. 2009, 7, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, P. Management of invasive fungal infections: A role for polyenes. J. Antimicrob. Chemother. 2011, 66, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.; Isaacs, D.; Halliday, R.; Australasian Study Group For Neonatal Infections. Oral nystatin prophylaxis and neonatal fungal infections. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F429–F433. [Google Scholar] [CrossRef] [PubMed]
- Gotzsche, P.C.; Johansen, H.K. Nystatin prophylaxis and treatment in severely immunodepressed patients. Cochrane Database Syst. Rev. 2002. [Google Scholar] [CrossRef]
- Lacerda, J.F.; Oliveira, C.M. Diagnosis and treatment of invasive fungal infections focus on liposomal amphotericin B. Clin. Drug Investig. 2013, 33, S5–S14. [Google Scholar] [CrossRef]
- Wingard, J.R.; White, M.H.; Anaissie, E.; Raffalli, J.; Goodman, J.; Arrieta, A. A randomized, double-blind comparative trial evaluating the safety of liposomal amphotericin B versus amphotericin B lipid complex in the empirical treatment of febrile neutropenia. L Amph/ABLC Collaborative Study Group. Clin. Infect. Dis. 2000, 31, 1155–1163. [Google Scholar] [CrossRef]
- Kelly, S.L.; Lamb, D.C.; Kelly, D.E.; Manning, N.J.; Loeffler, J.; Hebart, H.; Schumacher, U.; Einsele, H. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol delta5,6-desaturation. FEBS Lett. 1997, 400, 80–82. [Google Scholar] [CrossRef]
- Sokol-Anderson, M.L.; Brajtburg, J.; Medoff, G. Amphotericin B-induced oxidative damage and killing of Candida albicans. J. Infect. Dis. 1986, 154, 76–83. [Google Scholar] [CrossRef]
- Castanheira, M.; Deshpande, L.M.; Davis, A.P.; Rhomberg, P.R.; Pfaller, M.A. Monitoring Antifungal Resistance in a Global Collection of Invasive Yeasts and Molds: Application of CLSI Epidemiological Cutoff Values and Whole-Genome Sequencing Analysis for Detection of Azole Resistance in Candida albicans. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C. Update on antifungal resistance in Aspergillus and Candida. Clin. Microbiol. Infect. 2014, 20, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Faria-Ramos, I.; Neves-Maia, J.; Ricardo, E.; Santos-Antunes, J.; Silva, A.T.; Costa-de-Oliveira, S.; Canton, E.; Rodrigues, A.G.; Pina-Vaz, C. Species distribution and in vitro antifungal susceptibility profiles of yeast isolates from invasive infections during a Portuguese multicenter survey. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Akins, R.A. An update on antifungal targets and mechanisms of resistance in Candida albicans. Med. Mycol. 2005, 43, 285–318. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Madison, V.; Chau, A.S.; Loebenberg, D.; Palermo, R.E.; McNicholas, P.M. Three-dimensional models of wild-type and mutated forms of cytochrome P450 14alpha-sterol demethylases from Aspergillus fumigatus and Candida albicans provide insights into posaconazole binding. Antimicrob. Agents Chemother. 2004, 48, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C.; Brown, A.J.; Gow, N.A. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Meyer, E.; Schwab, F.; Gastmeier, P.; Ruden, H.; Heininger, A. Antifungal use in intensive care units. J. Antimicrob. Chemother. 2007, 60, 619–624. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Diekema, D.J.; Fothergill, A.; Johnson, E.; Pelaez, T.; Pfaller, M.A.; Rinaldi, M.G.; Canton, E.; Turnidge, J. Wild-type MIC distributions and epidemiological cutoff values for the triazoles and six Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). J. Clin. Microbiol. 2010, 48, 3251–3257. [Google Scholar] [CrossRef]
- Nagappan, V.; Deresinski, S. Reviews of anti-infective agents: Posaconazole: A broad-spectrum triazole antifungal agent. Clin. Infect. Dis. 2007, 45, 1610–1617. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Messer, S.A.; Rhomberg, P.R.; Jones, R.N.; Castanheira, M. In vitro activities of isavuconazole and comparator antifungal agents tested against a global collection of opportunistic yeasts and molds. J. Clin. Microbiol. 2013, 51, 2608–2616. [Google Scholar] [CrossRef]
