Correlation between the Antimicrobial Activity and Metabolic Profiles of Cell Free Supernatants and Membrane Vesicles Produced by Lactobacillus reuteri DSM 17938
Abstract
1. Introduction
2. Material and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Isolation and Size Fractionation of L. reuteri Cell Free Supernatant
2.3. Biofilm Formation Assay and MVs Isolation
2.4. Characterization of the Bacterial Strains Used
2.5. Determination of Minimum Inhibitory Concentration and Minimum Bactericidal Concentration of SurP, SurE 10K, and SurM 10K
2.6. XTT Metabolic Assay
2.7. Determination of Minimum Inhibitory Concentration and Minimum Bactericidal Concentration of pMVs and bMVs
2.8. Samples Preparation and FT-ICR Untargeted Metabolomics
2.9. Cytotoxicity Studies in Human Cell Lines
2.10. Statistical Analysis
3. Results
3.1. Evaluation of L. reuteri Biofilm Formation and Characterization of the Bacterial Strains Used in the Study
3.2. Determination of Minimum Inhibitory Concentration and Minimum Bactericidal Concentration of bMVs and pMVs
3.3. Determination of the MIC and MBC of SurP, SurE 10K, and SurM 10K Versus the Different Bacterial Strains Used in the Study
3.4. Evaluation Assay of pH on SurP and Proteinase K on SurE 10K Antimicrobial Activity
3.5. Metabolic Fingerprint
3.6. Cytotoxicity Studies in Human Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in human health and diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Giraffa, G.; Chanishvili, N.; Widyastuti, Y. Importance of lactobacilli in food and feed biotechnology. Res. Microbiol. 2010, 161, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C. Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera; emended description of the genus Lactobacillus Beijerinck 1901; and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Walter, J.; Britton, R.A.; Roos, S. Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc. Natl. Acad. Sci. USA 2011, 108, 4645–4652. [Google Scholar] [CrossRef]
- Frese, S.A.; Benson, A.K.; Tannock, G.W.; Loach, D.M.; Kim, J.; Zhang, M.; Oh, P.L.; Heng, N.C.; Patil, P.B.; Juge, N.; et al. The evolution of host specialization in the vertebrate gut symbiont Lactobacillus reuteri. PLoS Genet. 2011, 7, e1001314. [Google Scholar] [CrossRef] [PubMed]
- Wassenaar, T.M.; Panigrahi, P. Is a foetus developing in a sterile environment? Appl. Microbiol. 2014, 59, 572–579. [Google Scholar] [CrossRef]
- Stinson, L.F.; Boyce, M.C.; Payne, M.S.; Keelan, J.A. The not-so sterile womb: Evidence that the human fetus is exposed to bacteria prior to birth. Front. Microbiol. 2019, 20, 1124. [Google Scholar] [CrossRef]
- He, Q.; Kwok, L.; Xi, X.; Zhong, Z.; Ma, T.; Xu, H.; Meng, H.; Zhao, F.; Zhang, H. The meconium microbiota shares more features with amniotic fluid microbiota than maternal fecal and vaginal microbiota. Gut Microbes. 2020, 12, 1794266. [Google Scholar] [CrossRef]
- Valeur, N.; Engel, P.; Carbajal, N.; Connolly, E.; Ladefoged, K. Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl. Environ. Microbiol. 2004, 70, 1176–1181. [Google Scholar] [CrossRef]
