Protective or Detrimental? Understanding the Role of Host Immunity in Leishmaniasis
Abstract
1. Introduction
2. Clinical Aspects of Leishmaniasis
3. The Immunobiology of Leishmaniasis
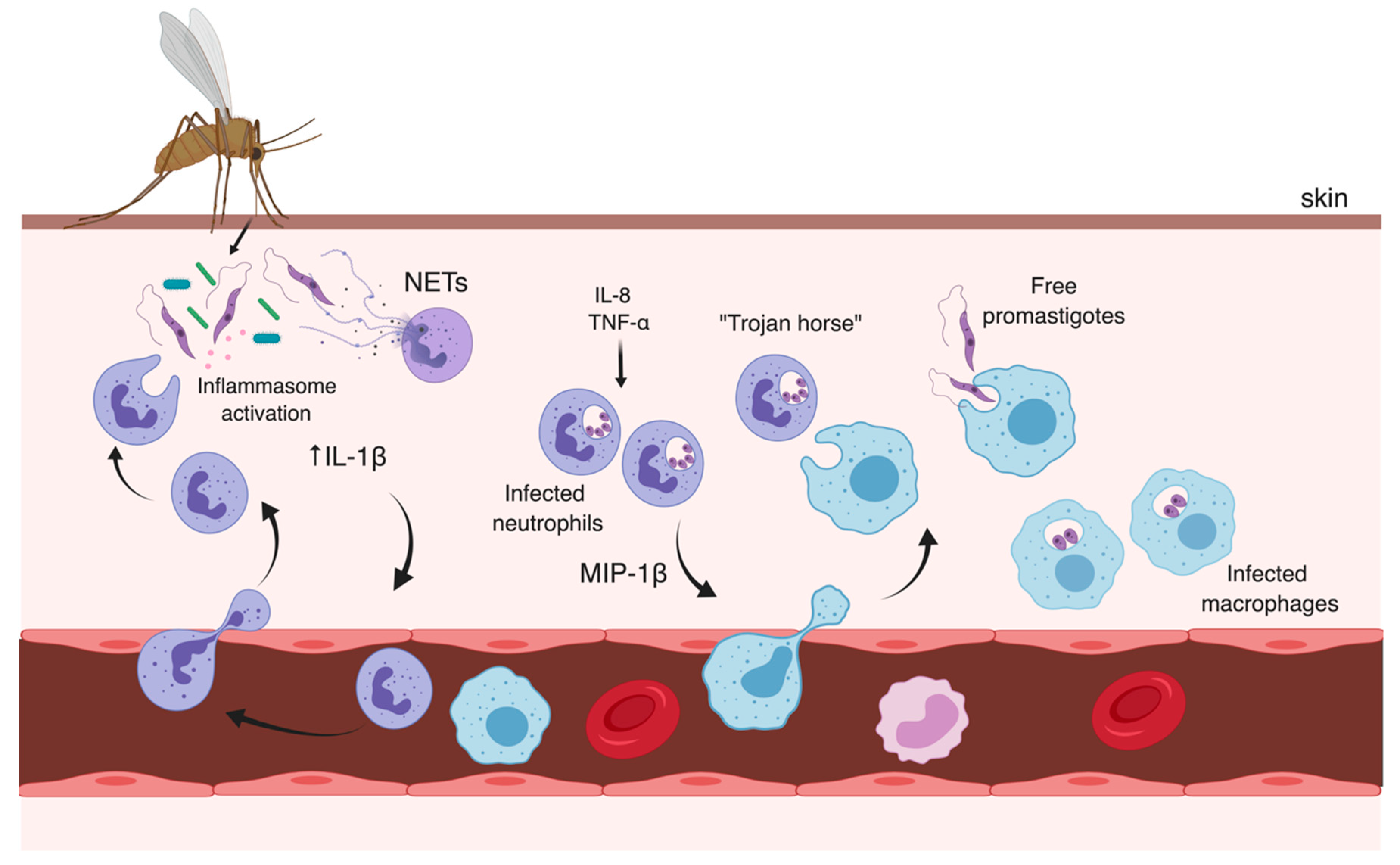
3.1. Early Events
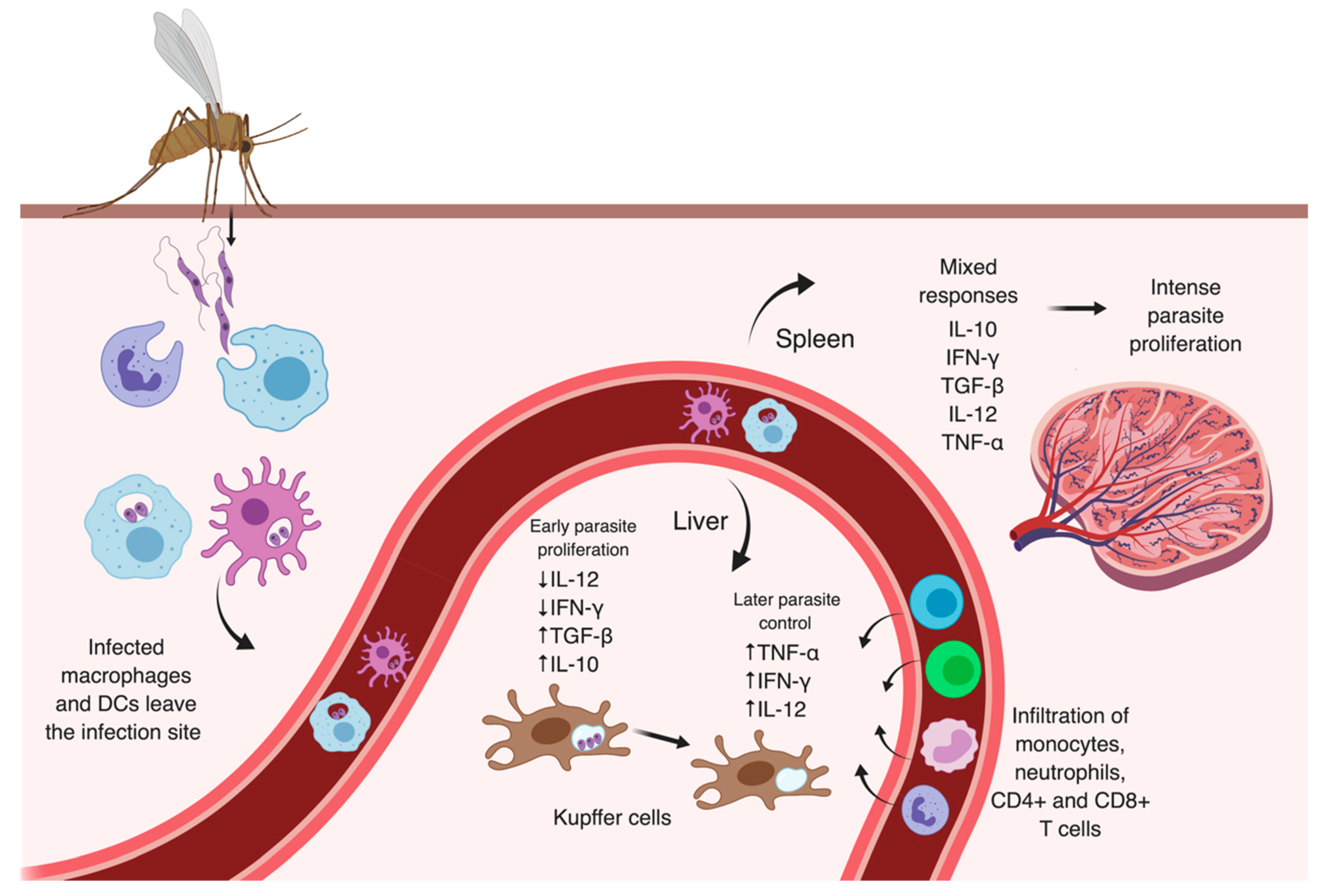
3.2. Later Moments After Infection
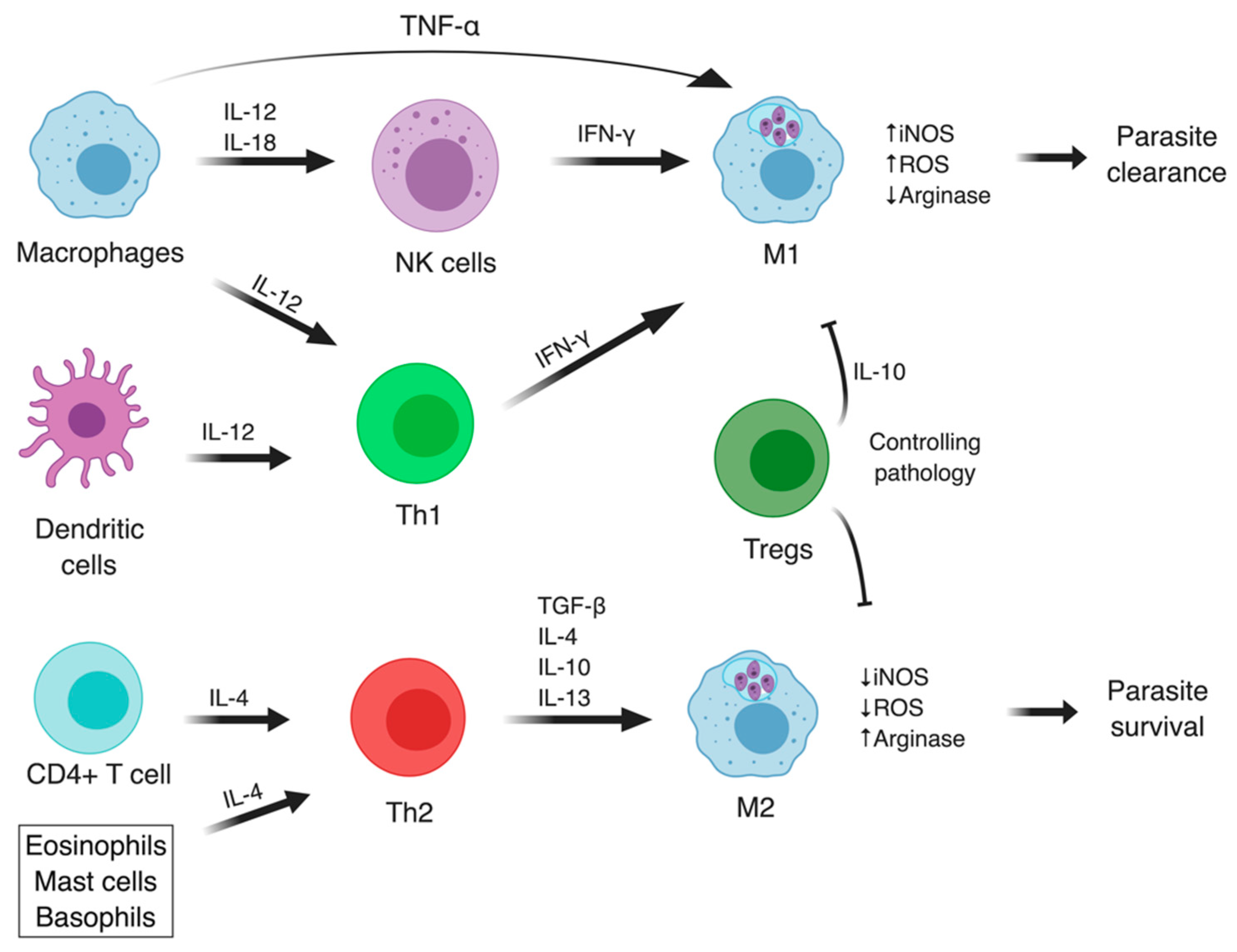
3.3. The Adaptive Immune Responses in Leishmaniasis
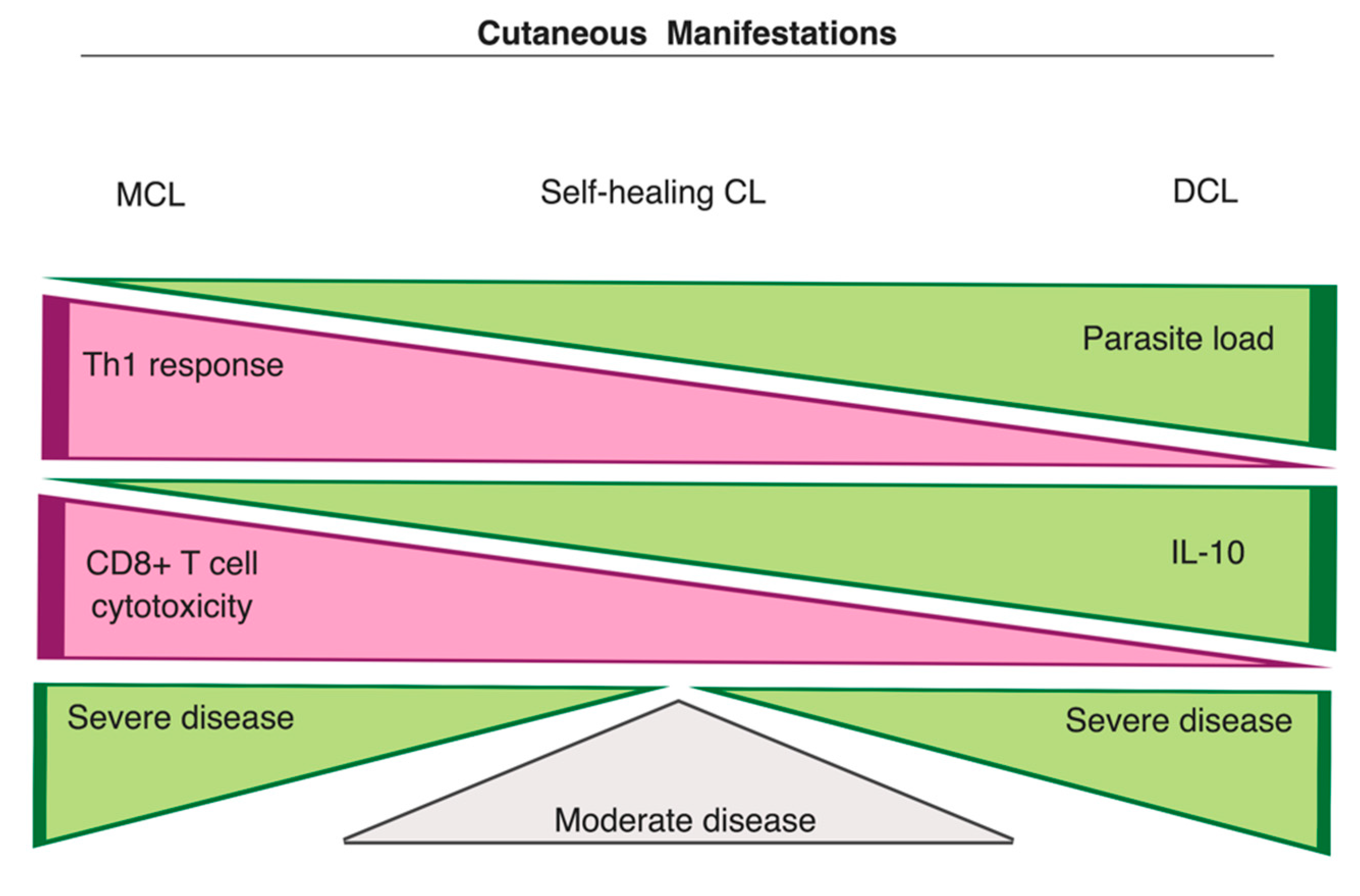
3.3.1. Cutaneous Manifestations
- Cathepsin-like cysteine proteases: papain-like cysteine proteases of Leishmania were shown to inhibit antigen presentation via major histocompatibility complex (MHC) class II and modulate IL-12 production in macrophages [73,74] and DCs [75]. Cathepsin B-like protease was also implicated in the activation of the latent TGF-β1 in L. infantum, and L. donovani infected macrophages, inhibiting IFN-γ-induced microbicidal activities [76,77].
