Modulation of the Immune Response to Improve Health and Reduce Foodborne Pathogens in Poultry
Abstract
1. Introduction
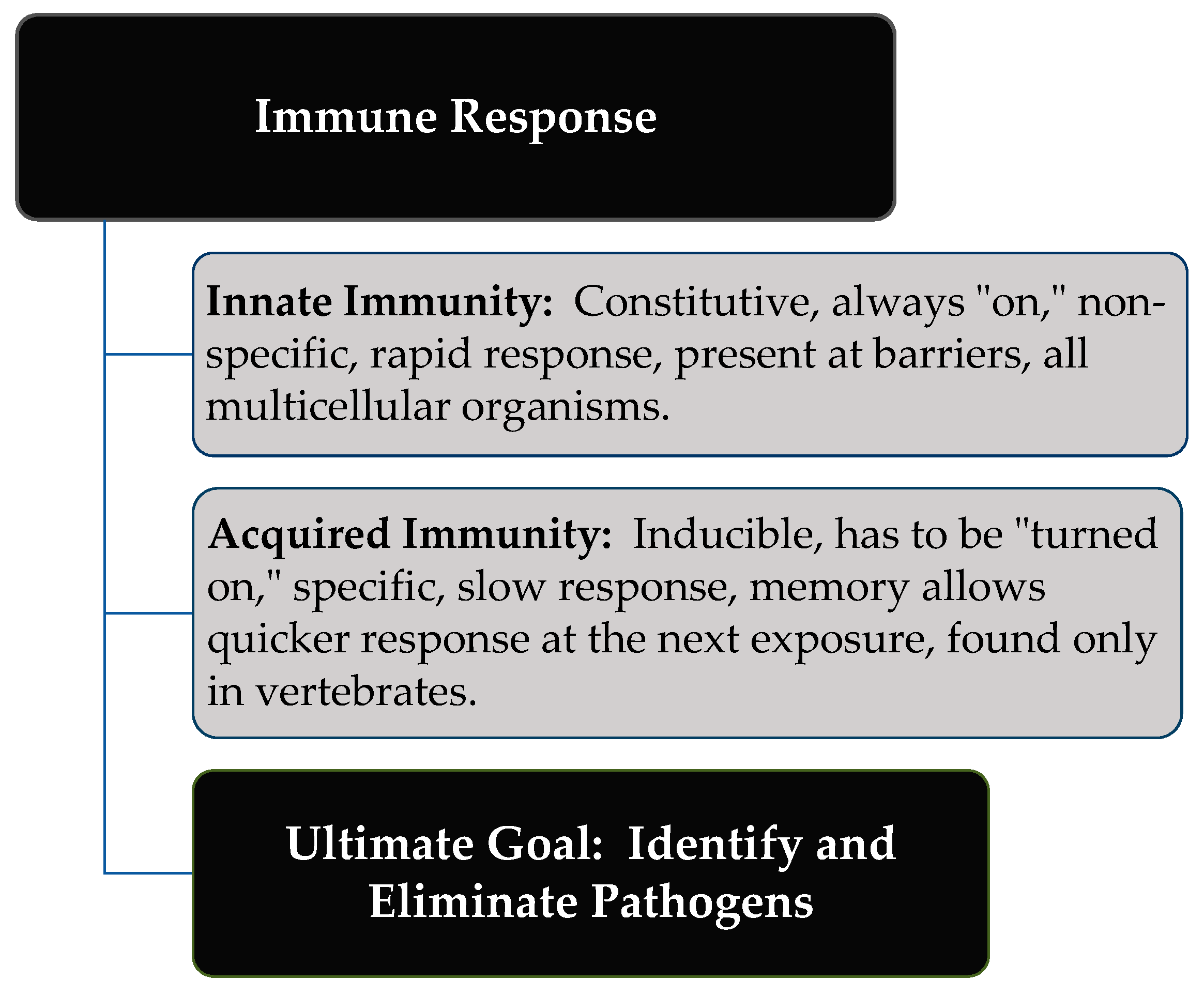
2. Immune Response
3. Nutrition
4. Immunomodulation in Poultry
5. Importance of Understanding the Mode-of-Action
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control. Surveillance for Foodborne Disease Outbreaks, United States, 2012, Annual Report; US Department of Health and Human Services: Atlanta, GA, USA, 2014.
