Contemporary Strategies and Current Trends in Designing Antiviral Drugs against Dengue Fever via Targeting Host-Based Approaches
Abstract
1. Introduction
1.1. History
1.2. Vector and Non-Vector Transmission of Dengue
1.3. Transmission Process
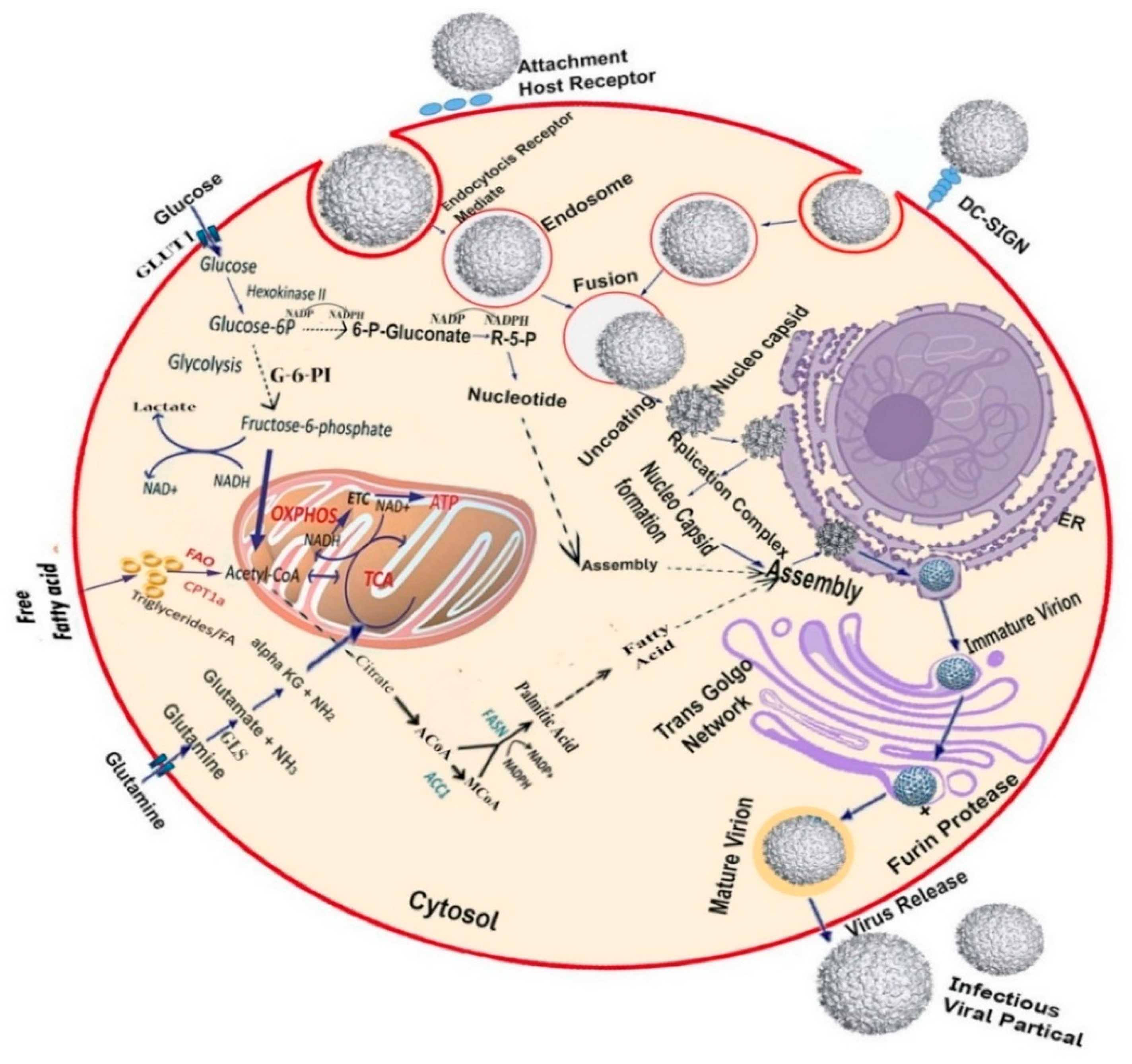
2. Targeting Host as an Antiviral Approach
3. Targeting Host Metabolic Pathway
3.1. Targeting the Host Glycolytic Pathway
3.2. Targeting the Host Lipid Biosynthesis Pathway
3.3. Targeting the Host Nucleoside Biosynthesis Pathway
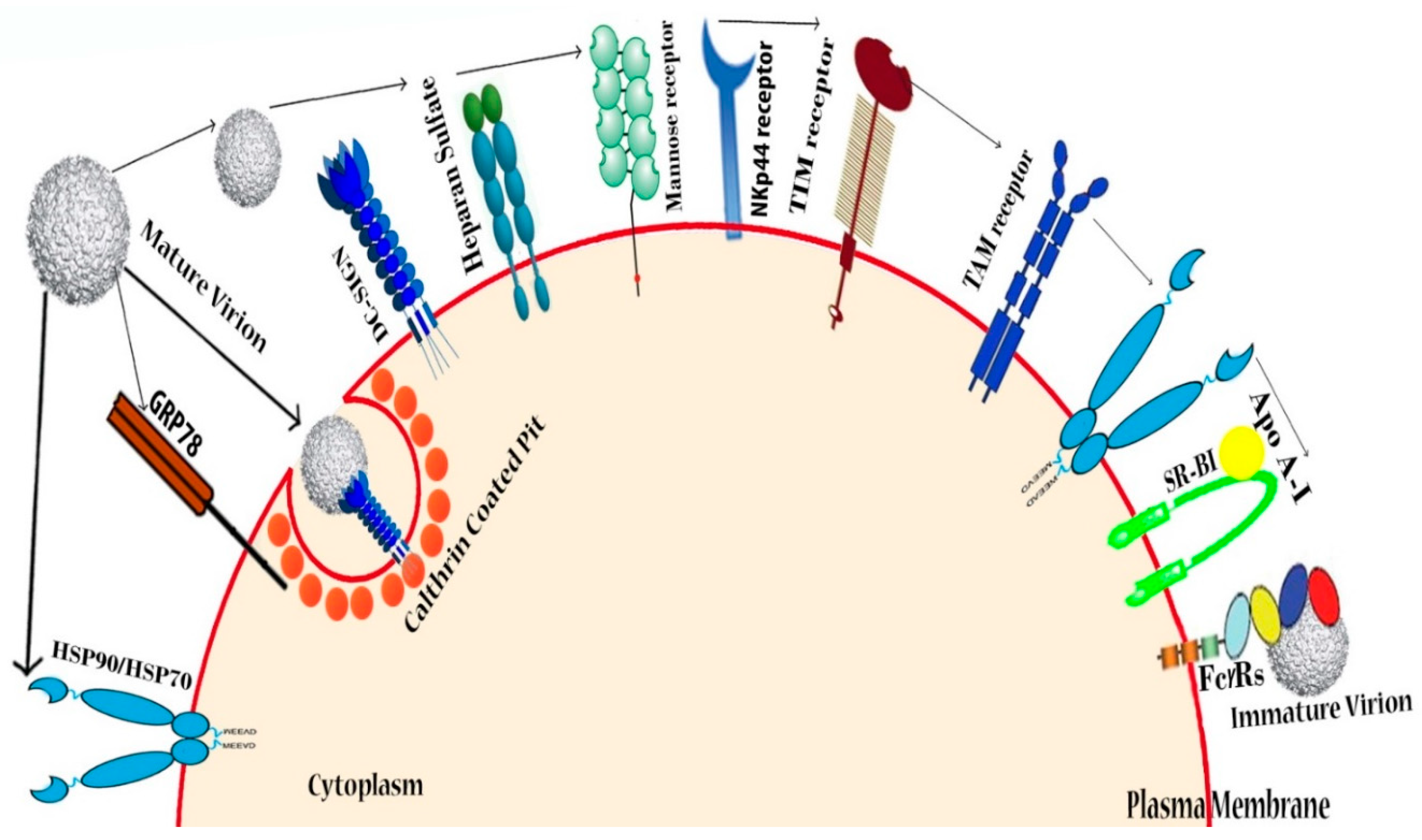
4. Targeting Host Cellular Receptors and Attachment Factors
4.1. Heparin and Heparan Sulfate (Glycosaminoglycans)
4.2. DC-SIGN
4.3. Other Possible Receptors
5. Targeting Host Proteins or Enzymes
5.1. Targeting Host Protease
5.2. Targeting Host Kinases
5.3. Glucosidase Inhibitors
6. Targeting Host Immunity and Inflammatory Pathways
6.1. Targeting Host Immune Factors Involved in DENV Sensing
6.2. Targeting Anti-Inflammatory Cytokine Populations
6.3. Targeting Host Plasma and Vascular Endothelium Leakage
6.4. Targeting Immune Factor Progress Disease after DENV Infection
7. Conclusions and Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buchmeier, M.J.; Peters, C.J.; de La, T.J.F.V. Arenaviridae: The Virus and Their Replication. Fields Virol. 2007, 1792–1827. [Google Scholar]
- Teles, F.R.R.; Prazeres, D.M.F.; Lima-Filho, J.L. Trends in Dengue Diagnosis. Rev. Med. Virol. 2005, 15, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.R.A.; Mohana-Borges, R.; de Alencastro, R.B.; Horta, B.A.C. The Flavivirus Capsid Protein: Structure, Function and Perspectives towards Drug Design. Virus Res. 2017, 227, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Miller, N. Recent Progress in Dengue Vaccine Research and Development. Curr. Opin. Mol. 2010, 12, 31–38. [Google Scholar]
- Fontaine, K.A.; Sanchez, E.L.; Camarda, R.; Lagunoff, M. Dengue Virus Induces and Requires Glycolysis for Optimal Replication. J. Virol. 2015, 89, 2358–2366. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, S.S.; Blaney, J.E.; Durbin, A.P.; Murphy, B.R. Prospects for a Dengue Virus Vaccine. Nat. Rev. Microbiol. 2007, 5, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Antibody, Macrophages, Dengue Virus Infection, Shock, and Hemorrhage: A Pathogenetic Cascade. Rev. Infect. Dis. 1989, 11, 830–839. [Google Scholar] [CrossRef]
- Christie, T. Remarks on “Kidinga Pepo”: A Peculiar Form of Exan-Thematous Disease. Br. Med. J. 1872, 1, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Epidemic Dengue/Dengue Hemorrhagic Fever as a Public Health, Social and Economic Problem in the 21st Century. Trends Microbiol. 2002, 10, 100–103. [Google Scholar] [CrossRef]
- Carey, D.E. Chikungunya and Dengue: A Case of Mistaken Identity? J. Hist. Med. Allied Sci. 1971, 26, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Mattingly, P.F. Ecological Aspects of the Evolution of Mosquito-Borne Virus Diseases. Trans. R. Soc. Trop. Med. Hyg. 1960, 54, 97–112. [Google Scholar] [CrossRef]
- Maas, O. On the Aetiology of Stuttering. J. Ment. Sci. 1946, 92, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Ashburn, P.M.; Craig, C.F.; Lieutenant, F.; SA, T. Experimental investigations regarding the etiology of dengue fever. Infect. Dis. 1907, 4, 440–475. [Google Scholar] [CrossRef]
- Cleland, J.B.; Bradley, B.; MacDonald, W. Further Experiments in the Etiology of Dengue Fever. J. Hyg. 1919, 18, 217–254. [Google Scholar] [CrossRef] [PubMed]
- Nishiura, H.; Halstead, S.B. Natural History of Dengue Virus (DENV) 1 and DENV 4 Infections. J. Infect. Dis. 2007, 195, 1007–1013. [Google Scholar] [CrossRef]
- Siler, J.F.; Hall, M.W.; Hitchens, A.P. Dengue: Its History, Epidemiology, Mechanism of Transmission, Etiology, Clinical Manifestations, Immunity, and Prevention. Philipp. J. Sci 1926, 29, 1–2. [Google Scholar]
- Davenport, F.M. Viral and Rickettsial Infections of Man. Am. J. Public Heal. Nations Heal. 2008, 49, 971. [Google Scholar] [CrossRef]
- Halstead, S.B. Mosquito-Borne Haemorrhagic Fevers of South and South-East Asia. Bull. World Health Organ. 1966, 35, 3–15. [Google Scholar]
- Konishi, E.; Kuno, G. In Memoriam: Susumu Hotta (1918–2011). Emerg. Infect. Dis. 2013, 19, 843–845. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Scott, T.W.; Zou, C.; Pigott, D.M.; Duda, K.A.; Simmons, C.P. Global Spread of Dengue Virus Types: Mapping the 70 Year History. Trends Microbiol. 2014, 22, 138–146. [Google Scholar] [CrossRef]
- Ebi, K.L.; Nealon, J. Dengue in a Changing Climate. Environ. Res. 2016, 151, 115–123. [Google Scholar] [CrossRef]
- Henchal, E.A.; Putnak, J.R. The dengue viruses. Clin. Microbiol. Rev. 1990, 3, 376–396. [Google Scholar] [CrossRef] [PubMed]
- Khetarpal, N.; Khanna, I. Dengue Fever: Causes, Complications, and Vaccine Strategies. J. Immunol. Res. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Mary, E. Non-Vector Transmission of Dengue and Other Mosquito-Borne Flaviviruses. Dengue Bull. 2005, 29, 18–31. [Google Scholar]
- Wazieres, D.; Elite, A.S.; Clinical, M.J. Nosocomial transmission of dengue from a needlestick injury. Lancet 1998, 351, 498. [Google Scholar] [CrossRef]
- Hirsch, J.F.; Deschamps, C.; Lhuillier, M. Transmission MŽtropolitaine d’une Dengue Par Inoculation Accidentelle Hospitalière. Ann. Med. Intern. 1990, 141, 629. [Google Scholar]
- Langgartner, J.; Audebert, F.; Schölmerich, J.; Glück, T. Dengue Virus Infection Transmitted by Needle Stick Injury. J. Infect. 2002, 44, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Nemes, Z.; Kiss, G.; Madarassi, E.P.; Peterfi, Z.; Ferenczi, E.; Bakonyi, T.; Ternak, G. Nosocomial Transmission of Dengue. Emerg. Infect. Dis. 2004, 10, 1880. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.; De With, K.; Huzly, D.; Hufert, F.; Weidmann, M.; Breisinger, S.; Eppinger, S.; Kern, W.V.; Bauer, T.M. Nosocomial Acquisition of Dengue. Emerg. Infect. Dis. 2004, 10, 1872–1873. [Google Scholar] [CrossRef]
- Berberian, G.; Farina, D.; Rosanova, M.T.; Hidalgo, S.; Enria, D.; Mitchenko, A.; Moreno, J.; Sánchez, I.S. Dengue Perinatal; Perinatal Dengue Infection. Arch. Argent. Pediatr. 2011, 109, 232–236. [Google Scholar]
- Rigau-Pérez, J.G.; Vorndam, A.V.; Clark, G.G. The Dengue and Dengue Hemorrhagic Fever Epidemic in Puerto Rico, 1994–1995. Am. J. Trop. Med. Hyg. 2001, 64, 67–74. [Google Scholar] [CrossRef]
- Chen, L.H.; Wilson, M.E. Transmission of Dengue Virus without a Mosquito Vector: Nosocomial Mucocutaneous Transmission and Other Routes of Transmission. Clin. Infect. Dis. 2004, 39, e56–e60. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Vasilakis, N. Dengue-Quo Tu et Quo Vadis? Viruses 2011, 3, 1562–1608. [Google Scholar] [CrossRef]
- Holmes, E.C.; Twiddy, S.S. The Origin, Emergence and Evolutionary Genetics of Dengue Virus. Infect. Genet. Evol. 2003, 3, 19–28. [Google Scholar] [CrossRef]
- Diallo, M.; Ba, Y.; Sall, A.A.; Diop, O.M.; Ndione, J.A.; Mondo, M.; Girault, L.; Mathiot, C. Amplification of the Sylvatic Cycle of Dengue Virus Type 2, Senegal, 1999-2000: Entomologic Findings and Epidemiologic Considerations. Emerg. Infect. Dis. 2003, 9, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Hervy, J.P.; Legros, F.; Roche, J.C.; Monteny, N.; Diaco, B. Circulation Du Virus Dengue 2 Dans Plusieurs Milieux Boisés des Savanes Soudaniennes de La Région de Bobo-Dioulasso (Burkina Faso). Cahiers Orstom. Série Entomologie Médicale et Parasitologie 1984, 22, 135–143. [Google Scholar]
