Effect of Schwertmannite Surface Modification by Surfactants on Adhesion of Acidophilic Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria Isolation and Growth
2.2. Synthesis of Schwertmannite
2.3. Surfactants and Biosurfactant
2.4. Electrokinetic Measurements
2.5. Particle Size Analysis
2.6. Contact Angle Measurements
2.7. Stability of Schwertmannite Suspensions
3. Results
3.1. Zeta Potentials
3.2. Particle Size Distribution
3.3. Stability Measurements
3.4. Surface Energy
3.5. Bacterial Cell Adhesion—Thermodynamic Approach
3.6. Bacterial Cell Adhesion—DLVO Approach
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miyata, N.; Takahashi, A.; Fujii, T.; Hashimoto, H.; Takada, J. Biosynthesis of schwertmannite and goethite in a bioreactor with acidophilic Fe(II)-oxidizing betaproteobacterium strain GJ-E10. Minerals 2018, 8, 98. [Google Scholar] [CrossRef]
- Wang, H.; Bigham, J.M.; Tuovinen, O.H. Formation of schwertmannite and its transformation to jarosite in the presence of acidophilic iron-oxidising microorganisms. Mater. Sci. Eng. C 2006, 26, 588–592. [Google Scholar]
- Bigham, J.M.; Schwertmann, U.; Traina, S.J.; Winland, R.L.; Wolf, M. Schwertmannite and the chemical modeling of iron in acid sulfate waters. Geochim. Cosmochim. Acta 1996, 60, 2111–2121. [Google Scholar] [CrossRef]
