SARS-CoV-2: From Structure to Pathology, Host Immune Response and Therapeutic Management
Abstract
1. Introduction
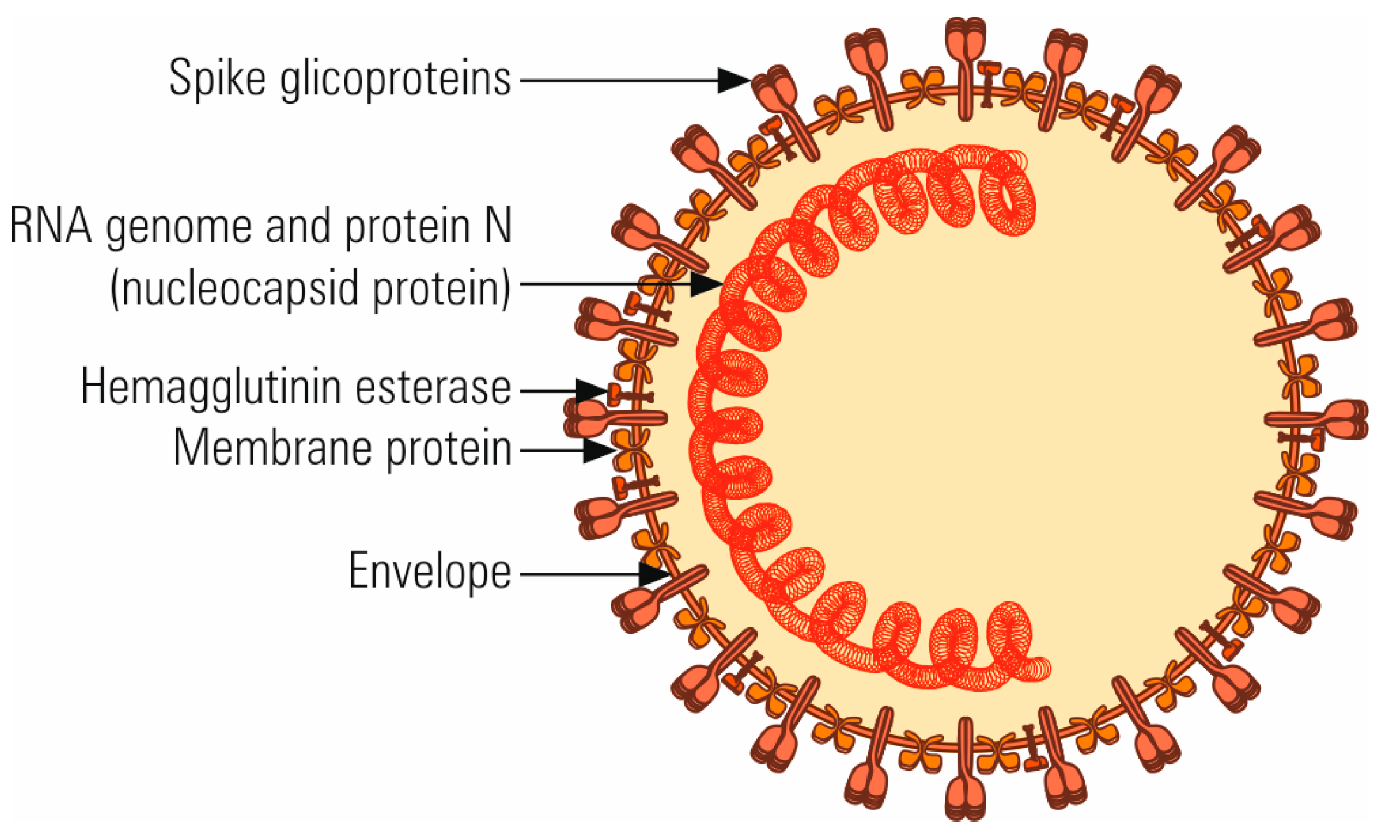
2. The Molecular Structure and Origin of SARS-CoV-2
3. Sensitivity to Physical and Chemical Agents
4. Viral Infection Cycle
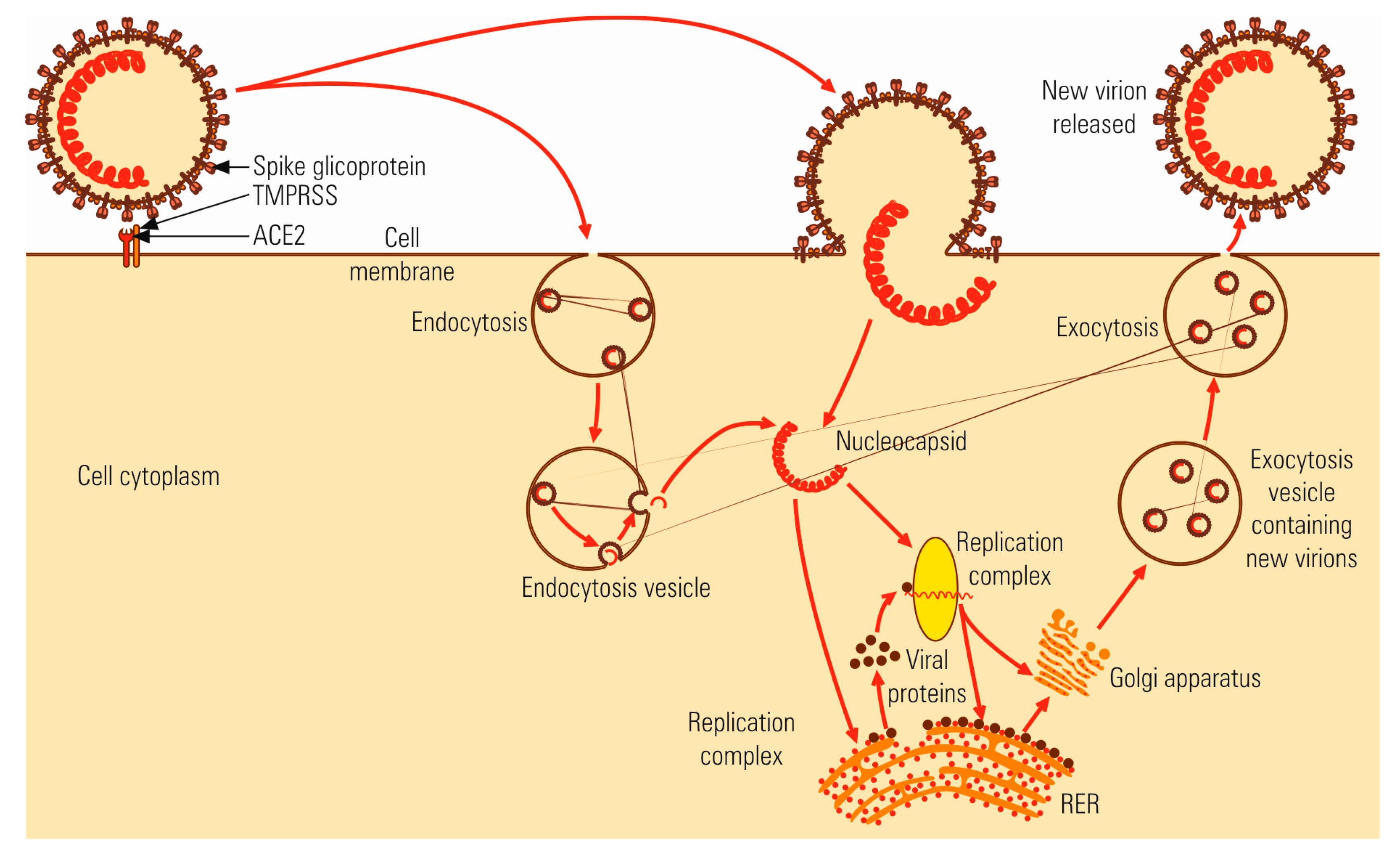
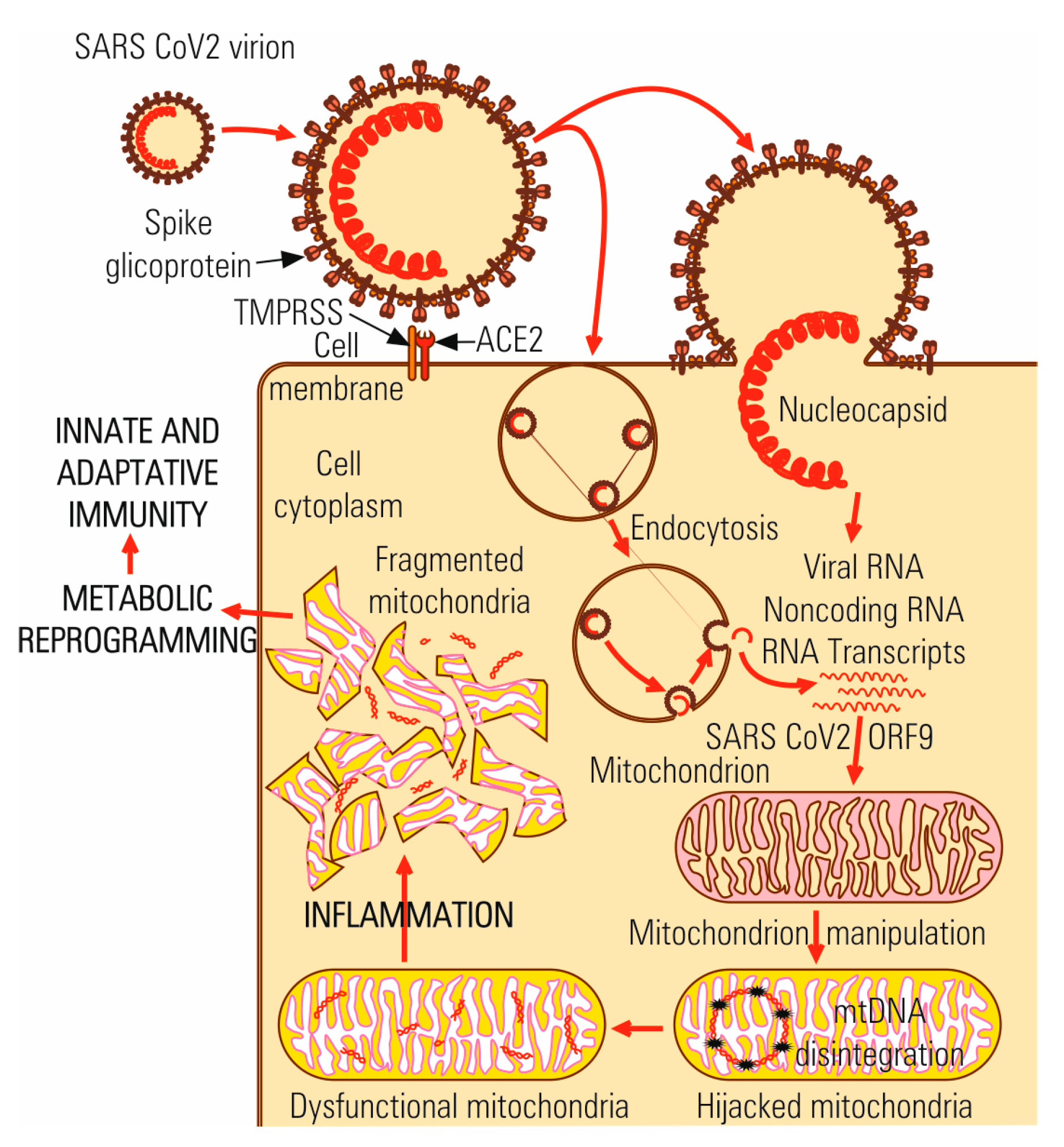
4.1. Virus-Cell Interaction
4.2. Multiplication Cycle
5. Genetic and Serologic Variability
6. COVID-19 Pathology
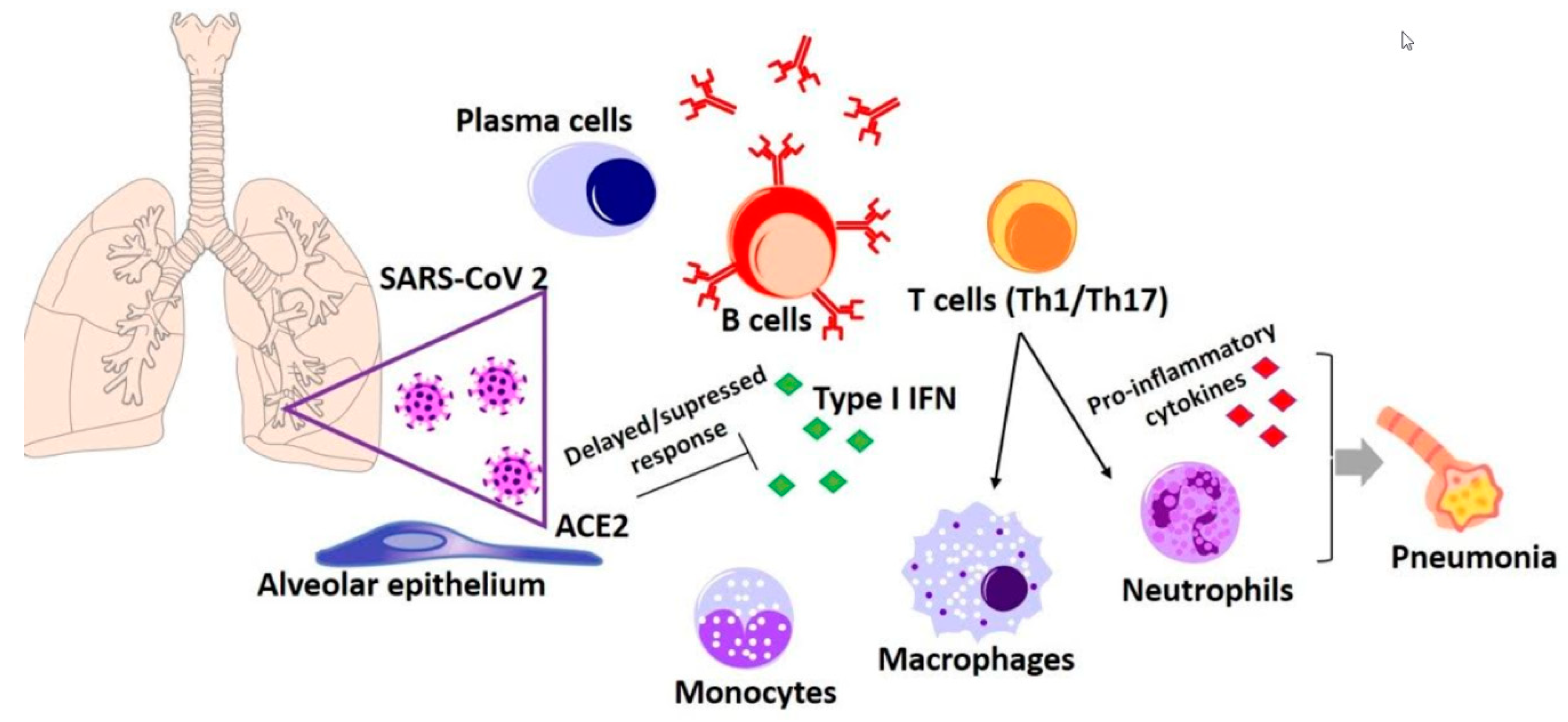
6.1. Immune Response to SARS-CoV-2 Infection
6.2. Inflammation-Triggering Risk Factors in COVID-19 Infection
Involvement of the Renin-Angiotensin System (RAS) in the Pulmonary Pathology of SARS-CoV-2
7. SARS-CoV-2 Diagnosis
8. Pharmacology
9. Prevention of SARS-CoV-2 Infection by Vaccination
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hamre, D. A new virus isolated from human respiratory tract. Proc. Soc. Exp. Biol. Med. 1966, 121, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, D.; Fielding, B.C. Coronavirus envelope protein: Current knowledge. Virology 2019, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Gaunt, E.R.; Hardie, A.; Claas, E.C.; Simmonds, P.; Templeton, K.E. Epidemiology and Clinical Presentations of the Four Human Years Using a Novel Multiplex Real-Time PCR Method. J. Clin. Microbiol. 2010, 48, 2940–2947. [Google Scholar] [CrossRef]
- Cascella, M.; Rajnik, M.; Cuomo, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation and Treatment Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Cui, J. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Wang, Q.; Gao, G.F. Bat-to human: Spike features determining ’host jump’of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. 2015, 23, 68. [Google Scholar] [CrossRef]
- Grifoni, A.; Sidney, J.; Zhang, Y.; Scheuermann, R.H.; Peters, B.; Sette, A. A Sequence Homology and Bioinformatic Approach Can Predict Candidate Targets for Immune Responses to SARS-CoV-2. Cell Host Microbe 2020, 27, 671–680.e2. [Google Scholar] [CrossRef]
- Andersen, K.G. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef]
- WHO. Modes of Transmission of Virus Causing COVID-19, Implications for Infection Prevention and Control (IPC) Precaution Recommendations. 2020. Available online: https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions (accessed on 17 September 2020).
- Guo, Y.R.; Cao, Q.D.; Hong, Z.S.; Tan, Y.Y.; Chen, S.D.; Jin, H.J.; Tan, K.S.; Wang, D.Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med. Res. 2020, 7, 11. [Google Scholar] [CrossRef]
- Chan, K.H.; Peiris, J.M.; Lam, S.Y.; Poon, L.L.M.; Yuen, K.Y.; Seto, W.H. The effects of temperature and relative humidity on the viability of the SARS Coronavirus. Adv. Virol. 2011, 2011, 734690. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Bushmaker, T.; Munster, V.J. Stability of middle east respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Eur. Surveill. 2013, 18, 20590. [Google Scholar]
- Gunthe, S.S.; Swain, B.; Patra, S.S.; Amte, A. On the global trends and spread of the COVID-19 outbreak: Preliminary assessment of the potential relation between location-specific temperature and UV index. Z. Gesundh Wiss. 2020, 1–10. [Google Scholar] [CrossRef]