- Rodhain, F. The role of monkeys in the biology of dengue and yellow fever. Comp. Immunol. Microbiol. Infect. Dis. 1991, 14, 9–19. [Google Scholar] [CrossRef]
- Robin, Y.; Cornet, M.; Heme, G.; Le Gonidec, G. Isolation of Dengue Virus from Senegal/Isolement Du Virus de La Dengue Au Senegal. Ann. Virol. E 1980, 131, 149–154. [Google Scholar]
- Roche, J.C.; Cordellier, R.; Hervy, J.P.; Digoutte, J.P.; Monteny, N. Isolement de 96 Souches de Virus Dengue 2 à Partir de Moustiques Capturés En Cote-d’ivoire et Haute-Volta. Ann. L’institut Pasteur Virol. 1983, 134, 233–244. [Google Scholar] [CrossRef]
- Rudnick, A. Dengue Virus Ecology in Malaysia. Inst. Med. Res. Malays. Bull. 1986, 23, 3. [Google Scholar]
- Black IV, W.C.; Bennett, K.E.; Gorrochótegui-Escalante, N.; Barillas-Mury, C.V.; Fernández-Salas, I.; Muñoz, M.D.L.; Farfán-Alé, J.A.; Olson, K.E.; Beaty, B.J. Flavivirus Susceptibility in Aedes Aegypti. Arch. Med. Res. 2002, 33, 379–388. [Google Scholar] [CrossRef]
- Yuwono, J.; Suharyono, W.; Koiman, I.; Tsuchiya, Y.; Tagaya, I. Seroepidemiological Survey on Dengue and Japanese Encephalitis Virus Infections in Asian Monkeys. South. Asian J. Trop. Med. Public Health 1984, 15, 194–200. [Google Scholar]
- Rosen, L. Observations on the Epidemiology of Dengue in Panama. Am. J. Epidemiol. 1958, 68, 45–58. [Google Scholar] [CrossRef]
- Roberts, D.; Peyton, E.; Pinheiro, F.; Balderrama, F. Associations of Arbovirus Vectors with Gallery Forests and Domestic Environments in Southeastern Bolivia; Department of Entomology, Walter Reed Army Institute of Research: Washington, DC, USA, 1984. [Google Scholar]
- Germain, M.; Mouchet, J.; Cordellier, R.; Chippaux, A.; Cornet, M.; Herve, J.P.; Sureau, P.; Fabre, J.; Robin, Y. Epidemiology of yellow fever in Africa. Med. Infect. Dis. 1978, 8, 69–77. [Google Scholar]
- Rudnick, A.L.B.E.R.T. Ecology of Dengue Virus. Asian J. Infect. Dis. 1978, 2, 156–160. [Google Scholar]
- Freier, J.E.; Rosen, L. Vertical Transmission of Dengue Viruses by Aedes Mediovittatus. Am. J. Trop. Med. Hyg. 1988, 39, 218–222. [Google Scholar] [CrossRef]
- WHO. Dengue and Dengue Haemorrhagic Fever. 1990–1994. Relev. Epidemiol. Hebd. 1995, 70, 334–335. [Google Scholar]
- Gubler, D.J. Dengue and Dengue Hemorrhagic Fever, 1996. Epidemiol. Bull. 1996, 17, 12–14. [Google Scholar]
- Tai, A.W.; Benita, Y.; Peng, L.F.; Kim, S.; Sakamoto, N.; Xavier, R.J.; Chung, R.T. A functional genomic screen identifies cellular cofactors of hepatitis C virus replication. Cell Host Microbe 2009, 5, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Aizaki, H.; Machida, K.; Ou, J.-H.J.; Lai, M.M.C. Hepatitis C Virus Translation Preferentially Depends on Active RNA Replication. PLoS ONE 2012, 7, e43600. [Google Scholar] [CrossRef]
- Brass, A.L.; Dykxhoorn, D.M.; Benita, Y.; Yan, N.; Engelman, A.; Xavier, R.J.; Lieberman, J.; Elledge, S.J. Identification of Host Proteins Required for HIV Infection through a Functional Genomic Screen. Science 2008, 319, 921–926. [Google Scholar] [CrossRef] [PubMed]
- König, R.; Zhou, Y.; Elleder, D.; Diamond, T.L.; Bonamy, G.M.C.; Irelan, J.T.; Chiang, C.-Y.; Tu, B.P.; De Jesus, P.D.; Lilley, C.E.; et al. Global Analysis of Host-Pathogen Interactions That Regulate Early-Stage HIV-1 Replication. Cell 2008, 135, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xu, M.; Huang, Q.; Gates, A.T.; Zhang, X.D.; Castle, J.C.; Stec, E.; Ferrer, M.; Strulovici, B.; Hazuda, D.J.; et al. Genome-Scale RNAi Screen for Host Factors Required for HIV Replication. Cell Host Microbe 2008, 4, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Stertz, S.; Zhou, Y.; Inoue, A.; Hoffmann, H.; Bhattacharyya, S.; Alamares, J.G.; Tscherne, D.M.; Ortigoza, M.B.; Liang, Y.; Gao, Q.; et al. Human Host Factors Required for Influenza Virus Replication. Nature 2010, 463, 813–817. [Google Scholar]
- Hao, L.; Sakurai, A.; Watanabe, T.; Sorensen, E.; Nidom, C.A.; Newton, M.A.; Ahlquist, P.; Kawaoka, Y. Drosophila RNAi Screen Identifies Host Genes Important for Influenza Virus Replication. Nature 2008, 454, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Martín-Acebes, M.A.; Blázquez, A.B.; Jiménez de Oya, N.; Escribano-Romero, E.; Saiz, J.C. West Nile Virus Replication Requires Fatty Acid Synthesis but Is Independent on Phosphatidylinositol-4-Phosphate Lipids. PLoS ONE 2011, 6, e24970. [Google Scholar] [CrossRef]
- Krishnan, M.N.; Ng, A.; Sukumaran, B.; Gilfoy, F.D.; Uchil, P.D.; Sultana, H.; Brass, A.L.; Adametz, R.; Tsui, M.; Qian, F.; et al. RNA Interference Screen for Human Genes Associated with West Nile Virus Infection. Nature 2008, 455, 242–245. [Google Scholar] [CrossRef]
- Sessions, O.M.; Barrows, N.J.; Souza-Neto, J.A.; Robinson, T.J.; Hershey, C.L.; Rodgers, M.A.; Ramirez, J.L.; Dimopoulos, G.; Yang, P.L.; Pearson, J.L.; et al. Discovery of Insect and Human Dengue Virus Host Factors. Nature 2009, 458, 1047–1050. [Google Scholar] [CrossRef]
- Jordan, T.X.; Randall, G. Flavivirus Modulation of Cellular Metabolism. Curr. Opin. Virol. 2016, 19, 7–10. [Google Scholar] [CrossRef]
- Yu, Y.; Clippinger, A.J.; Alwine, J.C. Viral effects on metabolism: Changes in glucose and glutamine utilization during human cytomegalovirus infection. Trends Microbiol. 2011, 19, 360–367. [Google Scholar] [CrossRef]
- Datan, E.; Roy, S.G.; Germain, G.; Zali, N.; McLean, J.E.; Golshan, G.; Harbajan, S.; Lockshin, R.A.; Zakeri, Z. Dengue-Induced Autophagy, Virus Replication and Protection from Cell Death Require ER Stress (PERK) Pathway Activation. Cell Death Dis. 2016, 7, e2127. [Google Scholar] [CrossRef] [PubMed]
- Heaton, N.S.; Randall, G. Dengue Virus-Induced Autophagy Regulates Lipid Metabolism. Cell Host Microbe 2010, 8, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Khakpoor, A.; Panyasrivanit, M.; Wikan, N.; Smith, D.R. A Role for Autophagolysosomes in Dengue Virus 3 Production in HepG2 Cells. J. Gen. Virol. 2009, 90, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Hu, H.Y.; Kuo, S.H.; Lei, H.Y.; Lin, Y.S.; Yeh, T.M.; Liu, C.C.; Liu, H.S. Dengue Virus Infection Induces Autophagy: An in Vivo Study. J. Biomed. Sci. 2013, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Mateo, R.; Nagamine, C.M.; Spagnolo, J.; Méndez, E.; Rahe, M.; Gale, M.; Yuan, J.; Kirkegaard, K. Inhibition of Cellular Autophagy Deranges Dengue Virion Maturation. J. Virol. 2013, 87, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Jordan, T.X.; Randall, G. Flaviviruses and Autophagy. In Autophagy, Infection, and the Immune Response; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 81–100. [Google Scholar]
- Rothwell, C.; LeBreton, A.; Young Ng, C.; Lim, J.Y.H.; Liu, W.; Vasudevan, S.; Labow, M.; Gu, F.; Gaither, L.A. Cholesterol Biosynthesis Modulation Regulates Dengue Viral Replication. Virology 2009, 389, 8–19. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Bushell, S.; Qing, M.; Xu, H.Y.; Bonavia, A.; Nunes, S.; Zhou, J.; Poh, M.K.; Florez de Sessions, P.; Niyomrattanakit, P.; et al. Inhibition of Dengue Virus through Suppression of Host Pyrimidine Biosynthesis. J. Virol. 2011, 85, 6548–6556. [Google Scholar] [CrossRef]