- Seyedmousavi, S.; Verweij, P.E.; Mouton, J.W. Isavuconazole, a broad-spectrum triazole for the treatment of systemic fungal diseases. Expert Rev. Anti-Infect. Ther. 2015, 13, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.R., 3rd; Wiederhold, N.P.; Sutton, D.A.; Fothergill, A.; Patterson, T.F. In vitro activity of isavuconazole against Trichosporon, Rhodotorula, Geotrichum, Saccharomyces and Pichia species. J. Antimicrob. Chemother. 2009, 64, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Florl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24, e1–e38. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.R.; Lin, Y.H.; Niimi, K.; Lamping, E.; Keniya, M.; Niimi, M.; Tanabe, K.; Monk, B.C.; Cannon, R.D. ABC transporter Cdr1p contributes more than Cdr2p does to fluconazole efflux in fluconazole-resistant Candida albicans clinical isolates. Antimicrob. Agents Chemother. 2008, 52, 3851–3862. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.D.; Barker, K.S. Genome-wide expression profile analysis reveals coordinately regulated genes associated with stepwise acquisition of azole resistance in Candida albicans clinical isolates. Antimicrob. Agents Chemother. 2003, 47, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Tudela, J.L.; Alcazar-Fuoli, L.; Cuesta, I.; Alastruey-Izquierdo, A.; Monzon, A.; Mellado, E.; Cuenca-Estrella, M. Clinical relevance of resistance to antifungals. Int. J. Antimicrob. Agents 2008, 32, S111–S113. [Google Scholar] [CrossRef]
- Clark, T.A.; Hajjeh, R.A. Recent trends in the epidemiology of invasive mycoses. Curr. Opin. Infect. Dis. 2002, 15, 569–574. [Google Scholar] [CrossRef]
- Ricardo, E.; Silva, A.P.; Goncalves, T.; Costa de Oliveira, S.; Granato, C.; Martins, J.; Rodrigues, A.G.; Pina-Vaz, C. Candida krusei reservoir in a neutropaenia unit: Molecular evidence of a foe? Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2011, 17, 259–263. [Google Scholar] [CrossRef]
- Cole, G.T.; Halawa, A.A.; Anaissie, E.J. The role of the gastrointestinal tract in hematogenous candidiasis: From the laboratory to the bedside. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1996, 22, S73–S88. [Google Scholar] [CrossRef]
- Sanglard, D.; Ischer, F.; Calabrese, D.; Majcherczyk, P.A.; Bille, J. The ATP binding cassette transporter gene CgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob. Agents Chemother. 1999, 43, 2753–2765. [Google Scholar] [CrossRef]
- Cowen, L.E. The evolution of fungal drug resistance: Modulating the trajectory from genotype to phenotype. Nat. Rev. Microbiol. 2008, 6, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, D.; Coste, A.; Ferrari, S. Antifungal drug resistance mechanisms in fungal pathogens from the perspective of transcriptional gene regulation. FEMS Yeast Res. 2009, 9, 1029–1050. [Google Scholar] [CrossRef] [PubMed]
- Borst, P.; Evers, R.; Kool, M.; Wijnholds, J. A family of drug transporters: The multidrug resistance-associated proteins. J. Natl. Cancer Inst. 2000, 92, 1295–1302. [Google Scholar] [CrossRef]
- Morschhauser, J. Regulation of multidrug resistance in pathogenic fungi. Fungal Genet. Biol. 2010, 47, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Baret, P.V.; Keniya, M.V.; Tanabe, K.; Niimi, M.; Goffeau, A.; Monk, B.C. Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 2009, 22, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Franz, R.; Kelly, S.L.; Lamb, D.C.; Kelly, D.E.; Ruhnke, M.; Morschhauser, J. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob. Agents Chemother. 1998, 42, 3065–3072. [Google Scholar] [CrossRef] [PubMed]
- Perea, S.; Lopez-Ribot, J.L.; Kirkpatrick, W.R.; McAtee, R.K.; Santillan, R.A.; Martinez, M.; Calabrese, D.; Sanglard, D.; Patterson, T.F. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 2001, 45, 2676–2684. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, E.; Costa-de-Oliveira, S.; Dias, A.S.; Guerra, J.; Rodrigues, A.G.; Pina-Vaz, C. Ibuprofen reverts antifungal resistance on Candida albicans showing overexpression of CDR genes. FEMS Yeast Res. 2009, 9, 618–625. [Google Scholar] [CrossRef]
- Prasad, R.; Banerjee, A.; Khandelwal, N.K.; Dhamgaye, S. The ABCs of Candida albicans Multidrug Transporter Cdr1. Eukaryot. Cell 2015, 14, 1154–1164. [Google Scholar] [CrossRef]
- Coste, A.T.; Karababa, M.; Ischer, F.; Bille, J.; Sanglard, D. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot. Cell 2004, 3, 1639–1652. [Google Scholar] [CrossRef]
- Coste, A.; Turner, V.; Ischer, F.; Morschhauser, J.; Forche, A.; Selmecki, A.; Berman, J.; Bille, J.; Sanglard, D. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics 2006, 172, 2139–2156. [Google Scholar] [CrossRef] [PubMed]
- Selmecki, A.; Forche, A.; Berman, J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science 2006, 313, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Lephart, P.; Forche, A.; Mueller, F.M.; Walsh, T.; Magee, P.T.; Magee, B.B. Homozygosity at the MTL locus in clinical strains of Candida albicans: Karyotypic rearrangements and tetraploid formation. Mol. Microbiol. 2004, 52, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.M.; Johnson, A.D. Identification of a mating type-like locus in the asexual pathogenic yeast Candida albicans. Science 1999, 285, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.; Selmecki, A.; Forche, A.; Diogo, D.; Bougnoux, M.E.; D’ENFERT, C.; Berman, J.; Sanglard, D. Genotypic evolution of azole resistance mechanisms in sequential Candida albicans isolates. Eukaryot. Cell 2007, 6, 1889–1904. [Google Scholar] [CrossRef]
