An Unexpectedly Broad Thermal and Salinity-Tolerant Estuarine Methanogen Community
Abstract
1. Introduction
2. Materials and Methods
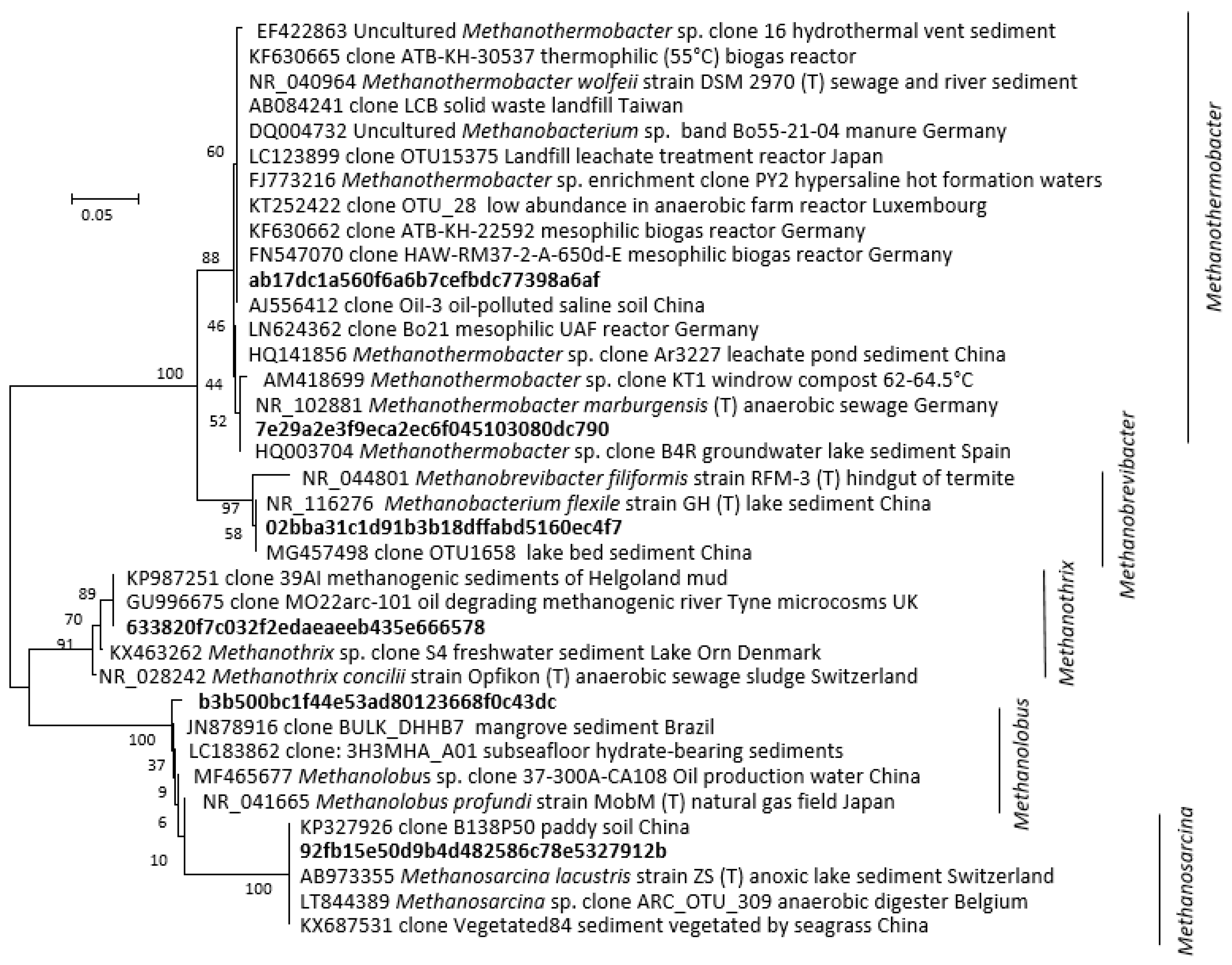
3. Results and Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2013–The Physical Science Basis; Intergovernmental Panel on Climate Change, Ed.; Cambridge University Press: Cambridge, UK, 2014; p. 509. [Google Scholar] [CrossRef]
- Cicerone, R.J.; Oremland, R.S. Biogeochemical aspects of atmospheric methane. Glob. Biogeochem. Cycles 1988, 2, 299–327. [Google Scholar] [CrossRef]
- Kan, S.Y.; Chen, B.; Wu, X.F.; Chen, Z.M.; Chen, G.Q. Natural gas overview for world economy: From primary supply to final demand via global supply chains. Energy Policy 2019, 124, 215–225. [Google Scholar] [CrossRef]
- Conrad, R. Importance of hydrogenotrophic, aceticlastic and methylotrophic methanogenesis for methane production in terrestrial, aquatic and other anoxic environments: A mini review. Pedosphere 2020, 30, 25–39. [Google Scholar] [CrossRef]
- Jabłońsk, S.; Rodowicz, P.; Lukaszewicz, M. Methanogenic archaea database containing physiological and biochemical characteristics. Int. J. Syst. Evol. Microbiol. 2015, 65, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Blake, L.I.; Tveit, A.; Ovreas, L.; Head, I.M.; Gray, N.D. Response of methanogens in Arctic sediments to temperature and methanogenic substrate availability. PLoS ONE 2015, 10, e0129733. [Google Scholar]
- Jerman, V.; Metje, M.; Mandic-Mulec, I.; Frenzel, P. Wetland restoration and methanogenesis: The activity of microbial populations and competition for substrate at different temperatures. Biogeosciences 2009, 6, 1127–1138. [Google Scholar] [CrossRef]
- Metje, M.; Frenzel, P. Effect of Temperature on Anaerobic Ethanol Oxidation and Methanogenesis in Acidic Peat from a Northern Wetland. Appl. Environ. Microbiol. 2005, 71, 8191–8200. [Google Scholar] [CrossRef]
- Metje, M.; Frenzel, P. Methanogenesis and methanogenic pathways in a peat from subarctic permafrost. Environ. Microbiol. 2007, 9, 954–964. [Google Scholar] [CrossRef]
- Zeikus, J.G.; Winfrey, M.R. Temperature limitation of methanogenesis in aquatic sediments. Appl. Environ. Microbiol. 1976, 1, 99–107. [Google Scholar] [CrossRef]
- Fey, A.; Conrad, R. Effect of temperature on carbon and electron flow and on the archaeal community in methanogenic rice field soil. Appl. Environ. Microbiol. 2000, 11, 4790–4797. [Google Scholar]
- Wu, X.-L.; Friedrich, M.W.; Conrad, R. Diversity and ubiquity of thermophilic methanogenic archaea in temperate anoxic soils. Environ. Microbiol. 2006, 8, 394–404. [Google Scholar] [CrossRef]
