Urine-Based Antigen (Protein) Detection Test for the Diagnosis of Visceral Leishmaniasis
Abstract
:1. Introduction
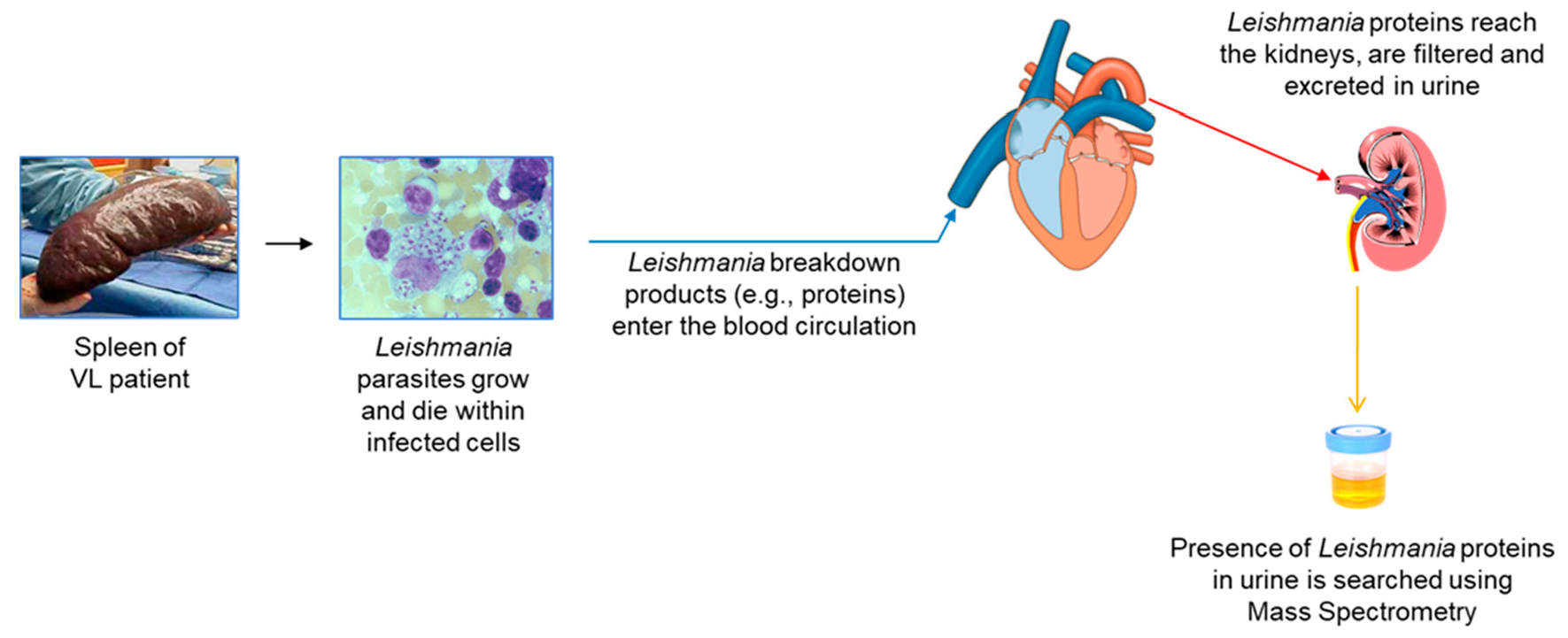
2. Overall Rationale
- Urine contains much less host proteins than plasma or serum, therefore small amounts of molecules from foreign organisms present in this excretion are much more likely to be identified by mass spectrometry than they would in blood.
- Urine is one of the easiest and least invasive samples that can be collected from a patient. This is particularly important for VL and for example tuberculosis (TB) in children. Gold standard diagnosis of VL requires invasive biopsy of organs like liver, spleen or bone marrow. In TB, because children in general do not spit sputum, invasive gastric lavage is the sample that is used to investigate the presence of Mycobacterium tuberculosis in this group of patients.
- Antigenemia and subsequent antigenuria occurs as a natural consequence of most infectious processes.
- The presence of pathogen’s antigens excreted in the urine may be detectable sooner than the detection of the pathogen itself in target organs of the disease.
- Antigen detection in urine is an attractive possibility to facilitate the diagnosis of VL in HIV-co-infected patients because these patients have in general reduced antibody response to L. infantum/L. donovani antigens.
- Antigens found in urine, theoretically, are likely to be highly stable because they have to resist the proteolytic enzymes present in both, target organs of infection and serum. Therefore, this stability makes them attractive candidates for a diagnostic assay.
- Urine collection is independent of medical facilities, does not require sterile conditions, medical equipment/personnel, and involves minimal sample preparation.
- Urine is a readily available sample for point-of-care diagnostic tests.
