Assessing the Risk of Occurrence of Bluetongue in Senegal
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
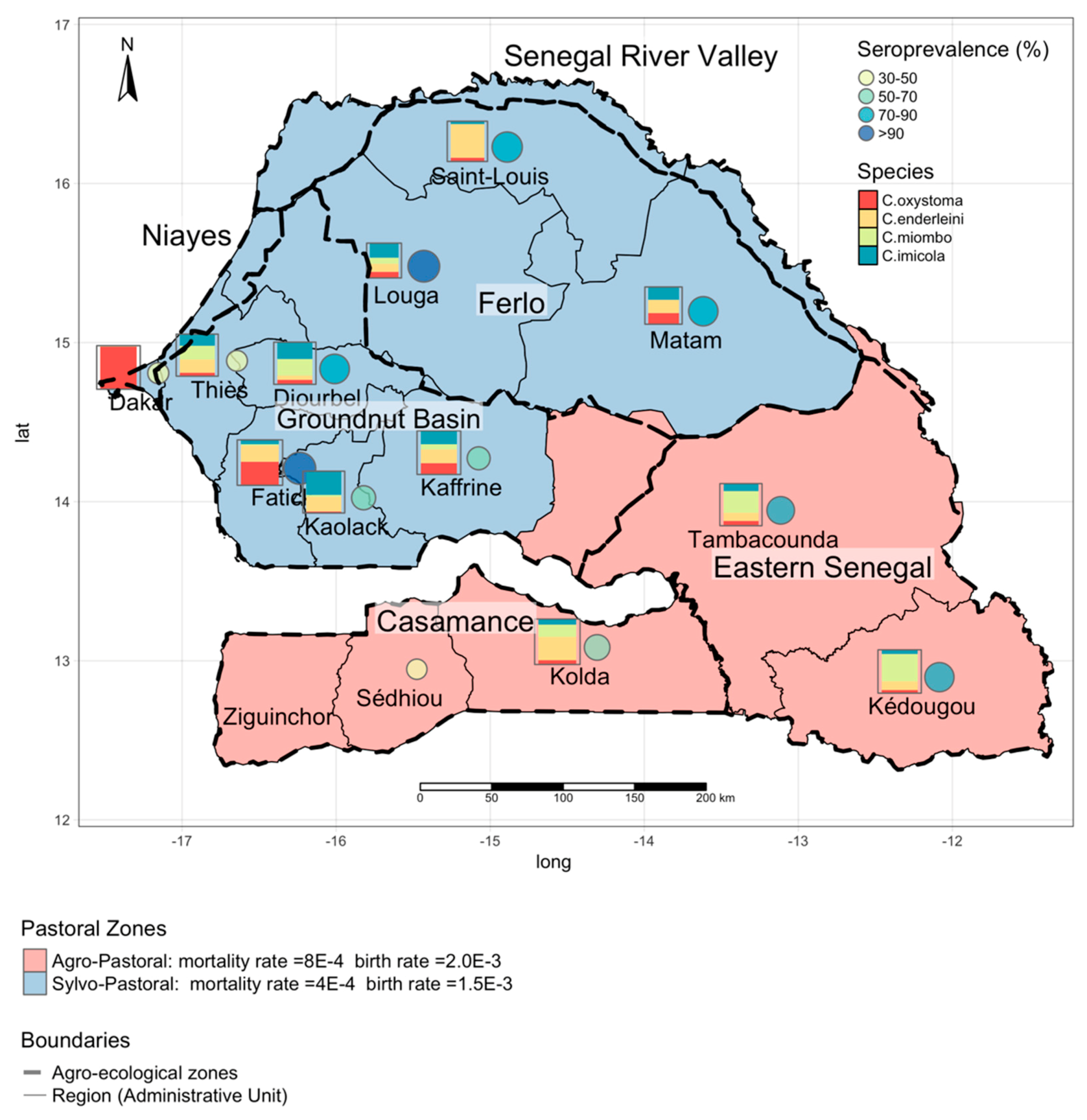
2.2.1. Entomological Data
2.2.2. Serological Data
2.2.3. Climatic, Environmental and Livestock Data
2.2.4. Small Ruminants Demographic Data
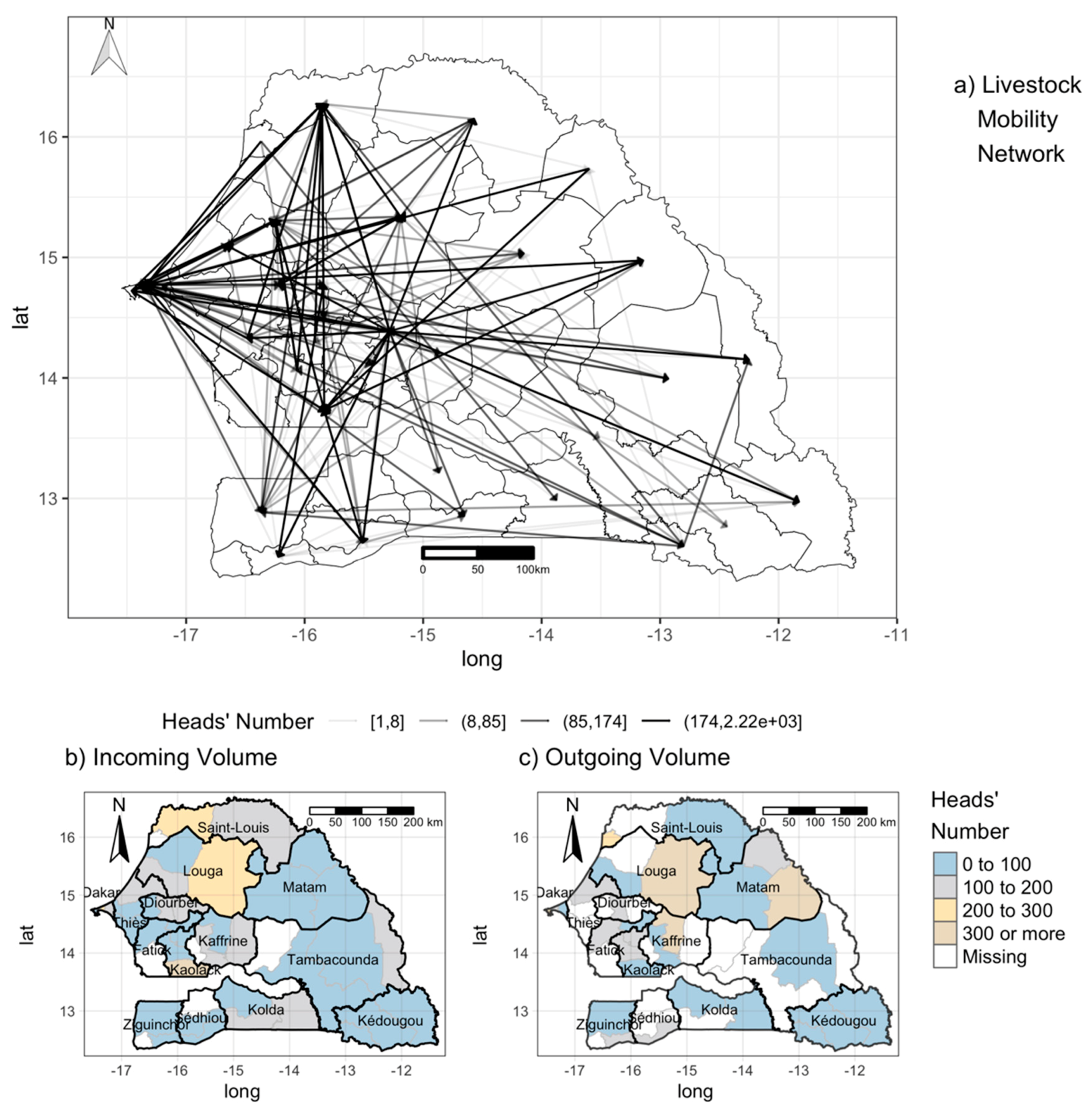
2.2.5. Mobility Data
2.3. Estimated Vector Abundance, R0, and the Probability of the Occurrence of an Outbreak
2.3.1. Abundance Modeling: Random Forest
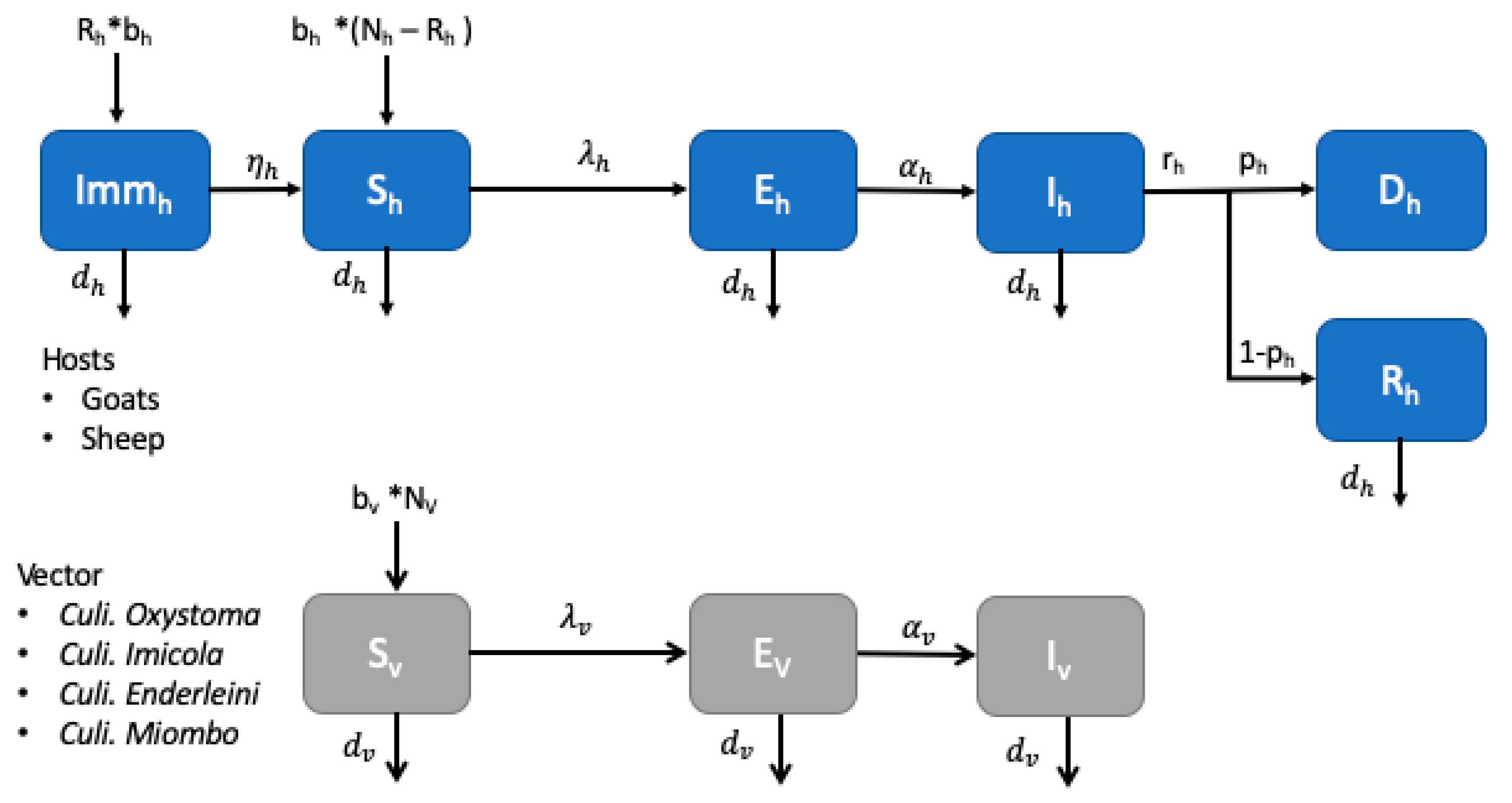
2.3.2. Model Host-Vector for Bluetongue Transmission
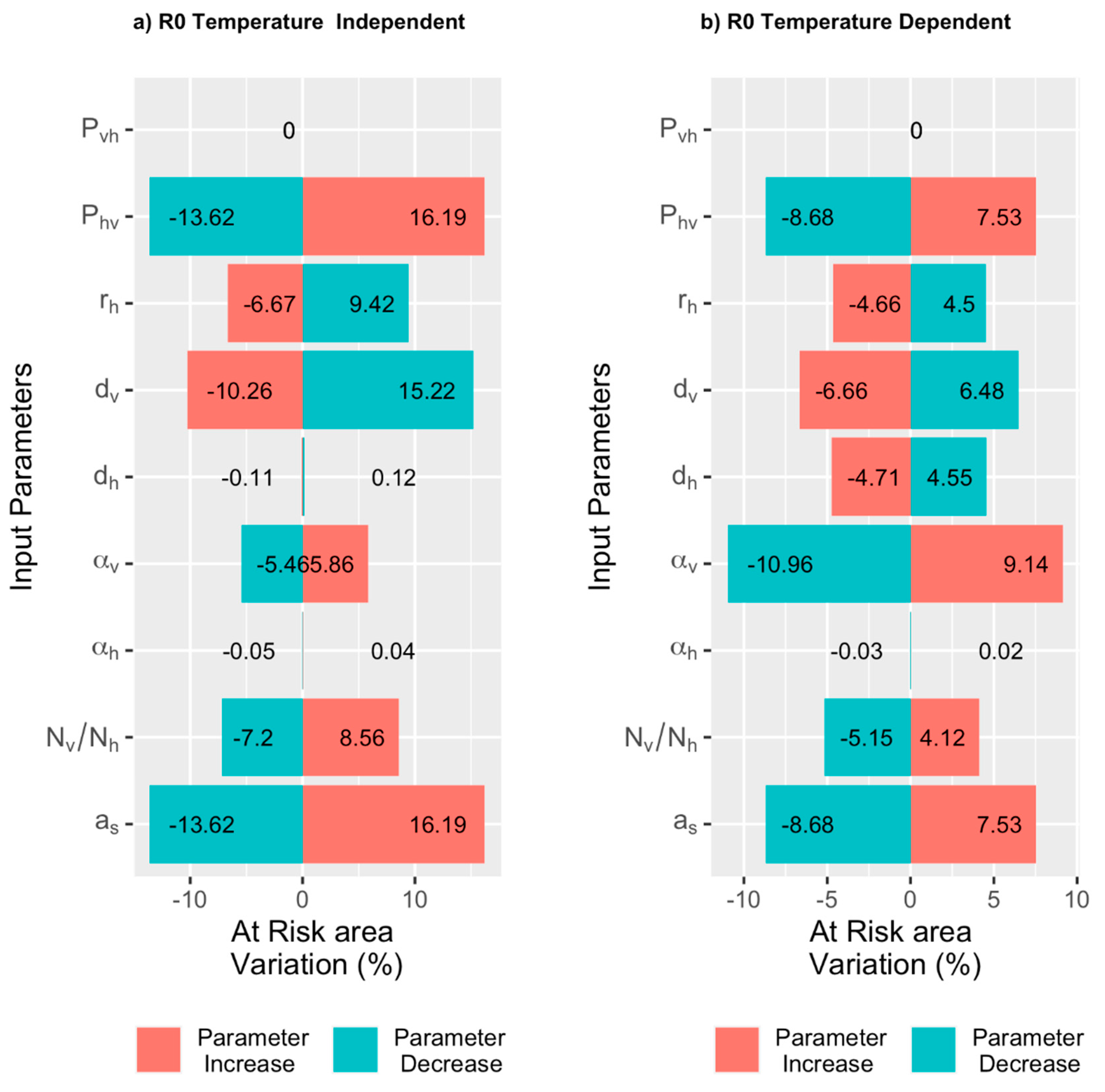
2.3.3. Sensitivity Analysis
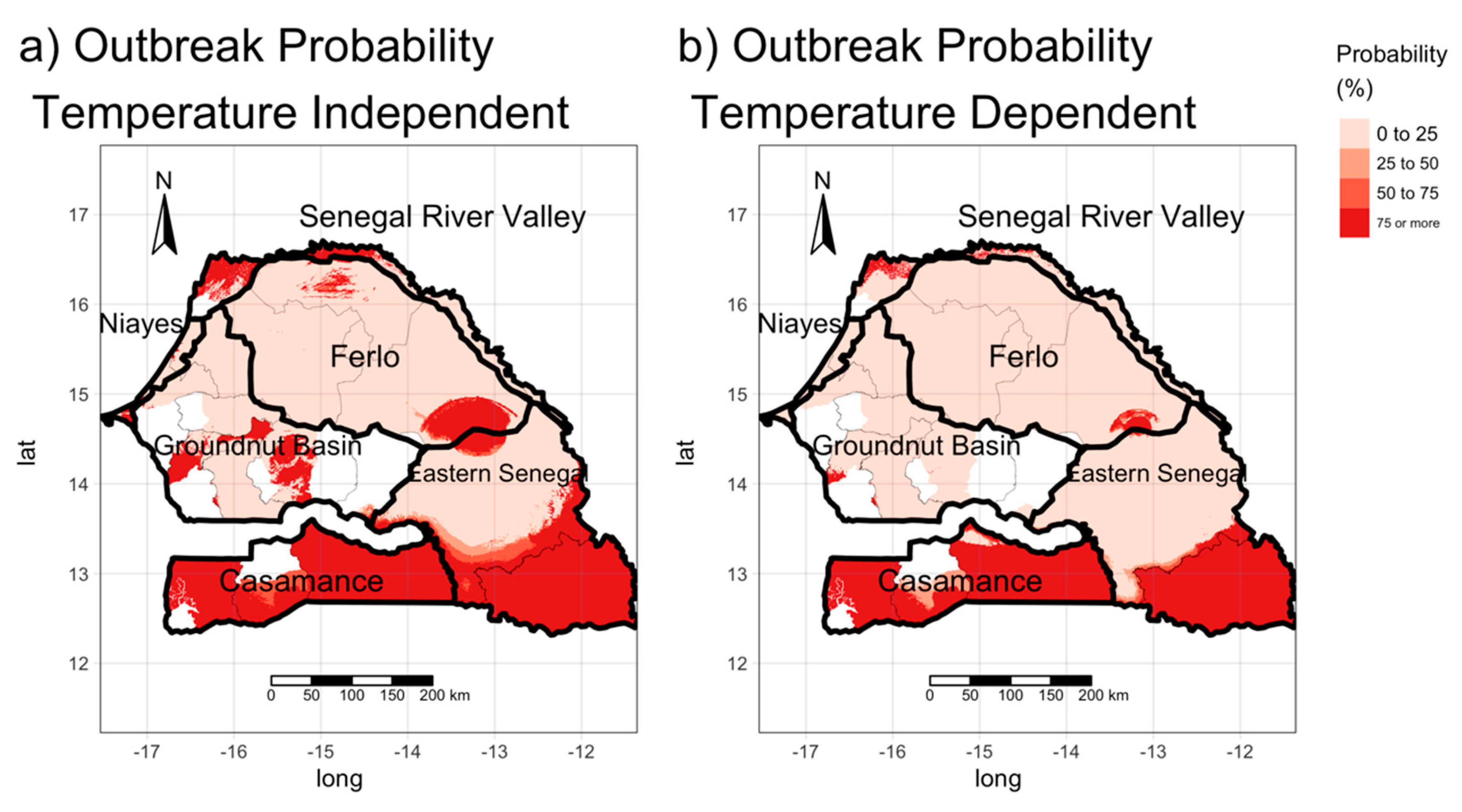
2.3.4. Occurrence Probability Map
3. Results
3.1. Estimating the Vector Spatial Abundance
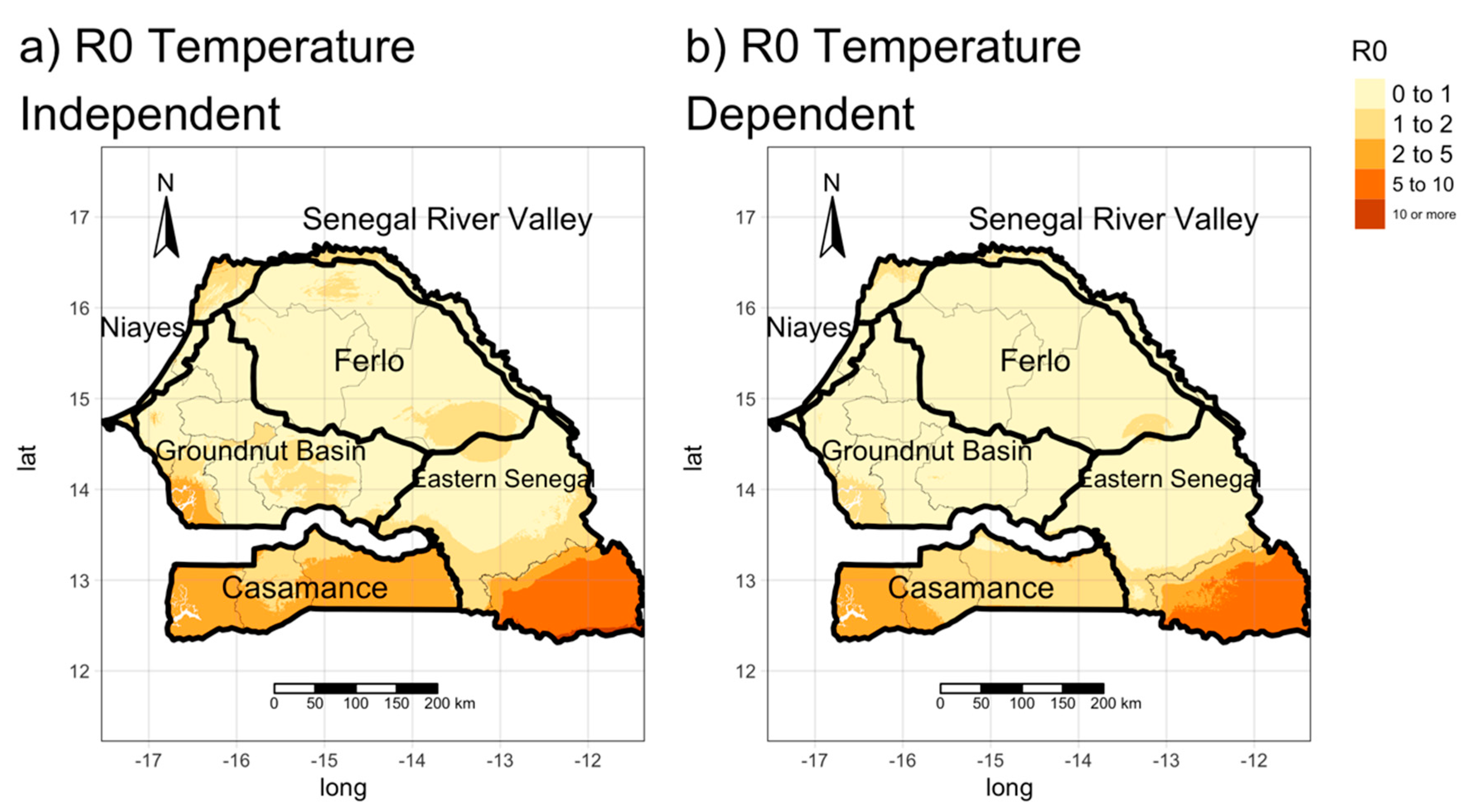
3.2. Estimating the Spatial Distribution of R0 (Transmission Maps)
3.3. Estimating the Risk of the Occurrence of BT Outbreaks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stear, M.J. OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Mammals, Birds and Bees) 5th Edn. Volumes 1 & 2. World Organization for Animal Health 2004. ISBN 92 9044 622 6. €140. Parasitology 2005, 130, 727. [Google Scholar] [CrossRef]
- Backx, A.; Heutink, R.; Van Rooij, E.; Van Rijn, P.A. Transplacental and Oral Transmission of Wild-Type Bluetongue Virus Serotype 8 in Cattle after Experimental Infection. Vet. Microbiol. 2009, 138, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Worwa, G.; Hilbe, M.; Ehrensperger, F.; Chaignat, V.; Hofmann, M.A.; Griot, C.; MacLachlan, N.J.; Thuer, B. Experimental Transplacental Infection of Sheep with Bluetongue Virus Serotype 8. Vet. Rec. 2009, 164, 499–500. [Google Scholar] [CrossRef] [PubMed]
