Adherence of Trichomonas vaginalis to SiHa Cells is Inhibited by Diphenyleneiodonium
Abstract
1. Introduction
2. Materials and Methods
2.1. Host Cell Culture
2.2. T. vaginalis Culture
2.3. Flow Cytometry Analysis
2.4. Measurement of ROS Production
2.5. Statistical Analysis
3. Results
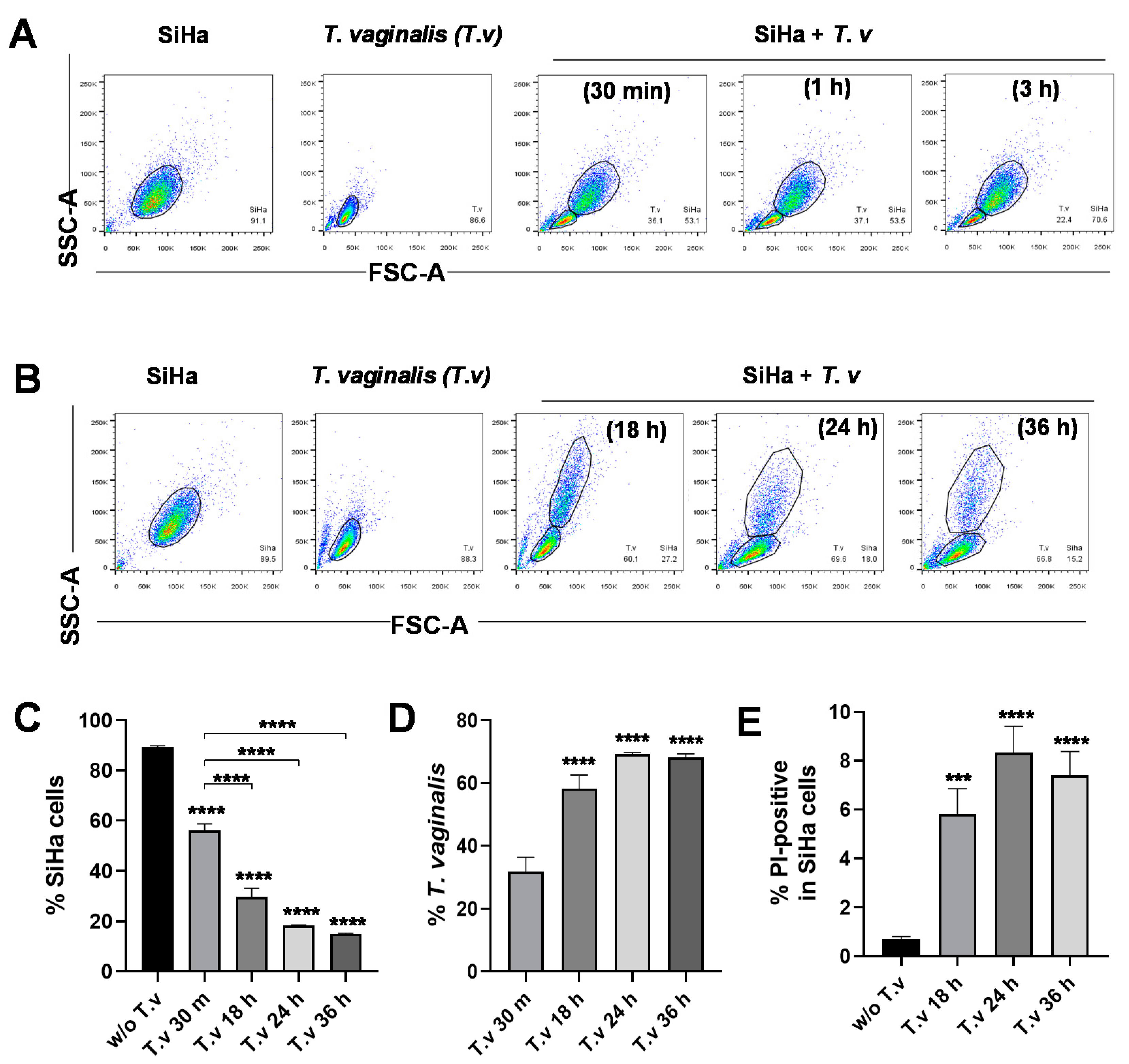
3.1. Flow Cytometric Analysis of Parasite–Host Cell Mixed Culture
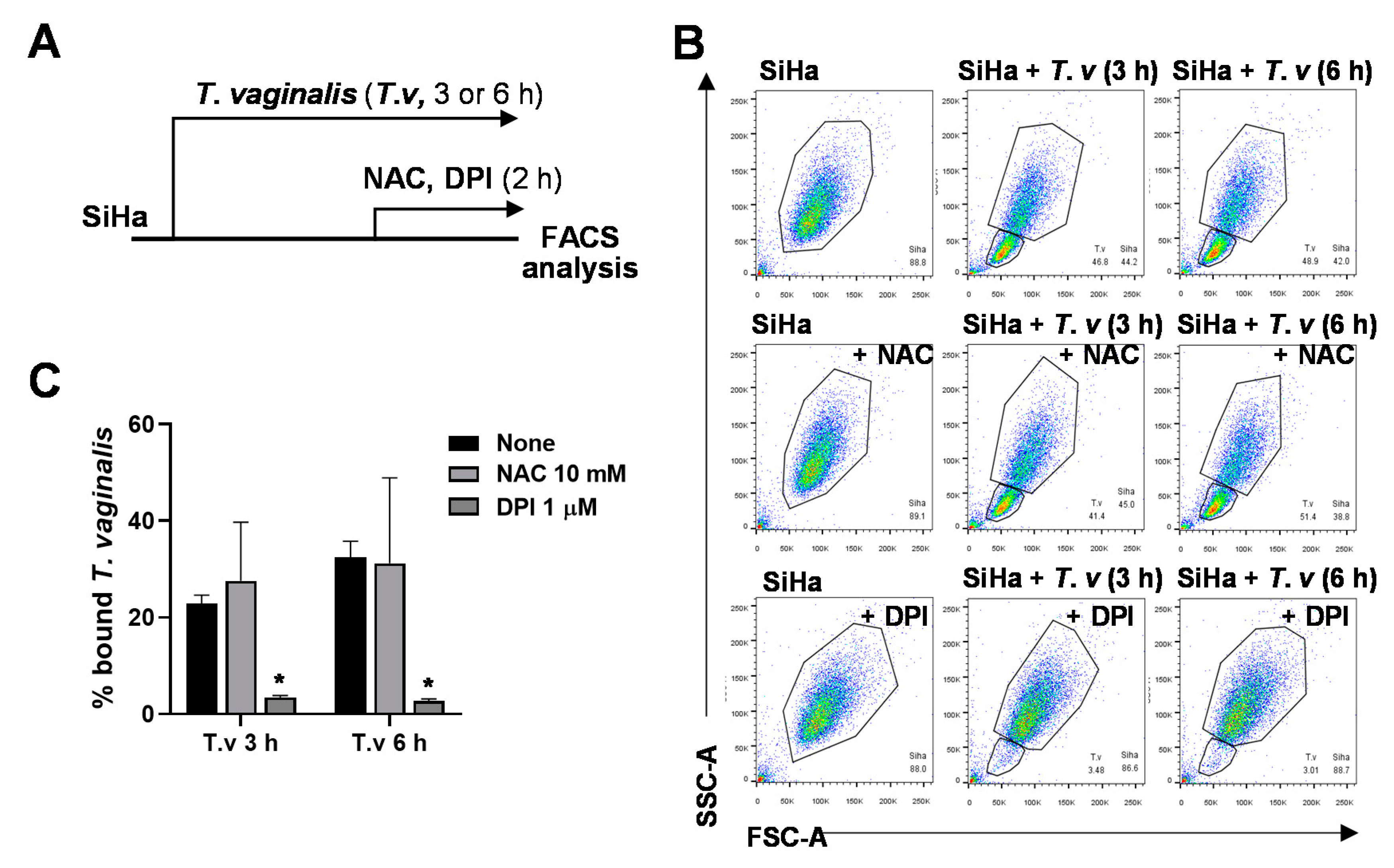
3.2. DPI Reduces Adherence of T. vaginalis to SiHa Cells
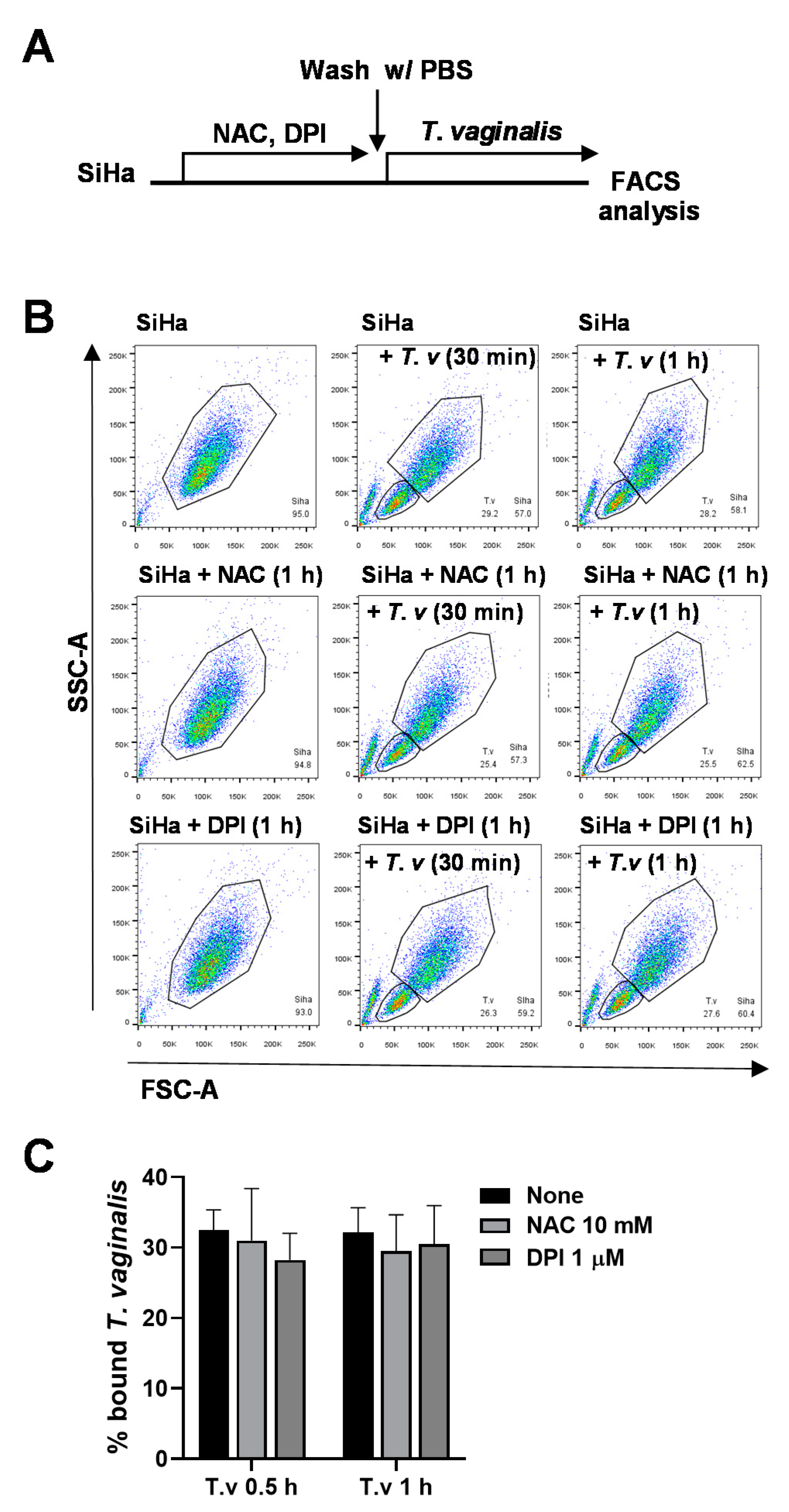
3.3. Antioxidant Pretreatment of SiHa Cells Does Not Affect T. vaginalis Adhesion
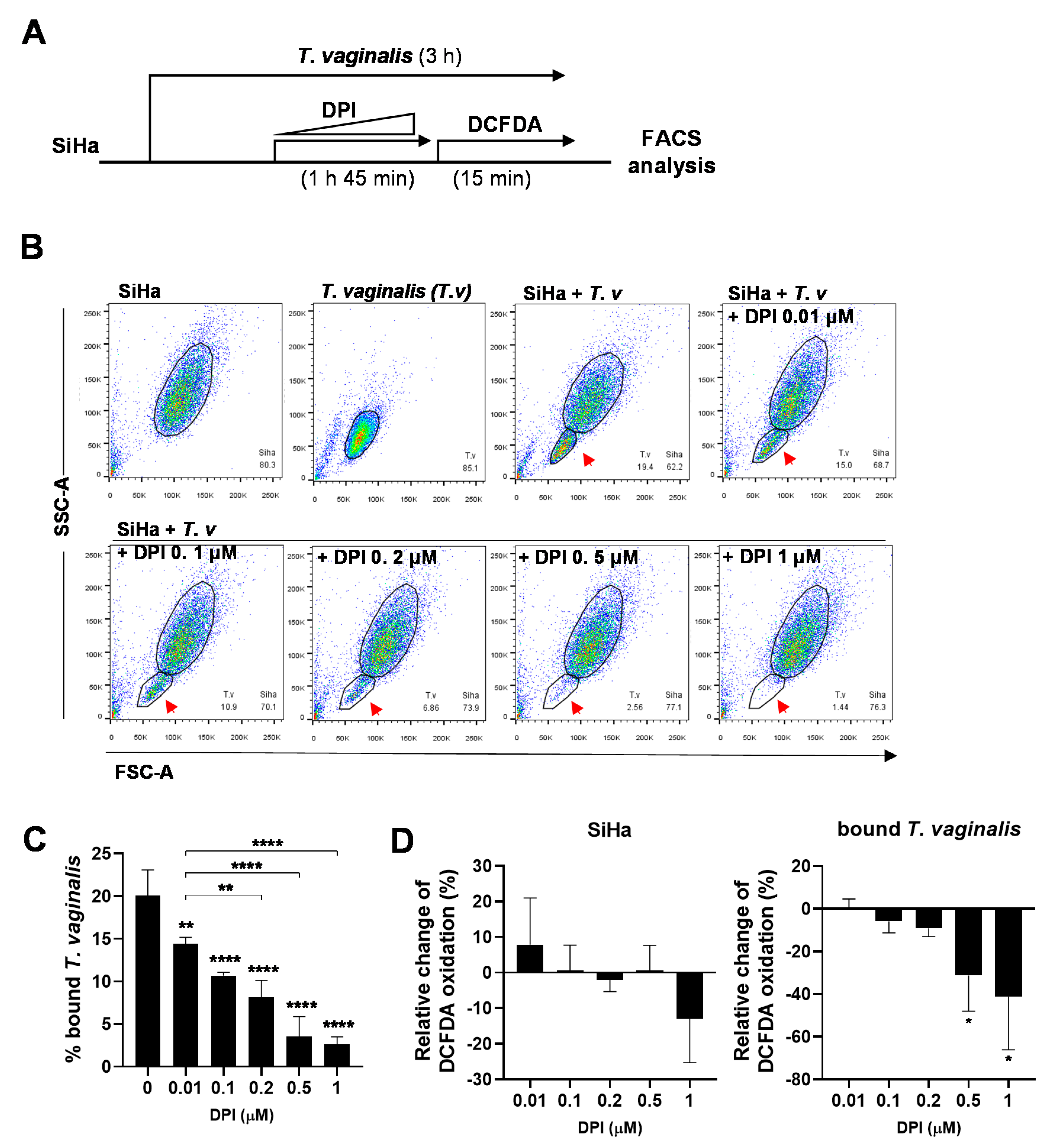
3.4. DPI Induces Detachment of T. vaginalis from SiHa Cells and Reduces ROS Generation
3.5. Kinetics of DPI-Induced Detachment of T. vaginalis from SiHa Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mercer, F.; Johnson, P.J. Trichomonas vaginalis: Pathogenesis, symbiont interactions, and host cell immune responses. Trends Parasitol. 2018, 34, 683–693. [Google Scholar] [CrossRef]
- Mielczarek, E.; Blaszkowska, J. Trichomonas vaginalis: Pathogenicity and potential role in human reproductive failure. Infection 2016, 44, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Bouchemal, K.; Bories, C.; Loiseau, P.M. Strategies for prevention and treatment of Trichomonas vaginalis infections. Clin. Microbiol. Rev. 2017, 30, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Cotch, M.F.; Pastorek, J.G.I.; Nugent, R.P.; Hillier, S.L.; Gibbs, R.S.; Martin, D.H.; Eschenbach, D.A.; Edelman, R.; Carey, C.J.; Regan, J.A. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex. Transm. Dis. 1997, 24, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Heine, P.; Mcgregor, J.A. Trichomonas vaginalis: A reemerging pathogen. Clin. Obstet. Gynecol. 1993, 36, 137–144. [Google Scholar] [CrossRef] [PubMed]