- Liao, Y.; Zhou, L.; Liang, J.; Xiong, H. Biosynthesis of schwertmannite by Acidithibacillus ferrooxidans cell suspensions under different pH condition. Mater. Sci. Eng. C 2009, 29, 211–215. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Y.; Wang, H. Comparison of the biological and chemical synthesis of schwertmannite at consistent Fe2+ oxidation efficiency and the effect of extracellular polymeric substances of Acidithiobacillus ferrooxidans on biomineralisation. Materials 2018, 11, 1739. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhou, L. Fe2+ oxidation rate drastically affect the formation and phase of secondary iron hydroxysulfate mineral occurred in acid mine drainage. Mater. Sci. Eng. C 2012, 32, 916–921. [Google Scholar] [CrossRef]
- Farahat, M.; Hirajima, T.; Sasaki, K. Adhesion of Ferroplasma acidophilum onto pyrite calculated from the extended DLVO theory using the van Oss-Good-Chaudhury approach. J. Colloid Interface Sci. 2010, 349, 594–601. [Google Scholar] [CrossRef]
- Alam, F.; Kumar, S.; Varadarajan, K.M. Quantification of adhesion force of bacteria on the surface of biomaterials: Techniques and assays. ACS Biomater. Sci. Eng. 2019, 5, 2093–2110. [Google Scholar] [CrossRef]
- Vilinska, A.; Rao, K.H. Surface thermodynamics and extended DLVO theory of Acidithiobacillus ferrooxidans cells adhesion on pyrite and chalcopyrite. Open Colloid Sci. J. 2009, 2, 1–14. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Yan, T.; Jiang, Z.; Zhang, X.; Zuo, Y.Y. Quantitatively predicting bacterial adhesion using surface free energy determined with a spectrophotometric method. Environ. Sci. Technol. 2015, 49, 6164–6171. [Google Scholar] [CrossRef]
