Antibacterial Activity of Three Extra Virgin Olive Oils of the Campania Region, Southern Italy, Related to Their Polyphenol Content and Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polyphenols Analysis
2.1.1. Standards and Reagents
2.1.2. Extraction and Determination of Total Polyphenols
2.1.3. Chromatographic Analysis
2.2. Antibacterial Activity
2.2.1. Microorganisms and Culture Conditions
2.2.2. Determination of the Antibacterial Susceptibility by Agar Diffusion
2.2.3. Minimal Inhibitory Concentration (MIC)
2.3. Statistical Analysis
3. Results and discussion
3.1. Antibacterial Activity of the Extracts
3.2. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ray, N.B.; Hilsabeck, K.D.; Karagiannis, T.C.; McCord, D.E. Bioactive Olive Oil Polyphenols in the Promotion of Health. In The Role of Functional Food Security in Global Health; Singh, R.B., Watson, R.R., Takahashi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 623–637. [Google Scholar]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [PubMed]
- Capasso, R.; Evidente, A.; Schivo, L.; Orru, G.; Marcialis, M.A.; Cristinzio, G. Antibacterial polyphenols from olive oil mill waste waters. J. Appl. Bacteriol. 1995, 79, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.; Medina, E.; Vargas, J.; Brenes, M.; De Castro, A. In vitro activity of olive oil polyphenols against Helicobacter pylori. J. Agric Food Chem. 2007, 55, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Karaosmanoglu, H.; Soyer, F.; Ozen, B.; Tokatli, F. Antimicrobial and antioxidant activities of Turkish extra virgin olive oils. J. Agric. Food Chem. 2010, 58, 8238–8245. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, P.O.; Aribisala, J.O.; Oladunmoye, M.K.; Arogunjo, A.O.; Ajayi-Moses, O.B. Therapeutic effect of goya extra virgin olive oil in albino rat oro-gastrically dosed with Salmonella Typhi. South Asian J. Res. Microbiol. 2019, 3, 1–9. [Google Scholar]
- Rubio, L.; Macia, A.; Castell-Auvi, A.; Pinent, M.; Blay, M.T.; Ardevol, A.; Romero, M.P.; Motilva, M.J. Effect of the co-occurring olive oil and thyme extracts on the phenolic bioaccessibility and bioavailability assessed by in vitro digestion and cell models. Food Chem. 2014, 149, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Deiana, M.; Serra, G.; Corona, G. Modulation of intestinal epithelium homeostasis by extra virgin olive oil phenolic compounds. Food Funct. 2018, 9, 4085–4099. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; d’Acierno, A.; Coppola, R. Gut Microbiota and Polyphenols: A Strict Connection Enhancing Human Health. In Advances in Food Biotechnology; Ravishankar Rai, V., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2015; pp. 335–350. [Google Scholar]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechn. 2012, 23, 129–135. [Google Scholar] [CrossRef]