- Spinler, J.K.; Taweechotipatr, M.; Rognerud, C.L.; Ou, C.N.; Tumwasorn, S.; Versalovic, J. Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe 2008, 14, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Talarico, T.L.; Dobrogosz, W.J. Chemical characterization of an antimicrobial substance produced by Lactobacillus reuteri. Antimicrob. Agents. Chemother. 1989, 33, 674–679. [Google Scholar] [CrossRef]
- Jones, S.E.; Versalovic, J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC. Microbiol. 2009, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Grande, R.; Celia, C.; Mincione, G.; Stringaro, A.; Di Marzio, L.; Colone, M.; Di Marcantonio, M.C.; Savino, L.; Puca, V.; Santoliquido, R.; et al. Detection and physicochemical characterization of Membrane Vesicles (MVs) of Lactobacillus reuteri DSM 17938. Front. Microbiol. 2017, 8, 1040. [Google Scholar] [CrossRef] [PubMed]
- Grande, R.; Nistico, L.; Sambanthamoorthy, K.; Longwell, M.; Iannitelli, A.; Cellini, L.; Di Stefano, A.; Hall-Stoodley, L.; Stoodley, P. Temporal expression of agrB; cidA; and alsS in the early development of Staphylococcus aureus UAMS-1 biofilm formation and the structural role of extracellular DNA and carbohydrates. Pathog. Dis. 2014, 70, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Grande, R.; Di Marcantonio, M.C.; Robuffo, I.; Pompilio, A.; Celia, C.; Di Marzio, L.; Paolino, D.; Codagnone, M.; Muraro, R.; Stoodley, P.; et al. Helicobacter pylori ATCC 43629/NCTC 11639 outer membrane vesicles (OMVs) from biofilm and planktonic phase associated with extracellular DNA (eDNA). Front. Microbiol. 2015, 6, 1369. [Google Scholar] [CrossRef]
- Forsberg, M.M.; Björkander, S.; Pang, Y.; Lundqvist, L.; Ndi, M.; Ott, M.; Buesa- Escribá, I.; Jaeger, M.C.; Roos, S.; Sverremark-Ekström, E. Extracellular membrane vesicles from Lactobacilli dampen IFN-γ responses in a monocyte-dependent manner. Sci. Rep. 2019, 9, 17109. [Google Scholar] [CrossRef]
- West, C.L.; Stanisz, A.M.; Mao, Y.K.; Champagne-Jorgensen, K.; Bienenstock, J.; Kunze, W.A. Microvesicles from Lactobacillus reuteri (DSM-17938) completely reproduce modulation of gut motility by bacteria in mice. PLoS ONE 2020, 15, e0225481. [Google Scholar] [CrossRef]
- Behzadi, E.; Mahmoodzadeh-Hosseini, H.; Imani-Fooladi, A.A. The inhibitory impacts of Lactobacillus rhamnosus GG-derived extracellular vesicles on the growth of hepatic cancer cells. Microb. Pathog. 2017, 110, 1–6. [Google Scholar] [CrossRef]
- Domínguez-Rubio, A.P.; Martínez, J.H.; Martínez-Casillas, D.C.; Coluccio- Leskow, F.; Piuri, M.; Pérez, O.E. Lactobacillus casei BL23 produces microvesicles carrying proteins that have been associated with its probiotic effect. Front. Microbiol. 2017, 8, 1783. [Google Scholar] [PubMed]
