Hafnia alvei HA4597 Strain Reduces Food Intake and Body Weight Gain and Improves Body Composition, Glucose, and Lipid Metabolism in a Mouse Model of Hyperphagic Obesity
Abstract
1. Introduction
2. Materials and Methods
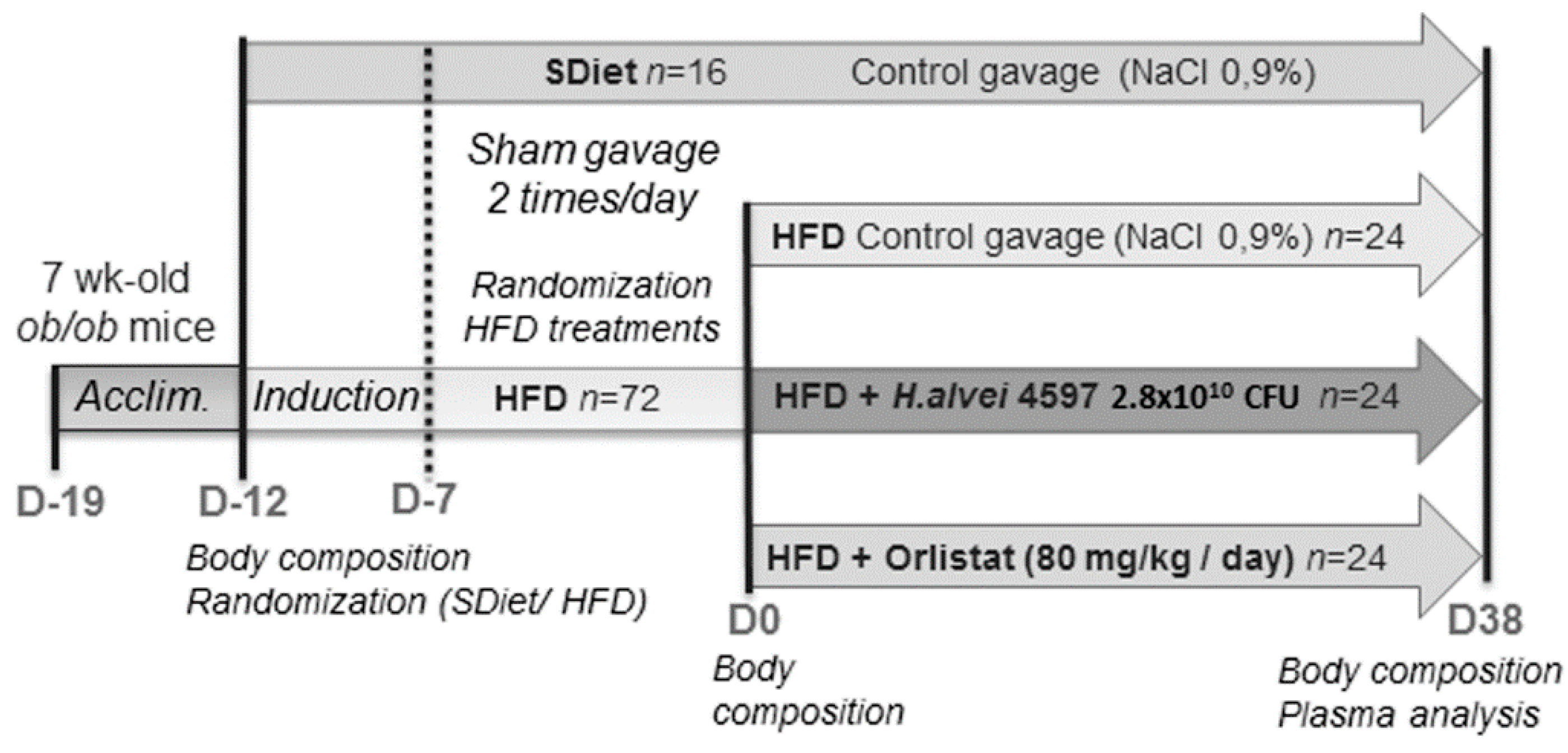
2.1. Animals and Experimental Procedure
2.2. Treatment Protocol
2.3. Plasma Dosage
2.4. Statistical Analysis
3. Results
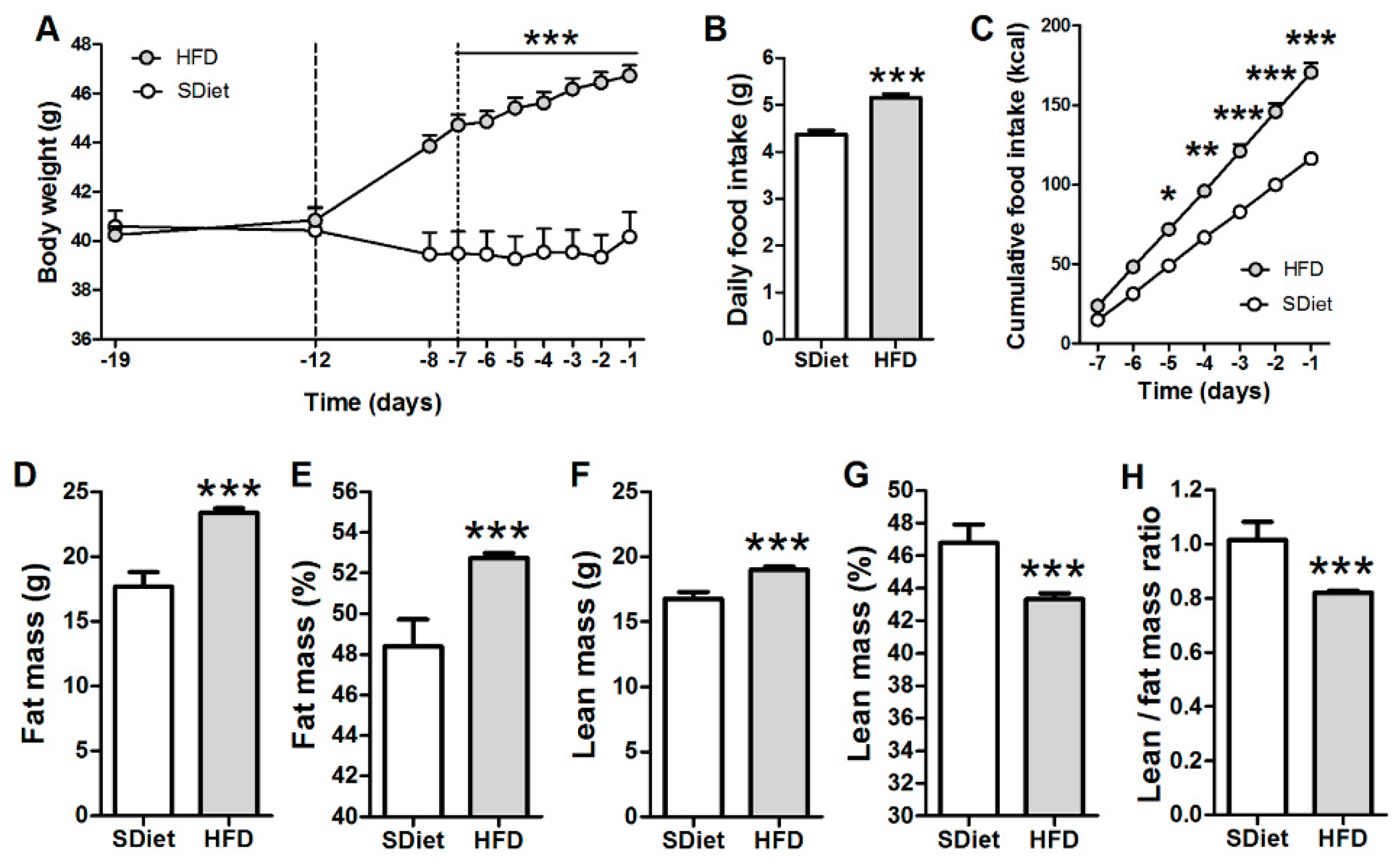
3.1. Induction of the DIO in ob/ob Mice
3.2. Effects of Hafnia alvei HA4597™ Supplementation on Obesity-Related Parameters
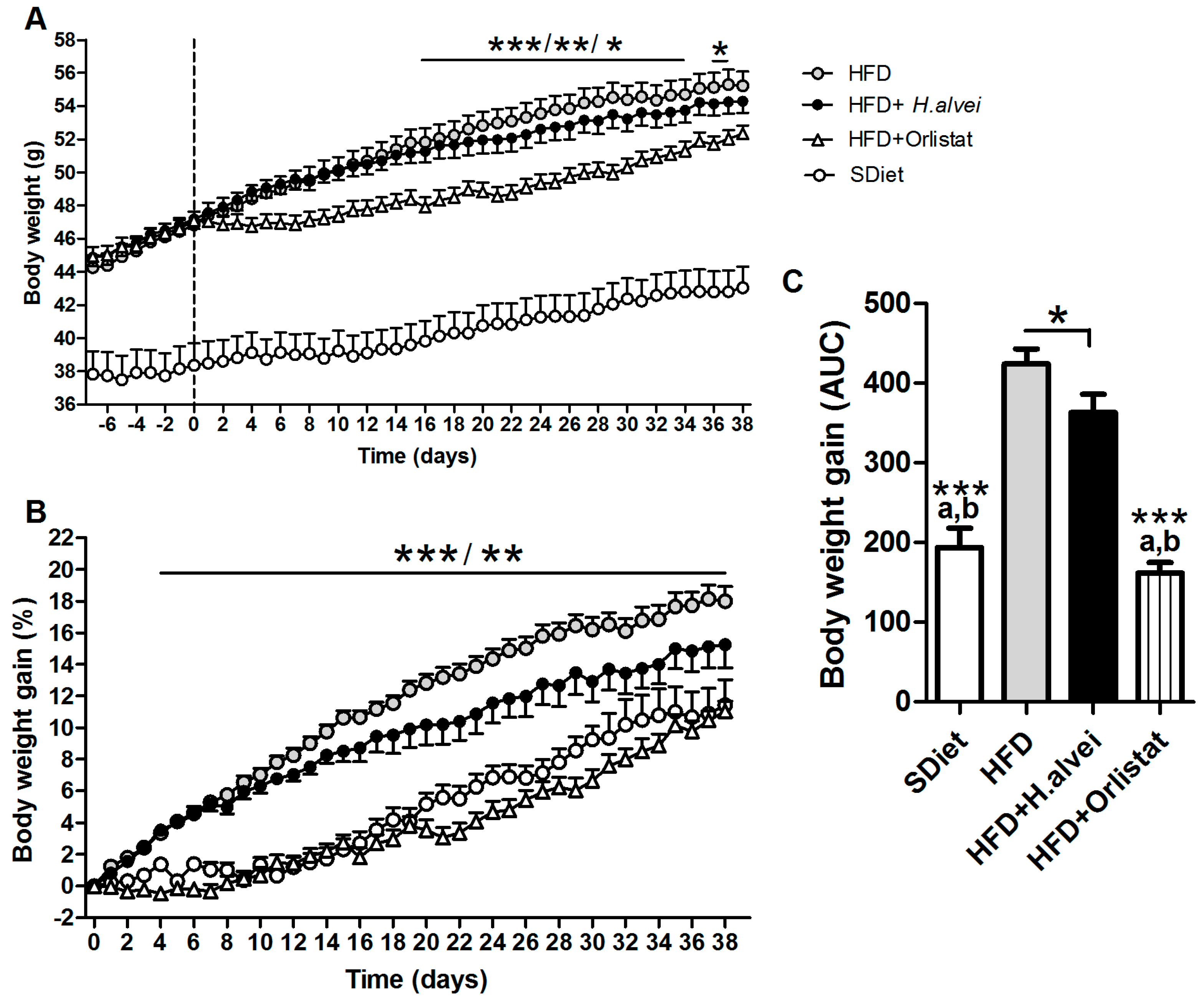
3.2.1. Effects on Body Weight
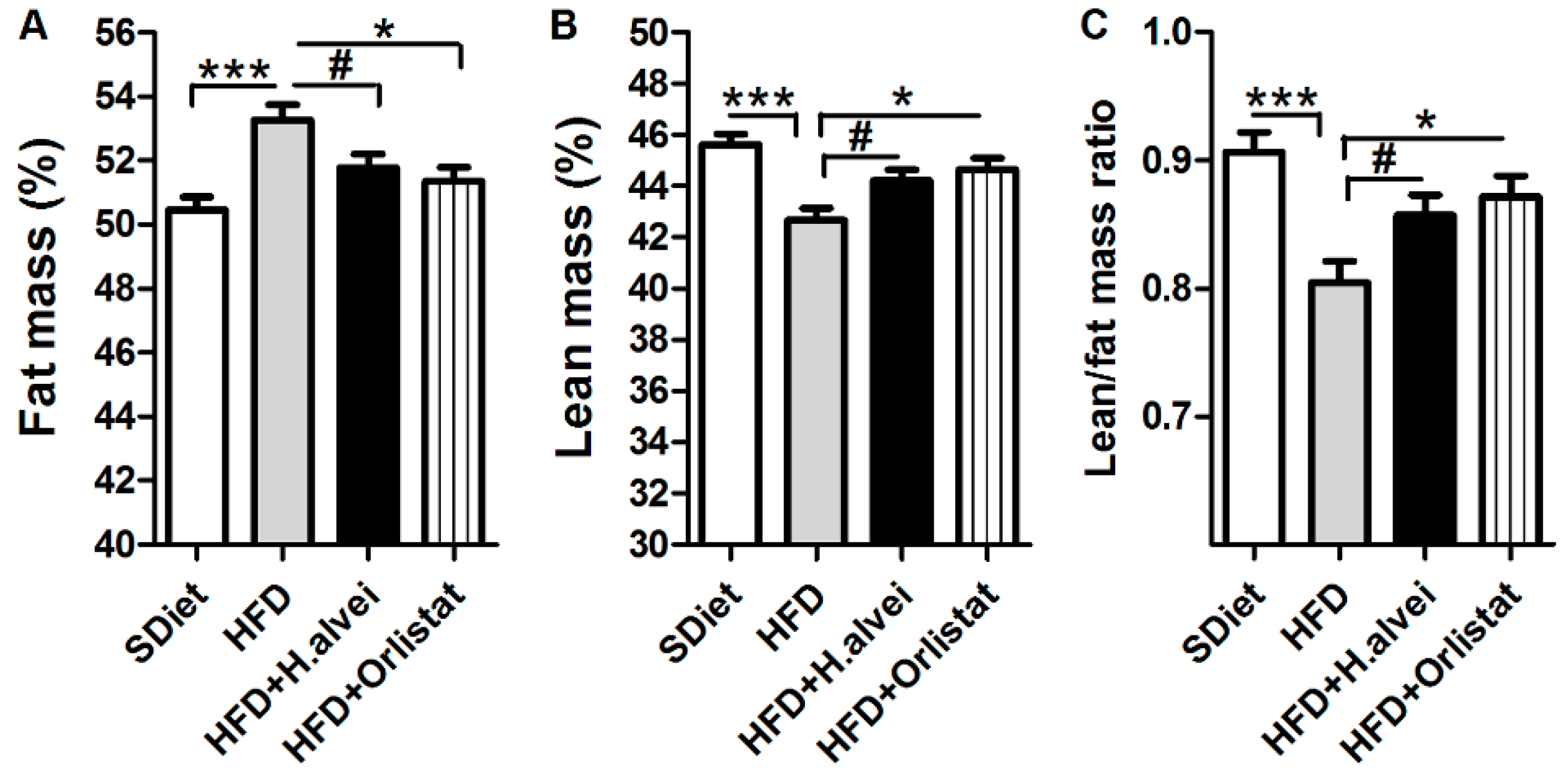
3.2.2. Effects on Body Composition
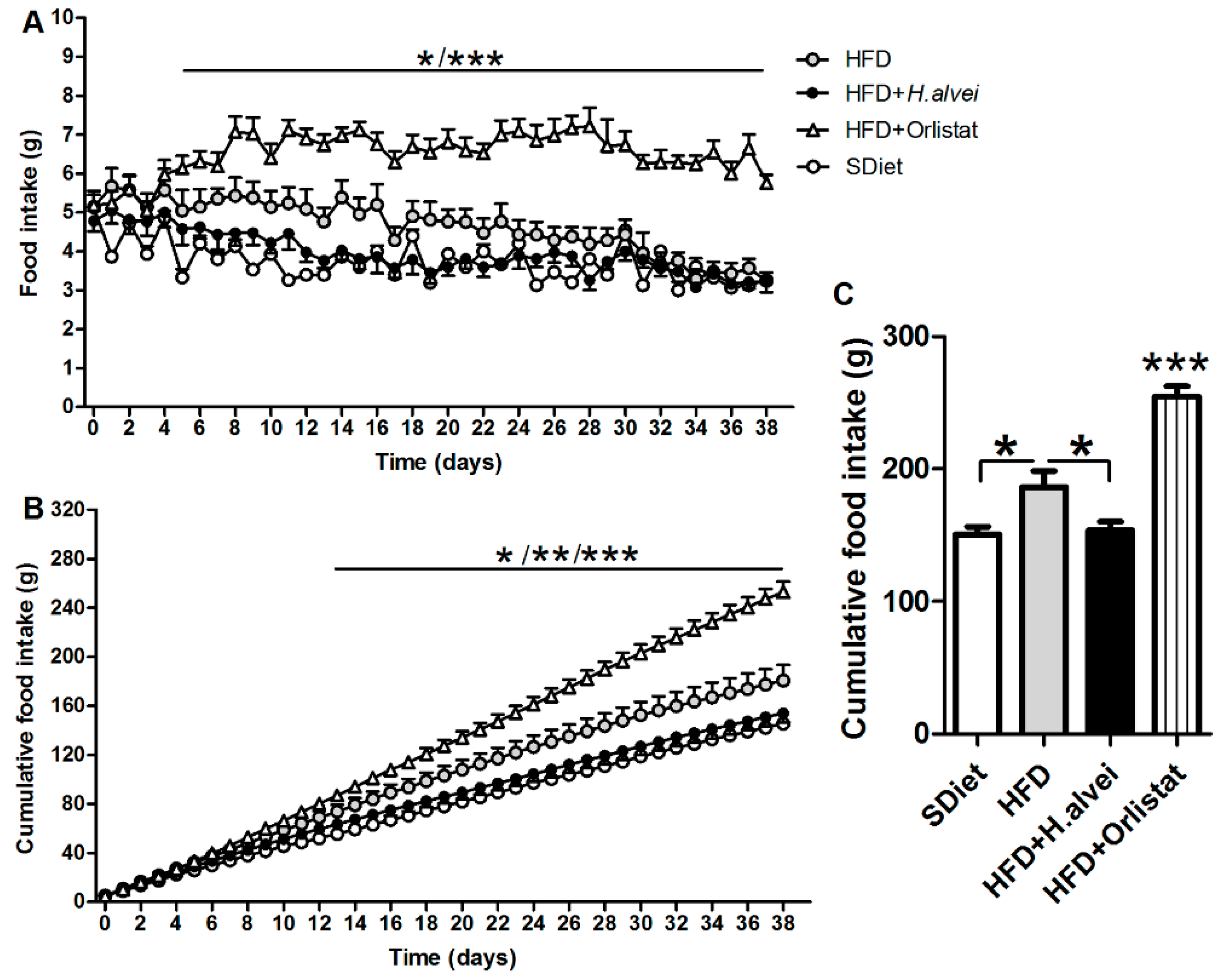
3.2.3. Effects on Food Intake
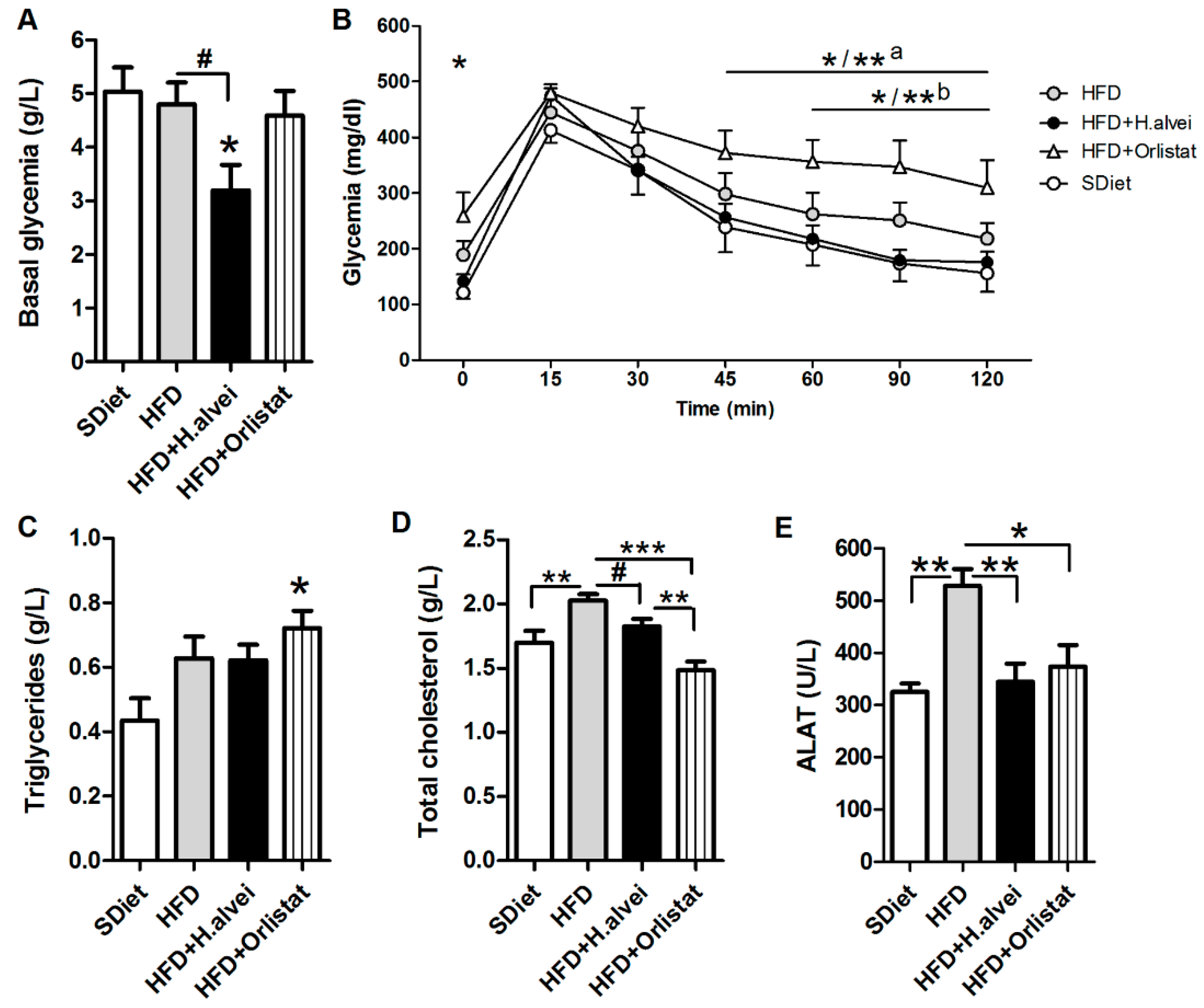
3.2.4. Effects on Glycemia
3.2.5. Effects on Blood Lipids and Alanine Aminotransferase
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Health Topics—Obesity. Available online: http://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 February 2018).
- Reilly, J.J.; Kelly, J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: Systematic review. Int. J. Obes. 2010, 35, 891. