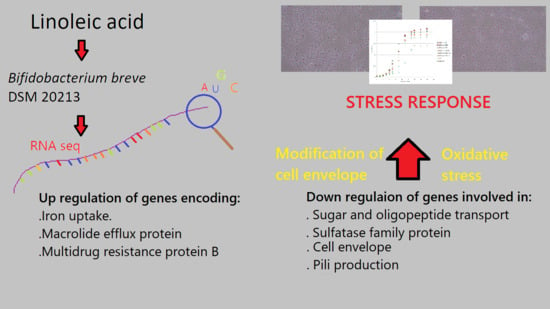
Effects of Linoleic Acid on Gut-Derived Bifidobacterium breve DSM 20213: A Transcriptomic Approach
Abstract
1. Introduction
2. Material and Methods
2.1. RNA Extraction and Sequencing
2.2. Real-Time Quantitative PCR Analysis
2.3. Chemicals, Strain, and Culture Conditions
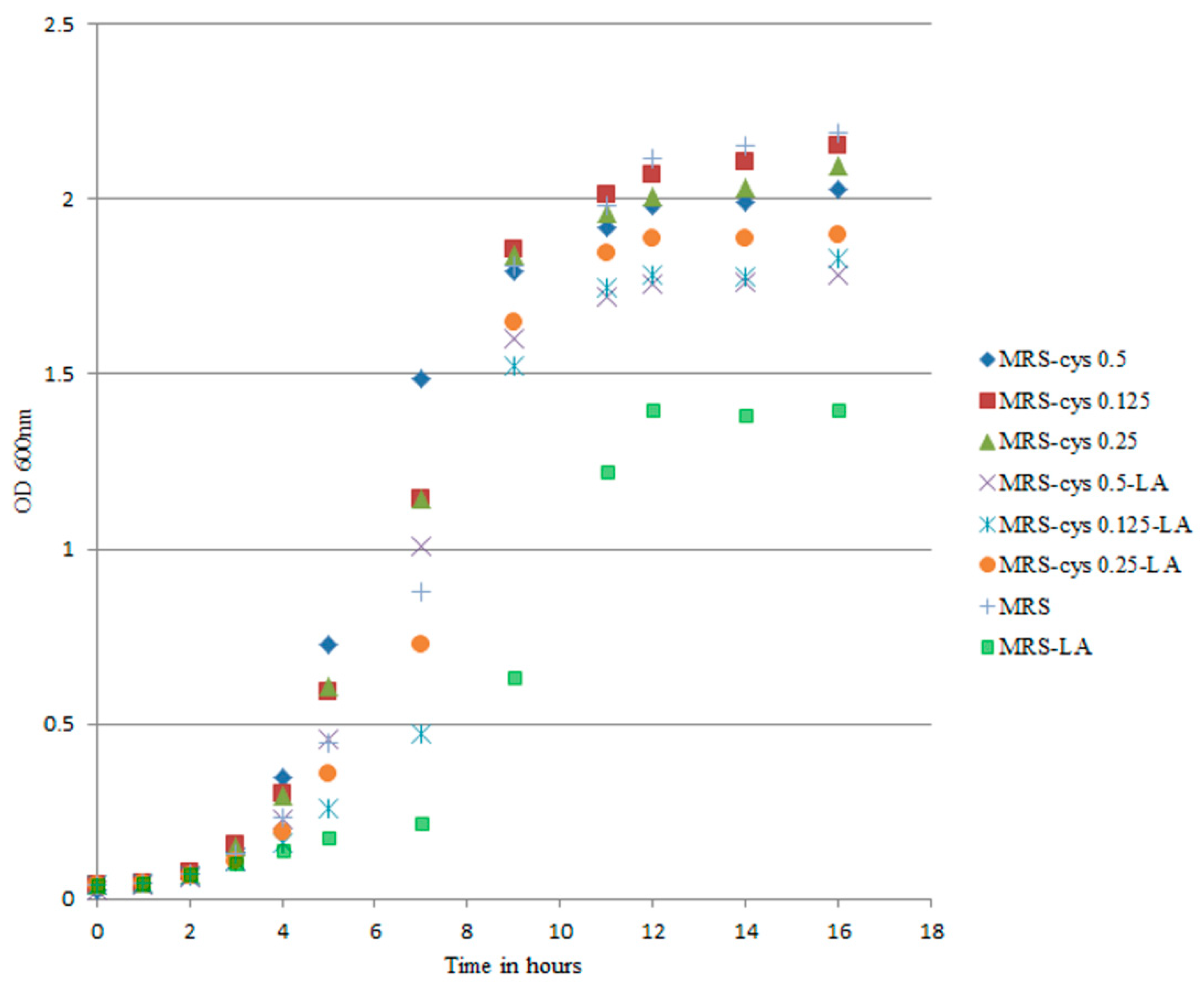
2.4. Growth of B. breve DSM 20213 at Different Cysteine Concentrations
2.5. Growth of B. breve DSM 20213 in Iron Salts-Supplemented Medium
2.6. Thiobarbituric Acid Reactive Substance (TBARS) Assay
2.7. Assessment of the Ability of LA to Chelate Fe2+
2.8. Extraction of Cell-Associated Lipids and Gas Chromatography (GC) Analysis
3. Results
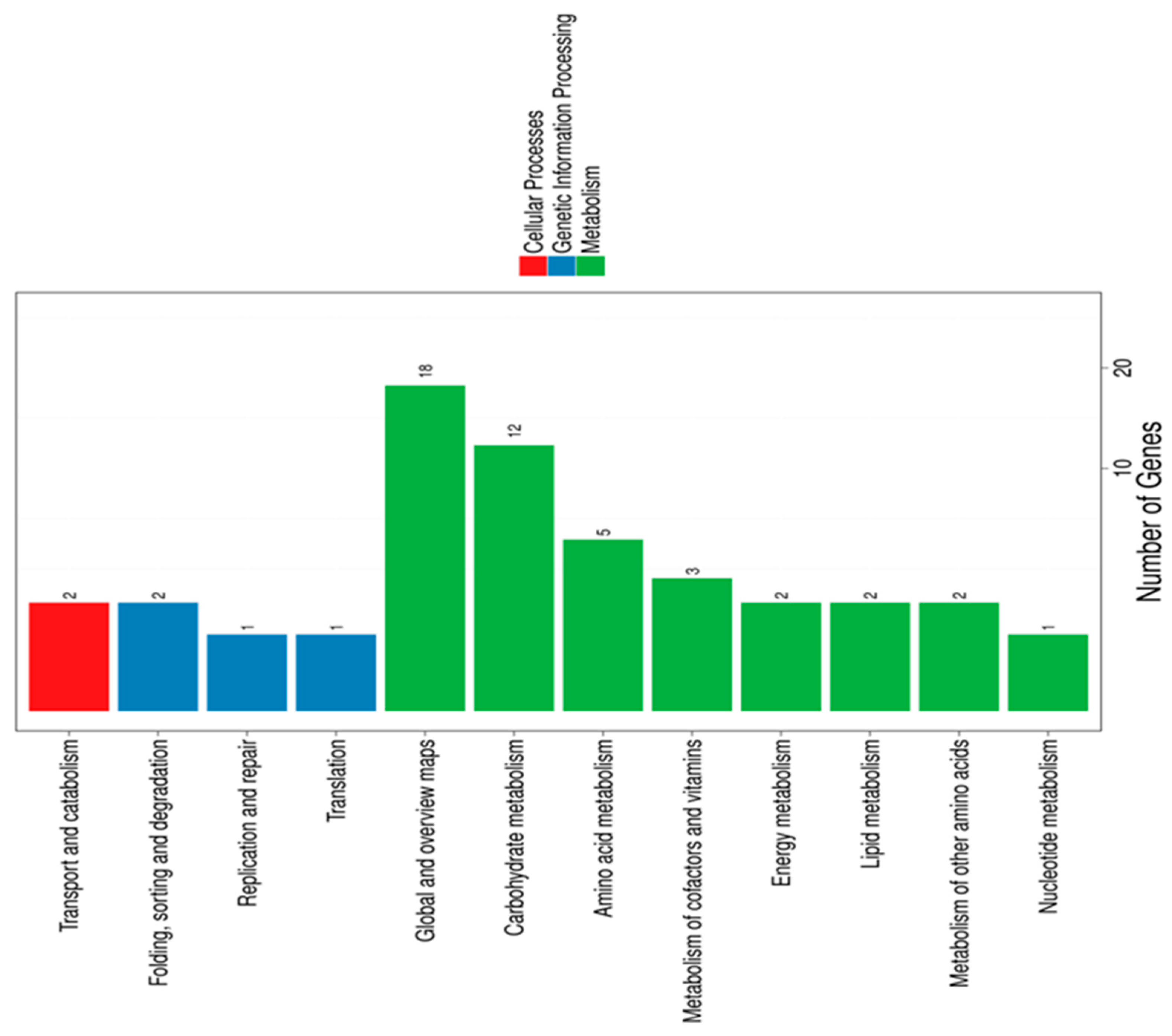
3.1. Gene Expression of B. breve DSM20213 in Response to LA Exposure
3.2. LA Effect on Iron Metabolism
3.3. LA Effect on ROS Production
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Delplanque, B.; Gibson, R.; Koletzko, B.; Lapillonne, A.; Strandvik, B. Lipid Quality in Infant Nutrition. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 1. [Google Scholar] [CrossRef]
- Gorissen, L.; Raes, K.; Weckx, S.; Dannenberger, D.; Leroy, F.; De Vuyst, L.; De Smet, S. Production of conjugated linoleic acid and conjugated linolenic acid isomers by Bifidobacterium species. Appl. Microbiol. Biotechnol. 2010, 87, 2257–2266. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Gu, Z.; Tian, F.; Ross, R.P.; Stanton, C.; Chen, Y.Q.; Chen, W.; Zhang, H. Synthesis of conjugated linoleic acid by the linoleate isomerase complex in food-derived lactobacilli. J. Appl. Microbiol. 2014, 117, 430–439. [Google Scholar] [CrossRef]
- Lim, J.N.; Oh, J.J.; Wang, T.; Lee, J.S.; Kim, S.H.; Kim, Y.J.; Lee, H.G. Trans−11 18:1 vaccenic acid (TVA) has a direct anti-carcinogenic effect on MCF−7 human mammary adenocarcinoma cells. Nutrients 2014, 6, 627–636. [Google Scholar] [CrossRef]
- Devillard, E.; McIntosh, F.M.; Duncan, S.H.; Wallace, R.J. Metabolism of Linoleic Acid by Human Gut Bacteria: Different Routes for Biosynthesis of Conjugated Linoleic Acid. J. Bacteriol. 2007, 189, 2566. [Google Scholar] [CrossRef]