- Li, M.; Lee, K.; Hsu, M.; Nau, G.; Mylonakis, E.; Ramratnam, B. Lactobacillus-derived extracellular vesicles enhance host immune responses against vancomycin-resistant enterococci. BMC Microbiol. 2017, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Simeone, P.; Bologna, G.; Lanuti, P.; Pierdomenico, L.; Guagnano, M.T.; Pieragostino, D.; Del Boccio, P.; Vergara, D.; Marchisio, M.; Miscia, S.; et al. Extracellular Vesicles as Signaling Mediators and Disease Biomarkers across Biological Barriers. Int. J. Mol. Sci. 2020, 21, E2514. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-S.; Ban, M.; Choi, E.J.; Moon, H.G.; Jeon, J.S.; Kim, D.K.; Park, S.K.; Jeon, S.G.; Roh, T.Y.; Myung, S.J.; et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS ONE 2013, 8, e76520. [Google Scholar] [CrossRef]
- Jang, S.C.; Kim, S.R.; Yoon, Y.J.; Park, K.S.; Kim, J.H.; Lee, J.; Kim, O.Y.; Choi, E.-J.; Kim, D.-K.; Choi, D.-S.; et al. In vivo kinetic biodistribution of nano-sized outer membrane vesicles derived from bacteria. Small 2015, 11, 456–461. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Rho, M.; You, Y.A.; Kwon, E.J.; Kim, M.H.; Kym, S.; Jee, Y.-K.; Kim, Y.-K.; Kim, Y.J. 16S rRNA gene-based metagenomic analysis reveals differences in bacteria-derived extracellular vesicles in the urine of pregnant and non-pregnant women. Exp. Mol. Med. 2016, 48, 208. [Google Scholar] [CrossRef]
- Thay, B.; Wai, S.N.; Oscarsson, J. Staphylococcus aureus α-toxin-dependent induction of host cell death by membrane-derived vesicles. PLoS ONE 2013, 8, e54661. [Google Scholar] [CrossRef]
- Liu, Y.; Defourny, K.A.Y.; Smid, E.J.; Abee, T. Gram-positive bacterial extracellular vesicles and their impact on health and disease. Front. Microbiol. 2018, 9, 1502. [Google Scholar] [CrossRef]
- Fuochi, V.; Coniglio, M.A.; Laghi, L.; Rescifina, A.; Caruso, M.; Stivala, A.; Furneri, P.M. Metabolic characterization of supernatants produced by Lactobacillus spp. with in vitro anti-Legionella activity. Front. Microbiol. 2019, 10, 1403. [Google Scholar] [CrossRef]
- Chen, C.C.; Lai, C.C.; Huang, H.L.; Su, Y.T.; Chiu, Y.H.; Toh, H.S.; Chiang, S.R.; Chuang, Y.C.; Lu, Y.C.; Tang, H.J. Antimicrobial ability and mechanism analysis of Lactobacillus species against carbapenemase-producing Enterobacteriaceae. J. Microbiol. Immunol. Infect. 2020. [Google Scholar] [CrossRef]
- Arrioja-Bretón, D.; Mani-López, E.; Bach, H.; López-Malo, A. Antimicrobial activity of protein-containing fractions isolated from Lactobacillus plantarum NRRL B-4496 culture. Braz. J. Microbiol. 2020, 51, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Ingallina, C.; Capitani, D.; Mannina, L.; Carradori, S.; Locatelli, M.; Di Sotto, A.; Di Giacomo, S.; Toniolo, C.; Pasqua, G.; Valletta, A.; et al. Phytochemical and biological characterization of Italian “sedano bianco di Sperlonga” Protected Geographical Indication celery ecotype: A multimethodological approach. Food Chem. 2020, 309, 125649. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L.; et al. Chromatographic analyses; in vitro biological activities and cytotoxicity of Cannabis sativa L. essential oil: A multidisciplinary study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Di Giacomo, S.; Rubini, E.; Macone, A.; Gulli, M.; Mammola, C.L.; Eufemi, M.; Mancinelli, R.; Mazzanti, G. Modulation of STAT3 signaling; cell redox defenses and cell cycle checkpoints by β-caryophyllene in cholangiocarcinoma cells: Possible mechanisms accounting for doxorubicin chemosensitization and chemoprevention. Cells 2020, 9, 858. [Google Scholar] [CrossRef]