- Nucleotidases: both parasite and vector-derived nucleotidases can modulate purinergic signaling mechanisms through increased generation of adenosine, which stimulates the production of IL-10 and inhibits inflammatory functions in neutrophils, DCs, and macrophages [29,78]. Enhanced activity of nucleotidases has been correlated with higher virulence of several Leishmania spp. and clinical isolates [79,80].
- Peroxiredoxins (Prxs): components of the unique antioxidant system of trypanosomatids. Prxs work in association with trypanothione, a glutathione analog, to reduce hydrogen peroxide, hydroperoxide, and hydroxyl radicals [81,82,83,84]. Cytosolic Prxs from Leishmania were demonstrated to confer protection against peroxides and increased virulence [85,86,87]. Prxs have also been linked to resistance against anti-leishmanial drugs [88].
- Superoxide dismutases (SODs): antioxidant metalloenzymes that convert superoxide to oxygen and hydrogen peroxide. The iron-dependent superoxide dismutase B1 (SODB1) of L. chagasi and L. major was correlated with parasite proliferation in human macrophages and mice models [89,90], while superoxide dismutase A (SODA) was associated with differentiation and virulence of L. amazonensis parasites [91]. The up-regulation of SODA has also been linked to anti-leishmanial drug-resistance (miltefosine) in L. donovani infections [92,93].
3.3.2. Leishmania RNA Viruses (LRV) and their Implications in Disease Severity
3.3.3. Visceral Manifestations
4. Promising Approaches for Drug Development: A Special Focus on the Host
5. Vaccines for Leishmaniasis
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- W.H.O. Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 1 October 2019).
- Desjeux, P. Leishmaniasis: Current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.O.; Coutinho, C.E.R.; Madeira, M.F.; Bottino, C.G.; Vieira, R.T.; Nascimento, S.B.; Bernardino, A.; Bourguignon, S.C.; Corte-Real, S.; Pinho, R.T.; et al. Leishmaniasis treatment—A challenge that remains: A review. Parasitol. Res. 2008, 103, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Akbari, M. Worldwide risk factors in leishmaniasis. Asian Pac. J. Trop. Med. 2016, 9, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, F.; Gradoni, L. The Leishmaniases: Old Neglected Tropical Diseases; Springer: Berlin, Germany, 2018. [Google Scholar]
- Bates, P.A.; Rogers, M.E. New insights into the developmental biology and transmission mechanisms of Leishmania. Curr. Mol. Med. 2004, 4, 601–609. [Google Scholar] [CrossRef]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef]
- Natarajan, G.; Oghumu, S.; Varikuti, S.; Thomas, A.; Satoskar, A. Mechanisms of immunopathology of leishmaniasis. In Pathogenesis of Leishmaniasis: New Developments in Research; Satoskar, A., Durvasula, R., Eds.; Springer New York: New York, NY, USA, 2014; pp. 1–13. [Google Scholar]
- Lainson, R.; Shaw, J.J. New World leishmaniasis. In Topley & Wilson’s Microbiology and Microbial Infections; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Scott, P.; Novais, F.O. Cutaneous leishmaniasis: Immune responses in protection and pathogenesis. Nat. Rev. Immunol. 2016, 16, 581. [Google Scholar] [CrossRef]
- Strazzulla, A.; Cocuzza, S.; Pinzone, M.R.; Postorino, M.C.; Cosentino, S.; Serra, A.; Cacopardo, B.; Nunnari, G. Mucosal leishmaniasis: An underestimated presentation of a neglected disease. Biomed. Res. Int. 2013, 2013, 7. [Google Scholar] [CrossRef]
- Wilson, M.E.; Jeronimo, S.M.B.; Pearson, R.D. Immunopathogenesis of infection with the visceralizing Leishmania species. Microb. Pathog. 2005, 38, 147–160. [Google Scholar] [CrossRef]
- Kaye, P.M. The immunology of visceral leishmaniasis: Current status. In Leishmania; Farrell, J.P., Ed.; Springer US: Boston, MA, USA, 2002; pp. 137–150. [Google Scholar]
- Zijlstra, E.E. The immunology of post-kala-azar dermal leishmaniasis (PKDL). Parasites Vectors 2016, 9, 464. [Google Scholar] [CrossRef]
- Zijlstra, E.E.; El-Hassan, A.M. Leishmaniasis in Sudan. 3. Visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, S27–S58. [Google Scholar] [CrossRef]
- Kaye, P.; Scott, P. Leishmaniasis: Complexity at the host–pathogen interface. Nat. Rev. Microbiol. 2011, 9, 604. [Google Scholar] [CrossRef] [PubMed]
- Hohman, L.S.; Peters, N.C. CD4+ T cell-mediated immunity against the phagosomal pathogen Leishmania: Implications for vaccination. Trends Parasitol. 2019, 35, 423–435. [Google Scholar] [CrossRef] [PubMed]
- W.H.O. Leishmaniasis-Situation and trends. Available online: https://www.who.int/gho/neglected_diseases/leishmaniasis/en/ (accessed on 20 November 2019).