- Fey, A.; Chin, K.J.; Conrad, R. Thermophilic methanogens in rice field soil. Environ. Microbiol. 2001, 3, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Kotsyurbenko, O.R.; Friedrich, M.W.; Simankova, M.V.; Nozhevnikova, A.N.; Golyshin, P.N.; Timmis, K.N.; Conrad, R. Shift from Acetoclastic to H2-dependant Methanogenesis in a West Siberian Peat Bog at low pH Values and Isolation of an Acidophilic Methanobacterium Strain. Appl. Environ. Microbiol. 2007, 73, 2344–2348. [Google Scholar] [CrossRef]
- Ganzert, L.; Jurgens, G.; Munster, U.; Wagner, D. Methanogenic communities in permafrost-affected soils of the Laptev Sea coast, Siberian Arctic, characterized by 16S rRNA gene fingerprints. FEMS Microbiol. Ecol. 2007, 59, 476–488. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nozhevnikova, A.N.; Holliger, C.; Ammann, A.; Zehnder, A.J.B. Methanogenesis in sediments from deep lakes at different temperatures (2–70 °C). Water Sci. Technol. 1997, 36, 57–64. [Google Scholar] [CrossRef]
- Angel, R.; Claus, P.; Conrad, R. Methanogenic archaea are globally ubiquitous in aerated soils and become active under wet anoxic conditions. ISME J. 2012, 6, 847–862. [Google Scholar] [CrossRef]
- Sherry, A.; Osborne, K.A.; Sidgwick, F.R.; Gray, N.D.; Talbot, H.M. A temperate river estuary is a sink for methanotrophs adapted to extremes of pH, temperature and salinity. Environ. Microbiol. Rep. 2015, 8, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Carbonero, F.; Oakley, B.B.; Hawkins, R.J.; Purdy, K.J. Genotypic Distribution of a Specialist Model Microorganism, Methanosaeta, along an Estuarine Gradient: Does Metabolic Restriction Limit Niche Differentiation Potential? Environ. Microbiol. 2012, 63, 856–864. [Google Scholar] [CrossRef]
- Torres-Alvarado, M.; Fernandez, J.F.; Vives, F.R.; Varona-Cordero, F. Dynamics of the Methanogenic Archaea in Tropical Estuarine Sediments. Archaea 2013, 2013, 582646. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Yang, S.; Horn, F.; Winkel, M.; Wagner, D.; Liebner, S. Global Biogeographic Analysis of Methanogenic Archaea Identifies Community-Shaping Environmental Factors of Natural Environments. Front. Microbiol. 2017, 8, 1339. [Google Scholar] [CrossRef]
- Bange, H.W. Nitrous oxide and methane in European coastal waters. Estuar. Coast. Shelf Sci. 2006, 70, 361–374. [Google Scholar] [CrossRef]
- Bange, H.W.; Bartell, U.H.; Rapsomaniks, S.; Andrae, M.O. Methane in the Baltic and North Seas and a reassessment of the marine emissions of methane. Glob. Biogeochem. Cycles 1994, 8, 465–480. [Google Scholar]
- Myllykangas, J.-P.; Hietanen, S.; Jilbert, T. Legacy Effects of Eutrophication on Modern Methane Dynamics in a Boreal Estuary. Estuar. Coasts 2020, 43, 189–206. [Google Scholar]
- Mejeha, O.K.; Head, I.M.; Sherry, A.; McCann, C.M.; Leary, P.; Jones, M.D.; Gray, N.D. Beyond N and P: The impact of Ni on crude oil biodegradation. Chemosphere 2019, 237, 124545. [Google Scholar] [CrossRef] [PubMed]