- Rustad, T.R.; Stevens, D.A.; Pfaller, M.A.; White, T.C. Homozygosity at the Candida albicans MTL locus associated with azole resistance. Microbiology 2002, 148, 1061–1072. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Pujol, C.; Daniels, K.J.; Miller, M.G.; Johnson, A.D.; Pfaller, M.A.; Soll, D.R. In Candida albicans, white-opaque switchers are homozygous for mating type. Genetics 2002, 162, 737–745. [Google Scholar]
- Pujol, C.; Messer, S.A.; Pfaller, M.; Soll, D.R. Drug resistance is not directly affected by mating type locus zygosity in Candida albicans. Antimicrob. Agents Chemother. 2003, 47, 1207–1212. [Google Scholar] [CrossRef][Green Version]
- White, T.C.; Holleman, S.; Dy, F.; Mirels, L.F.; Stevens, D.A. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob. Agents Chemother. 2002, 46, 1704–1713. [Google Scholar] [CrossRef]
- Morschhauser, J.; Barker, K.S.; Liu, T.T.; Bla, B.W.J.; Homayouni, R.; Rogers, P.D. The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans. PLoS Pathog. 2007, 3, e164. [Google Scholar] [CrossRef]
- Dunkel, N.; Blass, J.; Rogers, P.D.; Morschhauser, J. Mutations in the multi-drug resistance regulator MRR1, followed by loss of heterozygosity, are the main cause of MDR1 overexpression in fluconazole-resistant Candida albicans strains. Mol. Microbiol. 2008, 69, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, D.; Ischer, F.; Koymans, L.; Bille, J. Amino acid substitutions in the cytochrome P-450 lanosterol 14alpha-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob. Agents Chemother. 1998, 42, 241–253. [Google Scholar] [PubMed]
- Sanglard, D.; Ischer, F.; Parkinson, T.; Falconer, D.; Bille, J. Candida albicans mutations in the ergosterol biosynthetic pathway and resistance to several antifungal agents. Antimicrob. Agents Chemother. 2003, 47, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Lopez-Ribot, J.L.; Kirkpatrick, W.R.; Bachmann, S.P.; Perea, S.; Ruesga, M.T.; Patterson, T.F. Heterogeneous mechanisms of azole resistance in Candida albicans clinical isolates from an HIV-infected patient on continuous fluconazole therapy for oropharyngeal candidosis. J. Antimicrob. Chemother. 2002, 49, 515–524. [Google Scholar] [CrossRef]
- Selmecki, A.; Gerami-Nejad, M.; Paulson, C.; Forche, A.; Berman, J. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol. Microbiol. 2008, 68, 624–641. [Google Scholar] [CrossRef]
- Silver, P.M.; Oliver, B.G.; White, T.C. Role of Candida albicans transcription factor Upc2p in drug resistance and sterol metabolism. Eukaryot. Cell 2004, 3, 1391–1397. [Google Scholar] [CrossRef]
- Dunkel, N.; Liu, T.T.; Barker, K.S.; Homayouni, R.; Morschhauser, J.; Rogers, P.D. A gain-of-function mutation in the transcription factor Upc2p causes upregulation of ergosterol biosynthesis genes and increased fluconazole resistance in a clinical Candida albicans isolate. Eukaryot. Cell 2008, 7, 1180–1190. [Google Scholar] [CrossRef]
- Heilmann, C.J.; Schneider, S.; Barker, K.S.; Rogers, P.D.; Morschhauser, J. An A643T mutation in the transcription factor Upc2p causes constitutive ERG11 upregulation and increased fluconazole resistance in Candida albicans. Antimicrob. Agents Chemother. 2010, 54, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Oliver, B.G.; Song, J.L.; Choiniere, J.H.; White, T.C. cis-Acting elements within the Candida albicans ERG11 promoter mediate the azole response through transcription factor Upc2p. Eukaryot. Cell 2007, 6, 2231–2239. [Google Scholar] [CrossRef][Green Version]
- Hoot, S.J.; Smith, A.R.; Brown, R.P.; White, T.C. An A643V amino acid substitution in Upc2p contributes to azole resistance in well-characterized clinical isolates of Candida albicans. Antimicrob. Agents Chemother. 2011, 55, 940–942. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.K.; Rogers, P.D.; Baerson, S.R.; Jacob, M.R.; Barker, K.S.; Cleary, J.D.; Walker, L.A.; Nagle, D.G.; Clark, A.M. Genome-wide expression profiling of the response to polyene, pyrimidine, azole, and echinocandin antifungal agents in Saccharomyces cerevisiae. J. Biol. Chem. 2003, 278, 34998–35015. [Google Scholar] [CrossRef]
- Denning, D.W. Echinocandin antifungal drugs. Lancet 2003, 362, 1142–1151. [Google Scholar] [CrossRef]
- Perlin, D.S. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updates 2007, 10, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Effron, G.; Park, S.; Perlin, D.S. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: Implications for interpretive breakpoints. Antimicrob. Agents Chemother. 2009, 53, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.; Pfaller, M.; Edlind, T. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 2006, 50, 2892–2894. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Effron, G.; Chua, D.J.; Tomada, J.R.; DiPersio, J.; Perlin, D.S.; Ghannoum, M.; Bonilla, H. Novel FKS mutations associated with echinocandin resistance in Candida species. Antimicrob. Agents Chemother. 2010, 54, 2225–2227. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species from 1997–2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef]