- Karygianni, L.; Cecere, M.; Argyropoulou, A.; Hellwig, E.; Skaltsounis, A.L.; Wittmer, A.; Tchorz, J.P.; Al-Ahmad, A. Compounds from Olea europaea and Pistacia lentiscus inhibit oral microbial growth. BMC Compl. Altern. Med. 2019, 19, 51. [Google Scholar] [CrossRef]
- Manuel Silvana, J.; Pinto-Bustillos, M.A.; Vásquez-Ponce, P.; Prodanov, M.; Martinez-Rodriguez, A.J. Olive mill wastewater as a potential source of antibacterial and anti-inflammatory compounds against the food-borne pathogen Campylobacter. Inn. Food Sci. Em. Technol. 2019, 51, 177–185. [Google Scholar] [CrossRef]
- Lazzez, A.; Perri, E.; Caravita, M.A.; Khlif, M.; Cossentini, M. Influence of olive maturity stage and geographical origin on some minor components in virgin olive oil of the Chemlali variety. J. Agric. Food Chem. 2008, 56, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Rotondi, A.; Bendini, A.; Cerretani, L.; Mari, M.; Lercker, G.; Toschi, T.G. Effect of olive ripening degree on the oxidative stability and organoleptic properties of cv. Nostrana di Brisighella extra virgin olive oil. J. Agric. Food Chem. 2004, 52, 3649–3654. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; Cozzolino, R.; Martignetti, A.; Malorni, L.; d’Acierno, A.; De Feo, V.; Cruz, A.G.; Nazzaro, F. Biochemical composition and antioxidant activity of three extra virgin olive oils from the Irpinia province, Southern Italy. Food Sci. Nutr. 2019, in press. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Ombra, M.; d’Acierno, A.; Nazzaro, F.; Riccardi, R.; Spigno, P.; Zaccardelli, M.; Pane, C.; Maione, M.; Fratianni, F. Phenolic composition and antioxidant and antiproliferative activities of the extracts of twelve common bean (Phaseolus vulgaris L.) endemic ecotypes of Southern Italy before and after cooking. Oxid. Med. Cell Longev. 2016, 2016, 1398298. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; Ombra, M.N.; Cozzolino, A.; Riccardi, R.; Spigno, P.; Tremonte, P.; Coppola, R.; Nazzaro, F. Phenolic constituents, antioxidant, antimicrobial and anti-proliferative activities of different endemic Italian varieties of garlic (Allium sativum L.). J. Funct. Foods 2016, 21, 240–248. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; de Castro, A.; Romero, C.; Brenes, M. Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: Correlation with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 4954–4961. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; d’Acierno, A.; De Feo, V.; Ayala Zavala, F.J.; Cruz, A.G.; Granato, D.; Coppola, R. Effect of Polyphenols on Microbial Cell-Cell Communications. In Quorum Sensing; Tommonaro, G., Ed.; Academic Press: New York, NY, USA, 2019; pp. 195–223. [Google Scholar]