- Quince, C.; Lanzen, A.; Davenport, R.J.; Turnhaugh, P.J. Removing noise from pyrosequenced amplicons. BMC Bioinform. 2011, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Hamady, M.; Walker, J.J.; Harris, J.K.; Gold, N.J.; Knight, R. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat. Methods 2008, 5, 235–237. [Google Scholar] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxanomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar]
- Shamurad, B.; Gray, N.D.; Petropoulos, E.; Tabriaz, S.; Acharya, K.; Quintela Baluja, M.; Sallis, P. Co-digestion of organic and mineral wastes for enhanced biogas production: Reactor performance and evolution of microbial community and function. Waste Manag. 2019, 87, 313–325. [Google Scholar] [CrossRef]
- Nozhevnikova, A.N.; Nekrasova, V.; Ammann, A.; Zehnder, A.J.B.; Wehrli, B.; Holliger, C. Influence of temperature and high acetate concentrations on methanogenesis in lake sediment slurries. FEMS Microbiol. Ecol. 2007, 62, 336–344. [Google Scholar] [CrossRef]
- Conrad, R.; Klose, M.; Noll, M. Functional and structural response of the methanogenic microbial community in rice field soil to temperature change. Environ. Microbiol. 2009, 11, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Noll, M.; Klose, M.; Conrad, R. Effect of temperature change on the composition of the bacterial and archaeal community potentially involved in the turnover of acetate and propionate in methanogenic rice field soil. FEMS Microbiol. Ecol. 2010, 73, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.; Klose, M.; Conrad, R. Temperature effects on structure and function of the methanogenic microbial communities in two paddy soils and one desert soil. Soil Biol. Biochem. 2018, 124, 236–244. [Google Scholar]
- Schink, B. Energetics of Syntrophic Cooperation in Methanogenic Degradation. Microbiol. Mol. Biol. Rev. 1997, 61, 262–280. [Google Scholar] [CrossRef] [PubMed]
- Wasserfallen, A.; Nolling, J.; Pfister, P.; Reeve, J.; Conway de Macario, E. Phylogenetic analysis of 18 thermophilic Methanobacterium isolates supports the proposals to create a new genus, Methanothermobacter gen. nov., and to reclassify several isolates in three species, Methanothermobacter thermoautotrophicus comb. nov., Methanothermobacter wolfeii comb. nov., and Methanothermobacter marburgensis sp. nov. Int. J. Syst. Evol. Microbiol. 2000, 50, 43–53. [Google Scholar]
- Ding, X.; Yang, W.-J.; Min, H.; Peng, X.-T.; Zhou, H.-Y.; Lu, Z.-M. Isolation and characterization of a new strain of Methanothermobacter marburgensis DX01 from hot springs in China. Anaerobe 2010, 16, 54–59. [Google Scholar] [CrossRef]