- Gonçalves-de-Albuquerque, S.D.C.; Pessoa-e-Silva, R.; Trajano-Silva, L.A.M.; de Goes, T.C.; de Morais, R.C.S.; da Oliveira, C.N.; de Lorena, V.M.B.; de Paiva-Cavalcanti, M. The equivocal role of Th17 cells and neutrophils on immunopathogenesis of leishmaniasis. Front. Immunol. 2017, 8, 1437. [Google Scholar] [CrossRef]
- Peters, N.C.; Egen, J.G.; Secundino, N.; Debrabant, A.; Kimblin, N.; Kamhawi, S.; Lawyer, P.; Fay, M.P.; Germain, R.N.; Sacks, D. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 2008, 321, 970. [Google Scholar] [CrossRef]
- Ritter, U.; Frischknecht, F.; van Zandbergen, G. Are neutrophils important host cells for Leishmania parasites? Trends Parasitol. 2009, 25, 505–510. [Google Scholar] [CrossRef]
- Gorak, P.M.A.; Engwerda, C.R.; Kaye, P.M. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur. J. Immunol. 1998, 28, 687–695. [Google Scholar] [CrossRef]
- Carneiro, M.B.; Hohman, L.S.; Egen, J.G.; Peters, N.C. Use of two-photon microscopy to study Leishmania major infection of the skin. Methods 2017, 127, 45–52. [Google Scholar] [CrossRef]
- Lestinova, T.; Rohousova, I.; Sima, M.; de Oliveira, C.I.; Volf, P. Insights into the sand fly saliva: Blood-feeding and immune interactions between sand flies, hosts, and Leishmania. PLoS Negl. Trop. Dis. 2017, 11, e0005600. [Google Scholar] [CrossRef]
- Abdeladhim, M.; Kamhawi, S.; Valenzuela, J.G. What’s behind a sand fly bite? The profound effect of sand fly saliva on host hemostasis, inflammation and immunity. Infect. Genet. Evol. 2014, 28, 691–703. [Google Scholar] [CrossRef]
- Atayde, V.D.; Suau, H.A.; Townsend, S.; Hassani, K.; Kamhawi, S.; Olivier, M. Exosome secretion by the parasitic protozoan Leishmania within the sand fly midgut. Cell Rep. 2015, 13, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M. The role of Leishmania proteophosphoglycans in sand fly transmission and infection of the mammalian host. Front. Microbiol. 2012, 3, 223. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.B.d.; Souza-Testasicca, M.C.; Afonso, L.C.C. Purinergic signaling and infection by Leishmania: A new approach to evasion of the immune response. Biomed. J. 2016, 39, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.; Joshi, A.B.; Oliveira, F.; Pereira, L.; Guimarães-Costa, A.B.; Serafim, T.D.; de Castro, W.; Coutinho-Abreu, I.V.; Bhattacharya, P.; Townsend, S.; et al. Gut microbes egested during bites of infected sand flies augment severity of leishmaniasis via inflammasome-derived IL-1β. Cell Host Microbe 2018, 23, 134–143.e136. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, B.P.; Schuster, S.; Grün, E.; Coutaz, M.; Williams, R.A.; Held, W.; Malissen, B.; Malissen, M.; Yousefi, S.; Simon, H.-U.; et al. Rapid sequestration of Leishmania mexicana by neutrophils contributes to the development of chronic lesion. PLoS Pathog. 2015, 11, e1004929. [Google Scholar] [CrossRef] [PubMed]
- Daboul, M. Role of neutrophils in cutaneous leishmaniasis. East. Mediterr. Health J. 2010, 16, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Morgado, F.N.; Schubach, A.; Rosalino, C.M.V.; Quintella, L.P.; Santos, G.; Salgueiro, M.; Conceição-Silva, F. Is the in Situ inflammatory reaction an important tool to understand the cellular immune response in American tegumentary leishmaniasis? Br. J. Dermatol. 2008, 158, 50–58. [Google Scholar] [CrossRef]
- Regli, I.B.; Passelli, K.; Hurrell, B.P.; Tacchini-Cottier, F. Survival mechanisms used by some Leishmania species to escape neutrophil killing. Front. Immunol. 2017, 8, 1558. [Google Scholar] [CrossRef]
- Ribeiro-Gomes, F.L.; Sacks, D. The influence of early neutrophil-Leishmania interactions on the host immune response to infection. Front. Cell. Infect. Microbiol. 2012, 2, 59. [Google Scholar] [CrossRef]
- Guimarães-Costa, A.B.; Nascimento, M.T.C.; Froment, G.S.; Soares, R.P.P.; Morgado, F.N.; Conceição-Silva, F.; Saraiva, E.M. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. USA 2009, 106, 6748. [Google Scholar] [CrossRef]
- Hurrell, B.P.; Regli, I.B.; Tacchini-Cottier, F. Different Leishmania species drive distinct neutrophil functions. Trends Parasitol. 2016, 32, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, B.P.; Beaumann, M.; Heyde, S.; Regli, I.B.; Müller, A.J.; Tacchini-Cottier, F. Frontline Science: Leishmania mexicana amastigotes can replicate within neutrophils. J. Leukoc. Biol. 2017, 102, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Heyde, S.; Philipsen, L.; Formaglio, P.; Fu, Y.; Baars, I.; Höbbel, G.; Kleinholz, C.L.; Seiß, E.A.; Stettin, J.; Gintschel, P.; et al. CD11c-expressing Ly6C+CCR2+ monocytes constitute a reservoir for efficient Leishmania proliferation and cell-to-cell transmission. PLoS Pathog. 2018, 14, e1007374. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Uzonna, J.E. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Front. Cell. Infect. Microbiol. 2012, 2, 83. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 1474–1741. [Google Scholar] [CrossRef]
- Brinkmann, V.; Zychlinsky, A. Beneficial suicide: Why neutrophils die to make NETs. Nat. Rev. Microbiol. 2007, 5, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Charmoy, M.; Brunner-Agten, S.; Aebischer, D.; Auderset, F.; Launois, P.; Milon, G.; Proudfoot, A.E.I.; Tacchini-Cottier, F. Neutrophil-derived CCL3 is essential for the rapid recruitment of dendritic cells to the site of Leishmania major inoculation in resistant mice. PLoS Pathog. 2010, 6, e1000755. [Google Scholar] [CrossRef]
- Olivier, M.; Gregory, D.J.; Forget, G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: A signaling point of view. Clin. Microbiol. Rev. 2005, 18, 293–305. [Google Scholar] [CrossRef]
- Romano, A.; Carneiro, M.B.H.; Doria, N.A.; Roma, E.H.; Ribeiro-Gomes, F.L.; Inbar, E.; Lee, S.H.; Mendez, J.; Paun, A.; Sacks, D.L.; et al. Divergent roles for Ly6C+CCR2+CX3CR1+ inflammatory monocytes during primary or secondary infection of the skin with the intra-phagosomal pathogen Leishmania major. PLoS Pathog. 2017, 13, e1006479. [Google Scholar] [CrossRef]
- Guy, R.A.; Belosevic, M. Comparison of receptors required for entry of Leishmania major amastigotes into macrophages. Infect. Immun. 1993, 61, 1553–1558. [Google Scholar]
- Kima, P.E.; Constant, S.L.; Hannum, L.; Colmenares, M.; Lee, K.S.; Haberman, A.M.; Shlomchik, M.J.; McMahon-Pratt, D. Internalization of Leishmania mexicana complex amastigotes via the fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J. Exp. Med. 2000, 191, 1063. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.A.; Karmakar, J.; Mandal, C.; Chattopadhyay, A. Leishmania donovani internalizes into host cells via caveolin-mediated endocytosis. Sci. Rep. 2019, 9, 12636. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Donelson, J.E.; Wilson, M.E. The major surface protease (MSP or GP63) of Leishmania sp. Biosynthesis, regulation of expression, and function. Mol. Biochem. Parasitol. 2003, 132, 1–16. [Google Scholar] [CrossRef]