- Flynn, D. USDA: U.S. Foodborne Illnesses Cost More than $15.6 Billion Annually. Food Safety News. 2014. Available online: http://www.foodsafetynews.com/2014/10/foodborne-illnesses-cost-usa-15-6-billion-annually/#.VDgGlr7tj3Q (accessed on 26 February 2019).
- Marder, E.P.; Cieslak, P.R.; Cronquist, A.B.; Dunn, J.; Lathrop, S.; Rabatsky-Ehr, T.; Ryan, P.; Smith, K.; Tobin-D’Angelo, M.; Vugia, D.J.; et al. Incidence and Trends of Infections with Pathogens Transmitted Commonly Through Food and the Effect of Increasing Use of Culture-Independent Diagnostic Tests on Surveillance—Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2013–2016. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.F.; Yackley, J. Foodborne Disease Outbreaks in the United States: A Historical Overview. Foodborne Pathog. Dis. 2018, 15, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Griffin, P.M.; McLean, H.Q.; Mahon, B.E. Hospitalisations due to bacterial gastroenteritis: A comparison of surveillance and hospital discharge data. Epidemiol. Infect. 2018, 146, 954–960. [Google Scholar] [CrossRef] [PubMed]
- National Chicken Council 2018. Available online: https://www.nationalchickencouncil.org/about-the-industry/statistics/u-s-broiler-production/ (accessed on 26 February 2019).
- Food Safety Inspection Service. New performance standards for Salmonella and Campylobacter in not-ready-to-eat comminuted chicken and turkey products and raw chicken parts and changes to related agency verification procedures: Response to comments and announcement of implementation schedule. Fed. Regist. 2016, 81, 7285–7300. [Google Scholar]
- Epps, S.V.; Harvey, R.B.; Hume, M.E.; Phillips, T.D.; Anderson, R.C.; Nisbet, D.J. Foodborne Campylobacter: Infections, metabolism, pathogenesis and reservoirs. Int. J. Environ. Res. Public Health 2013, 10, 6292–6304. [Google Scholar] [CrossRef] [PubMed]
- Chlebicz, A.; Slizewska, K. Campylobacteriosis, Salmonellosis, Yersiniosis, and Listeriosis as Zoonotic Foodborne Diseases: A Review. Int. J. Environ. Res. Public Health 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Wales, A.D.; Vidal, A.B.; Davies, R.H.; Rodgers, J.D. Field Interventions Against Colonization of Broilers by Campylobacter. Compr. Rev. Food Sci. Food Saf. 2019, 18, 167–188. [Google Scholar] [CrossRef]
- Awad, W.A.; Ghareeb, K. Some aspects of control of salmonella infection in poultry for minimising contamination in the food chain. Worlds Poult. Sci. J. 2014, 70, 519–530. [Google Scholar] [CrossRef]
- Moore, J.E.; Corcoran, D.; Dooley, J.S.; Fanning, S.; Lucey, B.; Matsuda, M.; McDowell, D.A.; Megraud, F.; Millar, B.C.; O’Mahony, R.; et al. Campylobacter. Vet. Res. 2005, 36, 351–382. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, H.; Cheng, Y.; Zhang, W.; Luo, Q.; Wen, G.; Wang, G.; Shao, H.; Zhang, T. Quinolone resistance phenotype and genetic characterization of Salmonella enterica serovar Pullorum isolates in China, during 2011 to 2016. BMC Microbiol. 2018, 18, 225. [Google Scholar] [CrossRef] [PubMed]
- Wigley, P. Salmonella enterica serovar Gallinarum: Addressing fundamental questions in bacteriology sixty years on from the 9R vaccine. Avian Pathol. 2017, 46, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Barrow, P.A.; Freitas Neto, O.C. Pullorum disease and fowl typhoid--new thoughts on old diseases: A review. Avian Pathol. 2011, 40, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial Resistance in Bacterial Poultry Pathogens: A Review. Front. Vet. Sci. 2017, 4, 126. [Google Scholar] [CrossRef] [PubMed]
- Flock, D.K.; Laughlin, K.F.; Bentley, J. Minimizing losses in poultry breeding and production: How breeding companies contribute to poultry welfare. Worlds Poult. Sci. J. 2005, 61, 227–237. [Google Scholar] [CrossRef]
- Havenstein, G.B.; Ferket, P.R.; Qureshi, M.A. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003, 82, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Han, P.F.; Smyth, J.R., Jr. The influence of growth rate on the development of Marek’s disease in chickens. Poult. Sci. 1972, 51, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Nestor, K.E.; Saif, Y.M.; Zhu, J.; Noble, D.O. Influence of growth selection in turkeys on resistance to Pasteurella multocida. Poult. Sci. 1996, 75, 1161–1163. [Google Scholar] [CrossRef] [PubMed]