- Kritikos, A.; Neofytos, D.; Khanna, N.; Schreiber, P.W.; Boggian, K.; Bille, J.; Schrenzel, J.; Muhlethaler, K.; Zbinden, R.; Bruderer, T.; et al. Accuracy of Sensititre YeastOne echinocandins epidemiological cut-off values for identification of FKS mutant Candida albicans and Candida glabrata: A ten year national survey of the Fungal Infection Network of Switzerland (FUNGINOS). Clin. Microbiol. Infect. 2018, 24, e1211–e1214. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Effron, G.; Lee, S.; Park, S.; Cleary, J.D.; Perlin, D.S. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-beta-D-glucan synthase: Implication for the existing susceptibility breakpoint. Antimicrob. Agents Chemother. 2009, 53, 3690–3699. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Najvar, L.K.; Bocanegra, R.; Molina, D.; Olivo, M.; Graybill, J.R. In vivo efficacy of anidulafungin and caspofungin against Candida glabrata and association with in vitro potency in the presence of sera. Antimicrob. Agents Chemother. 2007, 51, 1616–1620. [Google Scholar] [CrossRef]
- Paderu, P.; Garcia-Effron, G.; Balashov, S.; Delmas, G.; Park, S.; Perlin, D.S. Serum differentially alters the antifungal properties of echinocandin drugs. Antimicrob. Agents Chemother. 2007, 51, 2253–2256. [Google Scholar] [CrossRef] [PubMed]
- Delarze, E.; Sanglard, D. Defining the frontiers between antifungal resistance, tolerance and the concept of persistence. Drug Resist. Updates 2015, 23, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Meletiadis, J.; Chanock, S.; Walsh, T.J. Human pharmacogenomic variations and their implications for antifungal efficacy. Clin. Microbiol. Rev. 2006, 19, 763–787. [Google Scholar] [CrossRef] [PubMed]
- Cowen, L.E.; Steinbach, W.J. Stress, drugs, and evolution: The role of cellular signaling in fungal drug resistance. Eukaryot. Cell 2008, 7, 747–764. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Akula, I.; Edlind, T. Cyclic AMP signaling pathway modulates susceptibility of candida species and Saccharomyces cerevisiae to antifungal azoles and other sterol biosynthesis inhibitors. Antimicrob. Agents Chemother. 2003, 47, 3195–3201. [Google Scholar] [CrossRef] [PubMed]
- Maidan, M.M.; De Rop, L.; Serneels, J.; Exler, S.; Rupp, S.; Tournu, H.; Thevelein, J.M.; Van Dijck, P. The G protein-coupled receptor Gpr1 and the Galpha protein Gpa2 act through the cAMP-protein kinase A pathway to induce morphogenesis in Candida albicans. Mol. Biol. Cell 2005, 16, 1971–1986. [Google Scholar] [CrossRef]
- Juvvadi, P.R.; Lee, S.C.; Heitman, J.; Steinbach, W.J. Calcineurin in fungal virulence and drug resistance: Prospects for harnessing targeted inhibition of calcineurin for an antifungal therapeutic approach. Virulence 2017, 8, 186–197. [Google Scholar] [CrossRef]
- Onyewu, C.; Wormley, F.L., Jr.; Perfect, J.R.; Heitman, J. The calcineurin target, Crz1, functions in azole tolerance but is not required for virulence of Candida albicans. Infect. Immun. 2004, 72, 7330–7333. [Google Scholar] [CrossRef]
- Sanglard, D.; Ischer, F.; Marchetti, O.; Entenza, J.; Bille, J. Calcineurin A of Candida albicans: Involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 2003, 48, 959–976. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Tang, R.J.; Wang, L.; Zhang, X.; Wang, Y.; Jia, X.M.; Jiang, Y.Y. Calcium-activated-calcineurin reduces the In vitro and In vivo sensitivity of fluconazole to Candida albicans via Rta2p. PLoS ONE 2012, 7, e48369. [Google Scholar] [CrossRef]
- Singh, S.D.; Robbins, N.; Zaas, A.K.; Schell, W.A.; Perfect, J.R.; Cowen, L.E. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog. 2009, 5, e1000532. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.A.; Munro, C.A.; de Bruijn, I.; Lenardon, M.D.; McKinnon, A.; Gow, N.A. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 2008, 4, e1000040. [Google Scholar] [CrossRef] [PubMed]
- Munro, C.A.; Selvaggini, S.; de Bruijn, I.; Walker, L.; Lenardon, M.D.; Gerssen, B.; Milne, S.; Brown, A.J.; Gow, N.A. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol. Microbiol. 2007, 63, 1399–1413. [Google Scholar] [CrossRef] [PubMed]