- Liu, S.; Neidhardt, E.A.; Grossman, T.H.; Ocain, T.; Clardy, J. Structures of Human Dihydroorotate Dehydrogenase in Complex with Antiproliferative Agents. Structure 2000, 8, 25–33. [Google Scholar] [CrossRef]
- McLean, J.E.; Neidhardt, E.A.; Grossman, T.H.; Hedstrom, L. Multiple Inhibitor Analysis of the Brequinar and Leflunomide Binding Sites on Human Dihydroorotate Dehydrogenase. Biochemistry 2001, 40, 2194–2200. [Google Scholar] [CrossRef]
- Navarro-Sanchez, E.; Altmeyer, R.; Amara, A.; Schwartz, O.; Fieschi, F.; Virelizier, J.L.; Arenzana-Seisdedos, F.; Desprès, P. Dendritic-Cell-Specific ICAM3-Grabbing Non-Integrin Is Essential for the Productive Infection of Human Dendritic Cells by Mosquito-Cell-Derived Dengue Viruses. EMBO Rep. 2003, 4, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Tassaneetrithep, B.; Burgess, T.H.; Granelli-Piperno, A.; Trumpfheller, C.; Finke, J.; Sun, W.; Eller, M.A.; Pattanapanyasat, K.; Sarasombath, S.; Birx, D.L.; et al. DC-SIGN (CD209) Mediates Dengue Virus Infection of Human Dendritic Cells. J. Exp. Med. 2003, 197, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.J.; Hsieh, M.T.; Young, M.J.; Kao, C.L.; King, C.C.; Chang, W. An External Loop Region of Domain III of Dengue Virus Type 2 Envelope Protein Is Involved in Serotype-Specific Binding to Mosquito but Not Mammalian Cells. J. Virol. 2004, 78, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Jindadamrongwech, S.; Thepparit, C.; Smith, D.R. Identification of GRP 78 (BiP) as a Liver Cell Expressed Receptor Element for Dengue Virus Serotype 2. Arch. Virol. 2004, 149, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Meertens, L.; Carnec, X.; Lecoin, M.P.; Ramdasi, R.; Guivel-Benhassine, F.; Lew, E.; Lemke, G.; Schwartz, O.; Amara, A. The TIM and TAM Families of Phosphatidylserine Receptors Mediate Dengue Virus Entry. Cell Host Microbe 2012, 12, 544–557. [Google Scholar] [CrossRef]
- Ichiyama, K.; Gopala Reddy, S.B.; Zhang, L.F.; Chin, W.X.; Muschin, T.; Heinig, L.; Suzuki, Y.; Nanjundappa, H.; Yoshinaka, Y.; Ryo, A.; et al. Sulfated Polysaccharide, Curdlan Sulfate, Efficiently Prevents Entry/Fusion and Restricts Antibody-Dependent Enhancement of Dengue Virus Infection In Vitro: A Possible Candidate for Clinical Application. PLoS Negl. Trop. Dis. 2013, 7, e2188. [Google Scholar] [CrossRef]
- Kato, D.; Era, S.; Watanabe, I.; Arihara, M.; Sugiura, N.; Kimata, K.; Suzuki, Y.; Morita, K.; Hidari, K.I.P.J.; Suzuki, T. Antiviral Activity of Chondroitin Sulphate E Targeting Dengue Virus Envelope Protein. Antivir. Res. 2010, 88, 236–243. [Google Scholar] [CrossRef]
- Chen, Y.; Maguire, T.; Hileman, R.; Fromm, J.; Esko, J.; Linhardt, R.; Marks, R. Dengue Virus Infectivity Depends Envelope Binding Heparan Sulfate. Nat. Med. 1997, 3, 866–871. [Google Scholar] [CrossRef]
- Lee, E.; Pavy, M.; Young, N.; Freeman, C.; Lobigs, M. Antiviral Effect of the Heparan Sulfate Mimetic, PI-88, against Dengue and Encephalitic Flaviviruses. Antivir. Res. 2006, 69, 31–38. [Google Scholar] [CrossRef]
- Hung, S.L.; Lee, P.L.; Chen, H.W.; Chen, L.K.; Kao, C.L.; King, C.C. Analysis of the Steps Involved in Dengue Virus Entry into Host Cells. Virology 1999, 257, 156–167. [Google Scholar] [CrossRef]
- Hidari, K.I.P.J.; Takahashi, N.; Arihara, M.; Nagaoka, M.; Morita, K.; Suzuki, T. Structure and Anti-Dengue Virus Activity of Sulfated Polysaccharide from a Marine Alga. Biochem. Biophys. Res. Commun. 2008, 376, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Vervaeke, P.; Alen, M.; Noppen, S.; Schols, D.; Oreste, P.; Liekens, S. Sulfated Escherichia Coli K5 Polysaccharide Derivatives Inhibit Dengue Virus Infection of Human Microvascular Endothelial Cells by Interacting with the Viral Envelope Protein E Domain III. PLoS ONE 2013, 8, e74035. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Tang, W.; Tong, X.; Ding, K.; Zuo, J. Structure Elucidation and Sulfated Derivatives Preparation of Two α-d-Glucans from Gastrodia Elata Bl. and Their Anti-Dengue Virus Bioactivities. Carbohydr. Res. 2007, 342, 2230–2236. [Google Scholar] [CrossRef] [PubMed]
- Alen, M.M.F.; Kaptein, S.J.F.; De Burghgraeve, T.; Balzarini, J.; Neyts, J.; Schols, D. Antiviral Activity of Carbohydrate-Binding Agents and the Role of DC-SIGN in Dengue Virus Infection. Virology 2009, 387, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Talarico, L.B.; Damonte, E.B. Interference in Dengue Virus Adsorption and Uncoating by Carrageenans. Virology 2007, 363, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Reyes-del Valle, J.; Salas-Benito, J.; Soto-Acosta, R.; del Angel, R.M. Dengue Virus Cellular Receptors and Tropism. Curr. Trop. Med. Rep. 2014, 1, 36–43. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.H.; Engering, A.; Van Kooyk, Y. DC-SIGN, a C-Type Lectin on Dendritic Cells That Unveils Many Aspects of Dendritic Cell Biology. J. Leukoc. Biol. 2002, 271, 921–931. [Google Scholar]
- Liu, P.; Ridilla, M.; Patel, P.; Betts, L.; Gallichotte, E.; Shahidi, L.; Thompson, N.L.; Jacobson, K. Beyond Attachment: Roles of DC-SIGN in Dengue Virus Infection. Traffic 2017, 18, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Flipse, J.; Wilschut, J.; Traffic, J.S. Molecular mechanisms involved in antibody-dependent enhancement of dengue virus infection in humans. Traffic 2013, 14, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Lozach, P.Y.; Burleigh, L.; Staropoli, I.; Navarro-Sanchez, E.; Harriague, J.; Virelizier, J.L.; Rey, F.A.; Desprès, P.; Arenzana-Seisdedos, F.; Amara, A. Dendritic Cell-Specific Intercellular Adhesion Molecule 3-Grabbing Non-Integrin (DC-SIGN)-Mediated Enhancement of Dengue Virus Infection Is Independent of DC-SIGN Internalization Signals. J. Biol. Chem. 2005, 280, 23698–23708. [Google Scholar] [CrossRef] [PubMed]
- Rodenhuis-Zybert, I.A.; Wilschut, J.; Smit, J.M. Dengue Virus Life Cycle: Viral and Host Factors Modulating Infectivity. Cell. Mol. Life Sci. 2010, 67, 2773–2786. [Google Scholar] [CrossRef] [PubMed]
- Pugach, P.; Ketas, T.J.; Michael, E.; Moore, J.P. Neutralizing Antibody and Anti-Retroviral Drug Sensitivities of HIV-1 Isolates Resistant to Small Molecule CCR5 Inhibitors. Virology 2008, 377, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Barragan, J.D.J.; del Angel, R.M. Identification of a Putative Coreceptor on Vero Cells That Participates in Dengue 4 Virus Infection. J. Virol. 2002, 75, 7818–7827. [Google Scholar] [CrossRef] [PubMed]
- Boonnak, K.; Slike, B.M.; Burgess, T.H.; Mason, R.M.; Wu, S.-J.; Sun, P.; Porter, K.; Rudiman, I.F.; Yuwono, D.; Puthavathana, P.; et al. Role of Dendritic Cells in Antibody-Dependent Enhancement of Dengue Virus Infection. J. Virol. 2008, 82, 3939–3951. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Zagórska, A.; Lew, E.D.; Shrestha, B.; Rothlin, C.V.; Naughton, J.; Diamond, M.S.; Lemke, G.; Young, J.A.T. Enveloped Viruses Disable Innate Immune Responses in Dendritic Cells by Direct Activation of TAM Receptors. Cell Host Microbe 2013, 14, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Perera-Lecoin, M.; Meertens, L.; Carnec, X.; Viruses, A.A. Flavivirus entry receptors: An update. Viruses 2014, 6, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Reyes-del Valle, J.; Chavez-Salinas, S.; Medina, F.; del Angel, R.M. Heat Shock Protein 90 and Heat Shock Protein 70 Are Components of Dengue Virus Receptor Complex in Human Cells. J. Virol. 2005, 79, 4557–4567. [Google Scholar] [CrossRef] [PubMed]