- Absolom, D.R.; Lamberti, F.V.; Policova, Z.; Zingg, W.; van Oss, C.J.; Neumann, A.W. Surface thermodynamics of bacterial adhesion. Appl. Environ. Microbiol. 1983, 46, 90–97. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, H. Bacterial adhesion to silica sand as related to Gibbs energy variations. Colloids Surf. B 2005, 44, 41–48. [Google Scholar] [CrossRef]
- Van der Westen, R.; Sjollema, J.; Molenaar, R.; Sharma, P.K.; van der Mei, H.C.; Busscher, H.J. Floating and tether-coupled adhesion of bacteria to hydrophobic and hydrophilic surfaces. Langmuir 2018, 34, 4937–4944. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Q. Influence of surface energy of modified surfaces on bacterial adhesion. Biophys. Chem. 2005, 117, 39–45. [Google Scholar] [CrossRef]
- Diao, M.; Taran, E.; Mahler, S.; Nguyen, T.A.H.; Nguyen, A.V. Quantifying adhesion of acidophilic bioleaching bacteria to silica and pyrite by atomic force microscopy with a bacterial probe. Colloids Surf. B Biointerfaces 2014, 115, 229–236. [Google Scholar] [CrossRef]
- Diao, M.; Taran, E.; Mahler, S.; Nguyen, A.V. A concise review of nanoscopic aspects of bioleaching bacteria-mineral interactions. Adv. Colloid Interface Sci. 2014, 212, 45–63. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Q.; Jiao, W.; Jiang, H.; Sand, W.; Xia, J.; Liu, X.; Qin, W.; Qiu, G.; Hu, Y.; et al. Adhesion forces between cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans or Leptospirillum ferrooxidans and chalcopyrite. Colloids Surf. B 2012, 94, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.H.P.; Chan, K.-Y.; Xu, L.-C. Quantification of bacterial adhesion forces using atomic force microscopy (AFM). J. Microbiol. Methods 2000, 40, 89–97. [Google Scholar] [CrossRef]