- El-Shazly, A.; El-Naggar, H.; Soliman, M.; El-Negeri, M.; El-Nemr, H.; Handousa, A.; Morsy, T. A study on Trichomonas vaginalis and female infertility. J. Egypt. Soc. Parasitol. 2001, 31, 545–553. [Google Scholar]
- Zhang, Z.-F.; Graham, S.; Yu, S.-Z.; Marshall, J.; Zielezny, M.; Chen, Y.-X.; Sun, M.; Tang, S.-L.; Liao, C.-S.; Xu, J.-L. Trichomonas vaginalis and cervical cancer: A prospective study in China. Ann. Epidemiol. 1995, 5, 325–332. [Google Scholar] [CrossRef]
- McClelland, R.S.; Sangaré, L.; Hassan, W.M.; Lavreys, L.; Mandaliya, K.; Kiarie, J.; Ndinya-Achola, J.; Jaoko, W.; Baeten, J.M. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J. Infect. Dis. 2007, 195, 698–702. [Google Scholar] [CrossRef]
- Edwards, T.; Burke, P.; Smalley, H.; Hobbs, G. Trichomonas vaginalis: Clinical relevance, pathogenicity and diagnosis. Crit. Rev. Microbiol. 2016, 42, 406–417. [Google Scholar] [CrossRef]
- Menezes, C.B.; Frasson, A.P.; Tasca, T. Trichomoniasis-are we giving the deserved attention to the most common non-viral sexually transmitted disease worldwide? Microb. Cell 2016, 3, 404. [Google Scholar] [CrossRef]
- de Brum Vieira, P.; Tasca, T.; Evan Secor, W. Challenges and persistent questions in the treatment of Trichomoniasis. Curr. Top. Med. Chem. 2017, 17, 1249–1265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirt, R.P.; de Miguel, N.; Nakjang, S.; Dessi, D.; Liu, Y.-C.; Diaz, N.; Rappelli, P.; Acosta-Serrano, A.; Fiori, P.-L.; Mottram, J.C. Trichomonas vaginalis pathobiology: New insights from the genome sequence. In Advances in Parasitology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 77, pp. 87–140. [Google Scholar]