- Raimondi, S.; Amaretti, A.; Leonardi, A.; Quartieri, A.; Gozzoli, C.; Rossi, M. Conjugated Linoleic Acid Production by Bifidobacteria: Screening, Kinetic, and Composition. Biomed Res. Int. 2016, 2016, 8654317. [Google Scholar] [CrossRef]
- O’Connell, K.J.; Motherway, M.O.; Hennessey, A.A.; Brodhun, F.; Ross, R.P.; Feussner, I.; Stanton, C.; Fitzgerald, G.F.; van Sinderen, D. Identification and characterization of an oleate hydratase-encoding gene from Bifidobacterium breve. Bioengineered 2013, 4, 313–321. [Google Scholar] [CrossRef]
- Rosberg-Cody, E.; Liavonchanka, A.; Göbel, C.; Ross, R.P.; O’Sullivan, O.; Fitzgerald, G.F.; Feussner, I.; Stanton, C. Myosin-cross-reactive antigen (MCRA) protein from Bifidobacterium breve is a FAD-dependent fatty acid hydratase which has a function in stress protection. BMC Biochem. 2011, 12, 9. [Google Scholar] [CrossRef]
- Koppová, I.; Lukáš, F.; Kopečný, J. Effect of fatty acids on growth of conjugated-linoleic-acids-producing bacteria in rumen. Folia Microbiol. 2006, 51, 291–293. [Google Scholar] [CrossRef]
- Fontes, A.L.; Pimentel, L.; Rodríguez-Alcalá, L.M.; Gomes, A. Effect of Pufa Substrates on Fatty Acid Profile of Bifidobacterium breve Ncimb 702258 and CLA/CLNA Production in Commercial Semi-Skimmed Milk. Sci. Rep. 2018, 8, 15591. [Google Scholar] [CrossRef]
- Coakley, M.; Ross, R.P.; Nordgren, M.; Fitzgerald, G.; Devery, R.; Stanton, C. Conjugated linoleic acid biosynthesis by human-derived Bifidobacterium species. J. Appl. Microbiol. 2003, 94, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Bottacini, F.; Zomer, A.; Milani, C.; Ferrario, C.; Lugli, G.A.; Egan, M.; Ventura, M.; van Sinderen, D. Global transcriptional landscape and promoter mapping of the gut commensal Bifidobacterium breve UCC2003. BMC Genomics 2017, 18, 991. [Google Scholar] [CrossRef]
- Analysis of malondialdehyde, chlorophyll proline, soluble sugar, and glutathione content in Arabidopsis seedlin-BOC Sciences. Available online: https://www.bocsci.com/publication/192 (accessed on 3 October 2019).
- Lin, X.; Li, J.; Ma, S.; Liu, G.; Yang, K.; Tong, M.; Lin, D. Toxicity of TiO2 Nanoparticles to Escherichia coli: Effects of Particle Size, Crystal Phase and Water Chemistry. PLoS ONE 2014, 9, e110247. [Google Scholar] [CrossRef]
- Carter, P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal. Biochem. 1971, 40, 450–458. [Google Scholar] [CrossRef]