- Lenardon, M.D.; Munro, C.A.; Gow, N.A. Chitin synthesis and fungal pathogenesis. Curr. Opin. Microbiol. 2010, 13, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.A.; Ichinomiya, M.; Koshi, Y.; Horiuchi, H. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for beta-1,6-glucan synthesis inhibition by caspofungin. Antimicrob. Agents Chemother. 2006, 50, 3160–3161. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, M.D.; Whyte, J.A.; Odds, F.C. Candida albicans and Candida dubliniensis respond differently to echinocandin antifungal agents in vitro. Antimicrob. Agents Chemother. 2007, 51, 1882–1884. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fleischhacker, M.; Radecke, C.; Schulz, B.; Ruhnke, M. Paradoxical growth effects of the echinocandins caspofungin and micafungin, but not of anidulafungin, on clinical isolates of Candida albicans and C. dubliniensis. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.A.; Gow, N.A.; Munro, C.A. Elevated chitin content reduces the susceptibility of Candida species to caspofungin. Antimicrob. Agents Chemother. 2013, 57, 146–154. [Google Scholar] [CrossRef]
- Costa-de-Oliveira, S.; Silva, A.P.; Miranda, I.M.; Salvador, A.; Azevedo, M.M.; Munro, C.A.; Rodrigues, A.G.; Pina-Vaz, C. Determination of chitin content in fungal cell wall: An alternative flow cytometric method. Cytom. Part 2013, 83, 324–328. [Google Scholar] [CrossRef]
- Lee, K.K.; Maccallum, D.M.; Jacobsen, M.D.; Walker, L.A.; Odds, F.C.; Gow, N.A.; Munro, C.A. Elevated Cell Wall Chitin in Candida albicans Confers Echinocandin Resistance In Vivo. Antimicrob. Agents Chemother. 2012, 56, 208–217. [Google Scholar] [CrossRef]
- Shields, R.K.; Nguyen, M.H.; Du, C.; Press, E.; Cheng, S.; Clancy, C.J. Paradoxical effect of caspofungin against Candida bloodstream isolates is mediated by multiple pathways but eliminated in human serum. Antimicrob. Agents Chemother. 2011, 55, 2641–2647. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.L.; Qin, L.; Miao, Z.; Grys, B.T.; Diaz, J.C.; Ting, K.; Krieger, J.R.; Tong, J.; Tan, K.; Leach, M.D.; et al. The Candida albicans transcription factor Cas5 couples stress responses, drug resistance and cell cycle regulation. Nat. Commun. 2017, 8, 499. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Kravets, A.; Bethlendy, G.; Welle, S.; Rustchenko, E. Chromosome 5 monosomy of Candida albicans controls susceptibility to various toxic agents, including major antifungals. Antimicrob. Agents Chemother. 2013, 57, 5026–5036. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, L.; Wakabayashi, H.; Myers, J.; Jiang, Y.; Cao, Y.; Jimenez-Ortigosa, C.; Perlin, D.S.; Rustchenko, E. Tolerance to Caspofungin in Candida albicans Is Associated with at Least Three Distinctive Mechanisms That Govern Expression of FKS Genes and Cell Wall Remodeling. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Suwunnakorn, S.; Wakabayashi, H.; Rustchenko, E. Chromosome 5 of Human Pathogen Candida albicans Carries Multiple Genes for Negative Control of Caspofungin and Anidulafungin Susceptibility. Antimicrob. Agents Chemother. 2016, 60, 7457–7467. [Google Scholar] [CrossRef]
- Lewis, R.E.; Liao, G.; Young, K.; Douglas, C.; Kontoyiannis, D.P. Macrophage reporter cell assay for screening immunopharmacological activity of cell wall-active antifungals. Antimicrob. Agents Chemother. 2014, 58, 1738–1743. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mora-Montes, H.M.; Netea, M.G.; Ferwerda, G.; Lenardon, M.D.; Brown, G.D.; Mistry, A.R.; Kullberg, B.J.; O’Callaghan, C.A.; Sheth, C.C.; Odds, F.C.; et al. Recognition and blocking of innate immunity cells by Candida albicans chitin. Infect. Immun. 2011, 79, 1961–1970. [Google Scholar] [CrossRef]
- Marakalala, M.J.; Vautier, S.; Potrykus, J.; Walker, L.A.; Shepardson, K.M.; Hopke, A.; Mora-Montes, H.M.; Kerrigan, A.; Netea, M.G.; Murray, G.I.; et al. Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1. PLoS Pathog. 2013, 9, e1003315. [Google Scholar] [CrossRef]
- Miranda, I.; Rocha, R.; Santos, M.C.; Mateus, D.D.; Moura, G.R.; Carreto, L.; Santos, M.A. A genetic code alteration is a phenotype diversity generator in the human pathogen Candida albicans. PLoS ONE 2007, 2, e996. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.M.; Paredes, J.A.; Moura, G.R.; Manadas, B.; Lima-Costa, T.; Rocha, R.; Miranda, I.; Gomes, A.C.; Koerkamp, M.J.; Perrot, M.; et al. Critical roles for a genetic code alteration in the evolution of the genus Candida. EMBO J. 2007, 26, 4555–4565. [Google Scholar] [CrossRef] [PubMed]
- D’ENFERT, C. Hidden killers: Persistence of opportunistic fungal pathogens in the human host. Curr. Opin. Microbiol. 2009, 12, 358–364. [Google Scholar] [CrossRef]
- Silva-Dias, A.; Miranda, I.M.; Branco, J.; Monteiro-Soares, M.; Pina-Vaz, C.; Rodrigues, A.G. Adhesion, biofilm formation, cell surface hydrophobicity, and antifungal planktonic susceptibility: Relationship among Candida spp. Front. Microbiol. 2015, 6, 205. [Google Scholar] [CrossRef] [PubMed]