3. Approach
4. Markers Discovery and Polyclonal Antibody-Based Test
4.1. L. infantum Markers
4.2. L. donovani Markers
5. Monoclonal Antibody-Based Test
5.1. Recombinant Single Domain Antibodies or VHH
5.2. Conventional mAbs
6. Overall Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; Boer, M.D. Who Leishmaniasis Control the WHO Leishmaniasis Control Team Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- WHO. Weekly Epidemiological Record; WHO: Geneva, Switzerland, 2019; Volume 95, pp. 1–12. [Google Scholar]
- Alvar, J.; Aparicio, P.; Aseffa, A.; Boer, M.D.; Cañavate, C.; Dedet, J.-P.; Gradoni, L.; Ter Horst, R.; López-Vélez, R.; Moreno, J. The Relationship between Leishmaniasis and AIDS: The Second 10 Years. Clin. Microbiol. Rev. 2008, 21, 334–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvar, J.; Canavate, C.; Gutierrez-Solar, B.; Jimenez, M.; Laguna, F.; Lopez-Velez, R.; Molina, R.; Moreno, J. Leishmania and human immunodeficiency virus coinfection: The first 10 years. Clin. Microbiol Rev. 1997, 10, 298–319. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.A.; Cruz, I.; Rubio, J.M.; Chicharro, C.; Cañavate, C.; Laguna, F.; Alvar, J. Relapses versus Reinfections in Patients Coinfected withLeishmania infantumand Human Immunodeficiency Virus Type 1. J. Infect. Dis. 2002, 185, 1533–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinnell, R.J.; Courtenay, O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology 2009, 136, 1915–1934. [Google Scholar] [CrossRef]
- Belazzoug, S. Leishmaniasis in Mediterranean countries. Vet. Parasitol. 1992, 44, 15–19. [Google Scholar] [CrossRef]
- Dujardin, J.-C.; Campino, L.; Cañavate, C.; Dedet, J.-P.; Gradoni, L.; Soteriadou, K.; Mazeris, A.; Özbel, Y.; Boelaert, M. Spread of Vector-borne Diseases and Neglect of Leishmaniasis, Europe. Emerg. Infect. Dis. 2008, 14, 1013–1018. [Google Scholar] [CrossRef]
- Boggiatto, P.M.; Gibson-Corley, K.N.; Metz, K.; Gallup, J.M.; Hostetter, J.M.; Mullin, K.; Petersen, C.A. Transplacental Transmission of Leishmania infantum as a Means for Continued Disease Incidence in North America. PLoS Negl. Trop. Dis. 2011, 5, e1019. [Google Scholar] [CrossRef]
- Eddlestone, S.M. Visceral leishmaniasis in a dog from Maryland. J. Am. Vet. Med Assoc. 2000, 217, 1686–1688. [Google Scholar] [CrossRef]
- Gaskin, A.A.; Schantz, P.; Jackson, J.; Birkenheuer, A.; Tomlinson, L.; Gramiccia, M.; Levy, M.; Steurer, F.; Kollmar, E.; Hegarty, B.C.; et al. Visceral leishmaniasis in a New York foxhound kennel. J. Vet. Intern Med. 2002, 16, 34–44. [Google Scholar] [CrossRef]
- Chappuis, F.; Sundar, S.; Hailu, A.; Ghalib, H.; Rijal, S.; Peeling, R.W.; Alvar, J.; Boelaert, M. Visceral leishmaniasis: What are the needs for diagnosis, treatment and control? Nat. Rev. Microbiol. 2007, 5, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.A.; Soong, T.-H.; Li, Y. Comparative merits of sternum, spleen and liver punctures in the study of human visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 1948, 41, 629–636. [Google Scholar] [CrossRef]
- Cota, G.F.; De Sousa, M.R.; Rabello, A. Predictors of Visceral Leishmaniasis Relapse in HIV-Infected Patients: A Systematic Review. PLoS Negl. Trop. Dis. 2011, 5, e1153. [Google Scholar] [CrossRef] [Green Version]