- Bastida-Corcuera, F.D.; Okumura, C.Y.; Colocoussi, A.; Johnson, P.J. Trichomonas vaginalis lipophosphoglycan mutants have reduced adherence and cytotoxicity to human ectocervical cells. Eukaryot. Cell 2005, 4, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Ardalan, S.; Lee, B.C.; Garber, G.E. Trichomonas vaginalis: The adhesins AP51 and AP65 bind heme and hemoglobin. Exp. Parasitol. 2009, 121, 300–306. [Google Scholar] [CrossRef]
- Noël, C.J.; Diaz, N.; Sicheritz-Ponten, T.; Safarikova, L.; Tachezy, J.; Tang, P.; Fiori, P.-L.; Hirt, R.P. Trichomonas vaginalis vast BspA-like gene family: Evidence for functional diversity from structural organisation and transcriptomics. BMC Genom. 2010, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Handrich, M.R.; Garg, S.G.; Sommerville, E.W.; Hirt, R.P.; Gould, S.B. Characterization of the BspA and Pmp protein family of trichomonads. Parasites Vectors 2019, 12, 1–15. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Riestra, A.M.; Rai, A.K.; Johnson, P.J. A novel cadherin-like protein mediates adherence to and killing of host cells by the parasite Trichomonas vaginalis. MBio 2019, 10. [Google Scholar] [CrossRef]
- Kusdian, G.; Woehle, C.; Martin, W.F.; Gould, S.B. The actin-based machinery of Trichomonas vaginalis mediates flagellate-amoeboid transition and migration across host tissue. Cell. Microbiol. 2013, 15, 1707–1721. [Google Scholar]