- Duan, S.M.; Zhao, X.S.; Wen, R.F.; Huang, J.J.; Pi, G.H.; Zhang, S.X.; Han, J.; Bi, S.L.; Ruan, L.; Dong, X.P. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed. Environ. Sci. 2003, 16, 246–255. [Google Scholar] [PubMed]
- Qiu, Y.; Zhao, Y.B.; Wang, Q.; Li, J.Y.; Zhou, Z.J.; Liao, C.H.; Ge, X.Y. Predicting the angiotensin converting enzyme 2 (ACE2) utilizing capability as the receptor of SARS-CoV-2. Microbes Infect. 2020. [Google Scholar] [CrossRef]
- Mei, Z.; Bingpeng, L.; Hongbin, G.; Xinhong, W.; Kaibin, W.; Mingxiao, L.; Chang, L.; Jianming, C.; Learn-han, L.; Cuiling, Q.; et al. Significant expression of FURIN and ACE2 on oral epithelial cells may facilitate the efficiency of 2019-nCov entry. BioRxiv 2020. [Google Scholar] [CrossRef]
- Dijkman, R.; Jebbink, M.F.; Koekkoek, S.M.; Deijs, M.; Jónsdóttir, H.R.; Molenkamp, R.; Ieven, M.; Goossens, H.; Thiel, V.; van der Hoek, L. Isolation and characterization of current human coronavirus strains in primary human epithelial cell cultures reveal differences in target cell tropism. J. Virol. 2013, 87, 6081–6090. [Google Scholar]
- Mina-Osorio, P. The moonlighting enzyme CD13, Old and new functions to target. Trends Mol. Med. 2008, 14, 361–371. [Google Scholar]
- Jung, K.; Hu, H.; Saif, L.J. Porcine delta coronavirus infection: Etiology, cell culture for virus isolation and propagation, molecular epidemiology and pathogenesis. Virus Res. 2016, 226, 50–59. [Google Scholar]
- Millet, J.K.; Whittaker, G.R. Host cell proteases: Critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015, 202, 120–134. [Google Scholar] [CrossRef]
- Felsenstein, S. Covid-19, Immunology and Treatment options. Clin. Immunol. 2020, 215, 108448. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, C.; Mihăescu, G.; Lazăr, V. Microbiologie şi Virologie Medicală; Editura Universităţii: Bucharest, Romania, 2011. [Google Scholar]
- Wang, H.; Yang, P.; Liu, K.; Guo, F.; Zhang, Y.; Zhang, G.; Jiang, C. SARS coronavirus entry into host cells through a novel clathrin-and caveolae-independent endocytic pathway. Cell Res. 2008, 18, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Millet, J.K.; Whittaker, G.R. Physiological and molecular triggers for SARS-CoV membrane fusion and entry into host cells. Virology 2018, 507, 3–8. [Google Scholar] [CrossRef]
- Xia, S.; Lan, Q.; Su, S.; Wang, X.; Xu, W.; Liu, Z.; Zhu, Y.; Wang, Q.; Lu, L.; Jiang, S. The role of furin cleavage site in SARS-CoV-2 spike protein-mediated membrane fusion in the presence or absence of trypsin. Signal. Transduct. Target. Ther. 2020, 5, 92. [Google Scholar] [CrossRef]
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432. [Google Scholar] [CrossRef]
- Weiss, S.R.; Navas-Martin, S. Coronavirus Pathogenesis and the Emerging Pathogen Severe Acute Respiratory Syndrome Coronavirus. Microbiol. Mol. Biol. Rev. 2005, 69, 635–664. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, F.; Wang, R.; Guan, K.; Jiang, T.; Xu, G.; Sun, J.; Chang, C. The deadly coronaviruses: The 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J. Autoimmun. 2020, 109, 102434. [Google Scholar] [CrossRef]
- Yang, D.; Leibowitz, J.L. The structure and functions of coronavirus genomic 3′ and 5′ ends. Virus Res. 2015, 206, 120–133. [Google Scholar] [CrossRef] [PubMed]