- Sudjana, A.N.; D’Orazio, C.; Ryan, V.; Rasool, N.; Ng, J.; Islam, N.; Riley, T.V.; Hammer, K.A. Antimicrobial activity of commercial Olea europaea (olive) leaf extract. Int. J. Antimicrob. Agents 2009, 33, 461–463. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R. Quorum sensing and phytochemicals. Int. J. Mol. Sci. 2013, 14, 12607–12619. [Google Scholar] [CrossRef]
- Cerulli, A.; Lauro, G.; Masullo, M.; Cantone, V.; Olas, B.; Kontek, B.; Nazzaro, F.; Bifulco, G.; Piacente, S. Cyclic diarylheptanoids from Corylus avellana green leafy covers: Determination of their absolute configurations and evaluation of their antioxidant and antimicrobial activities. J. Nat. Prod. 2017, 80, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomat. 2008, 4, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.A.; Pereira, A.P.G.; Ferreira, I.C.F.R.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A. Table olives from Portugal: Phenolic compounds, antioxidant potential and antimicrobial activity. J. Agric. Food Chem. 2006, 54, 8425–8431. [Google Scholar] [CrossRef] [PubMed]
- Proestos, C.; Chorianopoulos, N.; Nychas, G.J.E.; Komaitis, M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J. Agric. Food Chem. 2005, 53, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Rauha, J.P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H.; Vuorela, P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, H.; Lo, R. Phenolic compounds from the leaf extract of artichoke (Cynara scolymus L.) and their antimicrobial activities. J. Agric. Food Chem. 2004, 52, 7272–7278. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimia, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.-M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Pereira, A.P.; Ferreira, I.C.F.R.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef]
- Borchers, A.T.; Keen, C.L.; Gerstiwin, M.E. Mushrooms, tumors, and immunity: An update. Exp. Biol. Med. 2004, 229, 393–406. [Google Scholar] [CrossRef]
- Liu, R.H. Health benefits of fruits and vegetables are from additive and synergistic combination of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef]
- Cushnie, T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Sharma, O.P.; Sehgal, M.; Bhargava, C.; Jain, M.; Goswami, A.; Singhvi, S.; Gupta, U.; Agarwal, R.; Sharma, P.; et al. Antimicrobial principles from tissue culture of some plant species. Indian J. Pharm. Sci. 1980, 4, 113–117. [Google Scholar]