- Santos, J.S.; Alvarenga Brizola, V.R.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef]
- Van Nieuwenhove, C.P.; Oliszewski, R.; González, S.N.; Pérez Chaia, A.B. Conjugated linoleic acid conversion by dairy bacteria cultured in MRS broth and buffalo milk. Lett. Appl. Microbiol. 2007, 44, 467–474. [Google Scholar] [CrossRef]
- Lanigan, N.; Bottacini, F.; Casey, P.G.; O’Connell Motherway, M.; van Sinderen, D. Genome-Wide Search for Genes Required for Bifidobacterial Growth under Iron-Limitation. Front. Microbiol. 2017, 8, 964. [Google Scholar] [CrossRef]
- Yeom, J.; Jeon, C.O.; Madsen, E.L.; Park, W. Ferredoxin-NADP+ Reductase from Pseudomonas putida Functions as a Ferric Reductase. J. Bacteriol. 2009, 191, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.; Zomer, A.; O’Connell-Motherway, M.; van Sinderen, D.; Margolles, A. Discovering Novel Bile Protection Systems in Bifidobacterium breve UCC2003 through Functional Genomics. Appl. Environ. Microbiol. 2012, 78, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Averina, O.V.; Zakharevich, N.V.; Danilenko, V.N. Identification and characterization of WhiB-like family proteins of the Bifidobacterium genus. Anaerobe 2012, 18, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Zomer, A.; van Sinderen, D. Intertwinement of stress response regulons in Bifidobacterium breve UCC2003. Gut Microbes 2010, 1, 100–102. [Google Scholar] [CrossRef]
- Lee, E.-M.; Ahn, S.-H.; Park, J.-H.; Lee, J.-H.; Ahn, S.-C.; Kong, I.-S. Identification of oligopeptide permease (opp) gene cluster in Vibrio fluvialis and characterization of biofilm production by oppA knockout mutation. FEMS Microbiol. Lett. 2004, 240, 21–30. [Google Scholar] [CrossRef]
- An, H.; Douillard, F.P.; Wang, G.; Zhai, Z.; Yang, J.; Song, S.; Cui, J.; Ren, F.; Luo, Y.; Zhang, B.; et al. Integrated Transcriptomic and Proteomic Analysis of the Bile Stress Response in a Centenarian-originated Probiotic Bifidobacterium longum BBMN68. Mol. Cell. Proteomics 2014, 13, 2558–2572. [Google Scholar] [CrossRef]
- Ruiz, L.; Sànchez, B.; Ruas-Madiedo, P.; de los Reyes-GavilÃàn, C.G.; Margolles, A. Cell envelope changes in Bifidobacterium animalis ssp. lactis as a response to bile. FEMS Microbiol. Lett. 2007, 274, 316–322. [Google Scholar] [CrossRef]