- McRoy, W.C.; Baric, R.S. Amino acid substitutions in the S2 subunit of mouse hepatitis virus variant V51 encode determinants of host range expansion. J. Virol. 2008, 82, 1414–1424. [Google Scholar] [CrossRef]
- Masters, P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006, 66, 193–292. [Google Scholar]
- McBride, R.; van Zyl, M.; Fielding, B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses 2014, 6, 2991–3018. [Google Scholar] [CrossRef]
- Roh, H.J.; Hilt, D.A.; Jackwood, M.W. Simultaneous detection of five major serotypes of Avian coronavirus by a multiplex microsphere-based assay. J. Vet. Diagn. Investig. 2013, 25, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [PubMed]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Ogando, N.; Ferron, F.; Decroly, E.; Bruno, C.; Posthuma, C.; Snijder, E. The curious case of the nidovirus exoribonuclease: Its role in RNA synthesis and replication fidelity-front. Microbiology 2019, 10, 1813. [Google Scholar] [CrossRef]
- Pachetti, M.; Marini, B.; Benedetti, F.; Giudici, F.; Mauro, E.; Storici, P.; Masciovecchio, C.; Angeletti, S.; Ciccozzi, M.; Gallo, R.C.; et al. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA-polymerase variant. J. Transl. Med. 2020, 18, 179. [Google Scholar] [CrossRef]
- Chan, J.F.; To, K.K.; Tse, H.; Jin, D.Y.; Yuan, K.Y. Interspecies transmission and emergence of novel viruses: Lessons from bats and birds. Trends Microbiol. 2013, 21, 544–555. [Google Scholar] [CrossRef]
- Banner, L.R.; Lai, M.M. Random nature of coronavirus RNA recombination in the absence of selection pressure. Virology 1991, 185, 441–445. [Google Scholar] [CrossRef]
- Wu, K. RNA-GPS Predicts SARS-CoV 2 RNA localization to Host Mitochondria and Intracellular Nucleolus. bioRxiv 2020. [Google Scholar] [CrossRef]
- Peiris, J.S.M.; Poon, L.L.M. Severe Acute Respiratory Syndrome (SARS). In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Totura, A.L.; Baric, R.S. SARS coronavirus pathogenesis: Host innate immune responses and viral antagonism of interferon. Curr. Opin. Virol. 2012, 2, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Y.; Shao, C.; Huang, J.; Gan, J.; Huang, X.; Bucci, E.; Piacentini, M.; Ippolito, G.; Melino, G. COVID-19 infection: The perspectives on immune responses. Cell Death Differ. 2020, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Guillot, L.; Nathan, N.; Tabary, O.; Thouvenin, G.; Le Rouzic, P.; Corvol, H.; Amselem, S.; Clement, A. Alveolar epithelial cells: Master regulators of lung homeostasis. Int. J. Biochem. Cell Biol. 2013, 45, 2568–2573. [Google Scholar] [CrossRef] [PubMed]
- Gralinski, L.E.; Sheahan, T.P.; Morrison, T.E.; Menachery, V.D.; Jensen, K.; Leist, S.R.; Whitmore, A.; Heise, M.T.; Baric, R.S. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. MBio 2018, 95. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (Lond. Engl.) 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Zu, Z.Y.; Jiang, M.D.; Xu, P.P.; Chen, W.; Ni, Q.Q.; Lu, G.M.; Zhang, L.J. Coronavirus disease 2019 (COVID-19): A perspective from China. Radiology 2020, 21, 200490. [Google Scholar] [CrossRef]
- Bocksberger, S.; Wagner, W.; Hummel, T.; Guggemos, W.; Seilmaier, M.; Hoelscher, M.; Wendtner, C.M. Temporary hyposmia in COVID-19 patients. HNO 2020, 68, 440–443. [Google Scholar] [CrossRef]
- Channappanavar, R. Dysregulated type I interferon and inflammatory monocyte-macrophage response cause lethal pneumonia in SARS-CoV infected mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef]
