Environmental Aspects of the Use of Hedera helix Extract in Bioremediation Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microorganisms
2.3. Ivy Extract Preparation and Characteristic
2.4. Effect of Ivy Extract on Metabolic Activity of the Cells
2.5. Biodegradability of the Ivy Extract
2.6. Impact of Ivy Extract on Microbial Cells
2.7. Toluene Derivative Biodegradation
2.8. Toxicity of Hydrocarbons in Presence of the Extract
2.9. Statistical Analysis
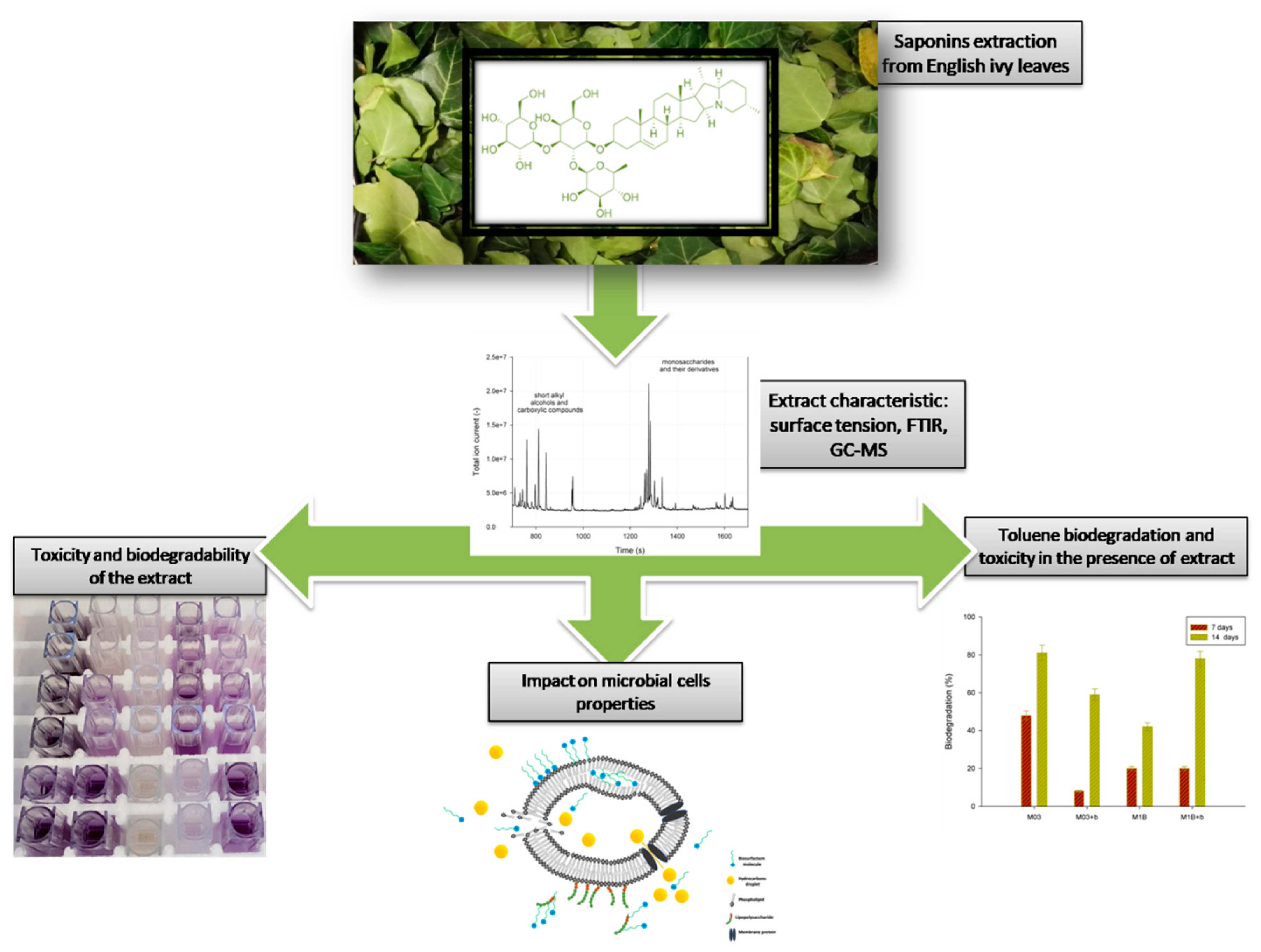
3. Results and Discussion
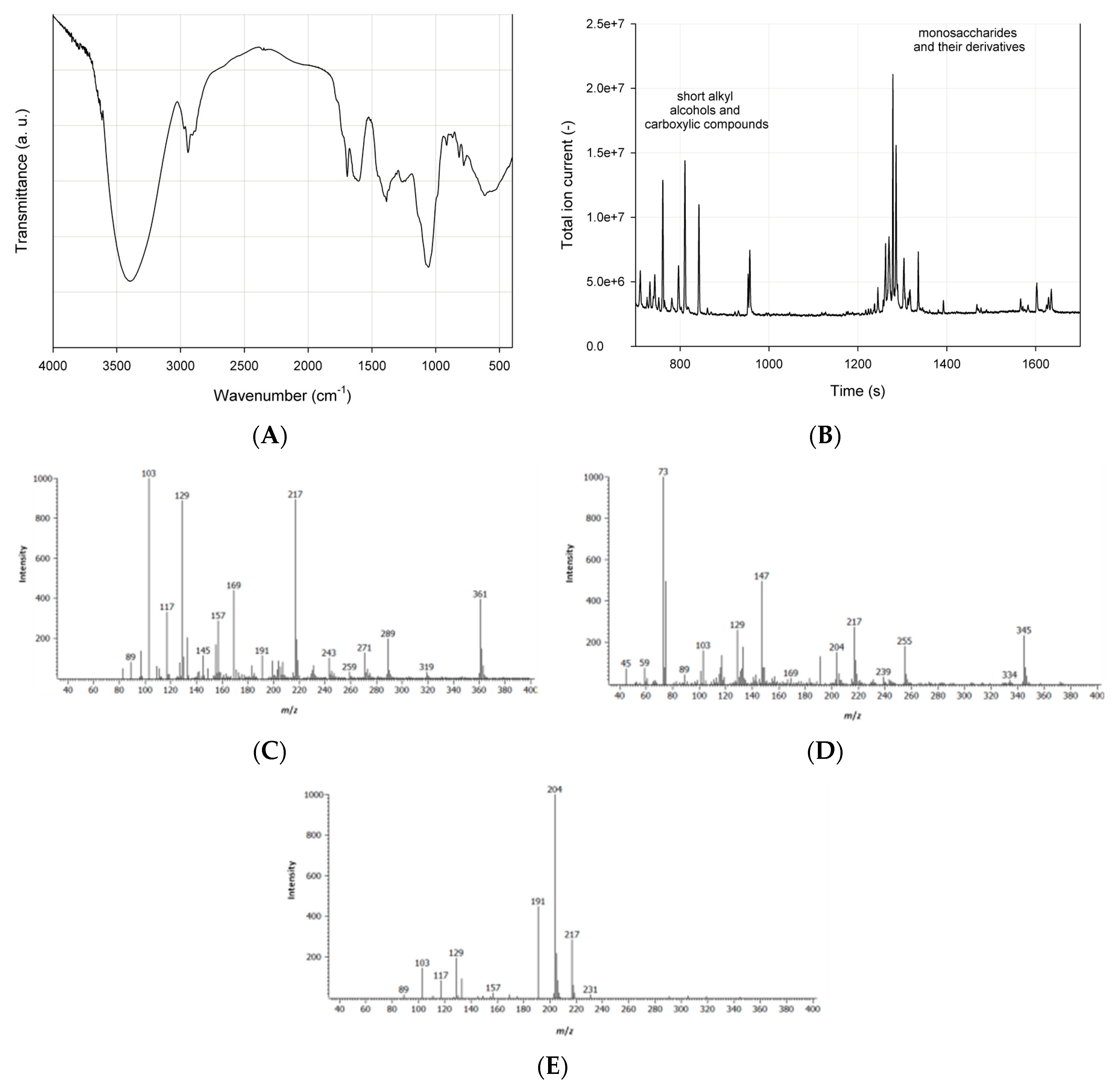
3.1. Saponins Extraction and Characteristic
3.2. Saponin Toxicity and Biodegradability
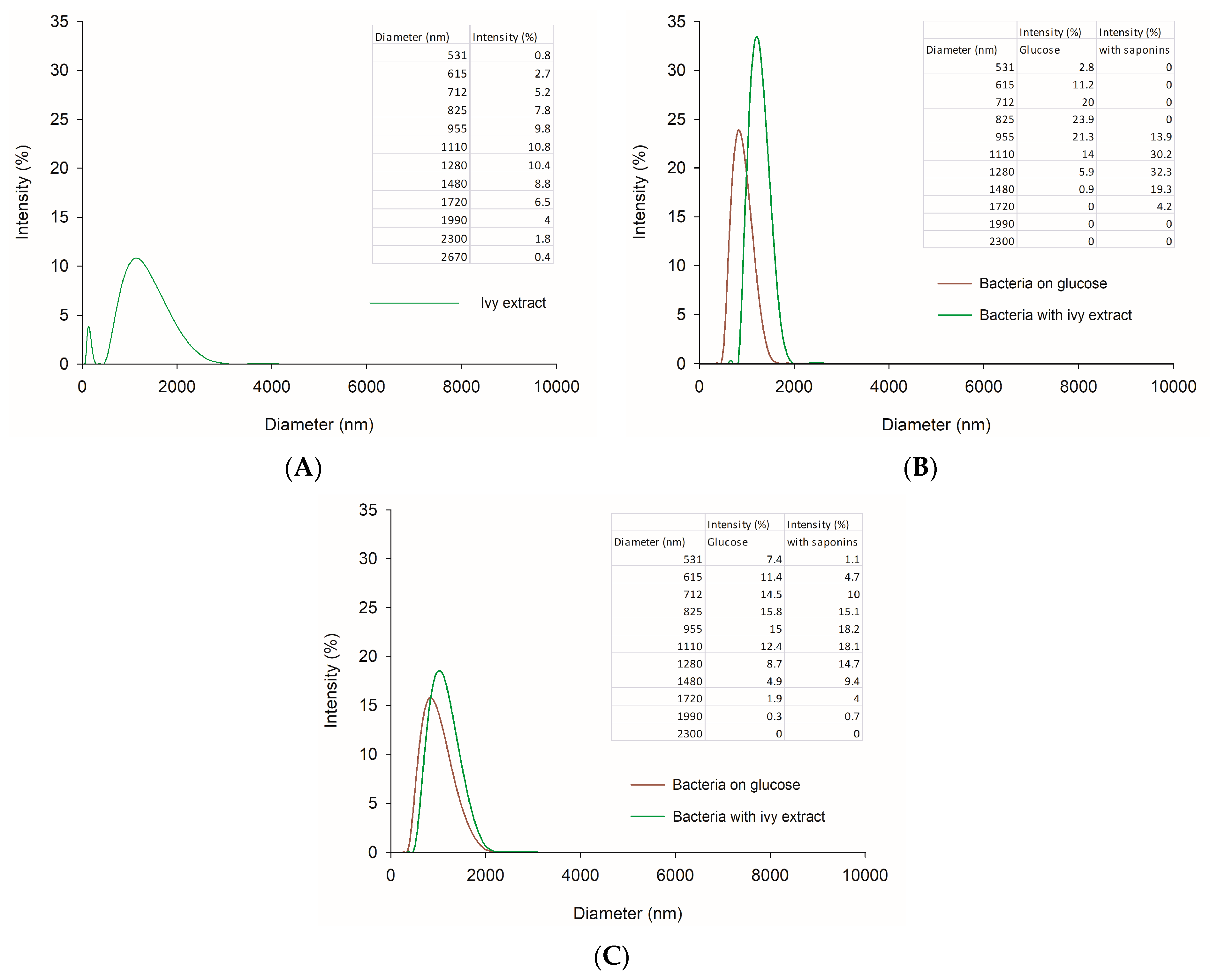
3.3. Impact of the H. helix Extract on Microbial Cells
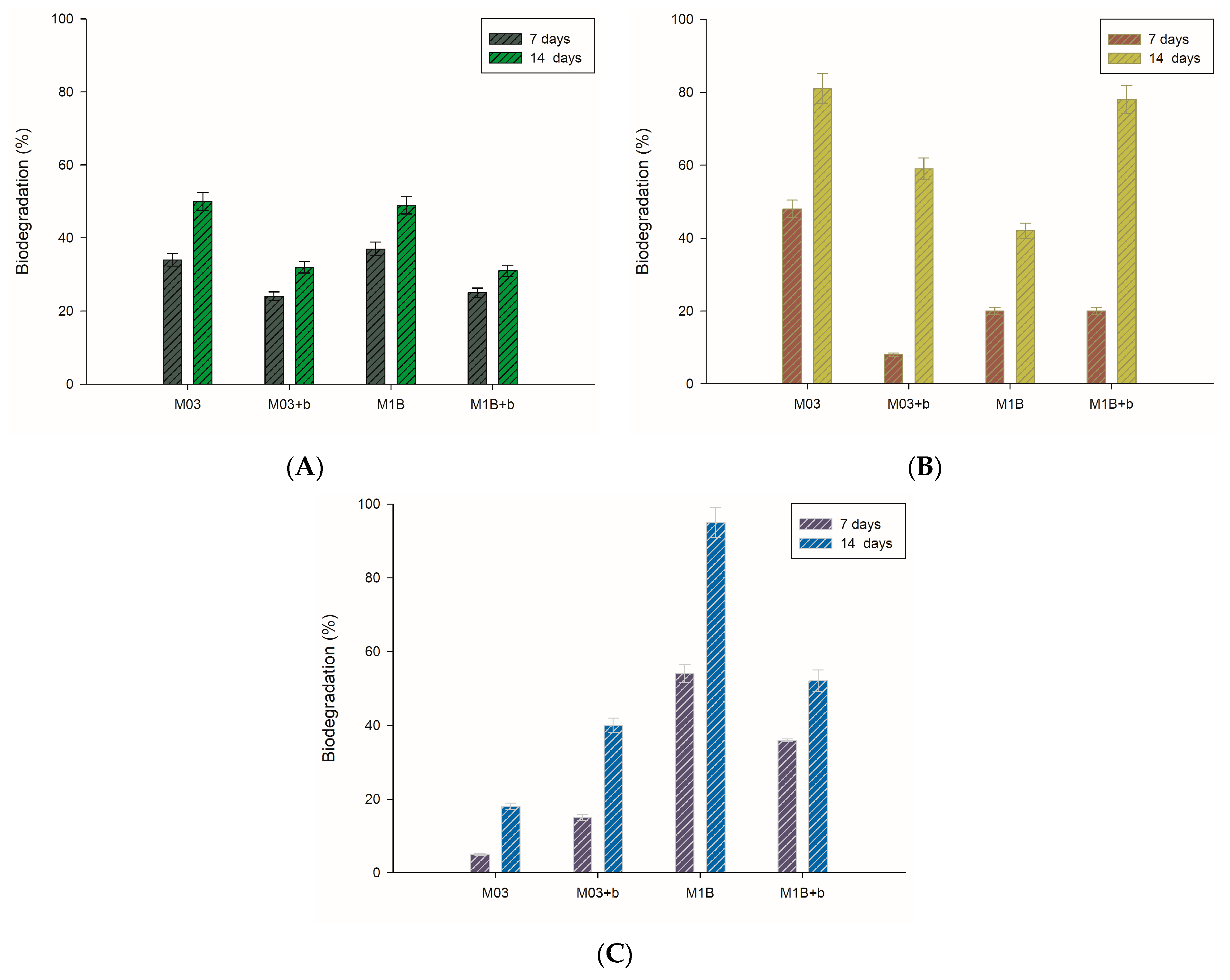
3.4. Biodegradation of Toluene Derivatives
3.5. Toxicity of Hydrocarbons in Presence of the Extract
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Montagnolli, R.N.; Lopes, P.R.M.; Cruz, J.M.; Claro, M.T.; Quiterio, G.M.; Bidoia, E.D. Metabolical shifts towards alternative BTEX biodegradation intermediates induced by perfluorinated compounds in firefighting foams. Chemosphere 2017, 173, 49–60. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Cheng, Z.; Yao, Y.; Chen, J. Biodegradation of benzene, toluene, ethylbenzene, and o-xylene by the bacterium Mycobacterium cosmeticum by f-4. Chemosphere 2013, 90, 1340–1347. [Google Scholar] [CrossRef]
- Heid, S.E.; Walker, M.K.; Swanson, H.I. Correlation of cardiotoxicity mediated by halogenated aromatic hydrocarbons to aryl hydrocarbon receptor activation. Toxicol. Sci. 2001, 61, 187–196. [Google Scholar] [CrossRef]