- Belbis, G.; Breard, E.; Cordonnier, N.; Moulin, V.; Desprat, A.; Sailleau, C.; Viarouge, C.; Doceul, V.; Zientara, S.; Millemann, Y. Evidence of Transplacental Transmission of Bluetongue Virus Serotype 8 in Goats. Vet. Microbiol. 2013, 166, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Zanella, G.; Durand, B.; Sellal, E.; Breard, E.; Sailleau, C.; Zientara, S.; Batten, C.; Mathevet, P.; Audeval, C. Bluetongue Virus Serotype 8: Abortion and Transplacental Transmission in Cattle in the Burgundy Region, France, 2008–2009. Theriogenology 2012, 77, 65–72. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, K.; De Leeuw, I.; Verheyden, B.; Vandemeulebroucke, E.; Vanbinst, T.; Herr, C.; Méroc, E.; Bertels, G.; Steurbaut, N.; Miry, C.; et al. Transplacental Infection and Apparently Immunotolerance Induced by a Wild-type Bluetongue Virus Serotype 8 Natural Infection. Transbound. Emerg. Dis. 2008, 55, 352–359. [Google Scholar] [CrossRef]
- Purse, B.V.; Mellor, P.S.; Rogers, D.J.; Samuel, A.R.; Mertens, P.P.C.; Baylis, M. Climate Change and the Recent Emergence of Bluetongue in Europe. Nat. Rev. Genet. 2005, 3, 171–181. [Google Scholar] [CrossRef]
- Purse, B.V.; Brown, H.E.; Harrup, L.; Mertens, P.P.C.; Rogers, D.J. Invasion of Bluetongue and other Orbivirus Infections into Europe: The Role of Biological and Climatic Processes. Rev. Sci. Tech. l’OIE 2008, 27, 427–442. [Google Scholar] [CrossRef]
- Wilson, A.J.; Mellor, P.S. Bluetongue in Europe: Past, Present and Future. Philos. Trans. R. Soc. B Boil. Sci. 2009, 364, 2669–2681. [Google Scholar] [CrossRef]
- Coetzee, P.; Stokstad, M.; Venter, E.; Myrmel, M.; Van Vuuren, M. Bluetongue: A Historical and Epidemiological Perspective with the Emphasis on South Africa. Virol. J. 2012, 9, 198. [Google Scholar] [CrossRef]