- Gould, S.B.; Woehle, C.; Kusdian, G.; Landan, G.; Tachezy, J.; Zimorski, V.; Martin, W.F. Deep sequencing of Trichomonas vaginalis during the early infection of vaginal epithelial cells and amoeboid transition. Int. J. Parasitol. 2013, 43, 707–719. [Google Scholar] [CrossRef]
- Lal, K.; Noel, C.J.; Field, M.C.; Goulding, D.; Hirt, R.P. Dramatic reorganisation of Trichomonas endomembranes during amoebal transformation: A possible role for G-proteins. Mol. Biochem. Parasitol. 2006, 48, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Bradford, W.; Buckholz, A.; Morton, J.; Price, C.; Jones, A.M.; Urano, D. Eukaryotic G protein signaling evolved to require G protein–coupled receptors for activation. Sci. Signal. 2013, 6, ra37. [Google Scholar] [CrossRef]
- Chiarugi, P.; Pani, G.; Giannoni, E.; Taddei, L.; Colavitti, R.; Raugei, G.; Symons, M.; Borrello, S.; Galeotti, T.; Ramponi, G. Reactive oxygen species as essential mediators of cell adhesion: The oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J. Cell Biol. 2003, 161, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, A.; Duran, C.; San Martin, A. The role of Nox-mediated oxidation in the regulation of cytoskeletal dynamics. Curr. Pharm. Des. 2015, 21, 6009–6022. [Google Scholar] [CrossRef] [PubMed]
- Goitre, L.; Pergolizzi, B.; Ferro, E.; Trabalzini, L.; Retta, S.F. Molecular crosstalk between integrins and cadherins: Do reactive oxygen species set the talk? J. Signal Transduct. 2012, 2012. [Google Scholar] [CrossRef]
- Quan, J.-H.; Kang, B.-H.; Cha, G.-H.; Zhou, W.; Koh, Y.-B.; Yang, J.-B.; Yoo, H.-J.; Lee, M.-A.; Ryu, J.-S.; Noh, H.-T. Trichonomas vaginalis metalloproteinase induces apoptosis of SiHa cells through disrupting the Mcl-1/Bim and Bcl-xL/Bim complexes. PLoS ONE 2014, 9, e110659. [Google Scholar] [CrossRef]