- Li, J.-L.; Chen, B.-H. Surfactant-mediated biodegradation of polycyclic aromatic hydrocarbons. Materials 2009, 2, 76–94. [Google Scholar] [CrossRef]
- Smułek, W.; Zdarta, A.; Łuczak, M.; Krawczyk, P.; Jesionowski, T.; Kaczorek, E. Sapindus saponins’ impact on hydrocarbon biodegradation by bacteria strains after short- and long-term contact with pollutant. Colloids Surf. B Biointerfaces 2016, 142, 207–213. [Google Scholar] [CrossRef]
- Manickam, N.; Bajaj, A.; Saini, H.S.; Shanker, R. Surfactant mediated enhanced biodegradation of hexachlorocyclohexane (HCH) isomers by Sphingomonas sp. NM05. Biodegradation 2012, 23, 673–682. [Google Scholar] [CrossRef]
- Kaczorek, E.; Moszyńska, S.; Olszanowski, A. Modification of cell surface properties of Pseudomonas alcaligenes S22 during hydrocarbon biodegradation. Biodegradation 2011, 22, 359–366. [Google Scholar] [CrossRef]
- Cui, X.; Mayer, P.; Gan, J. Methods to assess bioavailability of hydrophobic organic contaminants: Principles, operations, and limitations. Environ. Pollut. 2013, 172, 223–234. [Google Scholar] [CrossRef]
- Santos, D.; Rufino, R.; Luna, J.; Santos, V.; Sarubbo, L. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef]
- Mulligan, C.N. Recent advances in the environmental applications of biosurfactants. Curr. Opin. Colloid Interface Sci. 2009, 14, 372–378. [Google Scholar] [CrossRef]
- Pacwa-Plociniczak, M.; Plaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar] [CrossRef]
- Moulin-Traffort, J.; Favel, A.; Elias, R.; Regli, P. Study of the action of alpha-hederin on the ultrastructure of Candida albicans. Mycoses 1998, 41, 411–416. [Google Scholar] [CrossRef]