- Naderer, T.; McConville, M.J. The Leishmania–macrophage interaction: A metabolic perspective. Cell. Microbiol. 2008, 10, 301–308. [Google Scholar] [CrossRef]
- Pelletier, I.; Hashidate, T.; Urashima, T.; Nishi, N.; Nakamura, T.; Futai, M.; Arata, Y.; Kasai, K.-i.; Hirashima, M.; Hirabayashi, J.; et al. Specific recognition of Leishmania major poly-β-galactosyl epitopes by galectin-9: Possible implication of galectin-9 in interaction between L. major and host cells. J. Biol. Chem. 2003, 278, 22223–22230. [Google Scholar] [CrossRef]
- Green, P.J.; Feizi, T.; Stoll, M.S.; Thiel, S.; Prescott, A.; McConville, M.J. Recognition of the major cell surface glycoconjugates of Leishmania parasites by the human serum mannan-binding protein. Mol. Biochem. Parasitol. 1994, 66, 319–328. [Google Scholar] [CrossRef]
- Miles, S.A.; Conrad, S.M.; Alves, R.G.; Jeronimo, S.M.B.; Mosser, D.M. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J. Exp. Med. 2005, 201, 747. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of human macrophage polarization in inflammation during infectious diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef]
- Tomiotto-Pellissier, F.; Bortoleti, B.T.d.S.; Assolini, J.P.; Gonçalves, M.D.; Carloto, A.C.M.; Miranda-Sapla, M.M.; Conchon-Costa, I.; Bordignon, J.; Pavanelli, W.R. Macrophage polarization in leishmaniasis: Broadening horizons. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Muxel, S.M.; Aoki, J.I.; Fernandes, J.C.R.; Laranjeira-Silva, M.F.; Zampieri, R.A.; Acuña, S.M.; Müller, K.E.; Vanderlinde, R.H.; Floeter-Winter, L.M. Arginine and polyamines fate in Leishmania infection. Front. Microbiol. 2018, 8, 2682. [Google Scholar] [CrossRef]
- Li, S.-N.; Wang, W.; Fu, S.-P.; Wang, J.-F.; Liu, H.-M.; Xie, S.-S.; Liu, B.-R.; Li, Y.; Lv, Q.-K.; Li, Z.-Q.; et al. IL-21 modulates release of proinflammatory cytokines in LPS-stimulated macrophages through distinct signaling pathways. Mediat. Inflamm. 2013, 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Brombacher, F. T helper1/T helper2 cells and resistance/susceptibility to Leishmania infection: Is this paradigm still relevant? Front. Immunol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Oghumu, S.; Natarajan, G.; Satoskar, A.R. Pathogenesis of leishmaniasis in humans. Hum. Emerg. Re-Emerg. 2015. [Google Scholar] [CrossRef]
- Alexander, J.; Bryson, K. T helper (h) 1/Th2 and Leishmania: Paradox rather than paradigm. Immunol. Lett. 2005, 99, 17–23. [Google Scholar] [CrossRef]
- Maspi, N.; Abdoli, A.; Ghaffarifar, F. Pro-and anti-inflammatory cytokines in cutaneous leishmaniasis: A review. Pathog. Glob. Health 2016, 110, 247–260. [Google Scholar] [CrossRef]
- Sharma, U.; Singh, S. Immunobiology of leishmaniasis. Indian J. Exp. Biol. 2009, 47, 412–423. [Google Scholar]
- Qi, H.; Ji, J.; Wanasen, N.; Soong, L. Enhanced replication of Leishmania amazonensis amastigotes in gamma interferon-stimulated murine macrophages: Implications for the pathogenesis of cutaneous leishmaniasis. Infect. Immun. 2004, 72, 988–995. [Google Scholar] [CrossRef]
- McMahon-Pratt, D.; Alexander, J. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol. Rev. 2004, 201, 206–224. [Google Scholar] [CrossRef]
- Pinheiro, R.O.; Rossi-Bergmann, B. Interferon-gamma is required for the late but not early control of Leishmania amazonensis infection in C57Bl/6 mice. Memórias Do Inst. Oswaldo Cruz 2007, 102, 79–82. [Google Scholar] [CrossRef]
- Zamboni, D.S.; Sacks, D.L. Inflammasomes and Leishmania: In good times or bad, in sickness or in health. Curr. Opin. Microbiol. 2019, 52, 70–76. [Google Scholar] [CrossRef]
- Lima-Junior, D.S.; Costa, D.L.; Carregaro, V.; Cunha, L.D.; Silva, A.L.N.; Mineo, T.W.P.; Gutierrez, F.R.S.; Bellio, M.; Bortoluci, K.R.; Flavell, R.A.; et al. Inflammasome-derived IL-1β production induces nitric oxide–mediated resistance to Leishmania. Nat. Med. 2013, 19, 909. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Figueroa, E.A.; Rangel-Escareño, C.; Espinosa-Mateos, V.; Carrillo-Sánchez, K.; Salaiza-Suazo, N.; Carrada-Figueroa, G.; March-Mifsut, S.; Becker, I. Disease severity in patients infected with Leishmania mexicana relates to IL-1β. PLoS Negl. Trop. Dis. 2012, 6, e1533. [Google Scholar]
- Desjardins, M.; Descoteaux, A. Inhibition of phagolysosomal biogenesis by the Leishmania lipophosphoglycan. J. Exp. Med. 1997, 185, 2061. [Google Scholar] [CrossRef] [PubMed]
- Vinet, A.F.; Fukuda, M.; Turco, S.J.; Descoteaux, A. The Leishmania donovani lipophosphoglycan excludes the vesicular proton-ATPase from phagosomes by impairing the recruitment of synaptotagmin V. PLoS Pathog. 2009, 5, e1000628. [Google Scholar] [CrossRef] [PubMed]
- Antonia, A.L.; Gibbs, K.D.; Trahair, E.D.; Pittman, K.J.; Martin, A.T.; Schott, B.H.; Smith, J.S.; Rajagopal, S.; Thompson, J.W.; Reinhardt, R.L.; et al. Pathogen evasion of chemokine response through suppression of CXCL10. Front. Cell. Infect. Microbiol. 2019, 9, 280. [Google Scholar] [CrossRef] [PubMed]
- Atayde, V.D.; Hassani, K.; da Silva Lira Filho, A.; Borges, A.R.; Adhikari, A.; Martel, C.; Olivier, M. Leishmania exosomes and other virulence factors: Impact on innate immune response and macrophage functions. Cell. Immunol. 2016, 309, 7–18. [Google Scholar] [CrossRef]
- Silva-Almeida, M.; Pereira, B.A.S.; Ribeiro-Guimarães, M.L.; Alves, C.R. Proteinases as virulence factors in Leishmania spp. infection in mammals. Parasites Vectors 2012, 5, 160. [Google Scholar] [CrossRef]
- Weinheber, N.; Wolfram, M.; Harbecke, D.; Aebischer, T. Phagocytosis of Leishmania mexicana amastigotes by macrophages leads to a sustained suppression of IL-12 production. Eur. J. Immunol. 1998, 28, 2467–2477. [Google Scholar] [CrossRef]
- Cameron, P.; McGachy, A.; Anderson, M.; Paul, A.; Coombs, G.H.; Mottram, J.C.; Alexander, J.; Plevin, R. Inhibition of lipopolysaccharide-induced macrophage IL-12 production by Leishmania mexicana amastigotes: The role of cysteine peptidases and the NF-κB signaling pathway. J. Immunol. 2004, 173, 3297. [Google Scholar] [CrossRef]
- Somanna, A.; Mundodi, V.; Gedamu, L. Functional analysis of cathepsin B-like cysteine proteases from Leishmania donovani complex: Evidence for the activation of latent transforming growth factor β. J. Biol. Chem. 2002, 277, 25305–25312. [Google Scholar] [CrossRef]