- Guerin, P.J.; Olliaro, P.; Sundar, S.; Boelaert, M.; Croft, S.L.; Desjeux, P.; Wasunna, M.K.; Bryceson, A.D.M. Visceral leishmaniasis: Current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect. Dis. 2002, 2, 494–501. [Google Scholar] [CrossRef]
- Melby, P.C. Recent developments in leishmaniasis. Curr. Opin. Infect. Dis. 2002, 15, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Dayama, A.; Mehrotra, S.; Sundar, S. Diagnosis of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, P.; Mehrotra, S.; Tiwary, P.; Chakravarty, J.; Sundar, S. Diagnosis of Indian Visceral Leishmaniasis by Nucleic Acid Detection Using PCR. PLoS ONE 2011, 6, e19304. [Google Scholar] [CrossRef]
- Sundar, S.; Rai, M. Laboratory Diagnosis of Visceral Leishmaniasis. Clin. Vaccine Immunol. 2002, 9, 951–958. [Google Scholar] [CrossRef] [Green Version]
- Antinori, S.; Calattini, S.; Longhi, E.; Bestetti, G.; Piolini, R.; Magni, C.; Orlando, G.; Gramiccia, M.; Acquaviva, V.; Foschi, A.; et al. Clinical Use of Polymerase Chain Reaction Performed on Peripheral Blood and Bone Marrow Samples for the Diagnosis and Monitoring of Visceral Leishmaniasis in HIV-Infected and HIV-Uninfected Patients: A Single-Center, 8-Year Experience in Italy and Review of the Literature. Clin. Infect. Dis. 2007, 44, 1602–1610. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.M.; Durand, R.; Deniau, M.; Rivollet, D.; Izri, M.; Houin, R.; Vidaud, M.; Bretagne, S. PCR enzyme-linked immunosorbent assay for diagnosis of leishmaniasis in human immunodeficiency virus-infected patients. J. Clin. Microbiol. 1996, 34, 1831–1833. [Google Scholar] [CrossRef] [Green Version]
- Riera, C.; Falcó, V.; Molina, I.; Ribera, E.; Fisa, R.; Portús, M.; Moner, L.; Gállego, M.; Carrió, J. Value of culture and nested polymerase chain reaction of blood in the prediction of relapses in patients co-infected with leishmania and human immunodeficiency virus. Am. J. Trop. Med. Hyg. 2005, 73, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Badaró, R.; Lobo, I.; Munõs, A.; Netto, E.M.; Modabber, F.; Campos-Neto, A.; Coler, R.N.; Reed, S.G. Immunotherapy for Drug-Refractory Mucosal Leishmaniasis. J. Infect. Dis. 2006, 194, 1151–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braz, R.F.S.; Martins, D.R.A.; Wilson, M.E.; Reed, S.G.; Pearson, R.D.; Jeronimo, S.M.B.; Nascimento, E.T. The sensitivity and specificity of Leishmania chagasi recombinant K39 antigen in the diagnosis of American visceral leishmaniasis and in differentiating active from subclinical infection. Am. J. Trop. Med. Hyg. 2002, 67, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.M.; Barral, A.; Pedral-Sampaio, D.; Barral-Netto, M.; Badaró, R.; Rocha, H.; Johnson, W.D. Immunologic Markers of Clinical Evolution in Children Recently Infected with Leishmania donovani chagasi. J. Infect. Dis. 1992, 165, 535–540. [Google Scholar] [CrossRef]
- Costa, C.H.N.; Stewart, J.M.; Dissanayake, S.; Maguire, J.H.; Shaw, J.J.; David, J.R.; Bozza, M.; Ramos, P.K.S.; Silva, M.R.B.; Santos, R.S.; et al. Asymptomatic human carriers of Leishmania chagasi. Am. J. Trop. Med. Hyg. 2002, 66, 334–337. [Google Scholar] [CrossRef]