- Fan, E.K.Y.; Fan, J. Regulation of alveolar macrophage death in acute lung inflammation. Respir Res. 2018, 19, 50. [Google Scholar] [CrossRef]
- Lester, S.N.; Li, K. Toll like receptors in antiviral innate immunity. J. Mol. Biol. 2014, 426, 1246–1264. [Google Scholar] [CrossRef]
- Conti, P.; Gallenga, C.E.; Tete, G.; Caraffa, A.; Ronconi, G.; Younes, A.; Toniato, E.; Ross, R.; Kritas, S.K. How to reduce the likelihood of COVID-19 infection and lung inflammation mediated by IL-1. J. Biol. Regul. Homeost. Agents 2020, 34. [Google Scholar] [CrossRef]
- Bauer, R.N.; Diaz-Sanchez, D.; Jaspers, I. Effects of air pollutants on innate immunity. The role of Toll-like receptors and nucleotide-binding oligomerizatiob domain-like receptors. J. Allergy Clin. Immunol. 2012, 129, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Prompetchara, E.; Ketloy, C.; Palaga, T. Allergy and Immunology Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020, 38, 1–9. [Google Scholar]
- Zhao, J.; Zhao, J.; Mangalam, A.K.; Channappanavar, R.; Fett, C.; Meyerholz, D.K.; Agnihothram, S.; Baric, R.S.; David, C.S.; Perlman, S. Airway memory CD4 T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity 2016, 44, 1379–1391. [Google Scholar] [CrossRef]
- Tang, F.; Quan, Y.; Xin, Z.T.; Wrammert, J.; Ma, M.J.; Lv, H.; Wang, T.B.; Yang, H.; Richardus, J.H.; Liu, W.; et al. Lack of peripheral memory B Cell responses in recovered patients with SARS: A six years follow up study. J. Immunol. 2011, 186, 7264–7268. [Google Scholar] [CrossRef]
- Grigore Mihaescu, C.C. Immunology and Immunopathology; Editura Medicala: Bucharest, Romania, 2015; pp. 506–508. [Google Scholar]
- Lau, Y.L.; Peiris, J.S. Pathogenesis of severe acute respiratory syndrome. Curr. Opin. Immunol. 2005, 17, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.F.; Ward, P.A. Role of C5a in inflammatory responses. Ann. Rev. Immunol. 2005, 23, 821–852. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Zhang, F.; Wang, Q.; Li, T.; Liu, Z.; Wang, J.; Qin, Y.; Zhang, X.; Yan, X.; et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The perspectives of clinical immunologists from China. Clin. Immunol. 2020, 214, 108393. [Google Scholar] [CrossRef]
- Weinberg, S.E. Mitochondria in the regulation of innate and adaptive immunity. Immunity 2015, 42, 406–417. [Google Scholar] [CrossRef]
- Mehta, M.M.; Weinberg, S.E.; Chandel, N.S. Mitochondrial control of Immunity: Beyond ATP. Nat. Rev. Immunol. 2017, 17, 608. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; O’Neill, L.A. Mitochondria are the powerhouse of immunity. Nat. Immunol. 2017, 18, 488. [Google Scholar]
- Rongvaux, A. Innate Immunity and tolerance toward mitochondria. Mitochondrion 2018, 41, 14–20. [Google Scholar]
- Arnoult, D.; Soares, F.; Tattoli, I.; Girardin, S.E. Mitochondria in Innate Immunity. EMBO Rep. 2011, 12, 901–910. [Google Scholar] [PubMed]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [PubMed]
- Chen, J.; Jiang, Q.; Xia, X.; Liu, K.; Yu, Z.; Tao, W.; Gong, W.; Han, J.D.J. Individual Variation of the SARS-CoV2 Receptor ACE2 Gene Expression and Regulation. Preprints 2020, 2020030191. [Google Scholar]