- He, Z.P.; Tan, W.Q.; Tang, Y.F.; Feng, M.F. Differentiation of putative hepatic stem cells derived from adult rats into mature hepatocytes in the presence of epidermal growth factor and hepatocyte growth factor. Differentiation 2003, 71, 281–290. [Google Scholar] [CrossRef]
- Hiemstra, P.S.; Grootaers, G.; van der Does, A.M.; Krul, C.A.M.; Kooter, I.M. Human lung epithelial cell cultures for analysis of inhaled toxicants: Lessons learned and future directions. Toxicol. In Vitro 2018, 47, 137–146. [Google Scholar] [CrossRef]
- Rosander, A.; Connolly, E.; Roos, S. Removal of antibiotic resistance gene-carrying plasmids from Lactobacillus reuteri ATCC 55730 and characterization of the resulting daughter strain; L. reuteri DSM 17938. Appl. Environ. Microbiol. 2008, 19, 6032–6040. [Google Scholar] [CrossRef]
- Puca, V.; Ercolino, E.; Celia, C.; Bologna, G.; Di Marzio, L.; Mincione, G.; Marchisio, M.; Miscia, S.; Muraro, R.; Lanuti, P.; et al. Detection and quantification of eDNA-associated bacterial membrane vesicles by flow cytometry. Int. J. Mol. Sci. 2019, 20, E5307. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Pérez, L.M.; Alvarez, B.L.; Codony, F.; Fittipaldi, M.; Adrados, B.; Peñuela, G.; Morató, J. A new microtitre plate screening method for evaluating the viability of aerobic respiring bacteria in high surface biofilms. Lett. Appl. Microbiol. 2010, 51, 331–337. [Google Scholar] [CrossRef]
- Tavares, L.J.; Klein, M.I.; Panariello, B.H.D.; Dorigatti de Avila, E.; Pavarina, A.C. An in vitro model of Fusobacterium nucleatum and Porphyromonas gingivalis in single-and dual-species biofilms. J. Periodontal Implant Sci. 2018, 48, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Banzi, E.C.F.; Costab, A.R.; Puppin-Rontani, R.M.; Babud, J.; García-Godoye, F. Inhibitory effects of a cured antibacterial bonding system on viability and metabolic activity of oral bacteria. Dent. Mater. 2014, 30, e238–e244. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.G.; Chen, T. 40 years of Fourier transform ion cyclotron resonance mass spectrometry. Int. J. Mass Spectrom. 2015, 377, 410–420. [Google Scholar] [CrossRef]
- Wägele, B.; Witting, M.; Suhre, K. MassTRIX reloaded: Combined analysis and visualization of transcriptome and metabolome data. PLoS ONE 2012, 7, e39860. [Google Scholar]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Sud, M.; Fahy, E.; Cotter, D.; Brown, A.; Dennis, E.A.; Glass, C.K.; Merrill, A.H., Jr.; Murphy, R.C.; Raetz, C.R.; Russell, D.W.; et al. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 2007, 35, D527–D532. [Google Scholar] [CrossRef]