- Cioacá, C.; Margineanu, C.; Cucu, V. The saponins of Hedera helix with antibacterial activity. Die Pharm. 1978, 33, 609–610. [Google Scholar]
- Süleyman, H.; Mshvildadze, V.; Gepdiremen, A.; Elias, R. Acute and chronic antiinflammatory profile of the ivy plant, Hedera helix, in rats. Phytomedicine 2003, 10, 370–374. [Google Scholar] [CrossRef]
- Majester-Savornin, B.; Elias, R.; Diaz-Lanza, A.; Balansard, G.; Gasquet, M.; Delmas, F. Saponins of the ivy plant, Hedera helix, and their leishmanicidic activity. Planta Med. 1991, 57, 260–262. [Google Scholar] [CrossRef]
- Delmas, F.; Di Giorgio, C.; Elias, R.; Gasquet, M.; Azas, N.; Mshvildadze, V.; Dekanosidze, G.; Kemertelidze, E.; Timon-David, P. Antileishmanial activity of three saponins isolated from ivy, α-hederin, β-hederin and hederacolchiside A 1, as compared to their action on mammalian cells cultured in vitro. Planta Med. 2000, 66, 343–347. [Google Scholar] [CrossRef]
- Cheng, L.; Xia, T.-S.; Shi, L.; Xu, L.; Chen, W.; Zhu, Y.; Ding, Q. D Rhamnose β-hederin inhibits migration and invasion of human breast cancer cell line MDA-MB-231. Biochem. Biophys. Res. Commun. 2018, 495, 775–780. [Google Scholar] [CrossRef]
- Dudášová, H.; Lukáčová, L.; Murínová, S.; Dercová, K. Effects of plant terpenes on biodegradation of polychlorinated biphenyls (PCBs). Int.Biodeterior. Biodegrad. 2012, 69, 23–27. [Google Scholar] [CrossRef]
- Murínová, S.; Dercová, K. Potential use of newly isolated bacterial strain Ochrobactrum anthropi in bioremediation of polychlorinated biphenyls. Water Air Soil Pollut. 2014, 225, 1980. [Google Scholar] [CrossRef]