- Roy, H. Tuning the properties of the bacterial membrane with aminoacylated phosphatidylglycerol. IUBMB Life 2009, 61, 940–953. [Google Scholar] [CrossRef]
- O’Connell Motherway, M.; Zomer, A.; Leahy, S.C.; Reunanen, J.; Bottacini, F.; Claesson, M.J.; O’Brien, F.; Flynn, K.; Casey, P.G.; Moreno Munoz, J.A.; et al. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc. Natl. Acad. Sci. USA 2011, 108, 11217–11222. [Google Scholar] [CrossRef]
- Westermann, C.; Gleinser, M.; Corr, S.C.; Riedel, C.U. A Critical Evaluation of Bifidobacterial Adhesion to the Host Tissue. Front. Microbiol. 2016, 7, 1220. [Google Scholar] [CrossRef]
- Zuo, F.; Yu, R.; Xiao, M.; Khaskheli, G.B.; Sun, X.; Ma, H.; Ren, F.; Zhang, B.; Chen, S. Transcriptomic analysis of Bifidobacterium longum subsp. longum BBMN68 in response to oxidative shock. Sci. Rep. 2018, 8, 17085. [Google Scholar] [CrossRef] [PubMed]
- Muto, M.; Abe, F.; Yaeshima, T.; Iwatsuki, K. Effect of Enumeration Method on Bifidobacterium Cell Counts in Commercial Powder Products. Biosci. Microflora 2010, 29, 143–148. [Google Scholar] [CrossRef]
- Turroni, F.; Milani, C.; Duranti, S.; Ferrario, C.; Lugli, G.A.; Mancabelli, L.; van Sinderen, D.; Ventura, M. Bifidobacteria and the infant gut: An example of co-evolution and natural selection. Cell. Mol. Life Sci. 2018, 75, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Lugli, G.A.; Duranti, S.; Turroni, F.; Mancabelli, L.; Ferrario, C.; Mangifesta, M.; Hevia, A.; Viappiani, A.; Scholz, M.; et al. Bifidobacteria exhibit social behavior through carbohydrate resource sharing in the gut. Sci. Rep. 2015, 5, 15782. [Google Scholar] [CrossRef]
- Gotoh, A.; Katoh, T.; Sakanaka, M.; Ling, Y.; Yamada, C.; Asakuma, S.; Urashima, T.; Tomabechi, Y.; Katayama-Ikegami, A.; Kurihara, S.; et al. Sharing of human milk oligosaccharides degradants within bifidobacterial communities in faecal cultures supplemented with Bifidobacterium bifidum. Sci. Rep. 2018, 8, 13958. [Google Scholar] [CrossRef]
- Matsuki, T.; Yahagi, K.; Mori, H.; Matsumoto, H.; Hara, T.; Tajima, S.; Ogawa, E.; Kodama, H.; Yamamoto, K.; Yamada, T.; et al. A key genetic factor for fucosyllactose utilization affects infant gut microbiota development. Nat. Commun. 2016, 7, 11939. [Google Scholar] [CrossRef]