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Guazzelli Marques, C.; de Piano Ganen, A.; de Barros, A.Z.; Thomatieli Dos Santos, R.V.; Dos Santos Quaresma, M.V.L. Weight loss probiotic supplementation effect in overweight and obesity subjects: A review. Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- Koutnikova, H.; Genser, B.; Monteiro-Sepulveda, M.; Faurie, J.M.; Rizkalla, S.; Schrezenmeir, J.; Clement, K. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related variables: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2019, 9, e017995. [Google Scholar] [CrossRef]
- Suzumura, E.A.; Bersch-Ferreira, A.C.; Torreglosa, C.R.; da Silva, J.T.; Coqueiro, A.Y.; Kuntz, M.G.F.; Chrispim, P.P.; Weber, B.; Cavalcanti, A.B. Effects of oral supplementation with probiotics or synbiotics in overweight and obese adults: A systematic review and meta-analyses of randomized trials. Nutr. Rev. 2019, 77, 430–450. [Google Scholar] [CrossRef]
- Jones, R.B.; Alderete, T.L.; Martin, A.A.; Geary, B.A.; Hwang, D.H.; Palmer, S.L.; Goran, M.I. Probiotic supplementation increases obesity with no detectable effects on liver fat or gut microbiota in obese Hispanic adolescents: A 16-week, randomized, placebo-controlled trial. Pediatr. Obes. 2018, 13, 705–714. [Google Scholar] [CrossRef]
- Fetissov, S.O. Role of the gut microbiota in host appetite control: Bacterial growth to animal feeding behaviour. Nat. Rev. Endocrinol. 2017, 13, 11–25. [Google Scholar] [CrossRef]