- Elshuber, S.; Allison, S.L.; Heinz, F.X.; Mandl, C.W. Cleavage of Protein PrM Is Necessary for Infection of BHK-21 Cells by Tick-Borne Encephalitis Virus. J. Gen. Virol. 2003, 84, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, T.L.; Fang, N.X.; Fairlie, D.P.; Young, P.R.; Martin, J.L. Catalytically Active Dengue Virus NS3 Protease Forms Aggregates That Are Separable by Size Exclusion Chromatography. Protein Expr. Purif. 2002, 25, 241–247. [Google Scholar] [CrossRef]
- Melino, S.; Paci, M. Progress for dengue virus diseases. FEBS J. 2007, 274, 2986–3002. [Google Scholar] [CrossRef]
- Gouvea, I.E.; Izidoro, M.A.; Judice, W.A.S.; Cezari, M.H.S.; Caliendo, G.; Santagada, V.; dos Santos, C.N.D.; Queiroz, M.H.; Juliano, M.A.; Young, P.R.; et al. Substrate Specificity of Recombinant Dengue 2 Virus NS2B-NS3 Protease: Influence of Natural and Unnatural Basic Amino Acids on Hydrolysis of Synthetic Fluorescent Substrates. Arch. Biochem. Biophys. 2007, 457, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Stadler, K.; Allison, S.L.; Schalich, J.; Heinz, F.X. Proteolytic activation of tick-borne encephalitis virus by furin. J. Virol. 1997, 71, 8475–8481. [Google Scholar] [PubMed]
- Chambers, T.; Nestorowicz, A.; Amberg, S.; Rice, C. Mutagenesis of the Yellow Fever Virus Nonstructural Polyportein: A Catalitically Active NS3 Proteinase Domain and NS2B Are Required for Cleavages at Dibasic Sites. J. Virol. 1993, 65, 6797–6807. [Google Scholar]
- Peng, M.; Watanabe, S.; Chan, K.W.K.; He, Q.; Zhao, Y.; Zhang, Z.; Lai, X.; Luo, D.; Vasudevan, S.G.; Li, G. Luteolin restricts dengue virus replication through inhibition of the proprotein convertase furin. Antivir. Res. 2017, 143, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.J.H.; Yang, P.L. C-Src Protein Kinase Inhibitors Block Assembly and Maturation of Dengue Virus. Proc. Natl. Acad. Sci. USA 2007, 104, 3520–3525. [Google Scholar] [CrossRef] [PubMed]
- de Wispelaere, M.; LaCroix, A.J.; Yang, P.L. The Small Molecules AZD0530 and Dasatinib Inhibit Dengue Virus RNA Replication via Fyn Kinase. J. Virol. 2013, 87, 7367–7381. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Hosoya, T.; Leong, K.M.; Onogi, H.; Okuno, Y.; Hiramatsu, T.; Koyama, H.; Suzuki, M.; Hagiwara, M.; Garcia-Blanco, M.A. The Kinase Inhibitor Sfv785 Dislocates Dengue Virus Envelope Protein from the Replication Complex and Blocks Virus Assembly. PLoS ONE 2011, 6, e23246. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.J.; Miduturu, C.; Schmidt, A.G.; Zhu, X.; Pitts, J.D.; Wang, J.; Potisopon, S.; Zhang, J.; Wojciechowski, A.; Hann Chu, J.J.; et al. GNF-2 Inhibits Dengue Virus by Targeting Abl Kinases and the Viral e Protein. Cell Chem. Biol. 2016, 23, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Courageot, M.P.; Frenkiel, M.P.; Dos Santos, C.D.; Deubel, V.; Desprès, P. Alpha-Glucosidase Inhibitors Reduce Dengue Virus Production by Affecting the Initial Steps of Virion Morphogenesis in the Endoplasmic Reticulum. J. Virol. 2000, 74, 564–572. [Google Scholar] [CrossRef]
- Chambers, T. Flavivirus Genome Organization, Expression, And Replication. Annu. Rev. Microbiol. 1990, 44, 649–688. [Google Scholar] [CrossRef]
- Whitby, K.; Pierson, T.C.; Geiss, B.; Lane, K.; Engle, M.; Zhou, Y.; Doms, R.W.; Diamond, M.S. Castanospermine, a Potent Inhibitor of Dengue Virus Infection In Vitro and In Vivo. J. Virol. 2005, 79, 8698–8706. [Google Scholar] [CrossRef] [PubMed]
- Hebert, D.N.; Foellmer, B.; Helenius, A. Calnexin and Calreticulin Promote Folding, Delay Oligomerization and Suppress Degradation of Influenza Hemagglutinin in Microsomes. EMBO J. 1996, 15, 2961–2968. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.F.; Lee, C.J.; Liao, C.L.; Dwek, R.A.; Zitzmann, N.; Lin, Y.L. Antiviral Effects of an Iminosugar Derivative on Flavivirus Infections. J. Virol. 2002, 76, 3596–3604. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Rathore, A.P.S.; Sung, C.; Lu, F.; Khoo, Y.M.; Connolly, J.; Low, J.; Ooi, E.E.; Lee, H.S.; Vasudevan, S.G. Dose- and Schedule-Dependent Protective Efficacy of Celgosivir in a Lethal Mouse Model for Dengue Virus Infection Informs Dosing Regimen for a Proof of Concept Clinical Trial. Antivir. Res. 2012, 96, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.P.; Paradkar, P.N.; Watanabe, S.; Tan, K.H.; Sung, C.; Connolly, J.E.; Low, J.; Ooi, E.E.; Vasudevan, S.G. Celgosivir Treatment Misfolds Dengue Virus NS1 Protein, Induces Cellular pro-Survival Genes and Protects against Lethal Challenge Mouse Model. Antivir. Res. 2011, 92, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.T.; Buck, M.D.; Plummer, E.M.; Penmasta, R.A.; Batra, H.; Stavale, E.J.; Warfield, K.L.; Dwek, R.A.; Butters, T.D.; Alonzi, D.S.; et al. An Iminosugar with Potent Inhibition of Dengue Virus Infection in Vivo. Antivir. Res. 2013, 98, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Schul, W.; Butters, T.D.; Yip, A.; Liu, B.; Goh, A.; Lakshminarayana, S.B.; Alonzi, D.; Reinkensmeier, G.; Pan, X.; et al. Combination of α-Glucosidase Inhibitor and Ribavirin for the Treatment of Dengue Virus Infection in Vitro and in Vivo. Antivir. Res. 2011, 89, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.F.; Saini, N.K.; Porcelli, S.A. Evasion of Innate and Adaptive Immunity by Mycobacterium Tuberculosis. Microbiol. Spectr. 2014, 2, 747–772. [Google Scholar] [CrossRef] [PubMed]
- Der, S.D.; Zhou, A.; Williams, B.R.G.; Silverman, R.H. Identification of Genes Differentially Regulated by Interferon α, β, or γ Using Oligonucleotide Arrays. Proc. Natl. Acad. Sci. USA 1998, 95, 15623–15628. [Google Scholar] [CrossRef] [PubMed]
- Meylan, E.; Tschopp, J.; Karin, M. Intracellular Pattern Recognition Receptors in the Host Response. Nature 2006, 442, 39–44. [Google Scholar] [CrossRef]
- Severa, M.; Fitzgerald, K.A. TLR-Mediated Activation of Type I IFN during Antiviral Immune Responses: Fighting the Battle to Win the War. Curr. Top. Microbiol. Immunol. 2007, 316, 167–192. [Google Scholar] [PubMed]
- Tsai, Y.T.; Chang, S.Y.; Lee, C.N.; Kao, C.L. Human TLR3 Recognizes Dengue Virus and Modulates Viral Replication in Vitro. Cell. Microbiol. 2009, 11, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Sen, G.C. dsRNA-activation of TLR3 and RLR signaling: gene induction-dependent and independent effects. J. Interferon Cytokine 2014, 34, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wu, S.; Li, Y.; He, L.; Wu, M.; Jiang, L.; Feng, L.; Zhang, P.; Huang, X. Activation of Toll-Like Receptor 3 Impairs the Dengue Virus Serotype 2 Replication through Induction of IFN-β in Cultured Hepatoma Cells. PLoS ONE 2011, 6, e23346. [Google Scholar] [CrossRef] [PubMed]
- Sariol, C.A.; Martínez, M.I.; Rivera, F.; Rodríguez, I.V.; Pantoja, P.; Abel, K.; Arana, T.; Giavedoni, L.; Hodara, V.; White, L.J.; et al. Decreased Dengue Replication and an Increased Anti-Viral Humoral Response with the Use of Combined Toll-Like Receptor 3 and 7/8 Agonists in Macaques. PLoS ONE 2011, 6, e19323. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Ortiz, Z.G.; Warke, R.V.; Pacheco, L.; Xhaja, K.; Sarkar, D.; Fisher, P.B.; Shaw, S.K.; Martin, K.J.; Bosch, I. Discovering Innate Immunity Genes Using Differential Display: A Story of RNA Helicases. J. Cell. Physiol. 2006, 209, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.M.; Fornek, J.; Crochet, N.; Bajwa, G.; Perwitasari, O.; Martinez-Sobrido, L.; Akira, S.; Gill, M.A.; García-Sastre, A.; Katze, M.G.; et al. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 2008, 82, 335–345. [Google Scholar] [CrossRef]