- Hong, Z.-N.; Jiang, J.; Li, J.-Y.; Xu, R.-K. Preferential adhesion of surface groups of Bacillus subtilis on gibbsite at different ionic strengths and pHs revealed by ATR-FTIR spectroscopy. Colloids Surf. B 2018, 165, 83–91. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Zhu, J.; Zhou, S.; Gan, M.; Jiang, H.; Sand, W. Effect of extracellular polymeric substances on surface properties and attachment behavior of Acidithiobacillus ferrooxidans. Minerals 2016, 6, 100. [Google Scholar] [CrossRef]
- Vardanyan, A.; Vardanyan, N.; Khachatryan, A.; Zhang, R.; Sand, W. Adhesion to mineral surface by cells of Leptospirillum, Acidithiobacillus and Sulfobacillus from Armenian sulfide ores. Minerals 2019, 9, 69. [Google Scholar] [CrossRef]
- Jacobs, A.; Lofolie, F.; Herry, J.M.; Debroux, M. Kinetic adhesion of bacterial cells to sand: Cell surface properties and adhesion rate. Colloids Surf. B 2007, 59, 35–45. [Google Scholar] [CrossRef]
- Vilinska, A.; Rao, K.H. Surface thermodynamics and extended DLVO theory of Leptospirillum ferrooxidans cells’ adhesion on sulfide minerals. Miner. Metall. Proc. 2011, 28, 151–158. [Google Scholar] [CrossRef]
- Lin, W.; Liu, S.; Tong, L.; Zhang, Y.; Yang, J.; Liu, W.; Guo, C.; Xie, Y.; Lu, G.; Dang, Z. Effects of rhamnolipids on the cell surface characteristics of Sphingomonas sp. GY2B and the biodegradation of phenanthrene. RSC Adv. 2017, 7, 24321–24330. [Google Scholar] [CrossRef]