- Legrand, R.; Lucas, N.; Dominique, M.; Azhar, S.; Deroissart, C.; Le Solliec, M.-A.; Rondeaux, J.; Nobis, S.; Guérin, C.; Léon, L.; et al. Commensal Hafnia alvei strain reduces food intake and fat mass in obese mice—A new potential probiotic for appetite and body weight management. Int. J. Obes. 2019, in press. [Google Scholar] [CrossRef]
- Tennoune, N.; Chan, P.; Breton, J.; Legrand, R.; Chabane, Y.N.; Akkermann, K.; Jarv, A.; Ouelaa, W.; Takagi, K.; Ghouzali, I.; et al. Bacterial ClpB heat-shock protein, an antigen-mimetic of the anorexigenic peptide [alpha]-MSH, at the origin of eating disorders. Transl. Psychiatry 2014, 4, e458. [Google Scholar] [CrossRef]
- Halaas, J.L.; Gajiwala, K.S.; Maffei, M.; Cohen, S.L.; Chait, B.T.; Rabinowitz, D.; Lallone, R.L.; Burley, S.K.; Friedman, J.M. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 1995, 269, 543–546. [Google Scholar] [CrossRef]
- Lin, P.Y.; Romsos, D.R.; Vander Tuig, J.G.; Leveille, G.A. Maintenance energy requirements, energy retention and heat production of young obese (ob/ob) and lean mice fed a high-fat or a high-carbohydrate diet. J. Nutr. 1979, 109, 1143–1153. [Google Scholar] [CrossRef]