- Viana, L.D.G.; De Assis, T.S.M.; Orsini, M.; Da Silva, A.R.; De Souza, G.F.; Caligiorne, R.; Da Silva, A.C.L.; Peruhype-Magalhães, V.; Marciano, A.P.V.; Martins-Filho, O.A.; et al. Combined diagnostic methods identify a remarkable proportion of asymptomatic Leishmania (Leishmania) chagasi carriers who present modulated cytokine profiles. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 548–555. [Google Scholar] [CrossRef]
- Romero, H.D.; Silva, L.D.A.; Silva-Vergara, M.L.; Rodrigues, V.; Costa, R.T.; Guimarães, S.F.; Alecrim, W.; Moraes-Souza, H.; Prata, A. Comparative study of serologic tests for the diagnosis of asymptomatic visceral leishmaniasis in an endemic area. Am. J. Trop. Med. Hyg. 2009, 81, 27–33. [Google Scholar]
- Silva, L.D.A.; Nascimento, E.; Carvalho, S.F.G.; Prata, A.; Romero, H.D.; Rodrigues, V.; Costa, R.T. IMMUNOLOGIC TESTS IN PATIENTS AFTER CLINICAL CURE OF VISCERAL LEISHMANIASIS. Am. J. Trop. Med. Hyg. 2006, 75, 739–743. [Google Scholar] [CrossRef] [Green Version]
- Hailu, A. Pre- and post-treatment antibody levels in visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 673–675. [Google Scholar] [CrossRef]
- Kumar, P.; Pai, K.; Tripathi, K.; Pandey, H.P.; Sundar, S. Immunoblot Analysis of the Humoral Immune Response to Leishmania donovani Polypeptides in Cases of Human Visceral Leishmaniasis: Its Usefulness in Prognosis. Clin. Vaccine Immunol. 2002, 9, 1119–1123. [Google Scholar] [CrossRef] [Green Version]
- Falqueto, A.; Cupolillo, E.; Ferreira, A.L.; Porrozzi, R.; Jr, G.G.; Teva, A.; Dos Santos, C.B.; Da Costa, M.V.S.; Campos-Neto, A. Cross-sectional and longitudinal epidemiologic surveys of human and canine Leishmania infantum visceral infections in an endemic rural area of southeast Brazil (Pancas, Espirito Santo). Am. J. Trop. Med. Hyg. 2009, 80, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Houghton, R.L.; Petrescu, M.; Benson, D.R.; Skeiky, Y.A.; Scalone, A.; Badaro, R.; Reed, S.G.; Gradoni, L. A cloned antigen (recombinant K39) of Leishmania chagasi diagnostic for visceral leishmaniasis in human immunodeficiency virus type 1 patients and a prognostic indicator for monitoring patients undergoing drug therapy. J. Infect. Dis. 1998, 177, 1339–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Gomes, G.; Gomes-Pereira, S.; Campino, L.; Araújo, M.D.A.; Abranches, P. Performance of Immunoblotting in Diagnosis of Visceral Leishmaniasis in Human Immunodeficiency Virus-Leishmania sp.-Coinfected Patients. J. Clin. Microbiol. 2000, 38, 175–178. [Google Scholar]
- Liu, Y.-P. Rapid and quantitative detection of hepatitis B virus. World J. Gastroenterol. 2015, 21. [Google Scholar] [CrossRef] [PubMed]
- Rysgaard, C.D.; Morris, C.S.; Drees, D.; Bebber, T.; Davis, S.R.; Kulhavy, J.; Krasowski, M.D. Positive hepatitis B surface antigen tests due to recent vaccination: A persistent problem. BMC Clin. Pathol. 2012, 12, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J.; Cohen, R.; Bidet, P.; Levy, C.; Deberdt, P.; D’Humières, C.; Liguori, S.; Corrard, F.; Thollot, F.; Mariani-Kurkdjian, P.; et al. Rapid-Antigen Detection Tests for Group A Streptococcal Pharyngitis: Revisiting False-Positive Results Using Polymerase Chain Reaction Testing. J. Pediatr. 2013, 162, 1282–1284.e1. [Google Scholar] [CrossRef] [PubMed]