- Gambles, R. Bluetongue of Sheep in Cyprus. J. Comp. Pathol. Ther. 1949, 59, 176–190. [Google Scholar] [CrossRef]
- Roy, P. Bluetongue Virus Genetics and Genome Structure. Virus Res. 1989, 13, 179–206. [Google Scholar] [CrossRef]
- Saegerman, C.; Berkvens, D.; Mellor, P.S. Bluetongue Epidemiology in the European Union. Emerg. Infect. Dis. 2008, 14, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Conraths, F.J.; Gethmann, J.; Staubach, C.; Mettenleiter, T.C.; Beer, M.; Hoffmann, B. Epidemiology of Bluetongue Virus Serotype 8, Germany. Emerg. Infect. Dis. 2009, 15, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Zientara, S.; Sailleau, C.; Bréard, E.; Viarouge, C.; Gorna, K.; Relmy, A.; Desprat, A. Intérêt des Outils Moléculaires Pour l’identification et de Typage des Orbivirus. Rencontres Autour Rech. Rumin. 2010, 17, 83–86. [Google Scholar]
- Lefevre, P.; Taylor, W. Epidemiological Situation of Bluetongue in Senegal. Rev. Elev. Med. Vet. Pays Trop. 1983, 36, 241–245. [Google Scholar]
- Lefèvre, P.-C.; Calvez, D. La Fièvre Catarrhale du Mouton (Bluetongue) en Afrique Intertropicale: Influence des Facteurs Écologiques sur la Prévalence de L’infection. Rev. Elev. Méd. vét. P. Trop. 1986, 39, 263–268. [Google Scholar]
- Bakhoum, M.T.; Fall, A.G.; Fall, M.; Bassene, C.K.; Baldet, T.; Seck, M.T.; Bouyer, J.; Garros, C.; Gimonneau, G. Insight on the Larval Habitat of Afrotropical Culicoides Latreille (Diptera: Ceratopogonidae) in the Niayes Area of Senegal, West Africa. Parasit. Vectors 2016, 9, 462. [Google Scholar] [CrossRef]
- Fall, M.; Fall, A.G.; Seck, M.T.; Bouyer, J.; Diarra, M.; Lancelot, R.; Gimonneau, G.; Garros, C.; Bakhoum, M.T.; Faye, O.; et al. Host Preferences and Circadian Rhythm of Culicoides (Diptera: Ceratopogonidae), Vectors of African Horse Sickness and Bluetongue Viruses in Senegal. Acta Trop. 2015, 149, 239–245. [Google Scholar] [CrossRef]