- Ghaste, M.; Mistrik, R.; Shulaev, V. Applications of Fourier Transform Ion Cyclotron Resonance (FT-ICR) and Orbitrap Based High Resolution Mass Spectrometry in metabolomics and lipidomics. Int. J. Mol. Sci. 2016, 17, 816. [Google Scholar] [CrossRef]
- Gowda, G.A.; Djukovic, D. Overview of mass spectrometry-based metabolomics: Opportunities and challenges. Methods Mol. Biol. 2014, 1198, 3–12. [Google Scholar]
- Guijas, C.; Montenegro-Burke, J.R.; Domingo-Almenara, X.; Palermo, A.; Warth, B.; Hermann, G.; Koellensperger, G.; Huan, T.; Uritboonthai, W.; Aisporna, A.E.; et al. METLIN: A technology platform for identifying knowns and unknowns. Anal. Chem. 2018, 90, 3156–3164. [Google Scholar] [CrossRef]
- Patiny, L.; Borel, A. ChemCalc: A building block for tomorrow’s chemical infrastructure. J. Chem. Inf. Model. 2013, 53, 1223–1228. [Google Scholar] [CrossRef]
- Kim, S.; Kramer, R.W.; Hatcher, P.G. Graphical method for analysis of ultrahigh-resolution broadband mass spectra of natural organic matter, the van Krevelen diagram. Anal. Chem. 2003, 75, 5336–5344. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, S.; Di Sotto, A.; El-Readi, M.Z.; Mazzanti, G.; Wink, M.S.; Di Sotto, A.; El-Readi, M.Z.; Mazzanti, G.; Wink, M. α-Hexylcinnamaldehyde synergistically increases doxorubicin cytotoxicity towards human cancer cell lines. Anticancer Res. 2016, 36, 3347–3351. [Google Scholar] [PubMed]
- Greifová, G.; Májeková, H.; Greif, G.; Body, P.; Greifová, M.; Dubničková, M. Analysis of antimicrobial and immunomodulatory substances produced by heterofermentative Lactobacillus reuteri. Folia Microbiol. 2017, 62, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.N.; Leary, D.H.; Sullivan, C.J.; Oh, E.; Walper, S.A. Isolation and characterization of Lactobacillus-derived membrane vesicles. Sci. Rep. 2019, 9, 877. [Google Scholar] [CrossRef]
- Aminnezhad, S.; Kermanshahi, R.K.; Ranjbar, R. Evaluation of Synergistic Interactions Between Cell-Free Supernatant of Lactobacillus Strains and Amikacin and Genetamicin Against Pseudomonas aeruginosa. Jundishapur J. Microbiol. 2015, 8, e16592. [Google Scholar] [CrossRef]
- Alakomi, H.L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef]
- Knysh, O.V.; Isayenko, O.Y.; Voyda, Y.V.; Kizimenko, O.O.; Babych, Y.M. Influence of cell-free extracts of Bifidobacterium bifidum and Lactobacillus reuteri on proliferation and biofilm formation by Escherichia coli and Pseudomonas aeruginosa. Regul. Mech. Biosyst. 2019, 10, 251–256. [Google Scholar] [CrossRef]
- Poppi, L.B.; Rivaldi, J.D.; Coutinho, T.S.; Astolfi-Ferreira, C.S.; Ferreira, A.J.P.; Mancilha, I.M. Effect of Lactobacillus sp. isolates supernatant on Escherichia coli O157:H7 enhances the role of organic acids production as a factor for pathogen control. Pesqui. Vet. Bras. 2015, 35, 353–359. [Google Scholar] [CrossRef]