- Basile, A.; Sorbo, S.; Giordano, S.; Ricciardi, L.; Ferrara, S.; Montesano, D.; Castaldo Cobianchi, R.; Vuotto, M.L.; Ferrara, L. Antibacterial and allelopathic activity of extract from Castanea sativa leaves. Fitoterapia 2000, 71, S110–S116. [Google Scholar] [CrossRef]
- Sakharkar, M.K.; Jayaraman, P.; Soe, W.M.; Chow, V.T.K.; Sing, L.C.; Sakharkar, K.R. In vitro combinations of antibiotics and phytochemicals against Pseudomonas aeruginosa. J. Microbiol. Immunol. Infect. 2009, 42, 364–370. [Google Scholar] [PubMed]
- Bisignano, G.; Tomaino, A.; Lo Cascio, R.; Crisafi, G.; Uccella, N.; Sajia, A. On the in-vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharmacol. 1999, 51, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Medina-Martínez, M.S.; Truchado, P.; Castro-Ibáñez, I.; Allende, A. Antimicrobial activity of hydroxytyrosol: A current controversy. Biosci. Biotechn. Biochem. 2016, 80, 801–810. [Google Scholar] [CrossRef] [PubMed]
| ‘Ogliarola’ | ‘Ravece’ | ‘Ruvea Antica’ | Tetracycline | ||||
|---|---|---|---|---|---|---|---|
| 2.5 µg | 4.9 µg | 2.5 µg | 4.9 µg | 2.5 µg | 4.9 µg | 7 µg | |
| E. coli | 7.30 (±0.57) | 13.30 (±0.57) | 7.00 (±0.57) | 13.67 (±0.28) | 5.30 (±0.52) | 10.00 (±0.00) | 12.67 (±0.57) |
| L. innocua | 5.67 (±0.57) | 10.67 (±0.57) | 6.67 (0.57) | 13.33 (±0.57) | 4.30 (±0.57) | 9.30 (±0.57) | 10.33 (±0.50) |
| S. aureus | 7.30 (±0.57) | 11.67 (±0.57) | 0.00 (±0.00) | 0.00 (±0.00) | 6.67 (±0.57) | 12.67 (±0.57) | 6.67 (±0.57) |
| B. cereus 4313 | 10.67 (±1.14) | 18.33 (±0.57) | 9.67 (±0.57) | 17.33 (±1.15) | 6.33 (±0.57) | 11.67 (±0.57) | 9.67 (±0.57) |
| B. cereus 4384 | 7.67 (±0.57) | 13.67 (±0.57) | 7.67 (±0.57) | 17.30 (±1.14) | 0.00 (±0.00) | 0.00 (±0.00) | 8.30 (±1.05) |
| P. aeruginosa | 6.33 (±0.57) | 11.33 (±0.57) | 8.67 (±0.57) | 16.33 (±0.57) | 4.33 (0.57) | 6.67 (±0.57) | 10.00 (±0.00) |
| E. faecalis | 5.67 (±0.57) | 11.33 (±0.57) | 7.67 (±0.57) | 17.33 (±1.14) | 0 00 (±0.00) | 0.00 (±0.00) | 12.33 (±0.57) |
| Ogliarola | Ravece | Ruvea Antica | |
|---|---|---|---|
| B. cereus 4313 | 1.00 | 1.00 | 1.00 |
| B. cereus 4384 | 1.00 | 1.00 | 2.00 |
| E.coli | 1.00 | 1.00 | 2.00 |
| P. aeruginosa | 1.00 | 1.00 | 2.00 |
| S. aureus | 1.00 | >15.00 | 2.00 |
| L. innocua | 2.00 | 2.00 | 2.00 |
| E. faecalis | 2.00 | 2.00 | >10.00 |
| Polyphenols (%) | ‘Ogliarola’ | ‘Ravece’ | ‘Ruvea Antica’ |
|---|---|---|---|
| Gallic acid | 0.00 | 0.00 | 0.00 |
| 3 Hydroxytirosol | 1.86 | 0.43 | 1.10 |
| Catechin | 1.08 | 0.00 | 0.43 |
| p-Coumaric acid | 0.00 | 0.28 | 0.11 |
| Quercetin-4-glucoside (spiraeoside) | 9.48 | 0.00 | 5.75 |
| Oleuropein | 15.77 | 5.92 | 12.82 |
| Dadzein | 4.13 | 0.00 | 2.36 |
| Luteolin | 0.00 | 6.22 | 1.57 |
| Quercetin | 24.06 | 18.03 | 10.61 |
| Apigenin | 0.00 | 0.00 | 3.18 |