- Urashima, T.; Asakuma, S.; Leo, F.; Fukuda, K.; Messer, M.; Oftedal, O.T. The Predominance of Type I Oligosaccharides Is a Feature Specific to Human Breast Milk. Adv. Nutr. 2012, 3, 473S–482S. [Google Scholar] [CrossRef]
- Bozzi Cionci, N.; Baffoni, L.; Gaggìa, F.; Di Gioia, D. Therapeutic Microbiology: The Role of Bifidobacterium breve as Food Supplement for the Prevention/Treatment of Paediatric Diseases. Nutrients 2018, 10, 1723. [Google Scholar] [CrossRef]
- Underwood, M.A.; Davis, J.C.C.; Kalanetra, K.M.; Gehlot, S.; Patole, S.; Tancredi, D.J.; Mills, D.A.; Lebrilla, C.B.; Simmer, K. Digestion of Human Milk Oligosaccharides by Bifidobacterium breve in the Premature Infant. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 449–455. [Google Scholar] [CrossRef]
- Patole, S.; Keil, A.D.; Chang, A.; Nathan, E.; Doherty, D.; Simmer, K.; Esvaran, M.; Conway, P. Effect of Bifidobacterium breve M−16V Supplementation on Fecal Bifidobacteria in Preterm Neonates-A Randomised Double Blind Placebo Controlled Trial. PLoS ONE 2014, 9, e89511. [Google Scholar] [CrossRef]
- Li, Y.; Shimizu, T.; Hosaka, A.; Kaneko, N.; Ohtsuka, Y.; Yamashiro, Y. Effects of Bifidobacterium breve supplementation on intestinal flora of low birth weight infants. Pediatr. Int. 2004, 46, 509–515. [Google Scholar] [CrossRef]
- Abrahamse-Berkeveld, M.; Alles, M.; Franke-Beckmann, E.; Helm, K.; Knecht, R.; Köllges, R.; Sandner, B.; Knol, J.; Ben Amor, K.; Bufe, A. Infant formula containing galacto-and fructo-oligosaccharides and Bifidobacterium breve M−16V supports adequate growth and tolerance in healthy infants in a randomised, controlled, double-blind, prospective, multicentre study. J. Nutr. Sci. 2016, 5, e42. [Google Scholar] [CrossRef] [PubMed]
- Aloisio, I.; Prodam, F.; Giglione, E.; Bozzi Cionci, N.; Solito, A.; Bellone, S.; Baffoni, L.; Mogna, L.; Pane, M.; Bona, G.; et al. Three-Month Feeding Integration With Bifidobacterium Strains Prevents Gastrointestinal Symptoms in Healthy Newborns. Front. Nutr. 2018, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, M.A.; Araújo, W.M.C.; Borgo, L.A.; Alencar, E.d.R. Lipid profile of different infant formulas for infants. PLoS ONE 2017, 12, e0177812. [Google Scholar] [CrossRef] [PubMed]