- Diarra, M.; Fall, M.; Fall, A.G.; Diop, A.; Lancelot, R.; Seck, M.T.; Rakotoarivony, I.; Allène, X.; Bouyer, J.; Guis, H. Spatial Distribution Modelling of Culicoides (Diptera: Ceratopogonidae) Biting Midges, Potential Vectors of African Horse Sickness and Bluetongue Viruses in Senegal. Parasit. Vectors 2018, 11, 341. [Google Scholar] [CrossRef]
- Ciss, M.; Biteye, B.; Fall, A.G.; Fall, M.; Gahn, M.C.B.; Leroux, L.; Apolloni, A. Ecological Niche Modelling to Estimate the Distribution of Culicoides, Potential Vectors of Bluetongue Virus in Senegal. BMC Ecol. 2019, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Akakpo, A.; Toukam, W.C.; Mankor, A.; Ly, C. Impact Économique de L’épizootie de Peste Équine de 2007 au Sénégal. Bull. Anim. Health Prod. Afr. 2011, 59, 1–16. [Google Scholar] [CrossRef]
- Diouf, N.D.; Etter, E.; Akakpo, A.J.; Lo, M.M. Outbreaks of African Horse Sickness in Senegal, and Methods of Control of the 2007 Epidemic. Vet. Rec. 2012, 172, 152. [Google Scholar] [CrossRef] [PubMed]
- Diarra, M.; Fall, M.; Fall, A.G.; Diop, A.; Seck, M.T.; Garros, C.; Balenghien, T.; Allène, X.; Rakotoarivony, I.; Lancelot, R.; et al. Seasonal Dynamics of Culicoides (Diptera: Ceratopogonidae) Biting Midges, Potential Vectors of African Horse Sickness and Bluetongue Viruses in the Niayes Area of Senegal. Parasites Vectors 2014, 7, 147. [Google Scholar] [CrossRef]
- Diarra, M.; Fall, M.; Lancelot, R.; Diop, A.; Fall, A.G.; Dicko, A.H.; Seck, M.T.; Garros, C.; Allène, X.; Rakotoarivony, I.; et al. Modelling the Abundances of Two Major Culicoides (Diptera: Ceratopogonidae) Species in the Niayes Area of Senegal. PLoS ONE 2015, 10, e0131021. [Google Scholar] [CrossRef] [PubMed]