- Brown, M.G.; McAlpine, S.M.; Huang, Y.Y.; Haidl, I.D.; Al-Afif, A.; Marshall, J.S.; Anderson, R. RNA Sensors Enable Human Mast Cell Anti-Viral Chemokine Production and IFN-Mediated Protection in Response to Antibody-Enhanced Dengue Virus Infection. PLoS ONE 2012, 7, e34055. [Google Scholar] [CrossRef]
- Nasirudeen, A.M.A.; Wong, H.H.; Thien, P.; Xu, S.; Lam, K.P.; Liu, D.X. RIG-I, MDA5 and TLR3 Synergistically Play an Important Role in Restriction of Dengue Virus Infection. PLoS Negl. Trop. Dis. 2011, 5, e926. [Google Scholar] [CrossRef]
- Kurane, I.; Innis, B.L.; Nimmannitya, S.; Nisalak, A.; Meager, A.; Ennis, F.A. High Levels of Interferon Alpha in the Sera of Children with Dengue Virus Infection. Am. J. Trop. Med. Hyg. 1993, 48, 222–229. [Google Scholar] [CrossRef]
- Perry, S.T.; Buck, M.D.; Lada, S.M.; Schindler, C.; Shresta, S. STAT2 mediates innate immunity to Dengue virus in the absence of STAT1 via the type I interferon receptor. PLoS Pathog. 2011, 7, e1001297. [Google Scholar] [CrossRef] [PubMed]
- Shresta, S.; Sharar, K. Critical roles for both STAT1-dependent and STAT1-independent pathways in the control of primary dengue virus infection in mice. J. Immunol. 2005, 175, 3946–3954. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.L.V.; Wee, L.J.K.; Khan, A.M.; Gil, L.H.V.G.; Marques, E.T.A.; Calzavara-Silva, C.E.; Tan, T.W. Classification of Dengue Fever Patients Based on Gene Expression Data Using Support Vector Machines. PLoS ONE 2010, 5, e11267. [Google Scholar] [CrossRef] [PubMed]
- Rothman, A.L. Immunity to Dengue Virus: A Tale of Original Antigenic Sin and Tropical Cytokine Storms. Nat. Rev. Immunol. 2011, 11, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Medin, C.L.; Fitzgerald, K.A.; Rothman, A.L. Dengue Virus Nonstructural Protein NS5 Induces Interleukin-8 Transcription and Secretion. J. Virol. 2005, 79, 11053–11061. [Google Scholar] [CrossRef]
- Chaturvedi, U.C.; Agarwal, R.; Elbishbishi, E.A.; Mustafa, A.S. Cytokine cascade in dengue hemorrhagic fever: Implications for pathogenesis. FEMS Immunol. Med. Microbiol. 2000, 28, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Lu, H.L.; Lai, S.L.; Campanella, G.S.; Sung, J.M.; Lu, M.Y.; Wu-Hsieh, B.A.; Lin, Y.L.; Lane, T.E.; Luster, A.D.; et al. Dengue Virus Induces Expression of CXC Chemokine Ligand 10/IFN-Gamma-Inducible Protein 10, Which Competitively Inhibits Viral Binding to Cell Surface Heparan Sulfate. J. Immunol. 2006, 177, 3185–3192. [Google Scholar] [CrossRef]
- Bozza, F.A.; Cruz, O.G.; Zagne, S.M.; Azeredo, E.L.; Nogueira, R.M.; Assis, E.F.; Bozza, P.T.; Kubelka, C.F. Multiplex Cytokine Profile from Dengue Patients: MIP-1beta and IFN-Gamma as Predictive Factors for Severity. BMC Infect. Dis. 2008, 8, 86. [Google Scholar] [CrossRef]
- Brasier, A.R.; Scott, T.W.; Morrison, A.C.; Kochel, T.J.; Spratt, H.M.; Bazan, I.; Forshey, B.M.; Garcia, J.; Victor, S.S.; Rocha, C.; et al. A Three-Component Biomarker Panel for Prediction of Dengue Hemorrhagic Fever. Am. J. Trop. Med. Hyg. 2012, 86, 341–348. [Google Scholar] [CrossRef]
- Tolfvenstam, T.; Lindblom, A.; Schreiber, M.J.; Ling, L.; Chow, A.; Ooi, E.E.; Hibberd, M.L. Characterization of Early Host Responses in Adults with Dengue Disease. BMC Infect. Dis. 2011, 11, 209. [Google Scholar] [CrossRef]
- Fagundes, C.T.; Costa, V.V.; Cisalpino, D.; Amaral, F.A.; Souza, P.R.S.; Souza, R.S.; Ryffel, B.; Vieira, L.Q.; Silva, T.A.; Atrasheuskaya, A.; et al. IFN-γ Production Depends on IL-12 and IL-18 Combined Action and Mediates Host Resistance to Dengue Virus Infection in a Nitric Oxide-Dependent Manner. PLoS Negl. Trop. Dis. 2011, 5, e1449. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.V.; Fagundes, C.T.; Valadão, D.F.; Cisalpino, D.; Dias, A.C.F.; Silveira, K.D.; Kangussu, L.M.; Ávila, T.V.; Bonfim, M.R.Q.; Bonaventura, D.; et al. A Model of DENV-3 Infection That Recapitulates Severe Disease and Highlights the Importance of IFN-γ in Host Resistance to Infection. PLoS Negl. Trop. Dis. 2012, 6, e1663. [Google Scholar] [CrossRef] [PubMed]
- Pacsa, A.S.; Agarwal, R.; Elbishbishi, E.A.; Chaturvedi, U.C.; Nagar, R.; Mustafa, A.S. Role of Interleukin-12 in Patients with Dengue Hemorrhagic Fever. FEMS Immunol. Med. Microbiol. 2000, 28, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Neves-Souza, P.C.; Azeredo, E.L.; Zagne, S.M.; Valls-de-Souza, R.; Reis, S.R.; Cerqueira, D.I.; Nogueira, R.M.; Kubelka, C.F. Inducible nitric oxide synthase (iNOS) expression in monocytes during acute Dengue Fever in patients and during in vitro infection. BMC Infect. Dis. 2005, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Charnsilpa, W.; Takhampunya, R.; Endy, T.P.; Mammen, M.P.; Libraty, D.H.; Ubol, S. Nitric Oxide Radical Suppresses Replication of Wild-Type Dengue 2 Viruses in Vitro. J. Med. Virol. 2005, 77, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Gunther, V.J.; Putnak, R.; Eckels, K.H.; Mammen, M.P.; Scherer, J.M.; Lyons, A.; Sun, W. A human challenge model for dengue infection reveals a possible protective role for sustained interferon gamma levels during the acute phase of illness. Vaccine 2011, 29, 3895–3904. [Google Scholar] [CrossRef] [PubMed]
- Mangada, M.M.; Endy, T.P.; Nisalak, A.; Chunsuttiwat, S.; Vaughn, D.W.; Libraty, D.H.; Green, S.; Ennis, F.A.; Rothman, A.L. Dengue-Specific T Cell Responses in Peripheral Blood Mononuclear Cells Obtained Prior to Secondary Dengue Virus Infections in Thai Schoolchildren. J. Infect. Dis. 2002, 185, 1697–1703. [Google Scholar] [CrossRef]
- Atrasheuskaya, A.; Petzelbauer, P.; Fredeking, T.M.; Ignatyev, G. Anti-TNF Antibody Treatment Reduces Mortality in Experimental Dengue Virus Infection. FEMS Immunol. Med. Microbiol. 2003, 35, 33–42. [Google Scholar] [CrossRef]
- Shresta, S.; Sharar, K.L.; Prigozhin, D.M.; Beatty, P.R.; Harris, E. Murine Model for Dengue Virus-Induced Lethal Disease with Increased Vascular Permeability. J. Virol. 2006, 80, 10208–10217. [Google Scholar] [CrossRef]
- Zellweger, R.M.; Prestwood, T.R.; Shresta, S. Supplemental Information Antibodies Enhance Infection of Liver Sinusoidal Endothelial Cells in a Mouse Model of Severe Dengue Disease. Cell Host Microbe 2010, 7, 128–139. [Google Scholar] [CrossRef]
- Assunção-Miranda, I.; Amaral, F.A.; Bozza, F.A.; Fagundes, C.T.; Sousa, L.P.; Souza, D.G.; Pacheco, P.; Barbosa-Lima, G.; Gomes, R.N.; Bozza, P.T.; et al. Contribution of Macrophage Migration Inhibitory Factor to the Pathogenesis of Dengue Virus Infection. FASEB J. 2010, 24, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Kyle, J.L.; Harris, E. Global Spread and Persistence of Dengue. Annu. Rev. Microbiol. 2008, 62, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Guabiraba, R.; Marques, R.E.; Besnard, A.G.; Fagundes, C.T.; Souza, D.G.; Ryffel, B.; Teixeira, M.M. Role of the Chemokine Receptors CCR1, CCR2 and CCR4 in the Pathogenesis of Experimental Dengue Infection in Mice. PLoS ONE. 2010, 5, e15680. [Google Scholar] [CrossRef] [PubMed]