- Baharfar, R.; Rahmani, Z.; Mohseni, M.; Azimi, R. Evaluation of the antioxidant and antibacterial properties of ethanol extracts from berries, leaves and stems of Hedera pastuchovii Woron. ex Grossh. Nat. Prod. Res. 2015, 29, 2145–2148. [Google Scholar] [CrossRef]
- Dobslaw, D.; Engesser, K.-H. Degradation of 2-chlorotoluene by Rhodococcus sp. OCT 10. Appl. Microbiol. Biotechnol. 2012, 93, 2205–2214. [Google Scholar] [CrossRef]
- Smułek, W.; Zdarta, A.; Kwiczak, J.; Zgoła-Grześkowiak, A.; Cybulski, Z.; Kaczorek, E. Environmental biodegradation of halophenols by activated sludge from two different sewage treatment plants. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 2017, 52, 1240–1246. [Google Scholar] [CrossRef]
- Jarzebski, M.; Smulek, W.; Kościński, M.; Bialopiotrowicz, T.; Kaczorek, E. Verbascum nigrum L. (mullein) extract as a natural emulsifier. Food Hydrocolloids 2018, 81, 341–350. [Google Scholar] [CrossRef]
- Walencka, E.; Rózalska, S.; Sadowska, B.; Rózalska, B. The influence of Lactobacillus acidophilus-derived surfactants on staphylococcal adhesion and biofilm formation. Folia Microbiol. 2008, 53, 61–66. [Google Scholar] [CrossRef]
- Hiai, S.; Oura, H.; Nakajima, T. Color reaction of some sapogenins and saponins with vanilin and sulfuric acid. Planta Med. 1976, 29, 116–122. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, L.; Li, F. Influences and mechanisms of surfactants on pyrene biodegradation based on interactions of surfactant with a Klebsiella oxytoca strain. Bioresour. Technol. 2013, 142, 454–461. [Google Scholar] [CrossRef]