- Davidson, W.F.; Haudenschild, C.; Kwon, J.; Williams, M.S. T cell receptor ligation triggers novel nonapoptotic cell death pathways that are Fas-independent or Fas-dependent. J. Immunol. 2002, 169, 6218–6230. [Google Scholar] [CrossRef]
- Kwon, J.; Shatynski, K.E.; Chen, H.; Morand, S.; De Deken, X.; Miot, F.; Leto, T.L.; Williams, M.S. The nonphagocytic NADPH oxidase Duox1 mediates a positive feedback loop during T cell receptor signaling. Sci. Signal. 2010, 3, ra59. [Google Scholar] [CrossRef]
- Brooks, A.E.; Parsamand, T.; Kelly, R.W.; Simoes-Barbosa, A. An improved quantitative method to assess adhesive properties of Trichomonas vaginalis to host vaginal ectocervical cells using flow cytometry. J. Microbiol. Methods 2013, 92, 73–78. [Google Scholar] [CrossRef]
- Lin, W.-C.; Chang, W.-T.; Chang, T.-Y.; Shin, J.-W. The pathogenesis of human cervical epithelium cells induced by interacting with Trichomonas vaginalis. PLoS ONE 2015, 10, e0124087. [Google Scholar] [CrossRef]
- Tasca, T.; De Carli, G.A. Shape variations of Trichomonas vaginalis in presence of different substrates. Parasitol. Latinoam. 2002, 57, 5–8. [Google Scholar] [CrossRef]
- Kirkcaldy, R.D.; Augostini, P.; Asbel, L.E.; Bernstein, K.T.; Kerani, R.P.; Mettenbrink, C.J.; Pathela, P.; Schwebke, J.R.; Secor, W.E.; Workowski, K.A. Trichomonas vaginalis antimicrobial drug resistance in 6 US cities, STD Surveillance Network, 2009–2010. Emerg. Infect. Dis. 2012, 18, 939. [Google Scholar] [CrossRef]