- Araujo, D.; Henriques, M.; Silva, S. Portrait of Candida Species Biofilm Regulatory Network Genes. Trends Microbiol. 2017, 25, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Fox, E.P.; Nett, J.E.; Sorrells, T.R.; Mitrovich, Q.M.; Hernday, A.D.; Tuch, B.B.; Andes, D.R.; Johnson, A.D. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 2012, 148, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.; Nett, J.; Oschel, P.; Albrecht, R.; Marchillo, K.; Pitula, A. Development and characterization of an in vivo central venous catheter Candida albicans biofilm model. Infect. Immun. 2004, 72, 6023–6031. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Chandra, J.; Kuhn, D.M.; Ghannoum, M.A. Mechanism of fluconazole resistance in Candida albicans biofilms: Phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 2003, 71, 4333–4340. [Google Scholar] [CrossRef]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal biofilm resistance. Int. J. Microbiol. 2012, 2012, 528521. [Google Scholar] [CrossRef]
- Ramage, G.; Bachmann, S.; Patterson, T.F.; Wickes, B.L.; Lopez-Ribot, J.L. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 2002, 49, 973–980. [Google Scholar] [CrossRef]
- Wang, J.F.; Xue, Y.; Zhu, X.B.; Fan, H. Efficacy and safety of echinocandins versus triazoles for the prophylaxis and treatment of fungal infections: A Meta-analysis of RCTs. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 651–659. [Google Scholar] [CrossRef]
- Katragkou, A.; Roilides, E.; Walsh, T.J. Role of Echinocandins in Fungal Biofilm-Related Disease: Vascular Catheter-Related Infections, Immunomodulation, and Mucosal Surfaces. Clin. Infect. Dis. 2015, 61, S622–S629. [Google Scholar] [CrossRef] [PubMed]
- Seidler, M.; Salvenmoser, S.; Muller, F.M. Liposomal amphotericin B eradicates Candida albicans biofilm in a continuous catheter flow model. FEMS Yeast Res. 2010, 10, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Larkin, E.L.; Dharmaiah, S.; Ghannoum, M.A. Biofilms and beyond: Expanding echinocandin utility. J. Antimicrob. Chemother. 2018, 73, i73–i81. [Google Scholar] [CrossRef] [PubMed]
- Brun, S.; Aubry, C.; Lima, O.; Filmon, R.; Berges, T.; Chabasse, D.; Bouchara, J.P. Relationships between respiration and susceptibility to azole antifungals in Candida glabrata. Antimicrob. Agents Chemother. 2003, 47, 847–853. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Inhibition of Candida parapsilosis mitochondrial respiratory pathways enhances susceptibility to caspofungin. Antimicrob. Agents Chemother. 2006, 50, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Costa-de-Oliveira, S.; Sampaio-Marques, B.; Barbosa, M.; Ricardo, E.; Pina-Vaz, C.; Ludovico, P.; Rodrigues, A.G. An alternative respiratory pathway on Candida krusei: Implications on susceptibility profile and oxidative stress. FEMS Yeast Res. 2012, 12, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Li, M.; Cao, Y.; Gao, P.; Wang, Y.; Jiang, Y. The alternative oxidase of Candida albicans causes reduced fluconazole susceptibility. J. Antimicrob. Chemother. 2009, 64, 764–773. [Google Scholar] [CrossRef]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Tanabe, K.; Niimi, M.; Monk, B.C. Candida albicans drug resistance another way to cope with stress. Microbiology 2007, 153, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Duvenage, L.; Munro, C.A.; Gourlay, C.W. The potential of respiration inhibition as a new approach to combat human fungal pathogens. Curr. Genet. 2019, 65, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Duvenage, L.; Walker, L.A.; Bojarczuk, A.; Johnston, S.A.; MacCallum, D.M.; Munro, C.A.; Gourlay, C.W. Inhibition of Classical and Alternative Modes of Respiration in Candida albicans Leads to Cell Wall Remodeling and Increased Macrophage Recognition. MBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Eggimann, P.; Garbino, J.; Pittet, D. Management of Candida species infections in critically ill patients. Lancet Infect. Dis. 2003, 3, 772–785. [Google Scholar] [CrossRef]
- Hachem, R.; Hanna, H.; Kontoyiannis, D.; Jiang, Y.; Raad, I. The changing epidemiology of invasive candidiasis: Candida glabrata and Candida krusei as the leading causes of candidemia in hematologic malignancy. Cancer 2008, 112, 2493–2499. [Google Scholar] [CrossRef] [PubMed]
- Goldani, L.Z.; Craven, D.E.; Sugar, A.M. Central venous catheter infection with Rhodotorula minuta in a patient with AIDS taking suppressive doses of fluconazole. J. Med. Vet. Mycol. Bi-Mon. Publ. Int. Soc. Hum. Anim. Mycol. 1995, 33, 267–270. [Google Scholar]
- Cutler, J.E. Putative virulence factors of Candida albicans. Annu. Rev. Microbiol. 1991, 45, 187–218. [Google Scholar] [CrossRef] [PubMed]