- Delgado, A.V.; González-Caballero, F.; Hunter, R.J.; Koopal, L.K.; Lyklema, J. Measurement and Interpretation of Electrokinetic Phenomena (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 1753–1805. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Krysztafkiewicz, A.; Jesionowski, T. Effect of inorganic oxides treatment on the titanium dioxide surface properties. Physicochem.Probl. Miner. Process. 2008, 42, 141–152. [Google Scholar]
- Liu, Z.; Li, Z.; Zhong, H.; Zeng, G.; Liang, Y.; Chen, M.; Wu, Z.; Zhou, Y.; Yu, M.; Shao, B. Recent advances in the environmental applications of biosurfactant saponins: A review. J. Environ. Chem. Eng. 2017, 5, 6030–6038. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Hashim, M.A.; Sahu, J.N.; Yusoff, I.; Gupta, B. Sen Comparison of a plant based natural surfactant with SDS for washing of As(V) from Fe rich soil. J. Environ. Sci. 2013, 25, 2247–2256. [Google Scholar] [CrossRef]
- Otzen, D.E. Biosurfactants and surfactants interacting with membranes and proteins: Same but different? Biochim. Biophys. Acta (BBA)–Biomembranes 2017, 1859, 639–649. [Google Scholar] [CrossRef]
- Kareru, P.G.; Keriko, J.M.; Gachanja, A.N.; Kenji, G.M. Direct detection of triterpenoid saponins in medicinal plants. Afr. J. Tradit. Complement. Altern. Med. 2007, 5, 56–60. [Google Scholar] [CrossRef]
- Murakami, T.; Hirano, K.; Yoshikawa, M. Medicinal foodstuffs. XXIII. Structures of new oleanane-type triterpene oligoglycosides, basellasaponins A, B, C, and D, from the fresh aerial parts of Basella rubra L. Chem. Pharm. Bull. 2001, 49, 776–779. [Google Scholar] [CrossRef]