- Druart, C.; Bindels, L.B.; Schmaltz, R.; Neyrinck, A.M.; Cani, P.D.; Walter, J.; Ramer-Tait, A.E.; Delzenne, N.M. Ability of the gut microbiota to produce PUFA-derived bacterial metabolites: Proof of concept in germ-free versus conventionalized mice. Mol. Nutr. Food Res. 2015, 59, 1603–1613. [Google Scholar] [CrossRef]
- De Weirdt, R.; Coenen, E.; Vlaeminck, B.; Fievez, V.; Van den Abbeele, P.; Van de Wiele, T. A simulated mucus layer protects Lactobacillus reuteri from the inhibitory effects of linoleic acid. Benef. Microbes 2013, 4, 299–312. [Google Scholar] [CrossRef]
- Alonso, L.; Cuesta, E.P.; Gilliland, S.E. Production of Free Conjugated Linoleic Acid by Lactobacillus acidophilus and Lactobacillus casei of Human Intestinal Origin. J. Dairy Sci. 2003, 86, 1941–1946. [Google Scholar] [CrossRef]
- Yang, B.; Gao, H.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, Y.Q.; Chen, H.; Chen, W. Bacterial conjugated linoleic acid production and their applications. Prog. Lipid Res. 2017, 68, 26–36. [Google Scholar] [CrossRef]
- Boyaval, P.; Corre, C.; Dupuis, C.; Roussel, E. Effects of free fatty acids on propionic acid bacteria. Lait 1995, 75, 17–29. [Google Scholar] [CrossRef]
- Oberg, T.S.; Ward, R.E.; Steele, J.L.; Broadbent, J.R. Genetic and Physiological Responses of Bifidobacterium animalis subsp. lactis to Hydrogen Peroxide Stress. J. Bacteriol. 2013, 195, 3743–3751. [Google Scholar]
- Duranti, S.; Turroni, F.; Lugli, G.A.; Milani, C.; Viappiani, A.; Mangifesta, M.; Gioiosa, L.; Palanza, P.; van Sinderen, D.; Ventura, M. Genomic Characterization and Transcriptional Studies of the Starch-Utilizing Strain Bifidobacterium adolescentis 22L. Appl. Environ. Microbiol. 2014, 80, 6080–6090. [Google Scholar] [CrossRef] [PubMed]
- Kishino, S.; Park, S.B.; Takeuchi, M.; Yokozeki, K.; Shimizu, S.; Ogawa, J. Novel multi-component enzyme machinery in lactic acid bacteria catalyzing CC double bond migration useful for conjugated fatty acid synthesis. Biochem. Biophys. Res. Commun. 2011, 416, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yang, B.