- Singh, K.K.; Chaubey, G.; Chen, J.Y.; Suravajhala, P. Decoding SARS-CoV-2 Hijacking of Host Mitochondria in Pathogenesis of COVID-19. Am. J. Physiol. Cell Physiol. 2020. [Google Scholar] [CrossRef]
- Cao, Y.; Li, L.; Feng, Z.; Wan, S.; Huang, P.; Sun, X.; Wen, F.; Huang, X.; Ning, G.; Wang, W. Comparative genetic analysis of 2019-nCoV receptor ACE2 in different populations. Cell Discov. 2020, 6, 1–4. [Google Scholar]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar]
- Steves, A.M.; Dowd, S.B.; Durick, D. Caring for the older patient, Part II: Age-related anatomic and physiologic changes and pathologies. J. Nucl. Med. Technol. 1997, 25, 86–105. [Google Scholar]
- Jobling, M.A.; Hurles, M.E.; Tayler-Smith, C. Human Evolutionary Genetics: Origins, Peoples and Disease; Garland Science: New York, NY, USA, 2004; 523p. [Google Scholar]
- Macciò, A.; Madeddu, C. Management of anemia of inflammation in the elderly. Anemia 2012, 2012, 563251. [Google Scholar]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Butcher, S.K.; Lord, J.M. Stress responses and innate immunity: Aging as a contributory factor. Aging Cell. 2004, 3, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Salvioli, S.; Capri, M.; Valensin, S.; Tieri, P.; Monti, D.; Ottaviani, E.; Franceschi, C. Inflamm-aging, cytokines and aging: State of the art, new hypotheses on the role of mitochondria and new perspectives from systems biology. Curr. Pharm. Des. 2006, 12, 3161–3171. [Google Scholar] [CrossRef] [PubMed]
- Andrews, C.N. The molecular basis of iron metabolism. In Molecular Hematology, 2nd ed.; Provan, D., Gribben, J., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2005. [Google Scholar]
- Pinti, M. Circulating mitochondrial DNA increases with age and is a familiar trait; Implication of “inflamm-aging”. Eur. J. Immunol. 2014, 44, 1552–1562. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Chen, Y.W.; Chenier, I.; Tran, S.L.M.; Zhang, S.L. Angiotensin II type II receptor deficiency accelerates the development of nephropathy in type I diabetes via oxidative stress and ACE2. Exp. Diabetes Res. 2011, 2011, 521076. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Coronavirus Infections and Type 2 Diabetes—Shared Pathways with Therapeutic Implications. Endocr. Rev. 2020, 41, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. 2020, 8, e21. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Dalan, R.; Hopkins, D.; Mingrone, G.; Boehm, B.O. Endocrine and metabolic link to coronavirus infection. Nat. Rev. Endocrinol. 2020, 16, 297–298. [Google Scholar] [CrossRef]
- Schett, G.; Sticherling, M.; Neurath, M.F. COVID-19, Risk for cytokine targeting in chronic inflammatory diseases? Nat. Rev. Immunol. 2020, 20, 271–272. [Google Scholar] [CrossRef]
- Batatinha, H.A.P.; Rosa Neto, J.C.; Krüger, K. Inflammatory features of obesity and smoke exposure and the immunologic effects of exercise. Exerc. Immunol. Rev. 2019, 25, 96–111. [Google Scholar]
- Kuba, K.; Imai, Y.; Ohto-Nakanishi, T.; Penninger, J.M. Pharmacology & Therapeutics Trilogy of ACE2: A peptidase in the renin—Angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010, 128, 119–128. [Google Scholar] [CrossRef] [PubMed]
- CDC. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19). 2020. Available online: https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html (accessed on 18 June 2020).