- Da Silva, B.P.; de Sousa, A.C.; Silva, G.M.; Mendes, T.P.; Parente, J.P. A new bioactive steroidal saponin from Agave attenuata. Z. Naturforschung. C J. Biosci. 2002, 57, 423–428. [Google Scholar] [CrossRef]
- Jiang, X.; Hansen, H.C.B.; Strobel, B.W.; Cedergreen, N. What is the aquatic toxicity of saponin-rich plant extracts used as biopesticides? Environ. Pollut. 2018, 236, 416–424. [Google Scholar] [CrossRef]
- Hirata, Y.; Ryu, M.; Oda, Y.; Igarashi, K.; Nagatsuka, A.; Furuta, T.; Sugiura, M. Novel characteristics of sophorolipids, yeast glycolipid biosurfactants, as biodegradable low-foaming surfactants. J. Biosci. Bioeng. 2009, 108, 142–146. [Google Scholar] [CrossRef]
- Mølgaard, P.; Chihaka, A.; Lemmich, E.; Furu, P.; Windberg, C.; Ingerslev, F.; Halling-Sørensen, B. Biodegradability of the molluscicidal saponins of Phytolacca dodecandra. Regul. Toxicol. Pharmacol. 2000, 32, 248–255. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Y.; Wei, Y.; Liu, J.; Lu, L.; Peng, K. Saponin-enhanced biomass accumulation and demulsification capability of the demulsifying bacteria Alcaligenes sp. S-XJ-1. RSC Adv. 2016, 6, 44758–44765. [Google Scholar] [CrossRef]
- Roshtkhari, S.J.; Mulligan, C.N. Application of microbial culture and rhamnolipid for improving the sedimentation of oil sand tailings. J. Bioremediat.Biodegrad. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Woertz, J.R.; Kinney, K.A. Influence of sodium dodecyl sulfate and Tween 20 on fungal growth and toluene degradation in a vapor-phase bioreactor. J. Environ. Eng. 2004, 130, 292–299. [Google Scholar] [CrossRef]