- Gerbaba, T.K.; Gedamu, L. Cathepsin B gene disruption induced Leishmania donovani proteome remodeling implies cathepsin B role in secretome regulation. PLoS ONE 2013, 8, e79951. [Google Scholar] [CrossRef] [PubMed]
- Paletta-Silva, R.; Meyer-Fernandes, J.R. Adenosine and immune imbalance in visceral leishmaniasis: The possible role of ectonucleotidases. J. Trop. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.K.; Thakur, C.P.; Velpandian, T.; Sharma, S.K.; Ghosh, B.; Mitra, D.K. High concentration of adenosine in human visceral leishmaniasis despite increased ADA and decreased CD73. Parasite Immunol. 2011, 33, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Amit, A.; Kumar, S.; Dikhit, M.R.; Jha, P.K.; Singh, A.K.; Sinha, K.K.; Pandey, K.; Das, V.N.R.; Das, P.; Bimal, S. Up regulation of A2B adenosine receptor on monocytes are crucially required for immune pathogenicity in Indian patients exposed to Leishmania donovani. Cytokine 2016, 79, 38–44. [Google Scholar]
- Castro, H.; Sousa, C.; Novais, M.; Santos, M.; Budde, H.; Cordeiro-da-Silva, A.; Flohé, L.; Tomás, A.M. Two linked genes of Leishmania infantum encode tryparedoxins localised to cytosol and mitochondrion. Mol. Biochem. Parasitol. 2004, 136, 137–147. [Google Scholar] [CrossRef]
- Dumas, C.; Ouellette, M.; Tovar, J.; Cunningham, M.L.; Fairlamb, A.H.; Tamar, S.; Olivier, M.; Papadopoulou, B. Disruption of the trypanothione reductase gene of Leishmania decreases its ability to survive oxidative stress in macrophages. EMBO J. 1997, 16, 2590. [Google Scholar] [CrossRef]
- Krauth-Siegel, R.L.; Meiering, S.K.; Schmidt, H. The parasite-specific trypanothione metabolism of Trypanosoma and Leishmania. Biol. Chem. 2003, 384, 539. [Google Scholar] [CrossRef]
- Tovar, J.; Cunningham, M.L.; Smith, A.C.; Croft, S.L.; Fairlamb, A.H. Down-regulation of Leishmania donovani trypanothione reductase by heterologous expression of a trans-dominant mutant homologue: Effect on parasite intracellular survival. Proc. Natl. Acad. Sci. USA 1998, 95, 5311. [Google Scholar] [CrossRef]
- Barr, S.D.; Gedamu, L. Role of peroxidoxins in Leishmania chagasi survival: Evidence of an enzymatic defense against nitrosative stress. J. Biol. Chem. 2003, 278, 10816–10823. [Google Scholar] [CrossRef]
- Barr, S.D.; Gedamu, L. Cloning and characterization of three differentially expressed peroxidoxin genes from Leishmania chagasi: Evidence for an enzymatic detoxification of hydroxyl radicals. J. Biol. Chem. 2001, 276, 34279–34287. [Google Scholar] [CrossRef]
- Acestor, N.; Masina, S.; Ives, A.; Walker, J.; Saravia, N.G.; Fasel, N. Resistance to oxidative stress is associated with metastasis in mucocutaneous leishmaniasis. J. Infect. Dis. 2006, 194, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Iyer, J.P.; Kaprakkaden, A.; Choudhary, M.L.; Shaha, C. Crucial role of cytosolic tryparedoxin peroxidase in Leishmania donovani survival, drug response and virulence. Mol. Microbiol. 2008, 68, 372–391. [Google Scholar] [CrossRef] [PubMed]
- Plewes, K.A.; Barr, S.D.; Gedamu, L. Iron superoxide dismutases targeted to the glycosomes of Leishmania chagasi are important for survival. Infect. Immun. 2003, 71, 5910. [Google Scholar] [CrossRef] [PubMed]
- Davenport, B.J.; Martin, C.G.; Beverley, S.M.; Orlicky, D.J.; Vazquez-Torres, A.; Morrison, T.E. SODB1 is essential for Leishmania major infection of macrophages and pathogenesis in mice. PLoS Negl. Trop. Dis. 2018, 12, e0006921. [Google Scholar] [CrossRef]
- Mittra, B.; Laranjeira-Silva, M.F.; Miguel, D.C.; Perrone Bezerra de Menezes, J.; Andrews, N.W. The iron-dependent mitochondrial superoxide dismutase SODA promotes Leishmania virulence. J. Biol. Chem. 2017, 292, 12324–12338. [Google Scholar] [CrossRef]
- Getachew, F.; Gedamu, L. Leishmania donovani mitochondrial iron superoxide dismutase A is released into the cytosol during miltefosine induced programmed cell death. Mol. Biochem. Parasitol. 2012, 183, 42–51. [Google Scholar] [CrossRef]
- Veronica, J.; Chandrasekaran, S.; Dayakar, A.; Devender, M.; Prajapati, V.K.; Sundar, S.; Maurya, R. Iron superoxide dismutase contributes to miltefosine resistance in Leishmania donovani. FEBS J. 2019, 286, 3488–3503. [Google Scholar] [CrossRef]
- Nylén, S.; Eidsmo, L. Tissue damage and immunity in cutaneous leishmaniasis. Parasite Immunol. 2012, 34, 551–561. [Google Scholar] [CrossRef]
- Akuffo, H.; Maasho, K.; Blostedt, M.; Jeberg, B.; Britton, S.; Bakhiet, M. Leishmania aethiopica derived from diffuse leishmaniasis patients preferentially induce mRNA for interleukin-10 while those from localized leishmaniasis patients induce interferon-gamma. J. Infect. Dis. 1997, 175, 737–741. [Google Scholar] [CrossRef]
- Rivera-Fernández, I.; Argueta-Donohué, J.; Wilkins-Rodríguez, A.A.; Gutiérrez-Kobeh, L. Effect of two different isolates of Leishmania mexicana in the production of cytokines and phagocytosis by murine dendritic cells. J. Parasitol. 2019, 105, 359–370. [Google Scholar]
- Barroso, P.A.; Marco, J.D.; Calvopina, M.; Kato, H.; Korenaga, M.; Hashiguchi, Y. A trial of immunotherapy against Leishmania amazonensis infection in vitro and In Vivo with Z-100, a polysaccharide obtained from Mycobacterium tuberculosis, alone or combined with meglumine antimoniate. J. Antimicrob. Chemother. 2007, 59, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.M.; França-Costa, J.; Van Weyenbergh, J.; dos-Santos, W.L.C.; Santos de Campos, D.C.; Malta-Santos, H.; Oliveira, M.C.S.; Luz, N.F.; Boaventura, V.S.; Barral, A.; et al. Arginase I, polyamine, and prostaglandin E2 pathways suppress the inflammatory response and contribute to diffuse cutaneous leishmaniasis. J. Infect. Dis. 2014, 211, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Bacellar, O.; Lessa, H.; Schriefer, A.; Machado, P.; Ribeiro de Jesus, A.; Dutra, W.O.; Gollob, K.J.; Carvalho, E.M. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect. Immun. 2002, 70, 6734. [Google Scholar] [CrossRef] [PubMed]
- Hartley, M.A.; Ronet, C.; Zangger, H.; Beverley, S.M.; Fasel, N. Leishmania RNA virus: When the host pays the toll. Front. Cell. Infect. Microbiol. 2012, 2, 99. [Google Scholar] [CrossRef] [PubMed]
- Faria, D.R.; Souza, P.E.A.; Durães, F.V.; Carvalho, E.M.; Gollob, K.J.; Machado, P.R.; Dutra, W.O. Recruitment of CD8+ T cells expressing granzyme A is associated with lesion progression in human cutaneous leishmaniasis. Parasite Immunol. 2009, 31, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, W.N.; Ribeiro, L.E.; Schrieffer, A.; Machado, P.; Carvalho, E.M.; Bacellar, O. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of human tegumentary leishmaniasis. Cytokine 2014, 66, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Novais, F.O.; Carvalho, L.P.; Passos, S.; Roos, D.S.; Carvalho, E.M.; Scott, P.; Beiting, D.P. Genomic profiling of human Leishmania braziliensis lesions identifies transcriptional modules associated with cutaneous immunopathology. J. Investig. Dermatol. 2015, 135, 94–101. [Google Scholar] [CrossRef]