- Renneson, J.; Guabiraba, R.; Maillet, I. A detrimental role for invariant natural killer T cells in the pathogenesis of experimental dengue virus infection. Am. J. Pathol. 2011, 179, 1872–1883. [Google Scholar] [CrossRef] [PubMed]
- Heaton, N.S.; Perera, R.; Berger, K.L.; Khadka, S.; LaCount, D.J.; Kuhn, R.J.; Randall, G. Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 17345–17350. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Miller, I.; Aja, S.; Landree, L.E.; Pinn, M.; McFadden, J.; Kuhajda, F.P.; Moran, T.H.; Ronnett, G.V. C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J. Biol. Chem. 2004, 279, 19970–19976. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.M.; Khromykh, A.A.; Parton, R.G. Cholesterol Manipulation by West Nile Virus Perturbs the Cellular Immune Response. Cell Host Microbe 2007, 2, 229–239. [Google Scholar] [CrossRef]
- Martínez-Gutierrez, M.; Castellanos, J.E.; Gallego-Gómez, J.C. Statins Reduce Dengue Virus Production via Decreased Virion Assembly. Intervirology 2011, 54, 202–216. [Google Scholar] [CrossRef]
- Poh, M.K.; Shui, G.; Xie, X.; Shi, P.Y.; Wenk, M.R.; Gu, F. U18666A, an Intra-Cellular Cholesterol Transport Inhibitor, Inhibits Dengue Virus Entry and Replication. Antivir. Res. 2012, 93, 191–198. [Google Scholar] [CrossRef]
- Soto-Acosta, R.; Bautista-Carbajal, P.; Syed, G.H.; Siddiqui, A.; Del Angel, R.M. Nordihydroguaiaretic Acid (NDGA) Inhibits Replication and Viral Morphogenesis of Dengue Virus. Antivir. Res. 2014, 109, 132–140. [Google Scholar] [CrossRef]
- Hyrina, A.; Meng, F.; McArthur, S.J.; Eivemark, S.; Nabi, I.R.; Jean, F. Human Subtilisin Kexin Isozyme-1 (SKI-1)/Site-1 Protease (S1P) Regulates Cytoplasmic Lipid Droplet Abundance: A Potential Target for Indirect-Acting Anti-Dengue Virus Agents. PLoS ONE 2017, 12, e0174483. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, M.B.; Talarico, L.B.; Vatansever, S.; Carro, A.C.; Fascio, M.L.; D’Accorso, N.B.; García, C.C.; Damonte, E.B. Antiviral Activity of an N-Allyl Acridone against Dengue Virus. J. Biomed. Sci. 2015, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.-H.; Kunz, A.; Simon, V.A.; Palese, P.; Shaw, M.L. Broad-Spectrum Antiviral That Interferes with de Novo Pyrimidine Biosynthesis. Proc. Natl. Acad. Sci. USA 2011, 108, 5777–5782. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Zachariah, M.; Harris, E. Mycophenolic Acid Inhibits Dengue Virus Infection by Preventing Replication of Viral RNA. Virology 2002, 304, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Takahashi, K.; Shiraki, K.; Sakamoto, K.; Smee, D.F.; Barnard, D.L.; Gowen, B.B.; Julander, J.G.; Morrey, J.D. T-705 (Favipiravir) and Related Compounds: Novel Broad-Spectrum Inhibitors of RNA Viral Infections. Antivir. Res. 2009, 82, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ono, L.; Wollinger, W.; Rocco, I.M.; Coimbra, T.L.M.; Gorin, P.A.J.; Sierakowski, M.R. In Vitro and in Vivo Antiviral Properties of Sulfated Galactomannans against Yellow Fever Virus (BeH111 Strain) and Dengue 1 Virus (Hawaii Strain). Antivir. Res. 2003, 60, 201–208. [Google Scholar] [CrossRef]
- Talarico, L.B.; Pujol, C.A.; Zibetti, R.G.M.; Faría, P.C.S.; Noseda, M.D.; Duarte, M.E.R.; Damonte, E.B. The Antiviral Activity of Sulfated Polysaccharides against Dengue Virus Is Dependent on Virus Serotype and Host Cell. Antivir. Res. 2005, 66, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Alen, M.M.F.; de Burghgraeve, T.; Kaptein, S.J.F.; Balzarini, J.; Neyts, J.; Schols, D. Broad Antiviral Activity of Carbohydrate-Binding Agents against the Four Serotypes of Dengue Virus in Monocyte-Derived Dendritic Cells. PLoS ONE 2011, 6, e21658. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; De Wispelaere, M.; Carocci, M.; Liu, Q.; Wang, J.; Yang, P.L.; Gray, N.S. Structure-Activity Relationship Study of QL47: A Broad-Spectrum Antiviral Agent. ACS Med. Chem. Lett. 2017, 8, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Varga, N.; Sutkeviciute, I.; Ribeiro-Viana, R.; Berzi, A.; Ramdasi, R.; Daghetti, A.; Vettoretti, G.; Amara, A.; Clerici, M.; Rojo, J.; et al. A Multivalent Inhibitor of the DC-SIGN Dependent Uptake of HIV-1 and Dengue Virus. Biomaterials 2014, 35, 4175–4184. [Google Scholar] [CrossRef] [PubMed]
- Kouretova, J.; Hammamy, M.Z.; Epp, A.; Hardes, K.; Kallis, S.; Zhang, L.; Hilgenfeld, R.; Bartenschlager, R.; Steinmetzer, T. Effects of NS2B-NS3 Protease and Furin Inhibition on West Nile and Dengue Virus Replication. J. Enzym. Inhib. Med. Chem. 2017, 32, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Hotokezaka, H.; Sakai, E.; Kanaoka, K.; Saito, K.; Matsuo, K.I.; Kitaura, H.; Nakayama, K. U0126 and PD98059, specific inhibitors of MEK, accelerate differentiation of RAW264. 7 cells into osteoclast-like cells. J. Biol. Chem. 2002, 277, 47366–47372. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, G.P.; Chuncharunee, A.; Sirimontaporn, A.; Panaampon, J.; Noisakran, S.; Yenchitsomanus, P.T.; Limjindaporn, T. SB203580 Modulates P38 MAPK Signaling and Dengue Virus-Induced Liver Injury by Reducing MAPKAPK2, HSP27, and ATF2 Phosphorylation. PLoS ONE 2016, 11, e0149486. [Google Scholar] [CrossRef] [PubMed]
- Roth, H.; Magg, V.; Uch, F.; Mutz, P.; Klein, P.; Haneke, K.; Lohmann, V.; Bartenschlager, R.; Fackler, O.T.; Locker, N.; et al. Flavivirus Infection Uncouples Translation Suppression from Cellular Stress Responses. MBio 2017, 8, e02150-16. [Google Scholar] [CrossRef] [PubMed]
- Kovackova, S.; Chang, L.; Bekerman, E.; Neveu, G.; Barouch-Bentov, R.; Chaikuad, A.; Heroven, C.; Šála, M.; De Jonghe, S.; Knapp, S.; et al. Selective Inhibitors of Cyclin G Associated Kinase (GAK) as Anti-Hepatitis C Agents. J. Med. Chem. 2015, 58, 3393–3410. [Google Scholar] [CrossRef] [PubMed]
- Bekerman, E.; Neveu, G.; Shulla, A.; Brannan, J.; Pu, S.Y.; Wang, S.; Xiao, F.; Barouch-Bentov, R.; Bakken, R.R.; Mateo, R.; et al. Anticancer Kinase Inhibitors Impair Intracellular Viral Trafficking and Exert Broad-Spectrum Antiviral Effects. J. Clin. Investig. 2017, 127, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.Y.; Xiao, F.; Schor, S.; Bekerman, E.; Zanini, F.; Barouch-Bentov, R.; Nagamine, C.M.; Einav, S. Feasibility and Biological Rationale of Repurposing Sunitinib and Erlotinib for Dengue Treatment. Antivir. Res. 2018, 155, 67–75. [Google Scholar] [CrossRef]
- Chen, H.H.; Chen, C.C.; Lin, Y.S.; Chang, P.C.; Lu, Z.Y.; Lin, C.F.; Chen, C.L.; Chang, C.P. AR-12 Suppresses Dengue Virus Replication by down-Regulation of PI3K/AKT and GRP78. Antivir. Res. 2017, 142, 158–168. [Google Scholar]
- Albarnaz, J.D.; De Oliveira, L.C.; Torres, A.A.; Palhares, R.M.; Casteluber, M.C.; Rodrigues, C.M.; Cardozo, P.L.; De Souza, A.M.R.; Pacca, C.C.; Ferreira, P.C.P.; et al. MEK/ERK Activation Plays a Decisive Role in Yellow Fever Virus Replication: Implication as an Antiviral Therapeutic Target. Antivir. Res. 2014, 111, 82–92. [Google Scholar] [CrossRef]
- Chang, J.; Schul, W.; Yip, A.; Xu, X.; Guo, J.T.; Block, T.M. Competitive Inhibitor of Cellular α-Glucosidases Protects Mice from Lethal Dengue Virus Infection. Antivir. Res. 2011, 92, 369–371. [Google Scholar] [CrossRef]