- Dowell, S.F.; Garman, R.L.; Liu, G.; Levine, O.S.; Yang, Y.-H. Evaluation of Binax NOW, an Assay for the Detection of Pneumococcal Antigen in Urine Samples, Performed among Pediatric Patients. Clin. Infect. Dis. 2001, 32, 824–825. [Google Scholar] [CrossRef] [Green Version]
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin. Infect. Dis. 2007, 44, S27–S72. [Google Scholar] [CrossRef]
- Dionne, M.; Hatchette, T.; Forward, K. Clinical utility of a Legionella pneumophila urinary antigen test in a large university teaching hospital. Can. J. Infect. Dis. 2003, 14, 85–88. [Google Scholar] [CrossRef] [Green Version]
- Flores, L.L.; Steingart, K.R.; Dendukuri, N.; Schiller, I.; Minion, J.; Pai, M.; Ramsay, A.; Henry, M.; Laal, S. Systematic Review and Meta-Analysis of Antigen Detection Tests for the Diagnosis of Tuberculosis. Clin. Vaccine Immunol. 2011, 18, 1616–1627. [Google Scholar] [CrossRef] [Green Version]
- Pollock, N.R.; Macovei, L.; Kanunfre, K.A.; Dhiman, R.; Restrepo, B.I.; Zarate, I.; Pino, P.A.; Mora-Guzman, F.; Fujiwara, R.T.; Michel, G.; et al. Validation of Mycobacterium tuberculosis Rv1681 Protein as a Diagnostic Marker of Active Pulmonary Tuberculosis. J. Clin. Microbiol. 2013, 51, 1367–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beadle, C.; Long, G.; McElroy, P.; Hoffman, S.; Weiss, W.; Maret, S.; Oloo, A. Diagnosis of malaria by detection of Plasmodium falciparum HRP-2 antigen with a rapid dipstick antigen-capture assay. Lancet 1994, 343, 564–568. [Google Scholar] [CrossRef] [Green Version]
- Jain, P.; Chakma, B.; Patra, S.; Goswami, P. Potential Biomarkers and Their Applications for Rapid and Reliable Detection of Malaria. BioMed Res. Int. 2014, 2014, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathison, B.A.; Pritt, B.S. Update on Malaria Diagnostics and Test Utilization. J. Clin. Microbiol. 2017, 55, 2009–2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanyuksel, M., Jr.; Petri, W.A. Laboratory Diagnosis of Amebiasis. Clin. Microbiol. Rev. 2003, 16, 713–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyosei, Y.; Namba, M.; Yamura, S.; Takeuchi, R.; Aoki, N.; Nakaishi, K.; Watabe, S.; Ito, E. Proposal of De Novo Antigen Test for COVID-19: Ultrasensitive Detection of Spike Proteins of SARS-CoV-2. Diagnostics 2020, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Mak, G.C.; Cheng, P.K.; Lau, S.S.; Wong, K.K.; Lau, C.S.; Lam, E.T.; Chan, R.C.; Tsang, D.N. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J. Clin. Virol. 2020, 129, 104500. [Google Scholar] [CrossRef]
- Nagura-Ikeda, M.; Imai, K.; Tabata, S.; Miyoshi, K.; Murahara, N.; Mizuno, T.; Horiuchi, M.; Kato, K.; Imoto, Y.; Iwata, M.; et al. Clinical Evaluation of Self-Collected Saliva by Quantitative Reverse Transcription-PCR (RT-qPCR), Direct RT-qPCR, Reverse Transcription–Loop-Mediated Isothermal Amplification, and a Rapid Antigen Test To Diagnose COVID-19. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef]
- Attar, Z.J.; Chance, M.L.; El-Safi, S.; Carney, J.; Azazy, A.; El-Hadi, M.; Dourado, C.; Hommel, M. Latex agglutination test for the detection of urinary antigens in visceral leishmaniasis. Acta Trop. 2001, 78, 11–16. [Google Scholar] [CrossRef]
- Sarkari, B.; Chance, M.; Hommel, M. Antigenuria in visceral leishmaniasis: Detection and partial characterisation of a carbohydrate antigen. Acta Trop. 2002, 82, 339–348. [Google Scholar] [CrossRef]