- Gómez-Arreaza, A.; Haenni, A.L.; Dunia, I.; Avilán, L. Viruses of parasites as actors in the parasite-host relationship: A “ménage à trois”. Acta Trop 2017, 166, 126–132. [Google Scholar] [CrossRef]
- Saberi, R.; Fakhar, M.; Mohebali, M.; Anvari, D.; Gholami, S. Global status of synchronizing Leishmania RNA virus in Leishmania parasites: A systematic review with meta-analysis. Transbound. Emerg. Dis. 2019, 0. [Google Scholar] [CrossRef]
- Grybchuk, D.; Kostygov, A.Y.; Macedo, D.H.; Avila-Levy, C.M.; Yurchenko, V. RNA viruses in trypanosomatid parasites: A historical overview. Memórias Inst. Oswaldo Cruz 2018, 113, e170487. [Google Scholar] [CrossRef]
- Zangger, H.; Hailu, A.; Desponds, C.; Lye, L.F.; Akopyants, N.S.; Dobson, D.E.; Ronet, C.; Ghalib, H.; Beverley, S.M.; Fasel, N. Leishmania aethiopica field isolates bearing an endosymbiontic dsRNA virus induce pro-inflammatory cytokine response. PLoS Negl. Trop. Dis. 2014, 8, e2836. [Google Scholar] [CrossRef] [PubMed]
- Lye, L.F.; Owens, K.; Shi, H.; Murta, S.M.F.; Vieira, A.C.; Turco, S.J.; Tschudi, C.; Ullu, E.; Beverley, S.M. Retention and loss of RNA interference pathways in trypanosomatid protozoans. PLoS Pathog. 2010, 6, e1001161. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, M.; Humann, J.; Penheiter, K.; Andreasen, K.; Lenz, L.L. Induction of IFN-αβ enables Listeria monocytogenes to suppress macrophage activation by IFN-γ. J. Exp. Med. 2010, 207, 327. [Google Scholar] [CrossRef] [PubMed]
- Ives, A.; Ronet, C.; Prevel, F.; Ruzzante, G.; Fuertes-Marraco, S.; Schutz, F.; Zangger, H.; Revaz-Breton, M.; Lye, L.F.; Hickerson, S.M.; et al. Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science 2011, 331, 775. [Google Scholar] [CrossRef]
- Fichorova, R.N.; Lee, Y.; Yamamoto, H.S.; Takagi, Y.; Hayes, G.R.; Goodman, R.P.; Chepa-Lotrea, X.; Buck, O.R.; Murray, R.; Kula, T.; et al. Endobiont viruses sensed by the human host – beyond conventional antiparasitic therapy. PLoS ONE 2012, 7, e48418. [Google Scholar] [CrossRef]
- Eren, R.O.; Reverte, M.; Rossi, M.; Hartley, M.-A.; Castiglioni, P.; Prevel, F.; Martin, R.; Desponds, C.; Lye, L.-F.; Drexler, S.K.; et al. Mammalian innate immune response to a Leishmania-resident RNA virus increases macrophage survival to promote parasite persistence. Cell Host Microbe 2016, 20, 318–328. [Google Scholar] [CrossRef]
- Ito, M.M.; Catanhêde, L.M.; Katsuragawa, T.H.; Silva Junior, C.F.d.; Camargo, L.M.A.; Mattos, R.d.G.; Vilallobos-Salcedo, J.M. Correlation between presence of Leishmania RNA virus 1 and clinical characteristics of nasal mucosal leishmaniosis. Braz. J. Otorhinolaryngol. 2015, 81, 533–540. [Google Scholar] [CrossRef]
- Cantanhêde, L.M.; da Silva Júnior, C.F.; Ito, M.M.; Felipin, K.P.; Nicolete, R.; Salcedo, J.M.V.; Porrozzi, R.; Cupolillo, E.; Ferreira, R.d.G.M. Further evidence of an association between the presence of Leishmania RNA virus 1 and the mucosal manifestations in tegumentary leishmaniasis patients. PLoS Negl. Trop. Dis. 2015, 9, e0004079. [Google Scholar] [CrossRef]
- Ginouvès, M.; Simon, S.; Bourreau, E.; Lacoste, V.; Ronet, C.; Couppié, P.; Nacher, M.; Demar, M.; Prévot, G. Prevalence and distribution of Leishmania RNA virus 1 in Leishmania parasites from French Guiana. Am. J. Trop. Med. Hyg. 2016, 94, 102–106. [Google Scholar] [CrossRef]
- Llanos-Cuentas, A.; Arevalo, J.; Adaui, V.; Zimic, M.; Garcia, L.; Maes, I.; De Doncker, S.; Dujardin, J.-C.; Dobson, D.E.; Lye, L.-F.; et al. Association of the endobiont double-stranded RNA virus LRV1 with treatment failure for human leishmaniasis caused by Leishmania braziliensis in Peru and Bolivia. J. Infect. Dis. 2015, 213, 112–121. [Google Scholar] [CrossRef]
- Bourreau, E.; Prévot, G.; Ginouves, M.; Couppié, P.; Bertolotti, A.; Sainte-Marie, D.; Dufour, J.; Ronet, C.; Hartley, M.-A.; Fasel, N.; et al. Presence of Leishmania RNA virus 1 in Leishmania guyanensis increases the risk of first-line treatment failure and symptomatic relapse. J. Infect. Dis. 2015, 213, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.D.O.R.; Maretti-Mira, A.C.; Rodrigues, K.M.; Lima, R.B.; Oliveira-Neto, M.P.D.; Cupolillo, E.; Pirmez, C.; Oliveira, M.P.D. Severity of tegumentary leishmaniasis is not exclusively associated with Leishmania RNA virus 1 infection in Brazil. Memórias Inst. Oswaldo Cruz 2013, 108, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Atayde, V.D.; da Silva Lira Filho, A.; Chaparro, V.; Zimmermann, A.; Martel, C.; Jaramillo, M.; Olivier, M. Exploitation of the Leishmania exosomal pathway by Leishmania RNA virus 1. Nat. Microbiol. 2019, 4, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.E.; Innes, D.J.; de Anastacio, Q.S.; Pearson, R.D. Early histopathology of experimental infection with Leishmania donovani in hamsters. J. Parasitol. 1987, 73, 55–63. [Google Scholar] [CrossRef]
- Wilson, M.E.; Sandor, M.; Blum, A.M.; Young, B.M.; Metwali, A.; Elliott, D.; Lynch, R.G.; Weinstock, J.V. Local suppression of IFN-gamma in hepatic granulomas correlates with tissue-specific replication of Leishmania chagasi. J. Immunol. 1996, 156, 2231–2239. [Google Scholar]
- Dutra, R.A.; Dutra, L.F.; de Reis, O.; Lambert, R.C. Splenectomy in a patient with treatment-resistant visceral leishmaniasis: A case report. Rev. Soc. Bras. Med. Trop 2012, 45, 130–131. [Google Scholar] [CrossRef][Green Version]
- McCall, L.I.; Zhang, W.W.; Matlashewski, G. Determinants for the development of visceral leishmaniasis disease. PLoS Pathog. 2013, 9, e1003053. [Google Scholar] [CrossRef]
- Farahmand, M.; Atashi Shirazi, H.; Nahrevanian, H.; Hajjaran, H. Molecular analysis of A2-genes encoding stage-specific S antigen-like proteins among isolates from Iranian cutaneous and visceral leishmaniasis. Iran. J. Basic Med. Sci. 2011, 14, 407–413. [Google Scholar]
- McCall, L.I.; Matlashewski, G. Localization and induction of the A2 virulence factor in Leishmania: Evidence that A2 is a stress response protein. Mol. Microbiol. 2010, 77, 518–530. [Google Scholar] [CrossRef]
- Garin, Y.J.F.; Meneceur, P.; Pratlong, F.; Dedet, J.-P.; Derouin, F.; Lorenzo, F. A2 gene of Old World cutaneous Leishmania is a single highly conserved functional gene. BMC Infect. Dis. 2005, 5, 18. [Google Scholar] [CrossRef]