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Pan, Y.; Cheng, S.M.; Hui, K.P.; Krishnan, P.; Liu, Y.; Ng, D.Y.; Wan, C.K.; Yang, P.; Wang, Q.; et al. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin. Chem. 2020, 66, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.W.; Yip, C.C.Y.; To, K.K.W.; Tang, T.H.C.; Wong, S.C.Y.; Leung, K.H.; Fung, A.Y.F.; Ng, A.C.K.; Zou, Z.; Tsoi, H.W.; et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-polymerase chain reaction assay validated in vitro and with clinical specimens. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef]
- Xpert® Xpress SARS-CoV-2. Available online: https://www.cepheid.com/coronavirus (accessed on 18 June 2020).
- Vivalytic Rapid Test for COVID-19. Available online: https://www.bosch.com/stories/vivalytic-rapid-test-for-covid-19/ (accessed on 5 July 2020).
- Abbott Launches Molecular Point-of-Care Test to Detect Novel Coronavirus in as Little as Five Minutes. Available online: https://www.abbott.com/corpnewsroom/product-and-innovation/detect-covid-19-in-as-little-as-5-minutes.html (accessed on 16 June 2020).
- Tan, W.; Lu, Y.; Zhang, J.; Wang, J.; Dan, Y.; Tan, Z.; He, X.; Qian, C.; Sun, Q.; Hu, Q.; et al. Viral kinetics and antibody responses in patients with COVID-19. MedRxiv 2020. [Google Scholar] [CrossRef]
- COVID-19 IgM/IgG Rapid Test. Available online: https://www.biomedomics.com/products/infectious-disease/covid-19-rt (accessed on 22 July 2020).
- Cao, J.; Tu, W.J.; Cheng, W.; Yu, L.; Tu, W.J.; Liu, Q. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020, 71, ciaa243. [Google Scholar] [CrossRef]
- Gautret, P.; Lagier, J.C.; Parola, P.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; Dupont, H.T.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19, Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020, 56, 105949. [Google Scholar] [CrossRef]
- Fantini, J.; Di Scala, C.; Chahinian, H.; Yahi, N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents. 2020, 55, 105960. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the treatment of covid-19—Preliminary report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Hoffmann, M.; Schroeder, S.; Kleine-Weber, H.; Muller, M.; Drosten, C.; Pohlmann, S. Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19. Antimicrob Agents Chemother. 2020, 64, e00754-20. [Google Scholar] [CrossRef]
- Li, M.; Mondrinos, M.J.; Gandhi, M.R.; Ko, F.K.; Weiss, A.S. Electrospun protein fibers as matrices for tissue engineering. Biomaterials 2016, 5, 5999–6008. [Google Scholar] [CrossRef]
- Sisk, J.M.; Frieman, M.B.; Machamer, C.E. Coronavirus S protein-induced fusion is blocked prior to hemifusion by Abl kinase inhibitors. J. Gen. Virol. 2018, 99, 619–630. [Google Scholar] [PubMed]
- Dong, L.; Hu, S.; Gao, J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov. Ther. 2020, 14, 58–60. [Google Scholar] [PubMed]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar]
- Blaising, J.; Polyak, S.J.; Pécheur, E.I. Arbidol as a broad-spectrum antiviral: An update. Antivir. Res. 2014, 107, 84–94. [Google Scholar]
- De Wilde, A.H.; Zevenhoven-Dobbe, J.C.; van der Meer, Y.; Thiel, V.; Narayanan, K.; Makino, S.; Snijder, E.J.; van Hemert, M.J. Cyclosporin A inhibits the replication of diverse coronaviruses. J. Gen. Virol. 2011, 92, 2542–2548. [Google Scholar]
- Luo, P.; Liu, Y.; Qiu, L.; Liu, X.; Liu, D.; Li, J. Tocilizumab treatment in COVID-19: A single center experience. J. Med. Virol. 2020, 92, 814–818. [Google Scholar]
- Ohe, M.; Shida, H.; Jodo, S.; Kusunoki, Y.; Seki, M.; Furuya, K.; Goudarzi, H. Macrolide treatment for COVID-19, Will this be the way forward? Biosci. Trends 2020, 14, 159–160. [Google Scholar]
- Elfiky, A.A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020, 253, 117592. [Google Scholar]
- Wagstaff, K.M.; Sivakumaran, H.; Heaton, S.M.; Harrich, D.; Jans, D.A. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and Dengue virus. Biochem. J. 2012, 443, 851–856. [Google Scholar]
- Jawhara, S. Could intravenous immunoglobulin collected from recovered coronavirus patients protect against COVID-19 and strengthen the immune system of new patients? Int. J. Mol. Sci. 2020, 21, 2272. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; Wang, F.; Li, D.; Yang, M.; Xing, L.; et al. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA 2020, 323, 1582–1589. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Zhou, Y.H.; Lu, Y.Q.; Sun, F.; Yang, S.; Harypursat, V.; Chen, Y.K. Effectiveness of glucocorticoid therapy in patients with severe coronavirus disease 2019, Protocol of a randomized controlled trial. Chin. Med. J. (Engl.) 2020, 133, 1080–1086. [Google Scholar] [CrossRef]
- Ali, M.J.; Hanif, M.; Haider, M.A.; Ahmed, M.U.; Sundas, F.; Hirani, A.; Khan, I.A.; Anis, K.; Karim, A.H. Treatment Options for COVID-19, A Review. Front. Med. 2020, 7, 480. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.; Zhang, Y.; Pandolfi, P.P. Virus against virus: A potential treatment for 2019-nCov (SARS-CoV-2) and other RNA viruses. Cell Res. 2020, 30, 189–190. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.; Pfeffer, M.A.; Solomon, S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.; Griffin, I.; Tucker, C.; Smith, D.; Oechsle, O.; Phelan, A.; Stebbing, J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020, 395, e30–e31. [Google Scholar] [CrossRef]
- Yang, N.; Shen, H.M. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int. J. Biol. Sci. 2020, 16, 1724. [Google Scholar] [CrossRef]
- Stebbing, J. COVID-19, Combining antiviral and anti-inflamatory treatments. Lancet 2020, 20, 400–402. [Google Scholar] [CrossRef]
- Agostini, M.L.; Andres, E.L.; Sims, A.C.; Graham, R.L.; Sheahan, T.P.; Lu, X.; Smith, E.C.; Case, J.B.; Feng, J.Y.; Jordan, R.; et al. Coronavirus susceptibility to the antiviral remdesivir (gs-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio 2018, 9, e00221-18. [Google Scholar] [CrossRef]