- Ahsan, M.M.; Islam, M.N.; Mollah, A.H.; Hoque, A.; Hossain, M.A.; Begum, Z. Evaluation of latex agglutination test (KAtex) for early diagnosis of kala-azar. Mymensingh Med. J. 2010, 19, 335–339. [Google Scholar]
- Chappuis, F.; Rijal, S.; Jha, U.K.; Desjeux, P.; Karki, B.M.S.; Koirala, S.; Loutan, L.; Boelaert, M. Field validity, reproducibility and feasibility of diagnostic tests for visceral leishmaniasis in rural Nepal. Trop. Med. Int. Health 2006, 11, 31–40. [Google Scholar] [CrossRef] [PubMed]
- El-Safi, S.H.; Abdel-Haleem, A.; Hammad, A.; El-Basha, I.; Omer, A.; Kareem, H.G.; Boelaert, M.; Chance, M.; Hommel, M. Field evaluation of latex agglutination test for detecting urinary antigens in visceral leishmaniasis in Sudan. East. Mediterr. Health J. 2003, 9, 844–855. [Google Scholar] [PubMed]
- Rijal, S.; Boelaert, M.; Regmi, S.; Karki, B.M.S.; Jacquet, D.; Singh, R.; Chance, M.L.; Chappuis, F.; Hommel, M.; Desjeux, P.; et al. Evaluation of a urinary antigen-based latex agglutination test in the diagnosis of kala-azar in eastern Nepal. Trop. Med. Int. Health 2004, 9, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.; Khan, G.; Mondal, D. Urine antigen detection by latex agglutination test for diagnosis and assessment of initial cure of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Agrawal, S.; Pai, K.; Chance, M.; Hommel, M. Detection of leishmanial antigen in the urine of patients with visceral leishmaniasis by a latex agglutination test. Am. J. Trop. Med. Hyg. 2005, 73, 269–271. [Google Scholar] [CrossRef]
- Boelaert, M.; El-Safi, S.; Hailu, A.; Mukhtar, M.; Rijal, S.; Sundar, S.; Wasunna, M.; Aseffa, A.; Mbui, J.; Menten, J.; et al. Diagnostic tests for kala-azar: A multi-centre study of the freeze-dried DAT, rK39 strip test and KAtex in East Africa and the Indian subcontinent. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 32–40. [Google Scholar] [CrossRef]
- Vallur, A.C.; Tutterrow, Y.L.; Mohamath, R.; Pattabhi, S.; Hailu, A.; Abdoun, A.O.; Ahmed, A.E.; Mukhtar, M.; Salam, A.; Almeida, M.L.; et al. Development and comparative evaluation of two antigen detection tests for Visceral Leishmaniasis. BMC Infect. Dis. 2015, 15, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sundar, S. Drug resistance in Indian visceral leishmaniasis. Trop. Med. Int. Health 2001, 6, 849–854. [Google Scholar] [CrossRef]
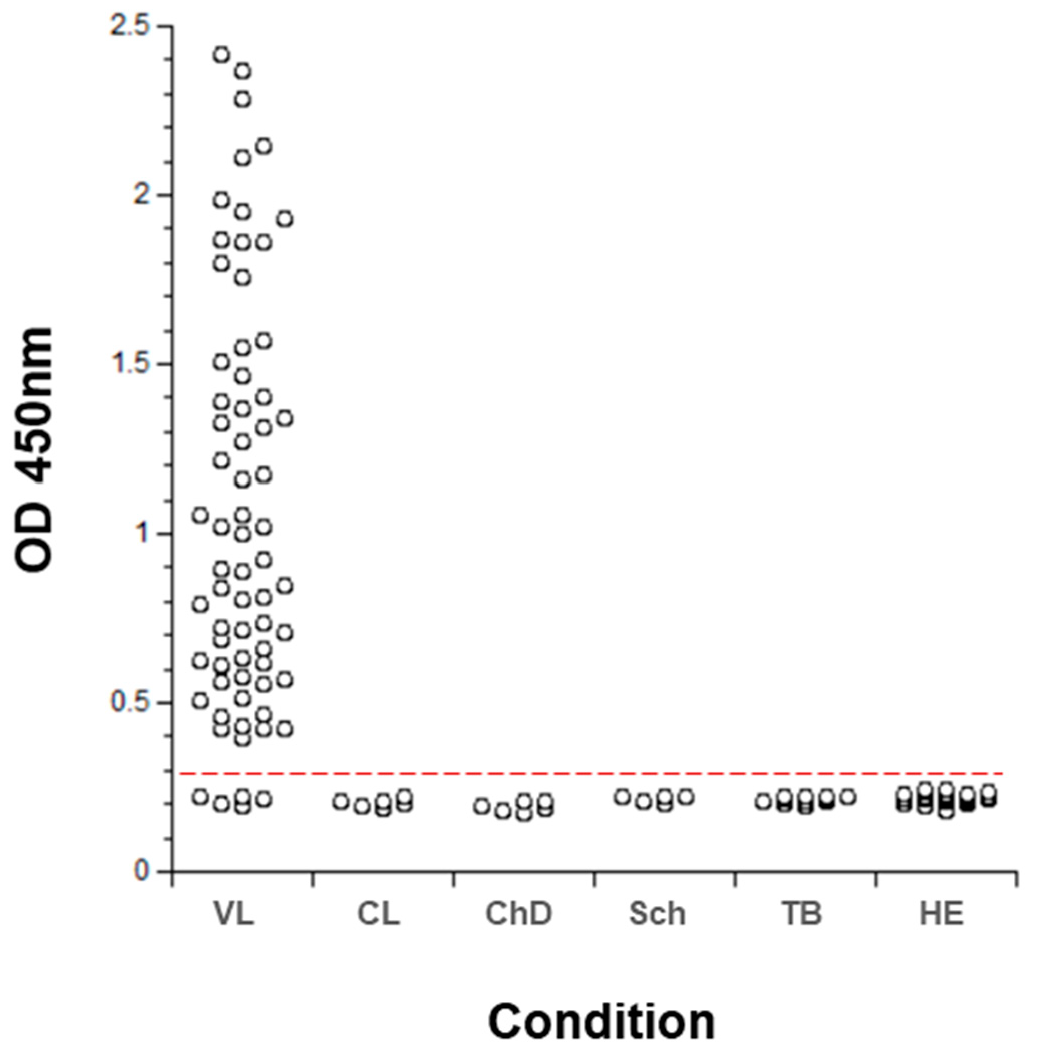
- Abeijon, C.; Alves, F.; Monnerat, S.; Mbui, J.; Viana, A.G.; Almeida, R.M.; Bueno, L.L.; Fujiwara, R.T.; Campos-Neto, A. Urine-based antigen detection assay for diagnosis of visceral leishmaniasis using monoclonal antibodies specific for six protein biomarkers of Leishmania infantum / Leishmania donovani. PLoS Negl. Trop. Dis. 2020, 14, e0008246. [Google Scholar] [CrossRef]
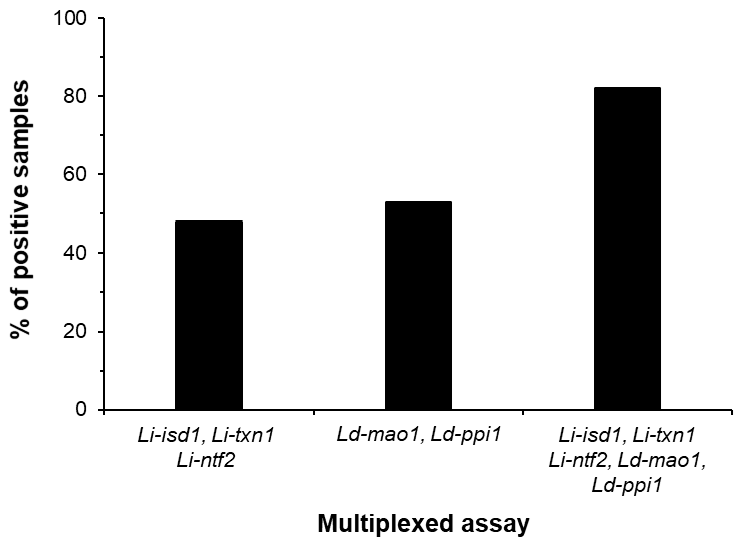
- Abeijon, C.; Alves, F.; Monnerat, S.; Wasunna, M.; Mbui, J.; Viana, A.G.; Bueno, L.L.; Siqueira, W.F.; Carvalho, S.G.; Agrawal, N.; et al. Development of a Multiplexed Assay for Detection of Leishmania donovani and Leishmania infantum Protein Biomarkers in Urine Samples of Patients with Visceral Leishmaniasis. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abeijon, C.; Campos-Neto, A. Potential Non-invasive Urine-Based Antigen (Protein) Detection Assay to Diagnose Active Visceral Leishmaniasis. PLoS Negl. Trop. Dis. 2013, 7, e2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abeijon, C.; Dilo, J.; Tremblay, J.M.; Viana, A.G.; Bueno, L.L.; Carvalho, S.F.G.; Fujiwara, R.T.; Shoemaker, C.B.; Campos-Neto, A. Use of VHH antibodies for the development of antigen detection test for visceral leishmaniasis. Parasite Immunol. 2018, 40, e12584. [Google Scholar] [CrossRef] [Green Version]