- Pacholak, A.; Smułek, W.; Jesionowski, T.; Kaczorek, E. The ability of Achromobacter sp. KW1 strain to biodegrade isomers of chlorotoluene. J. Chem. Technol. Biotechnol. 2017, 92, 2134–2141. [Google Scholar] [CrossRef]
- Santos, I.C.; Martin, M.S.; Carlton, D.D.; Amorim, C.L.; Castro, P.M.L.; Hildenbrand, Z.L.; Schug, K.A. MALDI-TOF MS for the Identification of Cultivable Organic-Degrading Bacteria in Contaminated Groundwater near Unconventional Natural Gas Extraction Sites. Microorganisms 2017, 5, 47. [Google Scholar] [CrossRef]
- Tian, W.; Yao, J.; Liu, R.; Zhu, M.; Wang, F.; Wu, X.; Liu, H. Effect of natural and synthetic surfactants on crude oil biodegradation by indigenous strains. Ecotoxicol. Environ. Saf. 2016, 121, 171–179. [Google Scholar] [CrossRef]
- Singh, A.; Van Hamme, J.D.; Ward, O.P. Surfactants in microbiology and biotechnology: Part 2. Application aspects. Biotechnol. Adv. 2007, 25, 99–121. [Google Scholar] [CrossRef]
- Nahar, N.; Alauddin, M.; Quilty, B. Toxic effects of toluene on the growth of activated sludge bacteria. World J. Microbiol. Biotechnol. 2000, 16, 307–311. [Google Scholar] [CrossRef]
- Segura, A.; Hurtado, A.; Rivera, B.; Lazaroaie, M.M. Isolation of new toluene-tolerant marine strains of bacteria and characterization of their solvent-tolerance properties. J. Appl. Microbiol. 2008, 104, 1408–1416. [Google Scholar] [CrossRef]
| Metabolic Activity (%) | Saponin Biodegradability after 27 Days (%) | ||
|---|---|---|---|
| Control | With Ivy Extract | ||
| M03 | 100.0 ± 2.4 | 160 ± 8.0 | 5.0 ± 0.3 |
| M1B | 100.0 ± 3.1 | 142.0 ± 7.1 | 31.0 ± 1.6 |
| CSH [%] | MP [μmol∙L−1min−1] | Z [mV] | ||||