- Vogel, M.; Hartmann, T.; Koberle, M.; Treiber, M.; Autenrieth, I.B.; Schumacher, U.K. Rifampicin induces MDR1 expression in Candida albicans. J. Antimicrob. Chemother. 2008, 61, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Stergiopoulou, T.; Meletiadis, J.; Sein, T.; Papaioannidou, P.; Tsiouris, I.; Roilides, E.; Walsh, T.J. Comparative pharmacodynamic interaction analysis between ciprofloxacin, moxifloxacin and levofloxacin and antifungal agents against Candida albicans and Aspergillus fumigatus. J. Antimicrob. Chemother. 2009, 63, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, F.; Sharghi, S.; Kuchler, K.; Lion, T. Pathogenetic Impact of Bacterial-Fungal Interactions. Microorganisms 2019, 7, 459. [Google Scholar] [CrossRef] [PubMed]
- Maki, N.; Moitra, K.; Silver, C.; Ghosh, P.; Chattopadhyay, A.; Dey, S. Modulator-induced interference in functional cross talk between the substrate and the ATP sites of human P-glycoprotein. Biochemistry 2006, 45, 2739–2751. [Google Scholar] [CrossRef] [PubMed]
- Modok, S.; Mellor, H.R.; Callaghan, R. Modulation of multidrug resistance efflux pump activity to overcome chemoresistance in cancer. Curr. Opin. Pharmacol. 2006, 6, 350–354. [Google Scholar] [CrossRef]
- Tsujimura, S.; Saito, K.; Nawata, M.; Nakayamada, S.; Tanaka, Y. Overcoming drug resistance induced by P-glycoprotein on lymphocytes in patients with refractory rheumatoid arthritis. Ann. Rheum. Dis. 2008, 67, 380–388. [Google Scholar] [CrossRef]
- Nim, S.; Rawal, M.K.; Prasad, R. FK520 interacts with the discrete intrahelical amino acids of multidrug transporter Cdr1 protein and acts as antagonist to selectively chemosensitize azole-resistant clinical isolates of Candida albicans. FEMS Yeast Res. 2014, 14, 624–632. [Google Scholar] [CrossRef][Green Version]
- Tanabe, K.; Bonus, M.; Tomiyama, S.; Miyoshi, K.; Nagi, M.; Niimi, K.; Chindamporn, A.; Gohlke, H.; Schmitt, L.; Cannon, R.D.; et al. FK506 Resistance of Saccharomyces cerevisiae Pdr5 and Candida albicans Cdr1 Involves Mutations in the Transmembrane Domains and Extracellular Loops. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Twentyman, P.R. Cyclosporins as drug resistance modifiers. Biochem. Pharmacol. 1992, 43, 109–117. [Google Scholar] [CrossRef]
- Kawamura, A.; Su, M.S. Interaction of FKBP12-FK506 with calcineurin A at the B subunit-binding domain. J. Biol. Chem. 1995, 270, 15463–15466. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Li, Y.; Guo, Q.; Shi, C.; Yu, J.; Ma, L. In vitro interactions between tacrolimus and azoles against Candida albicans determined by different methods. Antimicrob. Agents Chemother. 2008, 52, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, O.; Entenza, J.M.; Sanglard, D.; Bille, J.; Glauser, M.P.; Moreillon, P. Fluconazole plus cyclosporine: A fungicidal combination effective against experimental endocarditis due to Candida albicans. Antimicrob. Agents Chemother. 2000, 44, 2932–2938. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steinbach, W.J.; Reedy, J.L.; Cramer, R.A., Jr.; Perfect, J.R.; Heitman, J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat. Rev. Microbiol. 2007, 5, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Byrne, S.T.; Denkin, S.M.; Zhang, Y. Aspirin and ibuprofen enhance pyrazinamide treatment of murine tuberculosis. J. Antimicrob. Chemother. 2007, 59, 313–316. [Google Scholar] [CrossRef]
- Arai, R.; Sugita, T.; Nishikawa, A. Reassessment of the in vitro synergistic effect of fluconazole with the non-steroidal anti-inflammatory agent ibuprofen against Candida albicans. Mycoses 2005, 48, 38–41. [Google Scholar] [CrossRef]
- Pina-Vaz, C.; Rodrigues, A.G.; Costa-de-Oliveira, S.; Ricardo, E.; Mardh, P.A. Potent synergic effect between ibuprofen and azoles on Candida resulting from blockade of efflux pumps as determined by FUN-1 staining and flow cytometry. J. Antimicrob. Chemother. 2005, 56, 678–685. [Google Scholar] [CrossRef][Green Version]
- Venturini, T.P.; Rossato, L.; Spader, T.B.; Tronco-Alves, G.R.; Azevedo, M.I.; Weiler, C.B.; Santurio, J.M.; Alves, S.H. In vitro synergisms obtained by amphotericin B and voriconazole associated with non-antifungal agents against Fusarium spp. Diagn. Microbiol. Infect. Dis. 2011, 71, 126–130. [Google Scholar] [CrossRef]
- Costa-de-Oliveira, S.; Miranda, I.M.; Silva-Dias, A.; Silva, A.P.; Rodrigues, A.G.; Pina-Vaz, C. Ibuprofen potentiates the in vivo antifungal activity of fluconazole against Candida albicans murine infection. Antimicrob. Agents Chemother. 2015, 59, 4289–4292. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zhang, M.; Wang, T.; Li, Y.; Wang, C. The roles of CDR1, CDR2, and MDR1 in kaempferol-induced suppression with fluconazole-resistant Candida albicans. Pharm. Biol. 2016, 54, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Monk, B.C.; Niimi, K.; Lin, S.; Knight, A.; Kardos, T.B.; Cannon, R.D.; Parshot, R.; King, A.; Lun, D.; Harding, D.R. Surface-active fungicidal D-peptide inhibitors of the plasma membrane proton pump that block azole resistance. Antimicrob. Agents Chemother. 