- Wang, H.; Sodagari, M.; Chen, Y.; He, X.; Newby, B.-M.Z.; Ju, L.-K. Initial bacterial attachment in slow flowing systems: Effects of cell and substrate surface properties. Colloids Surf. B 2011, 87, 415–422. [Google Scholar] [CrossRef]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Katsikogianni, M.; Missirlis, Y.F. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cell. Mater. 2004, 8, 37–57. [Google Scholar]
- Bendersky, M.; Davis, J.M. DLVO interaction of colloid particles with topographically and chemically heterogeneous surfaces. J. Colloid Interface Sci. 2011, 353, 87–97. [Google Scholar] [CrossRef]
- Li, G.L.; Zhou, C.H.; Fiore, S.; Yu, W.H. Interactions between microorganisms and clay minerals: New insights and broader applications. Appl. Clay Sci. 2019, 177, 91–113. [Google Scholar] [CrossRef]
- van Loosdrecht, M.C.M.; Lyklema, J.; Norde, W.; Zehnder, A.J.B. Bacterial adhesion: A physicochemical approach. Microb. Ecol. 1989, 17, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Rong, X.; Cai, P.; Dai, K.; Liang, W.; Chen, W.; Huang, Q. Initial adhesion of Bacillus subtilis on soil minerals as related to their surface properties. Eur. J. Soil Sci. 2012, 63, 457–466. [Google Scholar] [CrossRef]
- Dockrey, J.W.; Lindsay, M.B.J.; Mayer, K.U.; Beckie, R.D.; Norlund, K.L.I.; Warren, L.A.; Southam, G. Acidic microenvironments in waste rock characterized by neutral drainage: Bacteria-mineral interactions at sulfide surfaces. Minerals 2014, 4, 170–190. [Google Scholar] [CrossRef]