- Diekmann, O.; Heesterbeek, H.; Roberts, M.G. The Construction of Next-Generation Matrices for Compartmental Epidemic Models. J. R. Soc. Interface 2009, 7, 873–885. [Google Scholar] [CrossRef]
- Brugger, K.; Rubel, F. Bluetongue Disease Risk Assessment Based on Observed and Projected Culicoides obsoletus spp. Vector Densities. PLoS ONE 2013, 8, e60330. [Google Scholar] [CrossRef]
- Colizza, V.; Barthelemy, M.; Barrat, A.; Vespignani, A. Epidemic Modeling in Complex Realities. Comptes Rendus Boil. 2007, 330, 364–374. [Google Scholar] [CrossRef]
- Fall, M.; Diarra, M.; Fall, A.G.; Balenghien, T.; Seck, M.T.; Bouyer, J.; Garros, C.; Gimonneau, G.; Allène, X.; Mall, I.; et al. Culicoides (Diptera: Ceratopogonidae) Midges, the Vectors of African Horse Sickness Virus--a Host/Vector Contact Study in the Niayes Area of Senegal. Parasit. Vectors 2015, 8, 39. [Google Scholar] [CrossRef]
- Vandenbussche, F.; Vanbinst, T.; Verheyden, B.; Van Dessel, W.; Demeestere, L.; Houdart, P.; Bertels, G.; Praet, N.; Berkvens, D.; Mintiens, K.; et al. Evaluation of Antibody-ELISA and Real-Time RT-PCR for the Diagnosis and Profiling of Bluetongue Virus Serotype 8 during the Epidemic in Belgium in 2006. Vet. Microbiol. 2008, 129, 15–27. [Google Scholar] [CrossRef]
- Gahn, M.C.B.; Seck, M.T.; Ciss, M.; Fall, A.G.; Lo, M.M.; Ndiaye, M.; Fall, M.; Biteye, B.; Sailleau, C.; Viarouge, C.; et al. Insight Bluetongue Virus Transmission in Small Ruminants in Senegal (to be submitted). Microorganism 2020. [Google Scholar]
- Lancelot, R.; Faye, B.; Juanes, X.; Ndiaye, M.; Pérochon, L.; Tillard, E. La Base de Données BAOBAB: Un Outil Pour Modéliser la Production et la Santé des Petits Ruminants dans les Systèmes D’élevage Traditionnels au Sénégal. Revue d’Elevage Méd. Vét. Pays Trop. 1998, 51, 135–146. [Google Scholar] [CrossRef]