2005, 49, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Dubikovskaya, E.A.; Thorne, S.H.; Pillow, T.H.; Contag, C.H.; Wender, P.A. Overcoming multidrug resistance of small-molecule therapeutics through conjugation with releasable octaarginine transporters. Proc. Natl. Acad. Sci. USA 2008, 105, 12128–12133. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, L.T.; Manavathu, E.K.; Cutright, J.L.; Alangaden, G.J.; Chandrasekar, P.H. In-vitro activity of nikkomycin Z alone and in combination with polyenes, triazoles or echinocandins against Aspergillus fumigatus. Clin. Microbiol. Infect. 2004, 10, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.A. Drug interaction studies of a glucan synthase inhibitor (LY 303366) and a chitin synthase inhibitor (Nikkomycin Z) for inhibition and killing of fungal pathogens. Antimicrob. Agents Chemother. 2000, 44, 2547–2548. [Google Scholar] [CrossRef]
- Verwer, P.E.; van Duijn, M.L.; Tavakol, M.; Bakker-Woudenberg, I.A.; van de Sande, W.W. Reshuffling of Aspergillus fumigatus cell wall components chitin and beta-glucan under the influence of caspofungin or nikkomycin Z alone or in combination. Antimicrob. Agents Chemother. 2012, 56, 1595–1598. [Google Scholar] [CrossRef]
- Fernandes, C.; Anjos, J.; Walker, L.A.; Silva, B.M.; Cortes, L.; Mota, M.; Munro, C.A.; Gow, N.A.; Goncalves, T. Modulation of Alternaria infectoria cell wall chitin and glucan synthesis by cell wall synthase inhibitors. Antimicrob. Agents Chemother. 2014, 58, 2894–2904. [Google Scholar] [CrossRef]
- Kovacs, R.; Nagy, F.; Toth, Z.; Bozo, A.; Balazs, B.; Majoros, L. Synergistic effect of nikkomycin Z with caspofungin and micafungin against Candida albicans and Candida parapsilosis biofilms. Lett. Appl. Microbiol. 2019, 69, 271–278. [Google Scholar] [CrossRef]
- Cheung, Y.Y.; Hui, M. Effects of Echinocandins in Combination with Nikkomycin Z against Invasive Candida albicans Bloodstream Isolates and the fks Mutants. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
| Antifungal Class | Antifungal Drug | Spectrum of Activity | Mechanism(s) of Action | Mechanism(s) of Resistance |
|---|---|---|---|---|
| Polyenes | Amphotericin B | Fungicidal | Polyene molecules links to ergosterol in the fungal membrane by inserting into the lipid bilayers, creating pores that disrupt plasma membrane; oxidative damage. | Mutations in the ERG3 gene affect ergosterol biosynthesis and content in the fungal membrane is responsible for a decrease access to the drug target; susceptibility to oxidative damage by increasing catalase activity. |
| Pyrimidine analogues | 5-Flucytosine | Fungicidal | Inhibition of cellular function and division by incorporating toxic fluorinated pyrimidine antimetabolites into DNA and RNA. | Mutations in the enzyme uracil phosphoribosyltransferase (Fur1p), decreasing the formation of toxic antimetabolites. |
| Azoles | Fluconazole Voriconazole Posaconazole | Fungistatic | Inhibition of the fungal cytochrome P450 14α-lanosterol demethylase and accumulation of toxic methylated intermediates, with resultant disruption of fungal cell membrane function and growth inhibition. | Overexpression of cell membrane efflux pumps, decreasing drug concentration (upregulation or overexpression CDR and MDR genes); alteration of the target enzyme, decreasing affinity to the binding site (point mutation in ERG11 gene); upregulation of the target enzyme (overexpression of ERG11 gene). |
| Echinocandins | Caspofungin Anidulafungin Micafungin | Fungicidal | Inhibition of β-(1,3) glucan synthase, decreasing the production of β-(1,3) glucan, which represents one of the major components of the fungal cell wall. | Point mutations in FKS1 and FKS2 genes. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa-de-Oliveira, S.; Rodrigues, A.G. Candida albicans Antifungal Resistance and Tolerance in Bloodstream Infections: The Triad Yeast-Host-Antifungal. Microorganisms 2020, 8, 154. https://doi.org/10.3390/microorganisms8020154
Costa-de-Oliveira S, Rodrigues AG. Candida albicans Antifungal Resistance and Tolerance in Bloodstream Infections: The Triad Yeast-Host-Antifungal. Microorganisms. 2020; 8(2):154. https://doi.org/10.3390/microorganisms8020154
Chicago/Turabian StyleCosta-de-Oliveira, Sofia, and Acácio G. Rodrigues. 2020. "Candida albicans Antifungal Resistance and Tolerance in Bloodstream Infections: The Triad Yeast-Host-Antifungal" Microorganisms 8, no. 2: 154. https://doi.org/10.3390/microorganisms8020154
APA StyleCosta-de-Oliveira, S., & Rodrigues, A. G. (2020). Candida albicans Antifungal Resistance and Tolerance in Bloodstream Infections: The Triad Yeast-Host-Antifungal. Microorganisms, 8(2), 154. https://doi.org/10.3390/microorganisms8020154