- Frederich, R.C.; Hamann, A.; Anderson, S.; Lollmann, B.; Lowell, B.B.; Flier, J.S. Leptin levels reflect body lipid content in mice: Evidence for diet-induced resistance to leptin action. Nat. Med. 1995, 1, 1311–1314. [Google Scholar] [CrossRef]
- Wang, J.H.; Shin, N.R.; Lim, S.K.; Im, U.; Song, E.J.; Nam, Y.D.; Kim, H. Diet Control More Intensively Disturbs Gut Microbiota Than Genetic Background in Wild Type and ob/ob Mice. Front. Microbiol. 2019, 10, 1292. [Google Scholar] [CrossRef]
- Kobyliak, N.; Conte, C.; Cammarota, G.; Haley, A.P.; Styriak, I.; Gaspar, L.; Fusek, J.; Rodrigo, L.; Kruzliak, P. Probiotics in prevention and treatment of obesity: A critical view. Nutr. Metab. 2016, 13, 14. [Google Scholar] [CrossRef]
- Franson, K.; Rössner, S. Fat intake and food choices during weight reduction with diet, behavioural modification and a lipase inhibitor. J. Intern. Med. 2000, 247, 607–614. [Google Scholar] [CrossRef]
- Ullrich, A.; Erdmann, J.; Margraf, J.; Schusdziarra, V. Impact of carbohydrate and fat intake on weight-reducing efficacy of orlistat. Aliment. Pharmacol. Ther. 2003, 17, 1007–1013. [Google Scholar] [CrossRef]
- Grilo, C.M.; White, M.A. Orlistat with behavioral weight loss for obesity with versus without binge eating disorder: Randomized placebo-controlled trial at a community mental health center serving educationally and economically disadvantaged Latino/as. Behav. Res. Ther. 2013, 51, 167–175. [Google Scholar] [CrossRef]