- O’Farrell, H.; Gourley, S.A. Modelling the Dynamics of Bluetongue Disease and the Effect of Seasonality. Bull. Math. Boil. 2014, 76, 1981–2009. [Google Scholar] [CrossRef] [PubMed]
- González-Parra, G.; Aranda, D.F.; Chen-Charpentier, B.; Díaz-Rodríguez, M.; Castellanos, J.E. Mathematical Modeling and Characterization of the Spread of Chikungunya in Colombia. Math. Comput. Appl. 2019, 24, 6. [Google Scholar] [CrossRef]
- Gubbins, S.; Carpenter, S.; Baylis, M.; Wood, J.L.; Mellor, P.S. Assessing the Risk of Bluetongue to UK Livestock: Uncertainty and Sensitivity Analyses of a Temperature-Dependent Model for the Basic Reproduction Number. J. R. Soc. Interface 2007, 5, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Hartemink, N.; Purse, B.; Meiswinkel, R.; Brown, H.E.; De Koeijer, A.; Elbers, A.; Boender, G.-J.; Rogers, D.; Heesterbeek, J. Mapping the Basic Reproduction Number (R0) for Vector-Borne Diseases: A Case Study on Bluetongue Virus. Epidemics 2009, 1, 153–161. [Google Scholar] [CrossRef]
- Santman-Berends, I.; Stegeman, J.; Vellema, P.; Van Schaik, G. Estimation of the Reproduction Ratio (R0) of Bluetongue Based on Serological Field Data and Comparison with Other BTV Transmission Models. Prev. Vet. Med. 2013, 108, 276–284. [Google Scholar] [CrossRef]
- Charron, M.V.; Seegers, H.; Langlais, M.; Ezanno, P. Seasonal Spread and Control of Bluetongue in Cattle. J. Theor. Boil. 2011, 291, 1–9. [Google Scholar] [CrossRef]
- Hammami, P.; Lancelot, R.; Lesnoff, M. Modelling the Dynamics of Post-Vaccination Immunity Rate in a Population of Sahelian Sheep after a Vaccination Campaign against Peste des Petits Ruminants Virus. PLoS ONE 2016, 11, e0161769. [Google Scholar] [CrossRef]