| Naringenin | 3.99 | 6.57 | 6.49 |
| Formononentin | 4.45 | 4.81 | 2.27 |
| Polyphenols | Corr-Values |
|---|---|
| Formononentin | 0.97 |
| Quercetin | 0.94 |
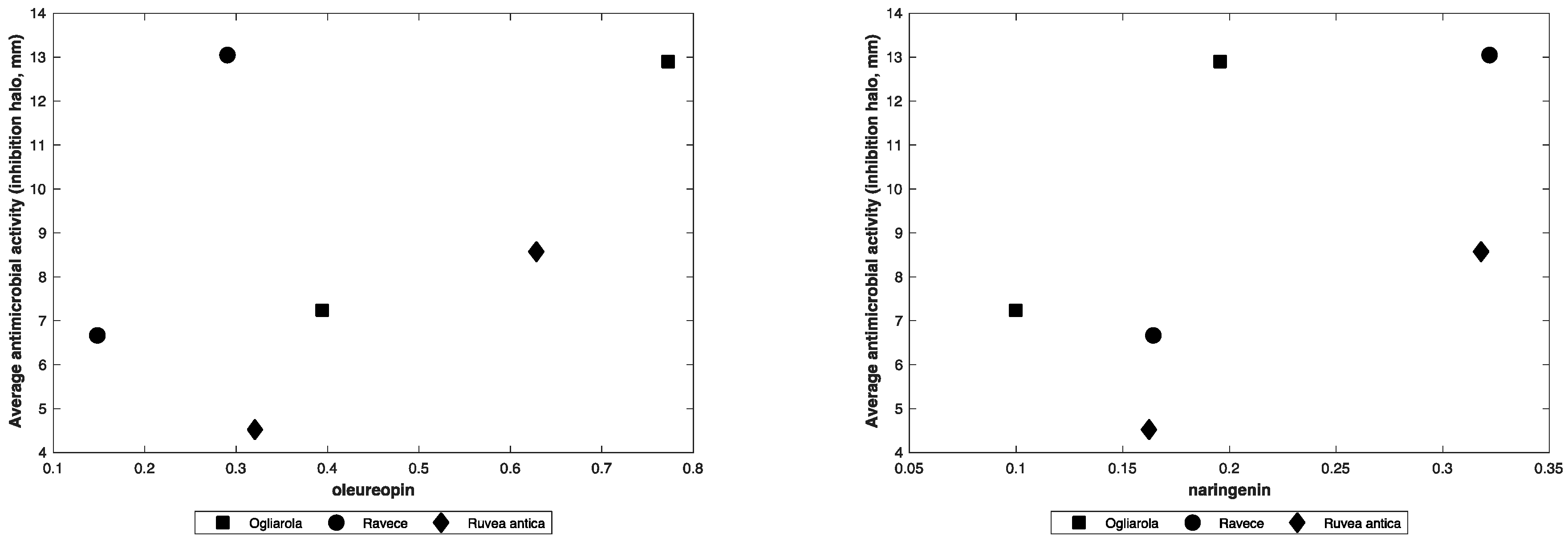
| Naringenin | 0.55 |
| Oleuropein | 0.47 |
| Luteolin | 0.37 |
| Catechin | 0.35 |
| p-Coumaric acid | 0.33 |
| Dadzein | 0.28 |
| Spiraeoside | 0.27 |
| Apigenin | −0.34 |
| Microorganisms | |||||||
|---|---|---|---|---|---|---|---|
| Polyphenol | BC 4313 | BC 4384 | EC | EF | LI | SA | PA |
| Formononentin | 0.97 | 0.95 | 0.94 | 0.91 | 0.91 | −0.16 | 0.95 |
| Quercetin | 0.96 | 0.93 | 0.90 | 0.75 | 0.77 | 0.18 | 0.74 |
| Naringenin | 0.47 | 0.57 | 0.65 | 0.26 | 0.78 | 0.02 | 0.55 |
| Oleuropein | 0.50 | 0.53 | 0.51 | −0.09 | 0.33 | 0.89 | 0.00 |
| Luteolin | 0.30 | 0.33 | 0.39 | 0.62 | 0.59 | −0.76 | 0.73 |
| Catechin | 0.41 | 0.38 | 0.33 | −0.04 | 0.086 | 0.80 | −0.10 |
| p-Coumaric acid | 0.25 | 0.30 | 0.36 | 0.52 | 0.58 | −0.69 | 0.66 |
| Dadzein | 0.34 | 0.33 | 0.29 | −0.19 | 0.06 | 0.90 | −0.19 |
| Spiraeoside | 0.32 | 0.32 | 0.27 | −0.21 | 0.05 | 0.91 | −0.21 |
| Apigenin | −0.38 | −0.27 | −0.21 | −0.75 | −0.15 | 0.56 | −0.51 |
| 3-Hydroxytyrosol | 0.51 | 0.51 | 0.47 | −0.01 | 0.25 | 0.84 | 0.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazzaro, F.; Fratianni, F.; Cozzolino, R.; Martignetti, A.; Malorni, L.; De Feo, V.; Cruz, A.G.; d’Acierno, A. Antibacterial Activity of Three Extra Virgin Olive Oils of the Campania Region, Southern Italy, Related to Their Polyphenol Content and Composition. Microorganisms 2019, 7, 321. https://doi.org/10.3390/microorganisms7090321
Nazzaro F, Fratianni F, Cozzolino R, Martignetti A, Malorni L, De Feo V, Cruz AG, d’Acierno A. Antibacterial Activity of Three Extra Virgin Olive Oils of the Campania Region, Southern Italy, Related to Their Polyphenol Content and Composition. Microorganisms. 2019; 7(9):321. https://doi.org/10.3390/microorganisms7090321
Chicago/Turabian StyleNazzaro, Filomena, Florinda Fratianni, Rosaria Cozzolino, Antonella Martignetti, Livia Malorni, Vincenzo De Feo, Adriano G. Cruz, and Antonio d’Acierno. 2019. "Antibacterial Activity of Three Extra Virgin Olive Oils of the Campania Region, Southern Italy, Related to Their Polyphenol Content and Composition" Microorganisms 7, no. 9: 321. https://doi.org/10.3390/microorganisms7090321
APA StyleNazzaro, F., Fratianni, F., Cozzolino, R., Martignetti, A., Malorni, L., De Feo, V., Cruz, A. G., & d’Acierno, A. (2019). Antibacterial Activity of Three Extra Virgin Olive Oils of the Campania Region, Southern Italy, Related to Their Polyphenol Content and Composition. Microorganisms, 7(9), 321. https://doi.org/10.3390/microorganisms7090321