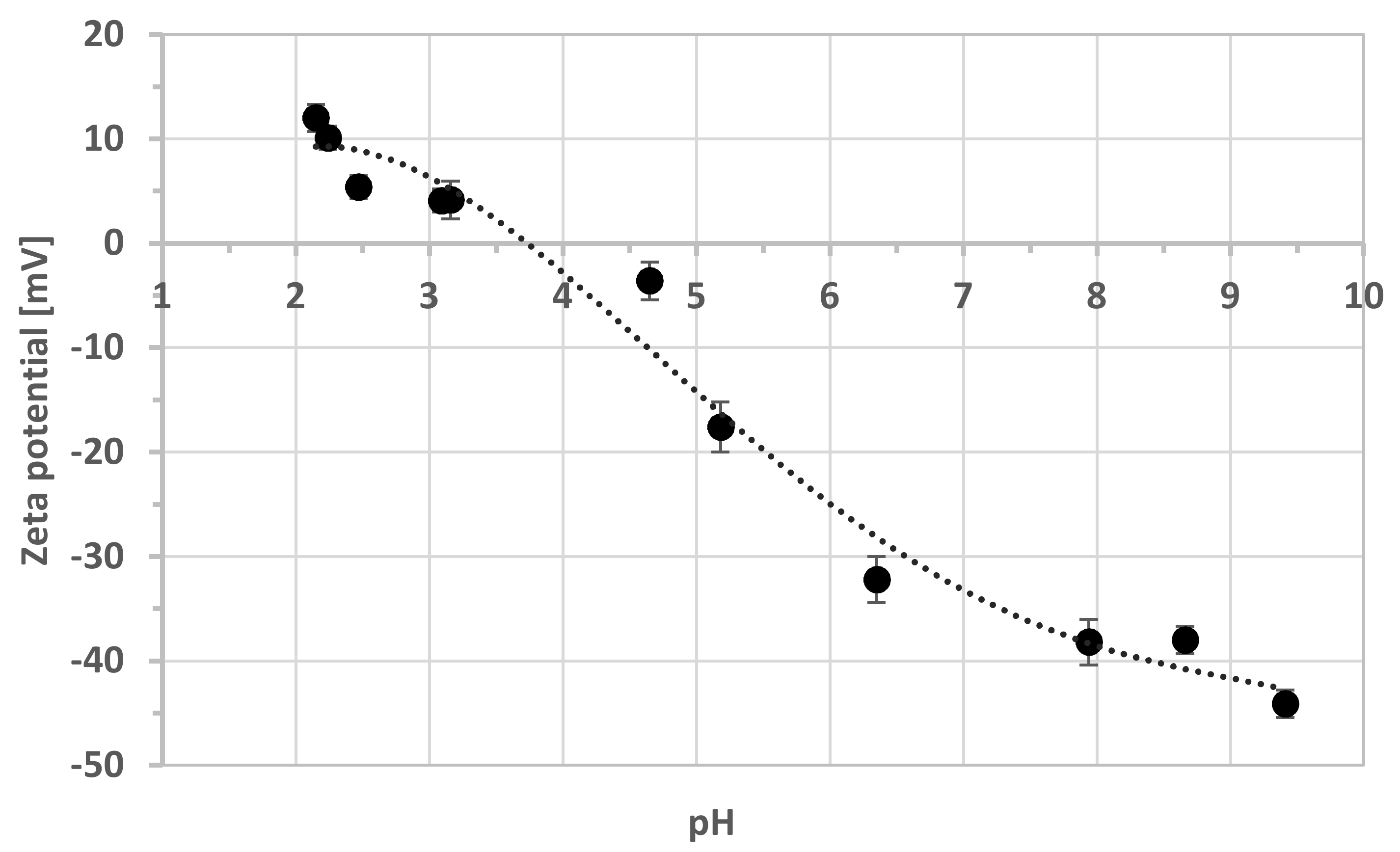
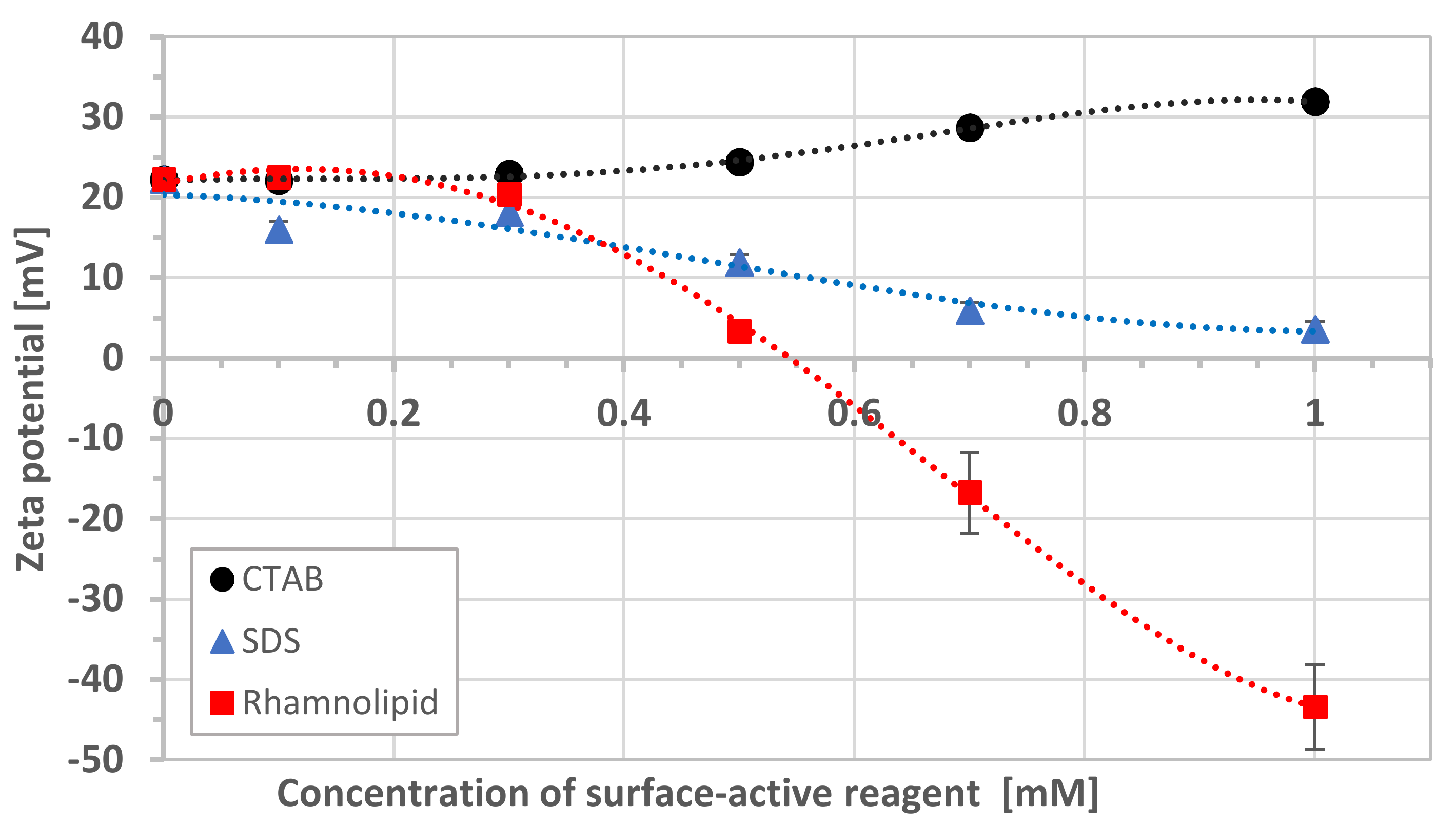
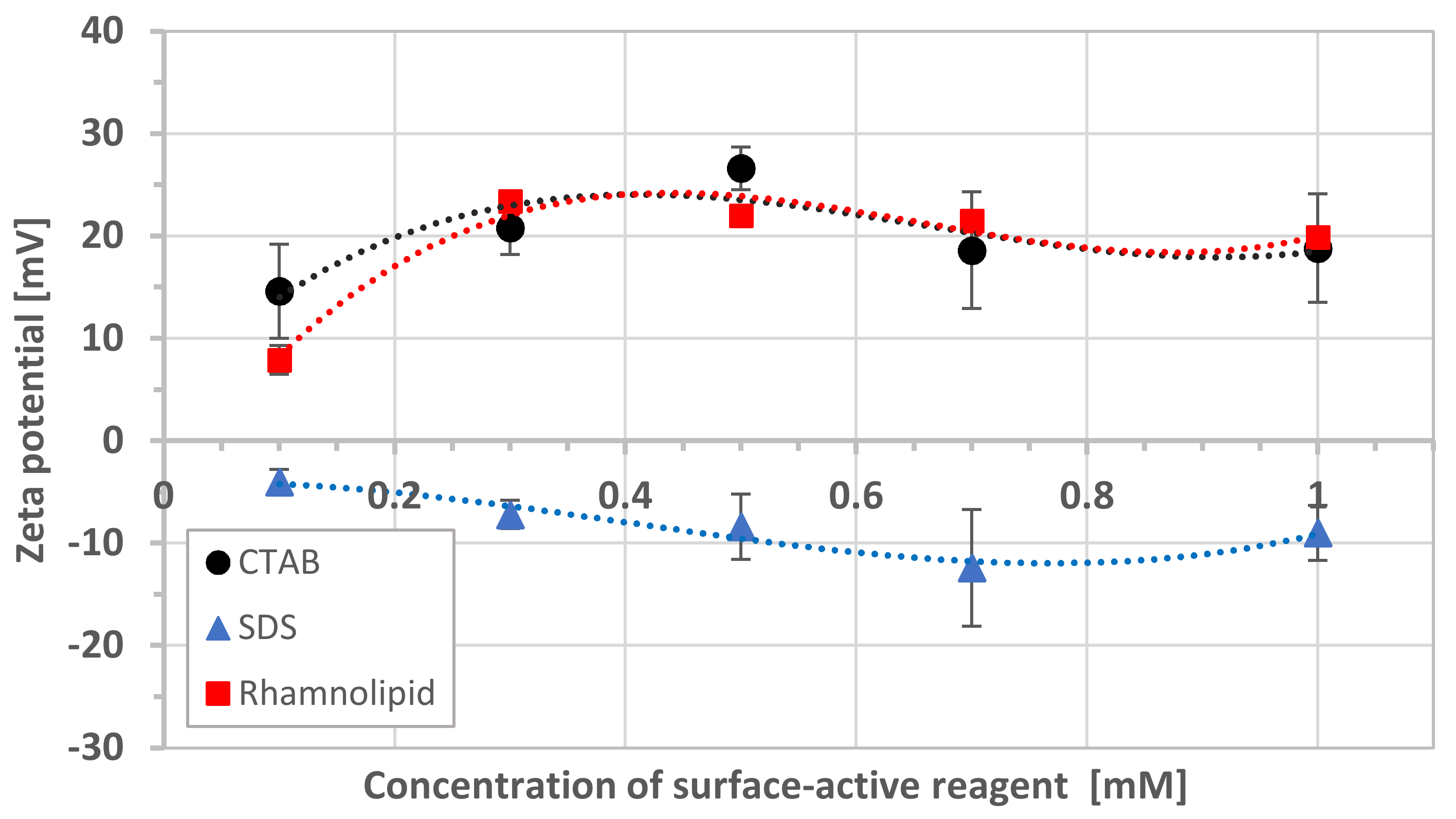

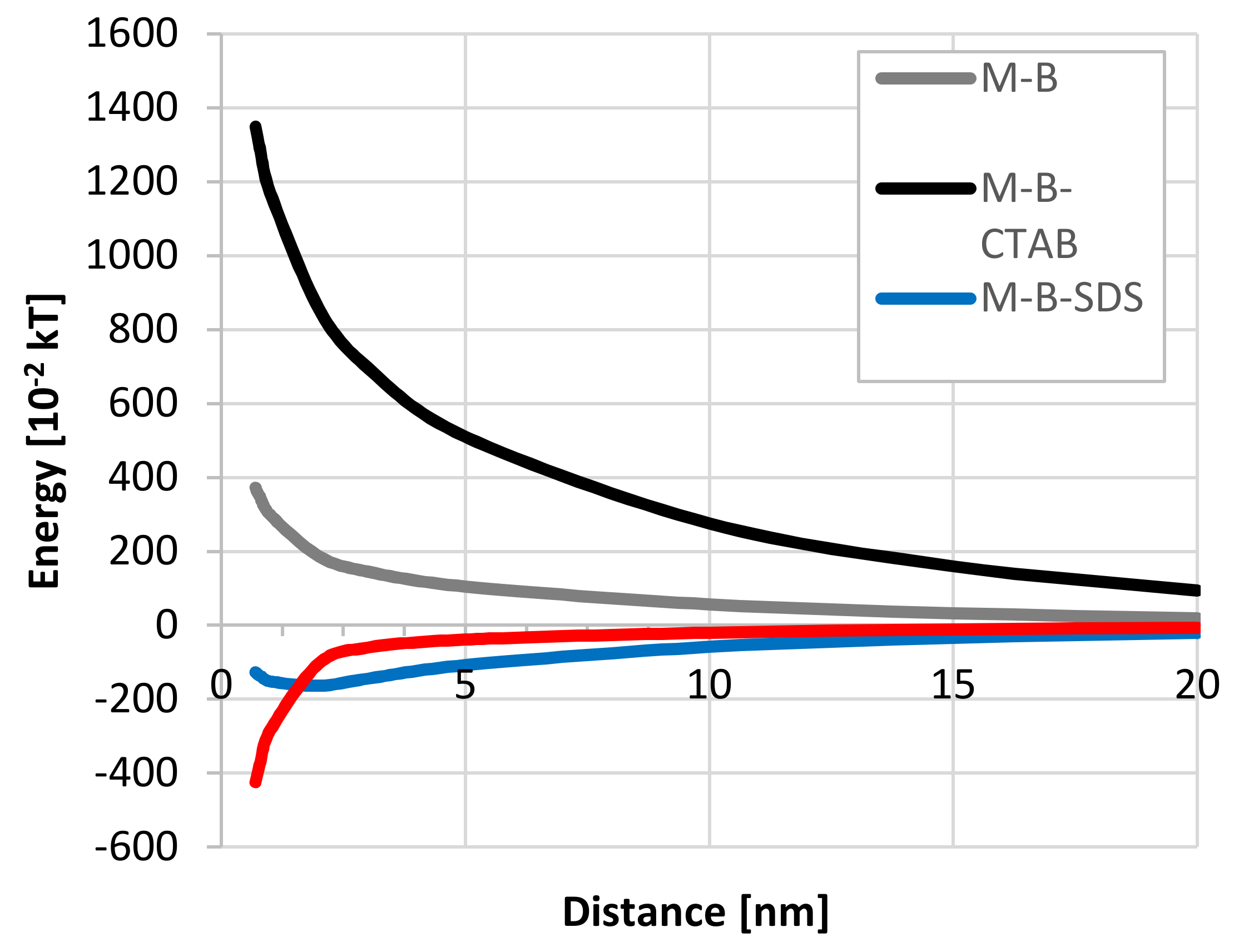
| pH | Zeta Potential [mV] | SD * |
|---|---|---|
| 1.91 | +4.8 | 1.1 |
| 2.33 | +4.6 | 1.1 |
| 2.57 | +4.4 | 1.4 |
| 3.01 | +5.0 | 1.2 |
| Size Distribution Parameter | The Concentration of Surface-Active Compound (mM) | ||||
|---|---|---|---|---|---|
| 0.1 | 0.3 | 0.5 | 0.7 | 1.0 | |
| Schwertmannite-Rhamnolipid | |||||
| Mean (μm) | 57.38 | 48.71 | 65.93 | 30.80 | 110.9 |
| Median (μm) | 41.16 | 29.20 | 51.17 | 22.41 | 58.49 |
| Mode (μm) | 37.97 | 28.70 | 80.08 | 31.51 | 80.07 |
| Span | 2.14 | 2.47 | 2.05 | 3.26 | 8.42 |
| Schwertmannite-SDS | |||||
| Mean (μm) | 24.61 | 25.70 | 25.19 | 25.32 | 25.87 |
| Median (μm) | 21.40 | 22.34 | 21.77 | 21.70 | 22.40 |
| Mode (μm) | 26.15 | 28.70 | 28.70 | 28.70 | 28.70 |
| Span | 1.82 | 1.84 | 1.78 | 1.905 | 1.916 |
| Schwertmannite-CTAB | |||||
| Mean (μm) | 25.25 | 27.46 | 26.32 | 29.86 | 28.93 |
| Median (μm) | 21.98 | 21.89 | 22.03 | 23.35 | 22.93 |
| Mode (μm) | 26.15 | 26.15 | 26.15 | 28.70 | 26.15 |
| Span | 1.80 | 1.95 | 1.86 | 2.04 | 2.03 |
| Sample | Contact Angle (o) | Surface Free Energy (mJ/m2) | ||||||
|---|---|---|---|---|---|---|---|---|
| Water | Diiodo-Methane | Form-Amide | γ− | γ+ | γAB | γLW | γTOT * | |
| Bacteria | 47.7 | 27.3 | 24.8 | 23.4 | 0.937 | 9.36 | 45.4 | 54.8 |
| Pure Mineral | 17.0 | 26.0 | 8.00 | 49.7 | 0.600 | 11.2 | 45.8 | 57.0 |