- Bayyari, G.R.; Huff, W.E.; Rath, N.C.; Balog, J.M.; Newberry, L.A.; Villines, J.D.; Skeeles, J.K.; Anthony, N.B.; Nestor, K.E. Effect of the genetic selection of turkeys for increased body weight and egg production on immune and physiological responses. Poult. Sci. 1997, 76, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Janss, L.L.G.; Bolder, N.M. Heritabilities of and genetic relationships between salmonella resistance traits in broilers. J. Anim. Sci. 2000, 78, 2287–2291. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dibner, J.J.; Richards, J.D. Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Castanon, J.I.R. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, C.G.; Macklin, K.S.; Kumar, S.; Bailey, M.; Ebner, P.E.; Oliver, H.F.; Martin-Gonzalez, F.S.; Singh, M. Prevalence and antimicrobial resistance patterns of Salmonella isolated from poultry farms in southeastern United States. Poult. Sci. 2018, 97, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- McDougald, L.R. Intestinal protozoa important to poultry. Poult. Sci. 1998, 77, 1156–1158. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H. Issues and consequences of using nutrition to modulate the avian immune response. J. Appl. Poult. Res. 2017, 26, 605–612. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Janeway, C.A., Jr. Innate immunity: The virtues of a nonclonal system of recognition. Cell 1997, 91, 295–298. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Signaling pathways activated by microorganisms. Curr. Opin. Cell Biol. 2007, 19, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Klasing, K.C. Nutrition and the immune system. Br. Poult. Sci. 2007, 48, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Blander, J.M.; Longman, R.S.; Iliev, I.D.; Sonnenberg, G.F.; Artis, D. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 2017, 18, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Blacher, E.; Elinav, E. Microbiome, metabolites and host immunity. Curr. Opin. Microbiol. 2017, 35, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M.T. Nutritional modulation of immune function in broilers. Poult. Sci. 2004, 83, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Korver, D.R. Implications of changing immune function through nutrition in poultry. Anim. Feed Sci. Technol. 2012, 173, 54–64. [Google Scholar] [CrossRef]
- Kogut, M.H. The gut microbiota and host innate immunity: Regulators of host metabolism and metabolic diseases in poulty? J. Appl. Poult. Res. 2013, 22, 637–646. [Google Scholar] [CrossRef]
- Kim, W.H.; Lillehoj, H.S. Immunity, immunomodulation, and antibiotic alternatives to maximize the genetic potential of poultry for growth and disease response. Anim. Feed Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Meunier, M.; Guyard-Nicodeme, M.; Dory, D.; Chemaly, M. Control strategies against Campylobacter at the poultry production level: Biosecurity measures, feed additives and vaccination. J. Appl. Microbiol. 2016, 120, 1139–1173. [Google Scholar] [CrossRef] [PubMed]
- Faber, T.A.; Dilger, R.N.; Iakiviak, M.; Hopkins, A.C.; Price, N.P.; Fahey, G.C., Jr. Ingestion of a novel galactoglucomannan oligosaccharide-arabinoxylan (GGMO-AX) complex affected growth performance and fermentative and immunological characteristics of broiler chicks challenged with Salmonella typhimurium. Poult. Sci. 2012, 91, 2241–2254. [Google Scholar] [CrossRef] [PubMed]
- Santini, C.; Baffoni, L.; Gaggia, F.; Granata, M.; Gasbarri, R.; Di Gioia, D.; Biavati, B. Characterization of probiotic strains: An application as feed additives in poultry against Campylobacter jejuni. Int. J. Food Microbiol. 2010, 141, S98–S108. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, G.R.; Syed, B.; Haldar, S.; Pender, C. Phytogenic Feed Additives as an Alternative to Antibiotic Growth Promoters in Broiler Chickens. Front. Vet. Sci. 2015, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Taha-Abdelaziz, K.; Hodgins, D.C.; Lammers, A.; Alkie, T.N.; Sharif, S. Effects of early feeding and dietary interventions on development of lymphoid organs and immune competence in neonatal chickens: A review. Vet. Immunol. Immunopathol. 2018, 201. [Google Scholar] [CrossRef] [PubMed]