- Abeijon, C.; Kashino, S.S.; Silva, F.O.; Costa, D.L.; Fujiwara, R.T.; Costa, C.H.N.; Campos-Neto, A. Identification and Diagnostic Utility of Leishmania infantum Proteins Found in Urine Samples from Patients with Visceral Leishmaniasis. Clin. Vaccine Immunol. 2012, 19, 935–943. [Google Scholar] [CrossRef] [Green Version]
- Marlais, T.; Bhattacharyya, T.; Pearson, C.; Gardner, B.L.; Marhoon, S.; Airs, S.; Hayes, K.; Falconar, A.K.; Singh, O.P.; Reed, S.G.; et al. Isolation and characterisation of Leishmania donovani protein antigens from urine of visceral leishmaniasis patients. PLoS ONE 2020, 15, e0238840. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; King, C.H.; Kittur, N.; Ramzy, R.M.R.; Secor, W.E.; Fredericks-James, M.; Ortu, G.; Clements, M.N.; Ruberanziza, E.; Umulisa, I.; et al. Evaluation, Validation, and Recognition of the Point-of-Care Circulating Cathodic Antigen, Urine-Based Assay for Mapping Schistosoma mansoni Infections. Am. J. Trop. Med. Hyg. 2020, 103, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, P.H.; Jørgensen, C.S.; Wolf, L.A. Performance of the ImmuView and BinaxNOW assays for the detection of urine and cerebrospinal fluid Streptococcus pneumoniae and Legionella pneumophila serogroup 1 antigen in patients with Legionnaires’ disease or pneumococcal pneumonia and meningitis. PLoS ONE 2020, 15, e0238479. [Google Scholar] [CrossRef]
- Fararouei, M.; Sarkari, B.; Khabisi, S.A.; Rezaei, Z. Diagnostic accuracy of urinary latex agglutination test (KAtex) for the diagnosis of visceral leishmaniasis: A meta-analysis. J. Infect. Dev. Ctries. 2018, 12, 1045–1051. [Google Scholar] [CrossRef] [Green Version]
- Nicol, M.P.; Schumacher, S.G.; Workman, L.; Broger, T.; Baard, C.; Prins, M.; Bateman, L.; Du Toit, E.; Van Heerden, J.; Szekely, R.; et al. Accuracy of a Novel Urine Test, Fujifilm SILVAMP Tuberculosis Lipoarabinomannan, for the Diagnosis of Pulmonary Tuberculosis in Children. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Samal, A.G.; Behera, P.K.; Mohanty, A.K.; Satpathi, S.; Kumar, A.; Panda, R.R.; Minz, A.M.; Mohanty, S.; Samal, A.; Van Der Pluijm, R.W. The sensitivity and specificity of a urine based Rapid Diagnostic Test for the diagnosis of plasmodium falciparum in a malaria endemic area in Odisha, India. Pathog. Glob. Health 2017, 111, 383–387. [Google Scholar] [CrossRef]
- Cravatt, B.F.; Simon, G.M.; Yates, J.R. The biological impact of mass-spectrometry-based proteomics. Nat. Cell Biol. 2007, 450, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, P.; Müller, M.; Appel, R.D. Automated protein identification by tandem mass spectrometry: Issues and strategies. Mass Spectrom. Rev. 2006, 25, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Siuzdak, G. The emergence of mass spectrometry in biochemical research. Proc. Natl. Acad. Sci. USA 1994, 91, 11290–11297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Arévalo, A.; El Baidouri, F.; Ravel, C.; Ballart, C.; Abras, A.; Lachaud, L.; Tebar, S.; Lami, P.; Pratlong, F.; Gállego, M.; et al. The Leishmania donovani species complex: A new insight into taxonomy. Int. J. Parasitol. 2020. [Google Scholar] [CrossRef]