- Kim, K.K.; Suh, H.S.; Hwang, I.C.; Ko, K.D. Influence of eating behaviors on short-term weight loss by orlistat and anorectic agent. Eat. Behav. 2014, 15, 87–90. [Google Scholar] [CrossRef]
- Panaro, B.L.; Tough, I.R.; Engelstoft, M.S.; Matthews, R.T.; Digby, G.J.; Møller, C.L.; Svendsen, B.; Gribble, F.; Reimann, F.; Holst, J.J.; et al. The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 in vivo. Cell Metab. 2014, 20, 1018–1029. [Google Scholar] [CrossRef]
- Dominique, M.; Breton, J.; Guérin, C.; Bole-Feysot, C.; Lambert, G.; Déchelotte, P.; Fetissov, S.O. Effects of macronutrients on the in vitro production of ClpB, a bacterial mimetic protein of α-MSH and its possible role in the satiety signaling. Nutrients 2019, 11, 2115. [Google Scholar] [CrossRef]
- Breton, J.; Tennoune, N.; Lucas, N.; François, M.; Legrand, R.; Jacquemot, J.; Goichon, A.; Guérin, C.; Peltier, J.; Pestel-Caron, M.; et al. Gut commensal E. coli proteins activate host satiety pathways following nutrient-induced bacterial growth. Cell Metab. 2016, 23, 324–334. [Google Scholar] [CrossRef]
- Caron, A.; Lee, S.; Elmquist, J.K.; Gautron, L. Leptin and brain–adipose crosstalks. Nat. Rev. Neurosci. 2018, 19, 153–165. [Google Scholar] [CrossRef]