- Gao, J.; Tian, Z.; Yang, X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends 2020, 14, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Sahraei, Z.; Shabani, M.; Shokouhi, S.; Saffaei, A. Aminoquinolines against coronavirus disease 2019 (COVID-19): Chloroquine or hydroxychloroquine. Int. J. Antimicrob. Agents 2020, 55, 105945. [Google Scholar] [CrossRef] [PubMed]
- Mégarbane, B.; Scherrmann, J.M. Hydroxychloroquine and Azithromycin to Treat Patients with COVID-19: Both Friends and Foes? J. Clin. Pharmacol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Geleris, J.; Sun, Y.; Platt, J.; Zucker, J.; Baldwin, M.; Hripcsak, G.; Labella, A.; Manson, D.K.; Kubin, C.; Barr, R.G.; et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020, 382, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Krammer, F. SARS-CoV-2 vaccines: Status report. Immunity 2020, 52, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Sarzi-Puttini, P.; Giorgi, V.; Sirotti, S.; Marotto, D.; Ardizzone, S.; Rizzardini, G.; Antinori, S.; Galli, M. COVID-19, cytokines and immunosuppression: What can we learn from severe acute respiratory syndrome? Clin. Exp. Rheumatol. 2020, 38, 337–342. [Google Scholar]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epitelial cells of oral mucosa. Int. J. Oral. Sci. 2020, 12, 8. [Google Scholar] [CrossRef]
- Hansson, M.; Nygren, P.A.; Sta˚hl, S. Design and production of recombinant subunit vaccines. Biotechnol. Appl. Biochem. 2000, 32, 95–107. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhao, M.; Liu, K.; Xu, K.; Wong, G.; Tan, W.; Gao, G.F. T cell immunity of SARS-CoV: Implications for vaccine development. Antivir. Res. 2017, 137, 82–92. [Google Scholar] [CrossRef]
- Schindewolf, C.; Menachery, V.D. Middle east respiratory syndrome vaccine candidates: Cautious optimism. Viruses 2019, 11, 74. [Google Scholar] [CrossRef]
- Riedel, S. Edward Jenner and the history of smallpox and vaccination. In Baylor University Medical Center Proceedings; Taylor & Francis: Abingdon-on-Thames, UK, 2005; Volume 18, pp. 21–25. [Google Scholar]
| Test | Specimen | Advantages | Limitations |
|---|---|---|---|
| Real time PCR | Nasopharyngeal and/or oropharyngeal swab, lower respiratory specimen | Currently, the gold standard High sensitivity and specificity | Requires special infrastructure and trained personnel Expensive Medium turnaround time (190 min) Incorrect sampling |
| RT-LAMP | Nasopharyngeal and/or oropharyngeal swab, lower respiratory specimen | Shorter turnaround time compared to RT-PCR (45-60 min) High sensitivity Can be used as substitute for RT-PCR when a reduced turnaround time is needed | Requires special infrastructure Incorrect sampling Expensive |
| Nucleoprotein antigen detection test | Nasopharyngeal and/or oropharyngeal swab, and/or lower respiratory specimen | Easier to use, suitable for labs which are less equipped | Low sensitivity Requires qualified personnel Incorrect sampling |
| ELISA | Serum, plasma, whole blood | Not very expensive Medium turnaround time | Requires special infrastructure and qualified personnel |
| Chemiluminescence immunoassay | Serum, plasma, whole blood | High sensitivity | Requires special infrastructure and qualified personnel |
| Rapid antibody (IgG and IgM) detection test | Fingerprick | Easy sampling Does not require special infrastructure Short turnaround time (max 30 min) | Low specificity and sensitivity More suitable for epidemiological screening rather than diagnosis per se |
| Drug | Mechanism of Action | Adverse Effects/Limitations | References |
|---|---|---|---|
| Chloroquine Hydroxychloroquine | Interferes with the terminal glycosylation of ACE2, and thus negatively influences the virus-receptor binding in SARS-CoV infection | Narrow therapeutic index Seizures Retinopathy Myopathy Bone marrow suppression | [99,100] |
| Remdesivir | An adenosine analog causing premature termination of the nascent viral RNA chains by incorporation into the viral genome | Kidney injury Elevated transaminases | [101] |
| Camostat/Nafamostat | TMPRSS2 inhibitors | Rash Diarrhea Nausea Hepatotoxicity | [102,103] |
| Imatinib | Abelson (Abl) kinase inhibitor, blocks the endocytic entry of other β-coronaviruses | GIT intolerance, Flu-like symptoms | [104] |
| Lopinavir & Ritonavir | Inhibits the activity of 3CLpro and is approved for the treatment of HIV/AIDS | GIT intolerance, vomiting, nausea Hepatotoxicity Pancreatitis | [105,106] |
| Arbidol | Inhibits virus entry/fusion of viral membranes with cellular membranes | Skin rash | [107] |
| Cyclosporin A | Approved immunosuppressant drug, interferes with protein interactions thereby affecting viral replication | nephrotoxicity, hypertension, increased blood urea nitrogen, increased serum creatinine, | [108] |
| Tocilizumab | Binds to both soluble and cell-associated IL-6R with high affinity. TCZ blocks IL-6 from initiating its pro-inflammatory downstream signaling, alleviating the host immune response | Hepatotoxicity Headache Hypertension Hematologic effects Increase in upper respiratory tract infections | [109] |
| Azithromycin | Inhibits protein synthesis in bacteria but harbors also anti-viral effects | QT interval prolongation | [110] |
| Favipiravir | Inhibits RNA-dependent RNA polymerase of RNA viruses which leads to chain termination | Diarrhea Neutropenia | [105] |
| Ribavirin | Nucleoside analog of guanosine inhibiting RNA polymerase and (chain terminator) | Hemolytic Anemia Teratogenic | [111] |
| Ivermectin | Anti-parasitic drug shown to inhibit replication of SARS-CoV-2 in vitro | Skin rash Muscle/joint pain | [112] |
| Immunoglobulin | Antibodies obtained from recovered patients | Headache Fever Malaise Thrombosis Renal impairment | [113,114] |
| Corticosteroids | Can reduce pathological damage caused by the infection harboring an anti -inflammatory role due to their various effects on various cytokines (1L-1, 1L-6, 1L-8, 1L-12, TNFα) | Long term use can cause; diabetes, hypertension, weight gain | [115] |