- Maass, D.R.; Sepulveda, J.; Pernthaner, A.; Shoemaker, C.B. Alpaca (Lama pacos) as a convenient source of recombinant camelid heavy chain antibodies (VHHs). J. Immunol. Methods 2007, 324, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Larsson, A.; Karlsson-Parra, A.; Sjöquist, J. Use of chicken antibodies in enzyme immunoassays to avoid interference by rheumatoid factors. Clin. Chem. 1991, 37, 411–414. [Google Scholar] [CrossRef]
- Ghahroudi, M.A.; Desmyter, A.; Wyns, L.; Hamers, R.; Muyldermans, S. Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 1997, 414, 521–526. [Google Scholar] [CrossRef] [Green Version]
- Moayeri, M.; Leysath, C.E.; Tremblay, J.M.; Vrentas, C.; Crown, D.; Leppla, S.H.; Shoemaker, C.B. A Heterodimer of a VHH (Variable Domains of Camelid Heavy Chain-only) Antibody That Inhibits Anthrax Toxin Cell Binding Linked to a VHH Antibody That Blocks Oligomer Formation Is Highly Protective in an Anthrax Spore Challenge Model. J. Biol. Chem. 2015, 290, 6584–6595. [Google Scholar] [CrossRef] [Green Version]
- Wesolowski, J.; Alzogaray, V.; Reyelt, J.; Unger, M.; Juarez, K.; Urrutia, M.; Cauerhff, A.; Danquah, W.; Rissiek, B.; Scheuplein, F.; et al. Single domain antibodies: Promising experimental and therapeutic tools in infection and immunity. Med. Microbiol. Immunol. 2009, 198, 157–174. [Google Scholar] [CrossRef] [Green Version]
- Abeijon, C.; Singh, O.P.; Campos-Neto, A.; Sundar, S.; Chakravarty, J. Novel Antigen Detection Assay to Monitor Therapeutic Efficacy of Visceral Leishmaniasis. Am. J. Trop. Med. Hyg. 2016, 95, 800–802. [Google Scholar] [CrossRef] [Green Version]

| Protein | Abbreviature | MW (kDa) | NCBI Accession |
|---|---|---|---|
| Iron superoxide dismutase | Li-isd1 | 21.53 | XP_001467866.1 |
| Tryparedoxin | Li-txn1 | 16.7 | XP_001466642.1 |
| Nuclear transport factor 2 | Li-ntf2 | 13.89 | XP_001463738.1 |
| MaoC family dehydratase | Ld-mao1 | 16.97 | XP_003858460.1 |
| Peptidyl-prolyl cis-trans isomerase | Ld-ppi1 | 12.62 | XP_003858557.1 |
| Malate dehydrogenase | Ld-mad1 | 33.28 | XP_003864180.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos-Neto, A.; Abeijon, C. Urine-Based Antigen (Protein) Detection Test for the Diagnosis of Visceral Leishmaniasis. Microorganisms 2020, 8, 1676. https://doi.org/10.3390/microorganisms8111676
Campos-Neto A, Abeijon C. Urine-Based Antigen (Protein) Detection Test for the Diagnosis of Visceral Leishmaniasis. Microorganisms. 2020; 8(11):1676. https://doi.org/10.3390/microorganisms8111676
Chicago/Turabian StyleCampos-Neto, Antonio, and Claudia Abeijon. 2020. "Urine-Based Antigen (Protein) Detection Test for the Diagnosis of Visceral Leishmaniasis" Microorganisms 8, no. 11: 1676. https://doi.org/10.3390/microorganisms8111676
APA StyleCampos-Neto, A., & Abeijon, C. (2020). Urine-Based Antigen (Protein) Detection Test for the Diagnosis of Visceral Leishmaniasis. Microorganisms, 8(11), 1676. https://doi.org/10.3390/microorganisms8111676