- Zeng, W.; Pirzgalska, R.M.; Pereira, M.M.; Kubasova, N.; Barateiro, A.; Seixas, E.; Lu, Y.H.; Kozlova, A.; Voss, H.; Martins, G.G.; et al. Sympathetic Neuro-adipose Connections Mediate Leptin-Driven Lipolysis. Cell 2015, 163, 84–94. [Google Scholar] [CrossRef]
- Huo, L.; Gamber, K.; Greeley, S.; Silva, J.; Huntoon, N.; Leng, X.H.; Bjorbaek, C. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 2009, 9, 537–547. [Google Scholar] [CrossRef]
- Fetissov, S.O.; Legrand, R.; Lucas, N. Bacterial protein mimetic of peptide hormone as a new class of protein-based drugs. Curr. Med. Chem. 2019, 26, 546–553. [Google Scholar] [CrossRef]
- Million, M.; Angelakis, E.; Maraninchi, M.; Henry, M.; Giorgi, R.; Valero, R.; Vialettes, B.; Raoult, D. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int. J. Obes. 2013, 37, 1460–1466. [Google Scholar] [CrossRef]
- Million, M.; Thuny, F.; Angelakis, E.; Casalta, J.P.; Giorgi, R.; Habib, G.; Raoult, D. Lactobacillus reuteri and Escherichia coli in the human gut microbiota may predict weight gain associated with vancomycin treatment. Nutr. Diabetes 2013, 3, e87. [Google Scholar] [CrossRef]
- Paganelli, F.L.; Luyer, M.; Hazelbag, C.M.; Uh, H.W.; Rogers, M.R.; Adriaans, D.; Berbers, R.M.; Hendrickx, A.P.A.; Viveen, M.C.; Groot, J.A.; et al. Roux-Y Gastric Bypass and Sleeve Gastrectomy directly change gut microbiota composition independent of surgery type. Sci. Rep. 2019, 9, 10979. [Google Scholar] [CrossRef]