- Dittoe, D.K.; Ricke, S.C.; Kiess, A.S. Organic Acids and Potential for Modifying the Avian Gastrointestinal Tract and Reducing Pathogens and Disease. Front. Vet. Sci. 2018, 5, 216. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Halley, J.; Silva, M. Can feeding the broiler breeder improve chick quality and offspring performance? Anim. Prod. Sci. 2016, 56, 1254–1262. [Google Scholar] [CrossRef]
- Jones, F.T. A review of practical Salmonella control measures in animal feed. J. Appl. Poult. Res. 2011, 20, 102–113. [Google Scholar] [CrossRef]
- Magossi, G.; Cernicchiaro, N.; Dritz, S.; Houser, T.; Woodworth, J.; Jones, C.; Trinetta, V. Evaluation of Salmonella presence in selected United States feed mills. MicrobiologyOpen 2018, e00711. [Google Scholar] [CrossRef] [PubMed]
- Genovese, K.J.; He, H.; Swaggerty, C.L.; Kogut, M.H. The avian heterophil. Dev. Comp. Immunol. 2013, 41, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Lowry, V.K.; Farnell, M.B.; Ferro, P.J.; Swaggerty, C.L.; Bahl, A.; Kogut, M.H. Purified beta-glucan as an abiotic feed adative up-regulates the innate immune response in immature chickens against Salmonella enterica serovar Enteritidis. Int. J. Food Microbiol. 2005, 98, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H.; Genovese, K.J.; He, H.; Swaggerty, C.L.; Jiang, Y.W. BT cationic peptides: Small peptides that modulate innate immune responses of chicken heterophils and monocytes. Vet. Immunol. Immunopathol. 2012, 145, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H.; Genovese, K.J.; He, H.; Swaggerty, C.L.; Jiang, Y. Modulation of Chicken Intestinal Immune Gene Expression by Small Cationic Peptides as Feed Additives During the First Week Post-Hatch. Clin. Vaccine Immunol. 2013, 20, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H. Impact of nutrition on the innate immune response to infection in poultry. J. Appl. Poult. Res. 2009, 18, 111–124. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, H.S.; Yim, J.H.; Kim, Y.J.; Kim, D.H.; Chon, J.W.; Kim, H.; Om, A.S.; Seo, K.H. Comparison of the isolation rates and characteristics of Salmonella isolated from antibiotic-free and conventional chicken meat samples. Poult. Sci. 2017, 96, 2831–2838. [Google Scholar] [CrossRef] [PubMed]
- Piva, A.; Pizzamiglio, V.; Morlacchini, M.; Tedeschi, M.; Piva, G. Lipid microencapsulation allows slow release of organic acids and natural identical flavors along the swine intestine. J. Anim. Sci. 2007, 85, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Grilli, E.; Tugnoli, B.; Formigoni, A.; Massi, P.; Fantinati, P.; Tosi, G.; Piva, A. Microencapsulated sorbic acid and nature-identical compounds reduced Salmonella Hadar and Salmonella Enteritidis colonization in experimentally infected chickens. Poult. Sci. 2011, 90, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Grilli, E.; Vitari, F.; Domeneghini, C.; Palmonari, A.; Tosi, G.; Fantinati, P.; Massi, P.; Piva, A. Development of a feed additive to reduce caecal Campylobacter jejuni in broilers at slaughter age: From in vitro to in vivo, a proof of concept. J. Appl. Microbiol. 2013, 114, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Huneau-Salaun, A.; Guyard-Nicodeme, M.; Benzoni, G.; Gautier, X.; Quesne, S.; Poezevara, T.; Chemaly, M. Randomized control trial to test the effect of a feed additive on Campylobacter contamination in commercial broiler flocks up to slaughter. Zoonoses Public Health 2018, 65, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Ferro, P.J.; Swaggerty, C.L.; Kaiser, P.; Pevzner, I.Y.; Kogut, M.H. Heterophils isolated from chickens resistant to extraintestinal Salmonella enteritidis infection express higher levels of pro-inflammatory cytokine mRNA following infection than heterophils from susceptible chickens. Epidemiol. Infect. 