| Mineral-CTAB | 21.0 | 35.0 | 16.0 | 48.8 | 0.900 | 13.4 | 42.0 | 55.4 |
| Mineral-SDS | 17.0 | 28.0 | 17.0 | 52.6 | 0.40 | 9.40 | 45.0 | 54.4 |
| Mineral-Rhamnolipid | 100 | 42.0 | 61.0 | 0.40 | 0.70 | 1.10 | 38.6 | 39.7 |
| Sample | γBS | γBL | γSL | |||
|---|---|---|---|---|---|---|
| Pure Mineral | −0.8570 | 6.061 | −12.70 | −30.99 | 14.47 | −16.52 |
| Mineral-CTAB | −0.0165 | 6.061 | −12.60 | −28.42 | 14.02 | −14.40 |
| Mineral-SDS | −1.623 | 6.061 | −15.30 | −33.28 | 16.06 | −17.22 |
| Mineral-Rhamnolipid | 1.380 | 6.061 | 39.60 | −49.75 | −37.89 | −87.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlowska, A.; Sadowski, Z. Effect of Schwertmannite Surface Modification by Surfactants on Adhesion of Acidophilic Bacteria. Microorganisms 2020, 8, 1725. https://doi.org/10.3390/microorganisms8111725
Pawlowska A, Sadowski Z. Effect of Schwertmannite Surface Modification by Surfactants on Adhesion of Acidophilic Bacteria. Microorganisms. 2020; 8(11):1725. https://doi.org/10.3390/microorganisms8111725
Chicago/Turabian StylePawlowska, Agnieszka, and Zygmunt Sadowski. 2020. "Effect of Schwertmannite Surface Modification by Surfactants on Adhesion of Acidophilic Bacteria" Microorganisms 8, no. 11: 1725. https://doi.org/10.3390/microorganisms8111725
APA StylePawlowska, A., & Sadowski, Z. (2020). Effect of Schwertmannite Surface Modification by Surfactants on Adhesion of Acidophilic Bacteria. Microorganisms, 8(11), 1725. https://doi.org/10.3390/microorganisms8111725