- Maghsood, F.; Mirshafiey, A.; Farahani, M.M.; Modarressi, M.H.; Jafari, P.; Motevaseli, E. Dual Effects of Cell Free Supernatants from Lactobacillus acidophilus and Lactobacillus rhamnosus GG in Regulation of MMP-9 by Up-Regulating TIMP-1 and Down-Regulating CD147 in PMA Differentiated THP-1 Cells. Cell J. 2018, 19, 559–568. [Google Scholar]
- Maroni, L.; Haibo, B.; Ray, D.; Zhou, T.; Wan, Y.; Meng, F.; Marzioni, M.; Alpini, G. Functional and structural features of cholangiocytes in health and disease. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 368–380. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Albon, J.; Boulton, M. The contribution of DNA repair and antioxidants in determining cell type-specific resistance to oxidative stress. Free Radic. Res. 2006, 40, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic Cell-Free Supernatants Exhibited Anti-Inflammatory and Antioxidant Activity on Human Gut Epithelial Cells and Macrophages Stimulated with LPS. Evid. Based Complement. Alternat. Med. 2018, 2018, 1756308. [Google Scholar] [CrossRef] [PubMed]
- Hajfarajollah, H.; Eslami, P.; Mokhtarani, B.; Akbari Noghabi, K. Biosurfactants from probiotic bacteria: A review. Biotechnol. Appl. Biochem. 2018, 65, 768–783. [Google Scholar] [CrossRef] [PubMed]
- Mnif, I.; Ghribi, D. Review lipopeptides biosurfactants: Mean classes and new insights for industrial, biomedical, and environmental applications. Biopolymers 2015, 104, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Marcia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar]
- Schaefer, L.; Auchtung, T.A.; Hermans, K.E.; Whitehead, D.; Borhan, B.; Britton, R.A. The antimicrobial compound reuterin (3-hydroxypropionaldehyde) induces oxidative stress via interaction with thiol groups. Microbiology (Reading) 2010, 156, 1589–1599. [Google Scholar] [CrossRef]
- Urrutia-Baca, V.H.; Escamilla-García, E.; de la Garza-Ramos, M.A.; Tamez-Guerra, P.; Gomez-Flores, R.; Urbina-Ríos, C.S. In vitro antimicrobial activity and downregulation of virulence gene expression on Helicobacter pylori by reuterin. Probiotics Antimicrob. Proteins 2018, 10, 168–175. [Google Scholar] [CrossRef]
- Zhang, J.; Sturla, S.; Lacroix, C.; Schwab, C. Gut microbial glycerol metabolism as an endogenous acrolein source. MBio 2018, 9, e01947-17. [Google Scholar] [CrossRef]
- Ñahui Palomino, R.A.; Vanpouille, C.; Laghi, L.; Parolin, C.; Melikov, K.; Backlund, P.; Vitali, B.; Margolis, L. Extracellular vesicles from symbiotic vaginal lactobacilli inhibit HIV-1 infection of human tissues. Nat. Commun. 2019, 10, 5656. [Google Scholar] [CrossRef]
- Heianza, Y.; Ma, W.; Manson, J.E.; Rexrode, K.M.; Qi, L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: A systematic review and meta-analysis of prospective studies. J. Am. Heart Assoc. 2017, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Hulme, H.; Meikle, L.M.; Strittmatter, N.; van der Hooft, J.J.J.; Swales, J.; Bragg, R.A.; Villar, V.H.; Ormsby, M.J.; Barnes, S.; Brown, S.L.; et al. Microbiome-derived carnitine mimics as previously unknown mediators of gut-brain axis communication. Sci. Adv. 2020, 6, eaax6328. [Google Scholar] [CrossRef] [PubMed]