- Richard, J.; Zadi, H. Inventaire de la flore bactérienne dominante des Camemberts fabriqués avec lait cru. Le Lait 1983, 63, 25–42. [Google Scholar] [CrossRef][Green Version]
- Micenková, L.; Bosák, J.; Smatana, S.; Novotný, A.; Budinská, E.; Šmajs, D. Administration of the Probiotic Escherichia coli Strain A0 34/86 Resulted in a Stable Colonization of the Human Intestine During the First Year of Life. Probiotics Antimicrob. Proteins 2019. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; van Hylckama Vlieg, J.E.T. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbial. 2015, 23, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.-R. Metabolic and hedonic drives in the neural control of appetite: Who is the boss? Curr. Opin. Neurobiol. 2011, 21, 888–896. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucas, N.; Legrand, R.; Deroissart, C.; Dominique, M.; Azhar, S.; Le Solliec, M.-A.; Léon, F.; do Rego, J.-C.; Déchelotte, P.; Fetissov, S.O.; et al. Hafnia alvei HA4597 Strain Reduces Food Intake and Body Weight Gain and Improves Body Composition, Glucose, and Lipid Metabolism in a Mouse Model of Hyperphagic Obesity. Microorganisms 2020, 8, 35. https://doi.org/10.3390/microorganisms8010035