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, H.; Chen, W. Role of 10-hydroxy-cis−12-octadecenic acid in transforming linoleic acid into conjugated linoleic acid by bifidobacteria. Appl. Microbiol. Biotechnol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Anaya, J.; Hernández-Santoyo, A. Production of bioactive conjugated linoleic acid by the multifunctional enolase from Lactobacillus plantarum. Int. J. Biol. Macromol. 2016, 91, 524–535. [Google Scholar] [CrossRef]

| Gene (Locus Tag) | Primers Sequence (5′→3′) | Product Size (bp) | |
|---|---|---|---|
| Forward | Reverse | ||
| recF (BBRE_0834) | GCACGCATCAATTCAGGAAC | GTCTTCAGGAGTAAACGAAAC | 80 |
| Cystathionine beta-lyase (BBRE_1404) | TGACCAATCCACTGCTCAAG | GCCAACACACCACCCAAAC | 264 |
| Permease protein of ABC transporter system (BBRE_1006) | CGCAGCAGATGAATGAGGAG | CCACCCAGTGATGATTGAGG | 245 |
| ATP-binding Mrp-like protein (BBRE_1432) | ACCAACCAGATCAACGGTGC | GCGGCAGAGAGAATCCATAG | 294 |
| Thioredoxin reductase (BBRE_1224) | CATTAACCATTGGCGGCCTG | GCTGACCACATCGGCAATGA | 164 |
| Lex A (BBRE_0527) | CGACTCCACATCGACGAAC | GCGTTCATGCGAATAAAGCC | 133 |
| Secreted protein probably involved in iron uptake (BBRE_1003) | CGCCAAGAAGGACGATTCAG | TTCACGGTCAGGTCAGGAAC | 281 |
| Ferredoxin--NADP reductase (BBRE_0908) | GTGACTGAATCTGAGAACACT | GATATCGGATGAGTAGACGC | 77 |
| Locus Tag | Protein’s Predicted Function | FC |
|---|---|---|
| BBRE_1003 | Secreted protein, probably involved in iron uptake | 4 |
| BBRE_1002 | Membrane-spanning protein with iron permease FTR1 family domain | 3 |
| BBRE_1006 | Permease protein of ABC transporter system | 3 |
| BBRE_1007 | ATP-binding protein of ABC transporter system | 3 |
| BBRE_1005 | Permease protein of ABC transporter system | 2 |
| BBRE_1004 | Membrane-spanning protein | 2 |
| BBRE_1008 | Hypothetical protein | 2 |
| BBRE_1612 | Hypothetical protein with methyl transferase domain | 2 |
| BBRE_1753 | ATP-binding protein of ABC transporter system | 4 |
| BBRE_0908 | Ferredoxin-NADP reductase | 2 |
| BBRE_1404 | Cystathionine beta-lyase metC | 3 |
| BBRE_1630 | Sulfatase family protein | −2 |
| BBRE_1618 | Macrolide-efflux protein, MFS member | 1 |
| BBRE_1621 | Multidrug resistance protein B, MFS member, bile efflux induced upon bile salt exposure | 2 |