- Meadows, J.A.; Wargo, M.J. Carnitine in bacterial physiology and metabolism. Microbiology (Reading) 2015, 161, 1161–1174. [Google Scholar] [CrossRef]
- Posner, M.G.; Upadhyay, A.; Bagby, S.; Hough, D.W.; Danson, M.J. A unique lipoylation system in the Archaea. Lipoylation in Thermoplasma acidophilum requires two proteins. FEBS J. 2009, 276, 4012–4022. [Google Scholar] [CrossRef] [PubMed]
- Lonvaud-Funel, A. Biogenic amines in wines: Role of lactic acid bacteria. FEMS Microbiol. Lett. 2001, 199, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Colosimo, D.A.; Kohn, J.A.; Luo, P.M.; Piscotta, F.J.; Han, S.M.; Pickard, A.J.; Rao, A.; Cross, J.R.; Cohen, L.J.; Brady, S.F. Mapping interactions of microbial metabolites with human G-Protein-Coupled Receptors. Cell Host Microbe 2019, 26, 273–282.e7. [Google Scholar] [CrossRef]
- Thomas, C.M.; Hong, T.; van Pijkeren, J.P.; Hemarajata, P.; Trinh, D.V.; Hu, W.; Britton, R.A.; Kalkum, M.; Versalovic, J. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS ONE 2012, 7, e31951. [Google Scholar] [CrossRef]
- Liu, Y.; Alookaran, J.J.; Rhoads, J.M. Probiotics in autoimmune and inflammatory disorders. Nutrients 2018, 10, 1537. [Google Scholar] [CrossRef]
- Peters, A.; Krumbholz, P.; Jäger, E.; Heintz-Buschart, A.; Çakir, M.V.; Rothemund, S.; Gaudl, A.; Ceglarek, U.; Schöneberg, T.; Stäubert, C. Metabolites of lactic acid bacteria present in fermented foods are highly potent agonists of human hydroxycarboxylic acid receptor 3. PLoS Genet. 2019, 15, e1008145. [Google Scholar]

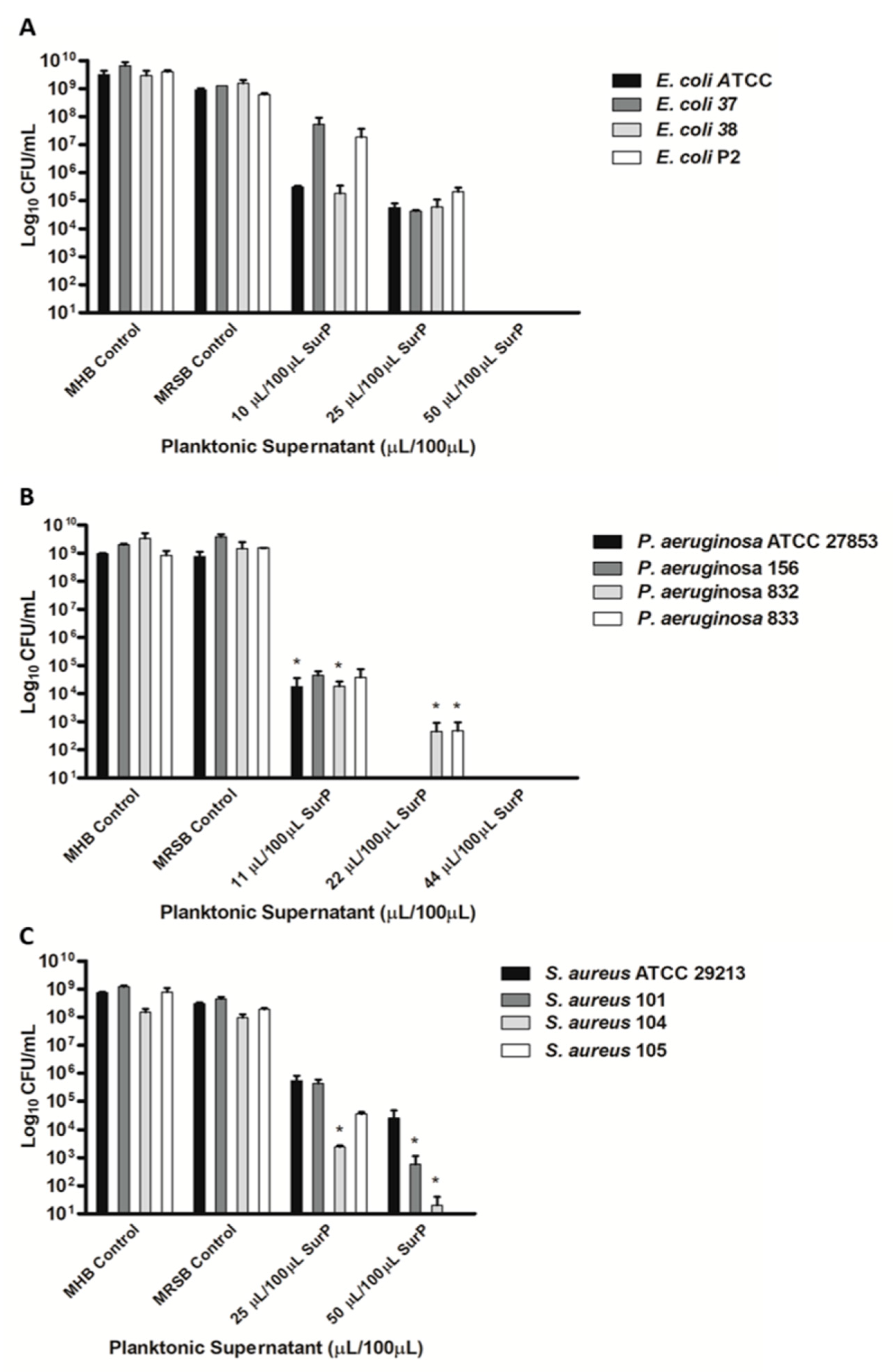
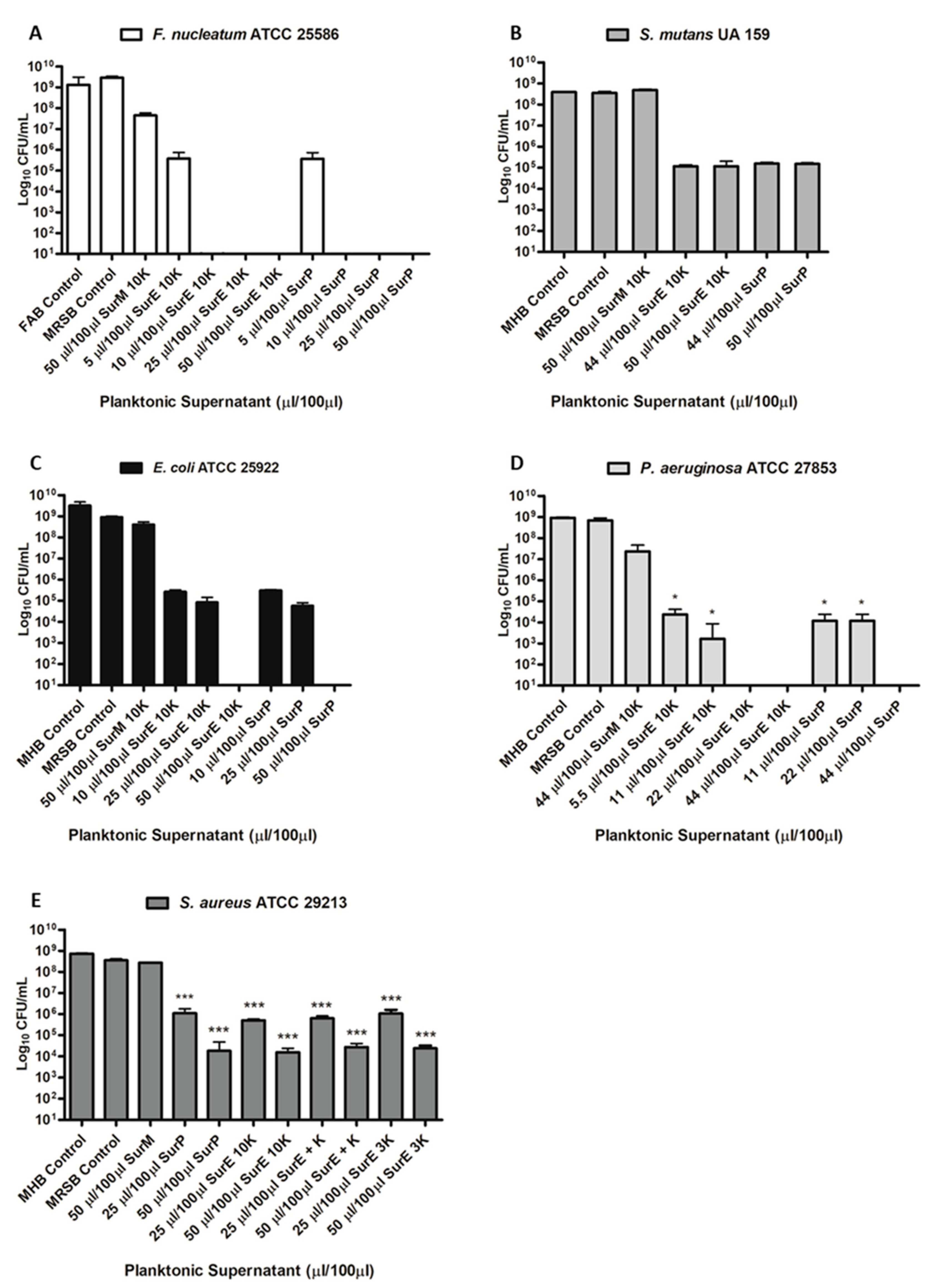
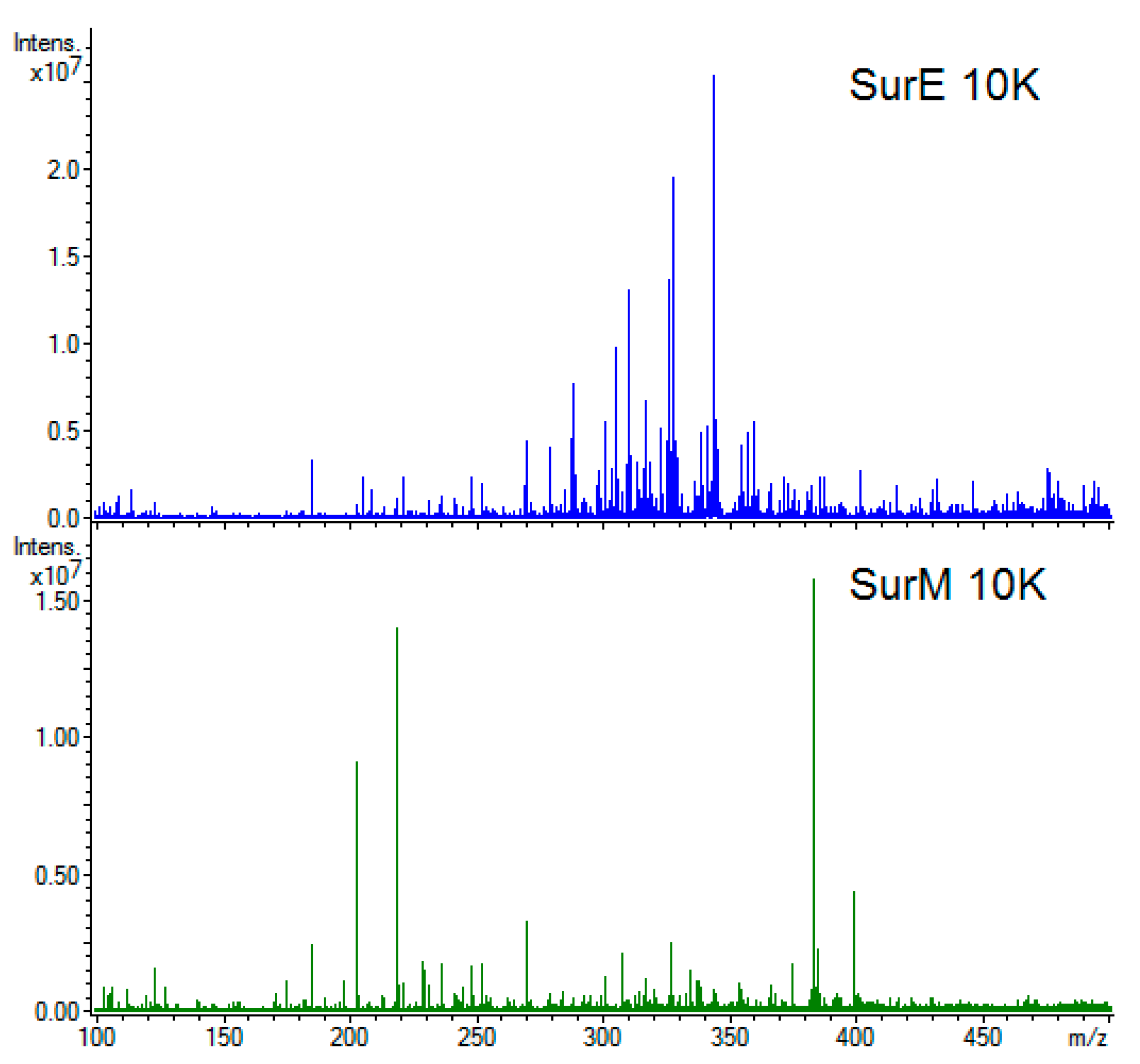
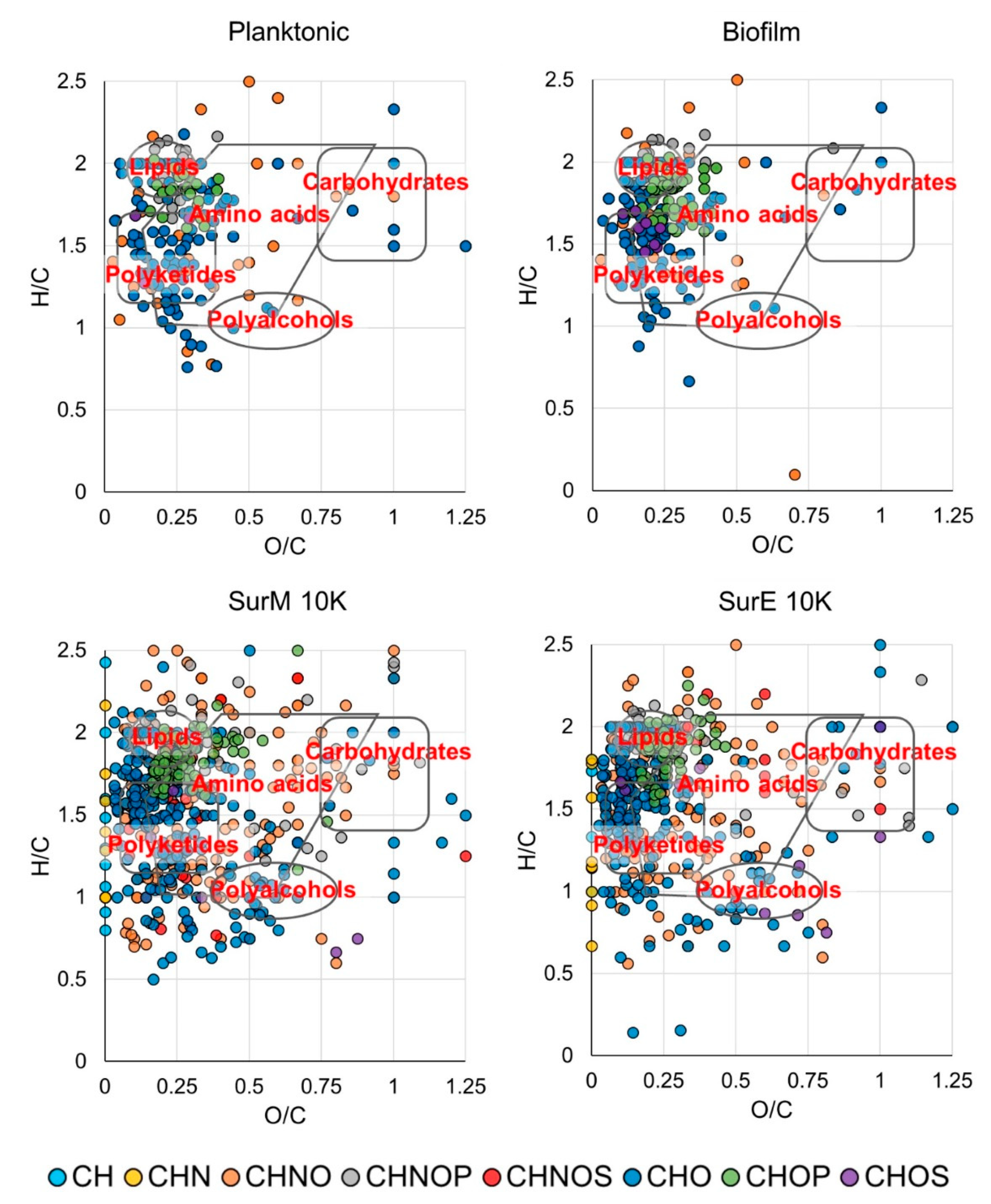
| Bacterial Strains | Clinical Isolation | Antimicrobial Susceptibility | SurP MIC/MBC (µL/100 µL) |
|---|---|---|---|
| E. coli ATCC 25922 | Clinical isolate | PRLR, AMLR | 10/50 |
| E. coli 37 | Urinary sample | CECR | 25/50 |
| E. coli 38 | Urinary sample | CROR, CECR, PRLR, CFMR, AMLR, LEVR | 10/50 |
| E. coli P2 | Urinary sample | PRLR, CECI | 25/50 |
| F. nucleatum ATCC 25586 | Oral cavity | MNZs, PENs | 5/10 |
| S. aureus ATCC 29213 | Wound | ERYS, TES, NETS, LEVS, FOXS, LNZS, RDS, CNI | 25/≥50 |
| S. aureus 101 | Vaginal swab | ERYS, TES, NETS, LEVS, FOXS, LNZS, RDS, CNS | 25/50 |
| S. aureus 104 | Pharyngeal swab | ERYR, TES, NETI, LEVR, FOXR, LNZS, RDS, CNS | 25/50 |
| S. aureus 105 | Urinary sample | ERYS, TES, NETS, LEVS, FOXS, LNZS, RDS, CNS | 25/50 |
| S. mutans UA 159 | Dental caries | AMPS | 22/>50 |
| P. aeruginosa ATCC 27853 | Blood culture | AMPR, CAZS, CXMR, CNS FR, LEVS, NORS, SXTR | 11/22 |
| P. aeruginosa 156 | Urinary sample | AMPR, CAZI, CXMR, CNS FR, LEVS, NORS, SXTR | 11/22 |
| P. aeruginosa 832 | Urinary sample | AMPR, CAZI, CXMR, CNI FR, LEVS, NORS, SXTR | 11/22 |
| P. aeruginosa 833 | Urinary sample | AMPR, CAZS, CXMR, CNS FR, LEVS, NORS, SXTR | 11/22 |