- Gu, B.; Mason, P.; Wang, L.; Norton, P.; Bourne, N.; Moriarty, R.; Mehta, A.; Despande, M.; Shah, R.; Block, T. Antiviral Profiles of Novel Iminocyclitol Compounds against Bovine Viral Diarrhea Virus, West Nile Virus, Dengue Virus and Hepatitis B Virus. Antivir. Chem. Chemother. 2007, 18, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.H.; Cheng, W.C.; Lee, Y.L.; Yu, H.P.; Wu, Y.T.; Lin, Y.L.; Wong, C.H. Novel Five-Membered Iminocyclitol Derivatives as Selective and Potent Glycosidase Inhibitors: New Structures for Antivirals and Osteoarthritis. ChemBioChem 2006, 7, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, L.; Ma, D.; Qu, X.; Guo, H.; Xu, X.; Mason, P.M.; Bourne, N.; Moriarty, R.; Gu, B.; et al. Novel Imino Sugar Derivatives Demonstrate Potent Antiviral Activity against Flaviviruses. Antimicrob. Agents Chemother. 2009, 53, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Gill, T.; Wang, L.; Du, Y.; Ye, H.; Qu, X.; Guo, J.-T.; Cuconati, A.; Zhao, K.; Block, T.M.; et al. Design, Synthesis, and Biological Evaluation of N -Alkylated Deoxynojirimycin (DNJ) Derivatives for the Treatment of Dengue Virus Infection. J. Med. Chem. 2012, 55, 6061–6075. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Plummer, E.; Buck, M.D.; Sanchez, M.; Greenbaum, J.A.; Turner, J.; Grewal, R.; Klose, B.; Sampath, A.; Warfield, K.L.; Peters, B.; et al. Dengue Virus Evolution under a Host-Targeted Antiviral. J. Virol. 2015, 89, 5592–5601. [Google Scholar] [CrossRef]
- Tseng, C.K.; Lin, C.K.; Wu, Y.H.; Chen, Y.H.; Chen, W.C.; Young, K.C.; Lee, J.C. Human Heme Oxygenase 1 Is a Potential Host Cell Factor against Dengue Virus Replication. Sci. Rep. 2016, 6, 32176. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Wu, Y.H.; Tseng, C.K.; Lin, C.K.; Hsu, Y.C.; Chen, Y.H.; Lee, J.C. Schisandrin A Inhibits Dengue Viral Replication via Upregulating Antiviral Interferon Responses through STAT Signaling Pathway. Sci. Rep. 2017, 7, 45171. [Google Scholar] [CrossRef]
- Yu, J.S.; Tseng, C.K.; Lin, C.K.; Hsu, Y.C.; Wu, Y.H.; Hsieh, C.L.; Lee, J.C. Celastrol Inhibits Dengue Virus Replication via Up-Regulating Type I Interferon and Downstream Interferon-Stimulated Responses. Antivir. Res. 2017, 137, 49–57. [Google Scholar] [CrossRef]
- Pryke, K.; Abraham, J.; Sali, T.; Gall, B.; Archer, I. A novel agonist of the TRIF pathway induces a cellular state refractory to replication of Zika, chikungunya, and dengue viruses. MBio 2027, 8, e00452-17. [Google Scholar] [CrossRef]
- Sharma, N.; Mishra, K. Salidroside Exhibits Anti-Dengue Virus Activity by Upregulating Host Innate Immune Factors. Arch. Virol. 2016, 161, 3331–3344. [Google Scholar] [CrossRef]
- Tsai, W.L.; Cheng, J.S.; Shu, C.W.; Lai, K.H.; Chan, H.H.; Wu, C.C.; Wu, J.M.; Hsu, P.I.; Chung, R.T.; Chang, T.H. Asunaprevir Evokes Hepatocytes Innate Immunity to Restrict the Replication of Hepatitis C and Dengue Virus. Front. Microbiol. 2017, 8, 668. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.; Beljanski, V.; Yin, K. Sequence-specific modifications enhance the broad-spectrum antiviral response activated by RIG-I agonists. J. Virol. 2015, 89, 8011–8025. [Google Scholar] [CrossRef] [PubMed]
- Shafee, N.; AbuBakar, S. Zinc Accelerates Dengue Virus Type 2-Induced Apoptosis in Vero Cells. FEBS Lett. 2002, 524, 20–24. [Google Scholar] [CrossRef]
- Corrêa, G.; Lindenberg, C.D.A.; Fernandes-Santos, C.; Gandini, M.; Paiva, F.P.; Coutinho-Silva, R.; Kubelka, C.F. The Purinergic Receptor P2X7 Role in Control of Dengue Virus-2 Infection and Cytokine/Chemokine Production in Infected Human Monocytes. Immunobiology 2016, 221, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Mello, C.D.S.; Valente, L.M.M.; Wolff, T.; Lima-Junior, R.S.; Fialho, L.G.; Marinho, C.F.; Azeredo, E.L.; Oliveira-Pinto, L.M.; Pereira, R.D.C.A.; Siani, A.C.; et al. Decrease in Dengue Virus-2 Infection and Reduction of Cytokine/Chemokine Production by Uncaria Guianensis in Human Hepatocyte Cell Line Huh-7. Memórias do Instituto Oswaldo Cruz 2017, 112, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.R.I.N.; Valente, L.M.M.; Sampaio, A.L.; Siani, A.C.; Gandini, M.; Azeredo, E.L.; D’Avila, L.A.; Mazzei, J.L.; Maria das Graças, M.H.; Kubelka, C.F. Immunomodulating and Antiviral Activities of Uncaria Tomentosa on Human Monocytes Infected with Dengue Virus-2. Int. Immunopharmacol. 2008, 8, 468–476. [Google Scholar] [CrossRef]
- Wagstaff, K.M.; Harrich, D.; Heaton, S.M.; Sivakumaran, H.; Jans, D.A. Ivermectin Is a Specific Inhibitor of Importin α/β-Mediated Nuclear Import Able to Inhibit Replication of HIV-1 and Dengue Virus. Biochem. J. 2012, 443, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.B.; Han, J.C.; Cong, X.; Wei, L. BST2/Tetherin Inhibits Dengue Virus Release from Human Hepatoma Cells. PLoS ONE 2012, 7, e51033. [Google Scholar] [CrossRef]
- Trung, D.T.; Wills, B. Systemic Vascular Leakage Associated with Dengue Infections—The Clinical Perspective; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Gröger, M.; Pasteiner, W.; Ignatyev, G.; Matt, U.; Knapp, S.; Atrasheuskaya, A.; Bukin, E.; Friedl, P.; Zinkl, D.; Hofer-Warbinek, R.; et al. Peptide Bβ15-42 Preserves Endothelial Barrier Function in Shock. PLoS ONE 2009, 4, e5391. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Lei, H.Y.; Liu, H.S.; Lin, Y.S.; Fu, T.F.; Yeh, T.M. Macrophage Migration Inhibitory Factor Induced by Dengue Virus Infection Increases Vascular Permeability. Cytokine 2011, 54, 222–231. [Google Scholar] [CrossRef]
- Kubelka, C.; Azeredo, E.; Gandini, M.; Pinto, L. Metalloproteinases Are Produced during Dengue Fever and MMP9 Is Associated with Severity; The British Infection Association: Preston, UK, 2010. [Google Scholar]
- Ong, S.P.; Lee, L.M.; Leong, Y.F.I.; Ng, M.L.; Chu, J.J.H. Dengue Virus Infection Mediates HMGB1 Release from Monocytes Involving PCAF Acetylase Complex and Induces Vascular Leakage in Endothelial Cells. PLoS ONE 2012, 7, e41932. [Google Scholar] [CrossRef]
- Costa, V.V.; Fagundes, C.T.; Souza, D.G.; Teixeira, M.M. Inflammatory and Innate Immune Responses in Dengue Infection. Am. J. Pathol. 2013, 182, 1950–1961. [Google Scholar] [CrossRef]
- Souza, D.; Fagundes, C. Essential Role of Platelet-Activating Factor Receptor in the Pathogenesis of Dengue Virus Infection. Proc. Natl. Acad. Sci. USA 2009, 106, 14138–14143. [Google Scholar] [CrossRef]
- Rahman, S.M.; Atikullah, M.; Islam, M.N.; Mohaimenul, M.; Ahammad, F.; Islam, M.S.; Saha, B.; Rahman, M.H. Anti-inflammatory, antinociceptive and antidiarrhoeal activities of methanol and ethyl acetate extract of Hemigraphis alternata leaves in mice. Clin. Phytosci. 2019, 5, 16. [Google Scholar] [CrossRef]

| Host Process | Inhibitor(s) | Target | DENV Types | Cell Line(s) Tested | Refs. |
|---|---|---|---|---|---|
| Glycolytic pathway | 2-deoxy-d-glucose (2DG) | Glycolysis | DENV-2 | HFFs Cell | [5] |
| Oxamate | Glycolysis | DENV-2 | HFFs Cell | [5] | |
| Lipid biosynthesis pathway | Cerulenin | Fatty acid biosynthesis | DENV-2 | Huh-7.5 Cell | [156,157] |
| C75 | Fatty acid biosynthesis | DENV-4 | C6/36 Cell | [156,157] | |
| Lovastatin (fluvastatin, lovastatin, mevastatin, and simvastatin) | Cholesterol biosynthesis | DENV-2 | Huh-7 Cell | [158,159] | |
| U18666A | Cholesterol biosynthesis | DENV-2 | C6/36 cell line | [160] | |
| Methyl b-cyclo dextrin | Cholesterol biosynthesis | DENV-1 to 4 | Huh-7 Cell | [158] | |
| Nordihydroguaiaretic acid | Fatty acid biosynthesis | DENV-4 | Huh-7 cell | [161] | |