| BBRE_1505 | WhiB protein, WblE | 3 |
| BBRE_0144 | Transcriptional regulator | 2 |
| BBRE_0799 | PTS system IIC component | −3 |
| BBRE_0967 | Oligopeptide transport system permease protein, oppB | −2 |
| BBRE_0966 | Oligopeptide-binding protein, oppA | −2 |
| BBRE_0806 | Ribonucleoside-diphosphate reductase alpha chain | −2 |
| BBRE_0137 | Acetyl-/propionyl-CoA carboxylase alpha chain | −2 |
| BBRE_0138 | Acetyl-/propionyl-CoA carboxylase beta chain | −2 |
| BBRE_0139 | Type I multifunctional fatty acid synthase | −2 |
| BBRE_0798 | Transcriptional regulator, GntR family | −2 |
| BBRE_0896 | Lysyl-cardiolipin synthase/Lysyl-transferase, mprF | 2 |
| BBRE_0949 | TadE-like protein | −3 |
| BBRE_0946 | TadA-like protein | −1 |
| BBRE_0948 | TadC-like protein | −1 |
| BBRE_0750 | Cell division protein, Fic | 2 |
| BBRE_0275 | Histidine kinase sensor of two-component system | −3 |
| BBRE_0440 | 60-kDa chaperonin, GroEL | −2 |
| BBRE_0182 | 10-kDa chaperonin, GroES | −2 |
| BBRE_1435 | ATP-binding Mrp-like protein | 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senizza, A.; Callegari, M.L.; Senizza, B.; Minuti, A.; Rocchetti, G.; Morelli, L.; Patrone, V. Effects of Linoleic Acid on Gut-Derived Bifidobacterium breve DSM 20213: A Transcriptomic Approach. Microorganisms 2019, 7, 710. https://doi.org/10.3390/microorganisms7120710
Senizza A, Callegari ML, Senizza B, Minuti A, Rocchetti G, Morelli L, Patrone V. Effects of Linoleic Acid on Gut-Derived Bifidobacterium breve DSM 20213: A Transcriptomic Approach. Microorganisms. 2019; 7(12):710. https://doi.org/10.3390/microorganisms7120710
Chicago/Turabian StyleSenizza, Alice, Maria Luisa Callegari, Biancamaria Senizza, Andrea Minuti, Gabriele Rocchetti, Lorenzo Morelli, and Vania Patrone. 2019. "Effects of Linoleic Acid on Gut-Derived Bifidobacterium breve DSM 20213: A Transcriptomic Approach" Microorganisms 7, no. 12: 710. https://doi.org/10.3390/microorganisms7120710
APA StyleSenizza, A., Callegari, M. L., Senizza, B., Minuti, A., Rocchetti, G., Morelli, L., & Patrone, V. (2019). Effects of Linoleic Acid on Gut-Derived Bifidobacterium breve DSM 20213: A Transcriptomic Approach. Microorganisms, 7(12), 710. https://doi.org/10.3390/microorganisms7120710