| Bacterial Strains | SurM 10K MIC (µL/100 µL) | SurM 10K MBC (µL/100 µL) | SurE 10K MIC (µL/100 µL) | SurE 10K MBC (µL/100 µL) | SurP MIC (µL/100 µL) | SurP MBC (µL/100 µL) |
|---|---|---|---|---|---|---|
| E. coli ATCC 25922 | ≥50 | ≥50 | 10 | 50 | 10 | 50 |
| S. aureus ATCC 29213 | ≥50/ | ≥50 | 25 | 50 | 25 | ≥50 |
| S. mutans UA 159 | ≥50 | 50 | 44 | >50 | 44 | >50 |
| P. aeruginosa ATCC 27853 | 44 | >44 | 5.5 | 22 | 11 | 22 |
| F. nucleatum ATCC 25586 | ≥50 | ≥50 | 5 | 10 | 5 | 10 |
| Sample | ESI(+) | ESI(−) | Total |
|---|---|---|---|
| SurE 10K | 304 | 205 | 489 |
| SurM 10K | 446 | 204 | 619 |
| Biofilm vesicles | 193 | 49 | 219 |
| Planktonic vesicles | 160 | 50 | 192 |
| Time Exposure | IC50 [µL/100 µL] (CL) | |||
|---|---|---|---|---|
| H69 | BEAS-2B | |||
| 24 h | 48 h | 24 h | 48 h | |
| MRSB | 32.6 (29.0–36.7) | 22.3 (14.7–34.0) | 3.2 (2.0–5.1) | 1.7 (1.2–2.3) |
| SurP | 37.8 (32.2–44.4) | 20.7 (15.7–27.3) | 2.9 (1.8–4.5) | 1.6 (1.2–2.2) |
| SurM 10K/MRSB | 31.4 (27.5–35.9) | 13.0 (10.5–16.4) | 1.3 (0.5–3.3) | 1.6 (1.2–2.2) |
| SurM 10K/RPMI | 69.7 (46.6–104.1) *** § | 26.2 (16.7–36.8) § | 11.3 (8.5–15.5) *** § | 9.1 (7.5–11.0) *** § |
| SurE 10K | 32.8 (26.9–40.0) | 20.7 (11.2–27.8) | 3.0 (2.1–4.1) | 1.5 (1.1–2.0) |
| SurE 3K | 39.2 (32.5–47.4) | 22.4 (17.5–28.7) | 2.1 (1.6–2.7) | 1.6 (1.2–2.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maccelli, A.; Carradori, S.; Puca, V.; Sisto, F.; Lanuti, P.; Crestoni, M.E.; Lasalvia, A.; Muraro, R.; Bysell, H.; Di Sotto, A.; et al. Correlation between the Antimicrobial Activity and Metabolic Profiles of Cell Free Supernatants and Membrane Vesicles Produced by Lactobacillus reuteri DSM 17938. Microorganisms 2020, 8, 1653. https://doi.org/10.3390/microorganisms8111653
Maccelli A, Carradori S, Puca V, Sisto F, Lanuti P, Crestoni ME, Lasalvia A, Muraro R, Bysell H, Di Sotto A, et al. Correlation between the Antimicrobial Activity and Metabolic Profiles of Cell Free Supernatants and Membrane Vesicles Produced by Lactobacillus reuteri DSM 17938. Microorganisms. 2020; 8(11):1653. https://doi.org/10.3390/microorganisms8111653
Chicago/Turabian StyleMaccelli, Alessandro, Simone Carradori, Valentina Puca, Francesca Sisto, Paola Lanuti, Maria Elisa Crestoni, Alba Lasalvia, Raffaella Muraro, Helena Bysell, Antonella Di Sotto, and et al. 2020. "Correlation between the Antimicrobial Activity and Metabolic Profiles of Cell Free Supernatants and Membrane Vesicles Produced by Lactobacillus reuteri DSM 17938" Microorganisms 8, no. 11: 1653. https://doi.org/10.3390/microorganisms8111653
APA StyleMaccelli, A., Carradori, S., Puca, V., Sisto, F., Lanuti, P., Crestoni, M. E., Lasalvia, A., Muraro, R., Bysell, H., Di Sotto, A., Roos, S., & Grande, R. (2020). Correlation between the Antimicrobial Activity and Metabolic Profiles of Cell Free Supernatants and Membrane Vesicles Produced by Lactobacillus reuteri DSM 17938. Microorganisms, 8(11), 1653. https://doi.org/10.3390/microorganisms8111653