- Wiegel, J. Temperature spans for growth: Hypothesis and discussion. FEMS Microbiol. Rev. 1990, 6, 155–169. [Google Scholar] [CrossRef]
- Gray, N.D.; Sherry, A.; Larter, S.R.; Erdmann, M.; Leyris, J.; Liengen, T.; Beeder, J.; Head, I.M. Biogenic methane production in formation waters from a large gas field in the North Sea. Extremophiles 2009, 13, 511–519. [Google Scholar] [CrossRef]
- Bell, E.; Blake, L.I.; Sherry, A.; Head, I.M.; Hubert, C.R.J. Distribution of thermophilic endospores in a temperate estuary indicate that dispersal history structures sediment microbial communities. Environ. Microbiol. 2018, 20, 1134–1147. [Google Scholar] [CrossRef]
- Peters, V.; Conrad, R. Methanogenic and other strictly anaerobic bacteria in desert soil and other oxic soils. Appl. Environ. Microbiol. 1995, 61, 1673–1676. [Google Scholar] [CrossRef]
- Gray, N.D.; Miskin, I.P.; Kornilova, O.; Curtis, T.P.; Head, I.M. Occurrence and activity of Archaea in aerated activated sludge wastewater treatment plants. Environl. Microbiol. 2002, 3, 158–168. [Google Scholar]
- Oren, A. Thermodynamic limits to life at high salt concentrations. Environ. Microbiol. 2011, 13, 1908–1923. [Google Scholar] [PubMed]
- Blaser, M.J.; Cardon, Z.G.; Cho, M.K.; Dang, J.L.; Donohue, T.J.; Green, J.L.; Knight, R.; Maxon, M.E.; Northen, T.R.; Pollard, K.S.; et al. Towards a Predictive Understanding of Earth’s Microbiomes to Address 21st Century Challenges. mBio 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pan, J.; Lui, Y.; Li, M.; Gu, J.D. Diversity and distribution of Archaea in global estuarine ecosystems. Sci. Total Environ. 2018, 637, 349–358. [Google Scholar]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blake, L.I.; Sherry, A.; Mejeha, O.K.; Leary, P.; Coombs, H.; Stone, W.; Head, I.M.; Gray, N.D. An Unexpectedly Broad Thermal and Salinity-Tolerant Estuarine Methanogen Community. Microorganisms 2020, 8, 1467. https://doi.org/10.3390/microorganisms8101467
Blake LI, Sherry A, Mejeha OK, Leary P, Coombs H, Stone W, Head IM, Gray ND. An Unexpectedly Broad Thermal and Salinity-Tolerant Estuarine Methanogen Community. Microorganisms. 2020; 8(10):1467. https://doi.org/10.3390/microorganisms8101467
Chicago/Turabian StyleBlake, Lynsay I., Angela Sherry, Obioma K. Mejeha, Peter Leary, Henry Coombs, Wendy Stone, Ian M. Head, and Neil D. Gray. 2020. "An Unexpectedly Broad Thermal and Salinity-Tolerant Estuarine Methanogen Community" Microorganisms 8, no. 10: 1467. https://doi.org/10.3390/microorganisms8101467
APA StyleBlake, L. I., Sherry, A., Mejeha, O. K., Leary, P., Coombs, H., Stone, W., Head, I. M., & Gray, N. D. (2020). An Unexpectedly Broad Thermal and Salinity-Tolerant Estuarine Methanogen Community. Microorganisms, 8(10), 1467. https://doi.org/10.3390/microorganisms8101467