| Interferon | Used for boosting the immune system | Fever Chills Flu-like symptoms such as headache, fatigue, and weakness | [116] |
| CRISPR/Cas13d | Knockdown system used in cleaving the SARS-CoV-2 RNA genome; the Cas13d effector can be delivered via an adeno-associated virus (AAV) to the SARS-CoV-2 infected lung | Experimental, Expensive | [117] |
| Vaccine Name | Developer (Country) | Description | Clinical Trial Details |
|---|---|---|---|
| IMP (CoVac-1) | University Hospital Tuebingen (Germany) | Multipeptide cocktail; SARS-CoV-2 HLA-DR peptides, XS15 emulsified in Montanide ISA 51 VG | NCT04546841 Phase 1 |
| TMV-083 | Institut Pasteur (France) | Live-attenuated recombinant measles vaccine virus vector expressing a modified surface glycoprotein of SARS-CoV2 | NCT04497298 Phase 1 |
| EpiVacCorona | Federal Budgetary Research Institution State Research Center of Virology and Biotechnology “Vector” (Russia) | Chemically synthesized peptide antigens of SARS-CoV-2 proteins, conjugated to a carrier protein and adsorbed on an aluminum-containing adjuvant | NCT04527575 Phase 1 |
| CoronaVac | Butantan Institute (Brazil) Sinovac Life Sciences Co., Ltd. | Adsorbed COVID-19 (inactivated) vaccine | NCT04456595 Phase 3 |
| aAPC | Shenzhen Geno-Immune Medical Institute (China) | Coronavirus Artificial Antigen Presenting Cell Vaccine | NCT04299724 Phase 1 |
| Gam-COVID-Vac | Gamaleya Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation (Russia) | adenoviral-based vaccine against SARS-CoV-2 | NCT04436471 |
| Inactivated SARS-CoV-2 vaccine (Vero cell) | China National Biotec Group Company Limited | Inactivated SARS-CoV-2 vaccine (Vero cell) | NCT04510207 Phase 3 |
| Recombinant SARS-CoV-2 vaccine (Sf9 cell) | Jiangsu Province Centers for Disease Control and Prevention (China) | recombinant SARS-CoV-2 vaccine (Sf9 Cell) | NCT04530656 (Phase 1) |
| Lentiviral Minigene vaccine (LV-SMENP) | Shenzhen Geno-Immune Medical Institute | Lentiviral-SMENP-dendritic cell vaccine and antigen-specific CTLs | NCT04276896 |
| UB-612 | United Biomedical Inc., Asia | S1-RBD-protein-based vaccine | NCT04545749 Phase i |
| Covax-19™ | GeneCure Biotechnologies | Therapeutic vaccine | NCT04428073 |
| Recombinant Coronavirus-Like Particle Vaccine | Medicago | Recombinant Coronavirus-Like Particle | NCT04450004 Phase 1 |
| mRNA-1273 | ModernaTX, Inc | Lipid nanoparticle-incapsulated mRNA-based vaccine encoding the S protein of SARS-CoV2 | NCT04283461 Phase 1 |
| CTCOVID-19 | CanSino Biologics Inc. (China) | Adenovirus Type 5 Vector | NCT04313127 Phase I |
| SCB-2019 | Clover Biopharmaceuticals AUS Pty Ltd. (Australia) | Recombinant Trimeric S Protein Subunit Vaccine | NCT04405908 (Phase I) |
| BNT162b3 | BioNTech RNA Pharmaceuticals GmbH (Germany) | Anti-viral RNA vaccine | NCT04537949 Phase 2 |
| AZD1222 | AstraZeneca | Non-replicating ChAdOx1 Vector vaccine | NCT04516746 Phase III |
| ChAdOx1 | University of Oxford (UK) | chimpanzee adenovirus vaccine vector | NCT04324606 |
| AG0302-COVID19 | AnGes, Inc (Japan) | DNA vaccine | NCT04527081 Phase 2 |
| CVnCoV | CureVac AG | mRNA vaccine | NCT04449276 Phase 1 |
| Ad26.COV2.S | Janssen Vaccines & Prevention B.V.(Netherlands) | adenovirus serotype 26 (Ad26) vector-based vaccine | NCT04505722 |
| AdimrSC-2f | Adimmune Corporation | the recombinant receptor-binding domain (RBD) of SARS-CoV-2 spike (S) protein amplified and purified using the baculovirus-insect cells expression system, | NCT04522089 Phase 1 |
| GRAd-COV2 | ReiThera Srl (Italy) | Encodes for SARS-COV-2 full length prefusion stabilized Spike protein gorilla-derived replication-defective adenoviral vector | NCT04528641 Phase 1 |
| V-SARS | Immunitor LLC (Canada) | pill derived from heat-inactivated plasma from COVID-19 patient | NCT04380532 Phase 2 |
| Spike nanoparticle with and without Matrix-MTM adjuvant | Novavax (Australia) | Stable, pre-fusion spike nanoparticle with and without Matrix-MTM adjuvant | NCT04368988 Phase 1 and 2 |
| Inactivated SARS-CoV-2 | Wuhan Institute of Biological Product (China) | Inactivated SARS-CoV-2 | ChiCTR2000031809 |
| PiCoVacc | Sinovac (China) | Inactivated SARS-CoV-2 (PiCoVacc) with an alum adjuvant | NCT04352608 Phase 2 |
| Ad5-nCoV encoding full-length spike protein | CanSino Biologics (China) | Ad5-nCoV encoding full-length spike protein | NCT04341389 (Phase 2) |
| COVAC1 | Imperial college London (UK) | mRNA SAM expressing spike protein in LNP | ISRCTN17072692 Phase 1 |
| INO-4800 | Inovio Pharmaceuticals (USA) | DNA expressing spike protein | NCT04336410 Phase 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihaescu, G.; Chifiriuc, M.C.; Iliescu, C.; Vrancianu, C.O.; Ditu, L.-M.; Marutescu, L.G.; Grigore, R.; Berteșteanu, Ș.; Constantin, M.; Gradisteanu Pircalabioru, G. SARS-CoV-2: From Structure to Pathology, Host Immune Response and Therapeutic Management. Microorganisms 2020, 8, 1468. https://doi.org/10.3390/microorganisms8101468
Mihaescu G, Chifiriuc MC, Iliescu C, Vrancianu CO, Ditu L-M, Marutescu LG, Grigore R, Berteșteanu Ș, Constantin M, Gradisteanu Pircalabioru G. SARS-CoV-2: From Structure to Pathology, Host Immune Response and Therapeutic Management. Microorganisms. 2020; 8(10):1468. https://doi.org/10.3390/microorganisms8101468
Chicago/Turabian StyleMihaescu, Grigore, Mariana Carmen Chifiriuc, Ciprian Iliescu, Corneliu Ovidiu Vrancianu, Lia-Mara Ditu, Luminita Gabriela Marutescu, Raluca Grigore, Șerban Berteșteanu, Marian Constantin, and Gratiela Gradisteanu Pircalabioru. 2020. "SARS-CoV-2: From Structure to Pathology, Host Immune Response and Therapeutic Management" Microorganisms 8, no. 10: 1468. https://doi.org/10.3390/microorganisms8101468
APA StyleMihaescu, G., Chifiriuc, M. C., Iliescu, C., Vrancianu, C. O., Ditu, L.-M., Marutescu, L. G., Grigore, R., Berteșteanu, Ș., Constantin, M., & Gradisteanu Pircalabioru, G. (2020). SARS-CoV-2: From Structure to Pathology, Host Immune Response and Therapeutic Management. Microorganisms, 8(10), 1468. https://doi.org/10.3390/microorganisms8101468