Lucas N, Legrand R, Deroissart C, Dominique M, Azhar S, Le Solliec M-A, Léon F, do Rego J-C, Déchelotte P, Fetissov SO, et al. Hafnia alvei HA4597 Strain Reduces Food Intake and Body Weight Gain and Improves Body Composition, Glucose, and Lipid Metabolism in a Mouse Model of Hyperphagic Obesity. Microorganisms. 2020; 8(1):35. https://doi.org/10.3390/microorganisms8010035
Chicago/Turabian StyleLucas, Nicolas, Romain Legrand, Camille Deroissart, Manon Dominique, Saïda Azhar, Marie-Anne Le Solliec, Fatima Léon, Jean-Claude do Rego, Pierre Déchelotte, Sergueï O. Fetissov, and et al. 2020. "Hafnia alvei HA4597 Strain Reduces Food Intake and Body Weight Gain and Improves Body Composition, Glucose, and Lipid Metabolism in a Mouse Model of Hyperphagic Obesity" Microorganisms 8, no. 1: 35. https://doi.org/10.3390/microorganisms8010035
APA StyleLucas, N., Legrand, R., Deroissart, C., Dominique, M., Azhar, S., Le Solliec, M.-A., Léon, F., do Rego, J.-C., Déchelotte, P., Fetissov, S. O., & Lambert, G. (2020). Hafnia alvei HA4597 Strain Reduces Food Intake and Body Weight Gain and Improves Body Composition, Glucose, and Lipid Metabolism in a Mouse Model of Hyperphagic Obesity. Microorganisms, 8(1), 35. https://doi.org/10.3390/microorganisms8010035