- Schwebke, J.R.; Barrientes, F.J. Prevalence of Trichomonas vaginalis isolates with resistance to metronidazole and tinidazole. Antimicrob. Agents Chemother. 2006, 50, 4209–4210. [Google Scholar] [CrossRef]
- Pandey, M.; Singh, A.K.; Thakare, R.; Talwar, S.; Karaulia, P.; Dasgupta, A.; Chopra, S.; Pandey, A.K. Diphenyleneiodonium chloride (DPIC) displays broad-spectrum bactericidal activity. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Grekov, I.; Pombinho, A.; Matyás, S.; Kobets, T.; Bartunek, P.; Lipoldová, M. Pharmaceutical Composition Comprising Diphenylenediodonium for the Treatment of Diseases Caused by Parasites Belonging to the Trypanosomatidae Family. Google Patents Application No. US20160220508A1, 4 August 2016. [Google Scholar]
- Yuan, J.; Johnson, R.L.; Huang, R.; Wichterman, J.; Jiang, H.; Hayton, K.; Fidock, D.A.; Wellems, T.E.; Inglese, J.; Austin, C.P. Genetic mapping of targets mediating differential chemical phenotypes in Plasmodium falciparum. Nat. Chem. Biol. 2009, 5, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Majander, A.; Finel, M.; Wikström, M. Diphenyleneiodonium inhibits reduction of iron-sulfur clusters in the mitochondrial NADH-ubiquinone oxidoreductase (Complex I). J. Biol. Chem. 1994, 269, 21037–21042. [Google Scholar] [PubMed]
- Smutná, T.; Pilarová, K.; Tarábek, J.; Tachezy, J.; Hrdý, I. Novel functions of an iron-sulfur flavoprotein from Trichomonas vaginalis hydrogenosomes. Antimicrob. Agents Chemother. 2014, 58, 3224–3232. [Google Scholar] [CrossRef]
- Schneider, R.E.; Brown, M.T.; Shiflett, A.M.; Dyall, S.D.; Hayes, R.D.; Xie, Y.; Loo, J.A.; Johnson, P.J. The Trichomonas vaginalis hydrogenosome proteome is highly reduced relative to mitochondria, yet complex compared with mitosomes. Int. J. Parasitol. 2011, 41, 1421–1434. [Google Scholar] [CrossRef]