- Dean, A.S.; Fournié, G.; Kulo, A.E.; Boukaya, G.A.; Schelling, E.; Bonfoh, B. Potential Risk of Regional Disease Spread in West Africa through Cross-Border Cattle Trade. PLoS ONE 2013, 8, e75570. [Google Scholar] [CrossRef]
- Cianci, D.; Hartemink, N.; Ibáñez-Justicia, A. Modelling the Potential Spatial Distribution of Mosquito Species Using Three Different Techniques. Int. J. Health Geogr. 2015, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.; De Baets, B.; Van Doninck, J.; Calvete, C.; Lucientes, J.; De Clercq, E.M.; Ducheyne, E.; Verhoest, N.E.C. Absence Reduction in Entomological Surveillance Data to Improve Niche-Based Distribution Models for Culicoides Imicola. Prev. Veter Med. 2011, 100, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Basille, M.; Calenge, C.; Marboutin, E.; Andersen, R.; Gaillard, J. Assessing Habitat Selection Using Multivariate Statistics: Some Refinements of the Ecological-Niche Factor Analysis. Ecol. Model. 2008, 211, 233–240. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]

| Parameter | Description | Temperature Independent | Temperature-Dependent | ||
|---|---|---|---|---|---|
| Values | References | Values | References | ||
| Vector mortality rate | 0.16 [0.1–0.5] | [36,37] | [36] | ||
| bv | Vector fertility rate | 6.1 eggs/year | [38] | ||
| αv | Latency rate for vector | 0.09 [0.06–0.1] | [33,36] | [35,36] | |
| Pvh | Transmission Vector-host | 0.9 [0.8–1.0] | [36] | ||
| as | Biting rate | 0.17 [0.05–0.4] | [36] | [36] | |
| dh | Host mortality rate | 0.0005 (Sylvo-pastoral area) 0.0008 (Agro-pastoral area) | [39] | ||
| bh | Host fertility rate | 0.002 (Sylvo-pastoral area) 0.0015 (Agro-pastoral area) | [39] | ||
| αh | Latency rate for host | 0.0625 | [33] | ||