| Orlistat | Fatty acid biosynthesis | DENV -4, -2 | HepG2 and HEK293T/17 Cell | [161] | |
| PF-429242 | Fatty acid biosynthesis | DENV-1–4 | Huh-7.5.1 Cell | [162] | |
| Hymeglusin | Cholesterol biosynthesis | DENV-2 | K562 cells | [68] | |
| Zaragozic acid | Cholesterol biosynthesis | DENV-2 | K562 cells | [68] | |
| Nucleotide biosynthesis pathways | Ribavirin | IMP dehydrogenase | DENV-2 | LLC-MK2 | [163] |
| N-ally acridones | IMP dehydrogenase (Partial) | DENV-2 | Vero cells | [163] | |
| Brequinar | Dihydroorotate dehydogenase | DENV-2 | Vero cells | [164] | |
| Mycophenolic acid | IMP dehydrogenase | DENV-2 | Huh-7, CRL-8024, and HepG2 | [165] | |
| NITD 982 | Dihydroorotate dehydogenase | DENV-2 | Vero cells | [69] | |
| ETAR | IMP dehydrogenase | DENV-2 | Vero cells | [166] | |
| IM18 | IMP dehydrogenase | DENV-2 | Vero cells | [166] | |
| Glycosaminoglycans | PI88 | Heparan sulfate | DENV-2 | BHK and in mice | [80] |
| Chondroitin sulfate | Heparan sulfate | DENV-1–4 | BHK-21 and Vero cells | [78] | |
| Curdlan sulfate | Heparan sulfate | DENV-1–4 | LLC-MK2 cells | [77] | |
| K5 polysaccharide from Escherichia coli | Heparan sulfate | DENV-2 | HMEC-1 and HMVEC-d cells | [83] | |
| Heparin | Heparan sulfate | DENV-2 | Vero, BHK, Hepatocytes | [79] | |
| Fucoidans | Heparan sulfate | DENV-2 | BHK | [82] | |
| GAG | Heparan sulfate | DENV-2 | Vero | [79] | |
| Sulfated galactomannan | Heparan sulfate | DENV-1 | C6/36 | [167] | |
| DL-galactan | Heparan sulfate | DENV-2, -3 | Vero, Hep-G2 | [141] | |
| Carrageenan | Heparan sulfate | DENV-2, -3 | Vero, Hep-G2 | [138] | |
| α-d-glucan | Heparan sulfate | DENV-2 | BHK | [84] | |
| Dextran sulfate 8000 | Heparan sulfate | DENV-2 | Hepatocytes, Vero | [168] | |
| Zosteric acid, CF-238 | Heparan sulfate | DENV-1–4 | LLC-MK2 | [77] | |
| DC-SIGN | PRM-S | Carbohydrate binding agent | DENV-2 | Raji/DC-SIGN and MDDC | [169] |
| QL-XII-47 (QL47) | DC-SIGN(BTK) | DENV-2 | Huh-7 Cell | [170] | |
| Plant lectins from Hippeastrum hybrid, Galanthus nivalis, Urtica dioica | DC-SIGN | DENV-1–4 | MDDC, Huh-7, U87/DC-SIGN | [169] | |
| Glycomimetic DC-SIGN ligand | DC-SIGN | DENV-2 | DC-SIGN/Raji cells | [171] | |
| DS (MW > 500,000 Da) | DC-SIGN | DENV-1–4 | C6/36 | [169] | |
| Host protease | 45 | Furin | DENV-2 | Huh-7 cells | [172] |
| 46 | Furin | DENV-2 | Huh-7 cells | [172] | |
| Peptidomimettic furin inhibitor, Luteolin | Furin | DENV-1–4 | Huh-7 cells | [105] | |
| Host kinase | Dasatinib | c-Src/Fyn | DENV-1–4 | Vero, Huh-7 | [106] |
| SaracatinibAZD0530 | c-Src/Fyn | DENV-1–4 | Vero, Huh-7 | [106] | |
| GNF-2 | Abl Kinases E Protein | DENV-2 | BHK-21 | [108] | |
| Imatinib | Abl Kinases | DENV-2 | BHK-21 | [108] | |
| Mitogen activated protein kinase | PD98059, U0126, FR180204 | MEK | DENV-2 | RAW264.7 | [173] |
| SB203580 | p38 pathway | DENV-2 | C6/36 | [174] | |
| CGP57380 | ERK and p38 pathways | DENV-2 | BHK-21 | [175] | |
| Imidazo[1,2-b] pyridazine | AAK1 | DENV-2 | Huh-7 | [176] | |
| Isothiazolo[5,4-b] pyridines | GAK | DENV-2 | Huh-7 | [177] | |
| Sunitinib and erlotinib | AAK1 and GAK | DENV-2 | Huh7 | [178] | |
| AR-12 | PI3K/JAKT pathway | DENV-1–4 | Huh 7 | [179] | |
| U0126 | Erk inhibitor | DENV-2, -3 | BHK-21 | [180] | |
| SFV785 | NTRK1 and MAPKAPK5 | DENV-2 | BHK-21 | [108] | |
| Host Glucosidase | CM-9-78 (DNJ derivative) | α-glucosidase | DENV-1 to 4 | BHK-21 | [181] |
| UV-4 (DNJ derivative) | α-glucosidase | DENV-2 | BHK-21 | [117] | |
| DNJ | α-glucosidase | DENV-1 | BHK-21 | [117] | |
| Celgosivir | α-glucosidase | DENV-1–4 | BHK | [116] | |
| Kotalanol | α-glucosidase | DENV-1–4 | BHK | [86] | |
| Castanospermine | α-glucosidase | DENV-1–4 | BHK, Huh-7 | [110,112] | |
| OSL-9511 | α-glucosidase | DENV-2 | BHK | [182] | |
| NN-DNJ | α-glucosidase | DENV-2 | BHK | [114] | |
| Compound 36 | α-glucosidase | DENV-2 | BHK-21 | [183] | |
| Compound 36 | α-glucosidase | DENV-2 | BHK-21 | [183] | |
| Compound 36 | α-glucosidase | DENV-2 | BHK-21 | [183] | |
| N-alkyl side chains 69(CST) | α-glucosidase | DENV-2 | BHK | [183] | |
| N-alkyl side chains 70(DNJ) | α-glucosidase | DENV-2 | BHK | [183] | |
| N-alkyl side chains 71 | α-glucosidase | DENV-2 | BHK | [182] | |
| N-alkyl side chains 72 | α-glucosidase | DENV-2 | BHK | [182] | |
| N-alkyl side chains 73 | α-glucosidase | DENV-2 | BHK | [182] | |
| N-alkyl side chains 74 | α-glucosidase | DENV-2 | BHK | [184] | |
| N-alkyl side chains 75 | α-glucosidase | DENV-2 | BHK | [184] | |
| N-alkyl side chains 76 | α-glucosidase | DENV-2 | BHK | [184] | |
| N-alkyl side chains 77 | α-glucosidase | DENV-2 | BHK | [185] | |
| N-alkyl side chains 78 | α-glucosidase | DENV-2 | BHK | [117] | |
| N-alkyl side chains 79 | α-glucosidase | DENV-2 | BHK | [186] | |
| SP173 | α-glucosidase | DENV-2 | BHK-21 | [112] | |
| SP169 | α-glucosidase | DENV-2 | BHK-21 | [112] | |
| 6-O-butanoyl castanospermine | α-glucosidase | DENV-2 | BHK-21 | [110] | |
| Host Immunity, and Inflammatory pathways | Human heme oxygenase I | Innate antiviral response | DENV-1–4 | Huh-7 | [187] |
| Schisandrin A | STAT1/2-mediated responses | DENV-1–4 | Huh-7 | [188] | |
| Celastrol | JAK–STAT signaling | DENV-1–4 | Huh-7 | [189] | |
| Agonists of IRF3-terminal pathways | TRIF Pathway | DENV-2 | Vero cells | [190] | |
| Salidroside | RIG-I | DENV-2 | THP-1 cell line | [191] | |
| Asunaprevir | MAVS pathways | DENV-2 | Huh 7.5.1, Hep-G2 cells, | [192] | |
| Sequence-specific RIG-I agonist | IRIG-I-mediated | DENV-2 | Lung epithelial A549 cells | [193] | |
| Helicase with zinc linger 2 | Innate antiviral response | DENV-2 | Vero cells | [194] | |
| Purinergic receptor P2X7 | Inflammatory process | DENV-2 | Human monocyte Cell | [195] | |
| Extract from Uncaria tomenrosa, N. brasiliensis Choisy, Uncaria guianensis | Cytokine/chemokine | DENV-2 | Huh-7 | [196,197] | |
| Extract from Cissampelos pareira Linn | Innate antiviral response | DENV-1–4 | C6/36, LLC-MK2, Vero, Hep-G2 | [198] | |
| Ivermectin | α/β-mediated transport | DENV-1–4 | HeLa | [198] | |
| BST2/tetherin | IFN induced | DENV-2 | Huh7 | [199] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahammad, F.; Tengku Abd Rashid, T.R.; Mohamed, M.; Tanbin, S.; Ahmad Fuad, F.A. Contemporary Strategies and Current Trends in Designing Antiviral Drugs against Dengue Fever via Targeting Host-Based Approaches. Microorganisms 2019, 7, 296. https://doi.org/10.3390/microorganisms7090296
Ahammad F, Tengku Abd Rashid TR, Mohamed M, Tanbin S, Ahmad Fuad FA. Contemporary Strategies and Current Trends in Designing Antiviral Drugs against Dengue Fever via Targeting Host-Based Approaches. Microorganisms. 2019; 7(9):296. https://doi.org/10.3390/microorganisms7090296
Chicago/Turabian StyleAhammad, Foysal, Tengku Rogayah Tengku Abd Rashid, Maizan Mohamed, Suriyea Tanbin, and Fazia Adyani Ahmad Fuad. 2019. "Contemporary Strategies and Current Trends in Designing Antiviral Drugs against Dengue Fever via Targeting Host-Based Approaches" Microorganisms 7, no. 9: 296. https://doi.org/10.3390/microorganisms7090296
APA StyleAhammad, F., Tengku Abd Rashid, T. R., Mohamed, M., Tanbin, S., & Ahmad Fuad, F. A. (2019). Contemporary Strategies and Current Trends in Designing Antiviral Drugs against Dengue Fever via Targeting Host-Based Approaches. Microorganisms, 7(9), 296. https://doi.org/10.3390/microorganisms7090296