- Xu, Q.; Huff, L.P.; Fujii, M.; Griendling, K.K. Redox regulation of the actin cytoskeleton and its role in the vascular system. Free Radic. Biol. Med. 2017, 109, 84–107. [Google Scholar] [CrossRef]
- Yeware, A.; Gample, S.; Agrawal, S.; Sarkar, D. Using diphenyleneiodonium to induce a viable but non-culturable phenotype in Mycobacterium tuberculosis and its metabolomics analysis. PLoS ONE 2019, 14, e0220628. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Lee, Y.H.; Choi, I.-W.; Heo, B.Y.; Kang, J.-G.; Yuk, J.-M.; Cha, G.-H.; Jo, E.-K.; Kwon, J. Adherence of Trichomonas vaginalis to SiHa Cells is Inhibited by Diphenyleneiodonium. Microorganisms 2020, 8, 1570. https://doi.org/10.3390/microorganisms8101570
Kim Y, Lee YH, Choi I-W, Heo BY, Kang J-G, Yuk J-M, Cha G-H, Jo E-K, Kwon J. Adherence of Trichomonas vaginalis to SiHa Cells is Inhibited by Diphenyleneiodonium. Microorganisms. 2020; 8(10):1570. https://doi.org/10.3390/microorganisms8101570
Chicago/Turabian StyleKim, Yeeun, Young Ha Lee, In-Wook Choi, Bu Yeon Heo, Ju-Gyeong Kang, Jae-Min Yuk, Guang-Ho Cha, Eun-Kyeong Jo, and Jaeyul Kwon. 2020. "Adherence of Trichomonas vaginalis to SiHa Cells is Inhibited by Diphenyleneiodonium" Microorganisms 8, no. 10: 1570. https://doi.org/10.3390/microorganisms8101570
APA StyleKim, Y., Lee, Y. H., Choi, I.-W., Heo, B. Y., Kang, J.-G., Yuk, J.-M., Cha, G.-H., Jo, E.-K., & Kwon, J. (2020). Adherence of Trichomonas vaginalis to SiHa Cells is Inhibited by Diphenyleneiodonium. Microorganisms, 8(10), 1570. https://doi.org/10.3390/microorganisms8101570