|---|---|---|---|---|---|---|
| Control | With Ivy Extract | Control | With Ivy Extract | Control | With Ivy Extract | |
| M03 | 17.7 ± 0.9 | 7.2 ± 0.4 | 15.6 ± 0.8 | 12.1 ± 0.6 | −18.6 ± 0.9 | −14.7 ± 0.7 |
| M1B | 3.3 ± 0.2 | 4.7 ± 0.2 | 8.3 ± 0.4 | 6.5 ± 0.3 | −11.5 ± 0.6 | −16.1 ± 0.8 |
| Metabolic Activity (%) | ||||||
|---|---|---|---|---|---|---|
| α,α,α-Trifluorotoluene | 4-Chlorotoluene | Toluene | ||||
| Control | With Ivy Extract | Control | With Ivy Extract | Control | With Ivy Extract | |
| M03 | 98.0 ± 5.2 | 100.4 ± 5.1 | 104.1 ± 5.3 | 101.2 ± 5.1 | 102.3 ± 5.0 | 100.0 ± 5.2 |
| M1B | 51.1 ± 3.3 | 114.0 ± 6.2 | 61.2 ± 3.0 | 113.4 ± 6.1 | 63.3 ± 3.2 | 140.1 ± 7.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdarta, A.; Smułek, W.; Pacholak, A.; Kaczorek, E. Environmental Aspects of the Use of Hedera helix Extract in Bioremediation Process. Microorganisms 2019, 7, 43. https://doi.org/10.3390/microorganisms7020043
Zdarta A, Smułek W, Pacholak A, Kaczorek E. Environmental Aspects of the Use of Hedera helix Extract in Bioremediation Process. Microorganisms. 2019; 7(2):43. https://doi.org/10.3390/microorganisms7020043
Chicago/Turabian StyleZdarta, Agata, Wojciech Smułek, Amanda Pacholak, and Ewa Kaczorek. 2019. "Environmental Aspects of the Use of Hedera helix Extract in Bioremediation Process" Microorganisms 7, no. 2: 43. https://doi.org/10.3390/microorganisms7020043
APA StyleZdarta, A., Smułek, W., Pacholak, A., & Kaczorek, E. (2019). Environmental Aspects of the Use of Hedera helix Extract in Bioremediation Process. Microorganisms, 7(2), 43. https://doi.org/10.3390/microorganisms7020043