| rh | Recovery rate for host | 0.125 | [27,36] | ||
| dh | Host mortality rate | 0.0005 (Sylvo-pastoral area) 0.0008 (Agro-pastoral area) | [39] | ||
| bh | Host fertility rate | 0.002 (Sylvo-pastoral area) 0.0015 (Agro-pastoral area) | [39] | ||
| αh | Latency rate for host | 0.0625 | [33] | ||
| rh | Recovery rate for host | 0.125 | [27,36] | ||
| Phv | Transmission Host Vector | 0.05 [0.001–0.15] | [36,37] | ||
| ph | Disease induced mortality | 0.01 [0.001–0.01] | [36] | ||
| Rate of immunity loss | 1/90 | [39] | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gahn, M.C.B.; Niakh, F.; Ciss, M.; Seck, I.; Lo, M.M.; Fall, A.G.; Biteye, B.; Fall, M.; Ndiaye, M.; Ba, A.; et al. Assessing the Risk of Occurrence of Bluetongue in Senegal. Microorganisms 2020, 8, 1766. https://doi.org/10.3390/microorganisms8111766
Gahn MCB, Niakh F, Ciss M, Seck I, Lo MM, Fall AG, Biteye B, Fall M, Ndiaye M, Ba A, et al. Assessing the Risk of Occurrence of Bluetongue in Senegal. Microorganisms. 2020; 8(11):1766. https://doi.org/10.3390/microorganisms8111766
Chicago/Turabian StyleGahn, Marie Cicille Ba, Fallou Niakh, Mamadou Ciss, Ismaila Seck, Modou Moustapha Lo, Assane Gueye Fall, Biram Biteye, Moussa Fall, Mbengué Ndiaye, Aminata Ba, and et al. 2020. "Assessing the Risk of Occurrence of Bluetongue in Senegal" Microorganisms 8, no. 11: 1766. https://doi.org/10.3390/microorganisms8111766
APA StyleGahn, M. C. B., Niakh, F., Ciss, M., Seck, I., Lo, M. M., Fall, A. G., Biteye, B., Fall, M., Ndiaye, M., Ba, A., Seck, M. T., Sall, B., Lo, M., Faye, C., Squarzoni-Diaw, C., Ka, A., Amevoin, Y., & Apolloni, A. (2020). Assessing the Risk of Occurrence of Bluetongue in Senegal. Microorganisms, 8(11), 1766. https://doi.org/10.3390/microorganisms8111766