2004, 132, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Swaggerty, C.L.; Ferro, P.J.; Pevzner, I.Y.; Kogut, M.H. Heterophils are associated with resistance to systemic Salmonella enteritidis infection in genetically distinct lines of chickens. FEMS Immunol. Med. Microbiol. 2005, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Redmond, S.B.; Chuammitri, P.; Andreasen, C.B.; Palic, D.; Lamont, S.J. Chicken heterophils from commercially selected and non-selected genetic lines express cytokines differently after in vitro exposure to Salmonella enteritidis. Vet. Immunol. Immunopathol. 2009, 132, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Swaggerty, C.L.; Kogut, M.H.; Chiang, H.; Wang, Y.; Genovese, K.J.; He, H.; Stern, N.J.; Pevzner, I.Y.; Zhou, H. The paternal effect of Campylobacter jejuni colonization in ceca in broilers. Poult. Sci. 2008, 87, 1742–1747. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.E.; Thiennimitr, P.; Winter, M.G.; Butler, B.P.; Huseby, D.L.; Crawford, R.W.; Russell, J.M.; Bevins, C.L.; Adams, L.G.; Tsolis, R.M.; et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 2010, 467, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Buber, M.T.; Yang, Q.; Cerne, R.; Cortes, R.Y.; Sprous, D.G.; Bryant, R.W. Thymol and related alkyl phenols activate the hTRPA1 channel. Br. J. Pharmacol. 2008, 153, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. TRP channels in the digestive system. Curr. Pharm. Biotechnol. 2011, 12, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006, 9, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Parenti, A.; De Logu, F.; Geppetti, P.; Benemei, S. What is the evidence for the role of TRP channels in inflammatory and immune cells? Br. J. Pharmacol. 2016, 173, 953–969. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Z.; Takats, Z.; Blake, T.A.; Gologan, B.; Guymon, A.J.; Wiseman, J.M.; Oliver, J.C.; Davisson, V.J.; Cooks, R.G. Preparing protein microarrays by soft-landing of mass-selected ions. Science 2003, 301, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-H. Progress in chemical proteomics-based kinome study. J. Int. Pharmaceut. Res. 2014, 41, 259–267. [Google Scholar]
- Arsenault, R.J.; Kogut, M.H. Chicken-specific peptide arrays for kinome analysis: Flight for the flightless. Curr. Top. Biotechnol. 2012, 7, 79–89. [Google Scholar]
- Arsenault, R.J.; Napper, S.; Kogut, M.H. Salmonella enterica Typhimurium infection causes metabolic changes in chicken muscle involving AMPK, fatty acid and insulin/mTOR signaling. Vet. Res. 2013, 44, 35. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, R.J.; Lee, J.T.; Latham, R.; Carter, B.; Kogut, M.H. Changes in immune and metabolic gut response in broilers fed beta-mannanase in beta-mannan-containing diets. Poult. Sci. 2017, 96, 4307–4316. [Google Scholar] [CrossRef] [PubMed]
- Hashim, M.M.; Arsenault, R.J.; Byrd, J.A.; Kogut, M.H.; Al-Ajeeli, M.; Bailey, C.A. Influence of different yeast cell wall preparations and their components on performance and immune and metabolic pathways in Clostridium perfringens-challenged broiler chicks. Poult. Sci. 2018, 97, 203–210. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swaggerty, C.L.; Callaway, T.R.; Kogut, M.H.; Piva, A.; Grilli, E. Modulation of the Immune Response to Improve Health and Reduce Foodborne Pathogens in Poultry. Microorganisms 2019, 7, 65. https://doi.org/10.3390/microorganisms7030065
Swaggerty CL, Callaway TR, Kogut MH, Piva A, Grilli E. Modulation of the Immune Response to Improve Health and Reduce Foodborne Pathogens in Poultry. Microorganisms. 2019; 7(3):65. https://doi.org/10.3390/microorganisms7030065
Chicago/Turabian StyleSwaggerty, Christina L., Todd R. Callaway, Michael H. Kogut, Andrea Piva, and Ester Grilli. 2019. "Modulation of the Immune Response to Improve Health and Reduce Foodborne Pathogens in Poultry" Microorganisms 7, no. 3: 65. https://doi.org/10.3390/microorganisms7030065
APA StyleSwaggerty, C. L., Callaway, T. R., Kogut, M. H., Piva, A., & Grilli, E. (2019). Modulation of the Immune Response to Improve Health and Reduce Foodborne Pathogens in Poultry. Microorganisms, 7(3), 65. https://doi.org/10.3390/microorganisms7030065