- Zhang, W.W.; Mendez, S.; Ghosh, A.; Myler, P.; Ivens, A.; Clos, J.; Sacks, D.L.; Matlashewski, G. Comparison of the A2 gene locus in Leishmania donovani and Leishmania major and its control over cutaneous infection. J. Biol. Chem. 2003, 278, 35508–35515. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Gurumurthy, S.; Duncan, R.; Nakhasi, H.L.; Salotra, P. Comparative in vivo expression of amastigote up regulated Leishmania genes in three different forms of leishmaniasis. Parasitol. Int. 2010, 59, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Melby, P.C.; Tabares, A.; Restrepo, B.I.; Cardona, A.E.; McGuff, H.S.; Teale, J.M. Leishmania donovani: Evolution and architecture of the splenic cellular immune response related to control of infection. Exp. Parasitol. 2001, 99, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Murray, H.W. Tissue granuloma structure-function in experimental visceral leishmaniasis. Int. J. Exp. Pathol. 2001, 82, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Beattie, L.; Peltan, A.; Maroof, A.; Kirby, A.; Brown, N.; Coles, M.; Smith, D.F.; Kaye, P.M. Dynamic imaging of experimental Leishmania donovani-induced hepatic granulomas detects kupffer cell-restricted antigen presentation to antigen-specific CD8+ T cells. PLoS Pathog. 2010, 6, e1000805. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.E.; Young, B.M.; Davidson, B.L.; Mente, K.A.; McGowan, S.E. The importance of TGF-β in murine visceral leishmaniasis. J. Immunol. 1998, 161, 6148. [Google Scholar] [PubMed]
- Gantt, K.R.; Goldman, T.L.; McCormick, M.L.; Miller, M.A.; Jeronimo, S.M.B.; Nascimento, E.T.; Britigan, B.E.; Wilson, M.E. Oxidative responses of human and murine macrophages during phagocytosis of Leishmania chagasi. J. Immunol. 2001, 167, 893–901. [Google Scholar] [CrossRef]
- Carvalho, E.M.; Barral, A.; Pedral-Sampaio, D.; Barral-Netto, M.; Badaro, R.; Rocha, H.; Johnson, W.D., Jr. Immunologic markers of clinical evolution in children recently infected with Leishmania donovani chagasi. J. Infect. Dis. 1992, 165, 535–540. [Google Scholar] [CrossRef]
- Ghalib, H.W.; Whittle, J.A.; Kubin, M.; Hashim, F.A.; El-Hassan, A.M.; Grabstein, K.H.; Trinchieri, G.; Reed, S.G. IL-12 enhances Th1-type responses in human Leishmania donovani infections. J. Immunol. 1995, 154, 4623. [Google Scholar]
- Bacellar, O.; Brodskyn, C.; Guerreiro, J.; Barral-Netto, M.; Costa, C.H.; Coffman, R.L.; Johnson, W.D.; Carvalho, E.M. Interleukin-12 restores interferon-γ production and cytotoxic responses in visceral leishmaniasis. J. Infect. Dis. 1996, 173, 1515–1518. [Google Scholar] [CrossRef]
- Singh, O.P.; Gidwani, K.; Kumar, R.; Nylén, S.; Jones, S.L.; Boelaert, M.; Sacks, D.; Sundar, S. Reassessment of immune correlates in human visceral leishmaniasis as defined by cytokine release in whole blood. Clin. Vaccine Immunol. 2012, 19, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Faleiro, R.J.; Kumar, R.; Hafner, L.M.; Engwerda, C.R. Immune regulation during chronic visceral leishmaniasis. PLoS Negl. Trop. Dis. 2014, 8, e2914. [Google Scholar] [CrossRef] [PubMed]
- Ato, M.; Maroof, A.; Zubairi, S.; Nakano, H.; Kakiuchi, T.; Kaye, P.M. Loss of dendritic cell migration and impaired resistance to Leishmania donovani infection in mice deficient in CCL19 and CCL21. J. Immunol. 2006, 176, 5486. [Google Scholar] [CrossRef] [PubMed]
- Hammami, A.; Abidin, B.M.; Heinonen, K.M.; Stäger, S. HIF-1α hampers dendritic cell function and Th1 generation during chronic visceral leishmaniasis. Sci. Rep. 2018, 8, 3500. [Google Scholar] [CrossRef]
- Hammami, A.; Charpentier, T.; Smans, M.; Stäger, S. IRF-5-mediated inflammation limits CD8+ T cell expansion by inducing HIF-1α and impairing dendritic cell functions during Leishmania Infection. PLoS Pathog. 2015, 11, e1004938. [Google Scholar] [CrossRef]
- Ato, M.; Stäger, S.; Engwerda, C.R.; Kaye, P.M. Defective CCR7 expression on dendritic cells contributes to the development of visceral leishmaniasis. Nat. Immunol. 2002, 3, 1185–1191. [Google Scholar] [CrossRef]
- Gasim, S.; Elhassan, A.M.; Khalil, E.A.G.; Ismail, A.; Kadaru, A.M.Y.; Kharazmi, A.; Theander, T.G. High levels of plasma IL-10 and expression of IL-10 by keratinocytes during visceral leishmaniasis predict subsequent development of post-kala-azar dermal leishmaniasis. Clin. Exp. Immunol. 1998, 111, 64–69. [Google Scholar] [CrossRef]
- Hartley, M.A.; Kohl, K.; Ronet, C.; Fasel, N. The therapeutic potential of immune cross-talk in leishmaniasis. Clin. Microbiol. Infect. 2013, 19, 119–130. [Google Scholar] [CrossRef][Green Version]
- Berg, M.; Mannaert, A.N.; Vanaerschot, M.; Van Der Auwera, G.; Dujardin, J.C. (Post-) Genomic approaches to tackle drug resistance in Leishmania. Parasitology 2013, 140, 1492–1505. [Google Scholar] [CrossRef]
- Lamotte, S.; Späth, G.F.; Rachidi, N.; Prina, E. The enemy within: Targeting host–parasite interaction for antileishmanial drug discovery. PLoS Negl. Trop. Dis. 2017, 11, e0005480. [Google Scholar] [CrossRef]
- Hendrickx, S.; Caljon, G.; Maes, L. Need for sustainable approaches in antileishmanial drug discovery. Parasitol. Res. 2019, 118, 2743–2752. [Google Scholar] [CrossRef] [PubMed]
- De Muylder, G.; Vanhollebeke, B.; Caljon, G.; Wolfe, A.R.; McKerrow, J.; Dujardin, J.C. Naloxonazine, an amastigote-specific compound, affects Leishmania parasites through modulation of host-encoded functions. PLoS Negl. Trop. Dis. 2016, 10, e0005234. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Mukherjee, B.; Mukhopadhyay, R.; Naskar, K.; Sundar, S.; Dujardin, J.C.; Roy, S. Imipramine exploits histone deacetylase 11 to increase the IL-12/IL-10 ratio in macrophages infected with antimony-resistant Leishmania donovani and clears organ parasites in experimental infection. J. Immunol. 2014, 193, 4083. [Google Scholar] [CrossRef] [PubMed]
- Carmen, C.; Tamara, L.; Antonio, G.R.; Rita, C.; Carlo, M.; Stefano, C. Imiquimod 5% cream use in dermatology, side effects and recent patents. Recent Pat. Inflamm. Allergy Drug Discov. 2012, 6, 65–69. [Google Scholar] [CrossRef]
- Buates, S.; Matlashewski, G. Treatment of experimental leishmaniasis with the immunomodulators imiquimod and S-28463: Efficacy and mode of action. J. Infect. Dis. 1999, 179, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, R.; Bou Youness, H.; Lachaud, L.; Bastien, P.; Masquefa, C.; Bonnet, P.-A.; El Hajj, H.; Khalifeh, I. EAPB0503: An imiquimod analog with potent in vitro activity against cutaneous leishmaniasis caused by Leishmania major and Leishmania tropica. PLoS Negl. Trop. Dis. 2018, 12, e0006854. [Google Scholar] [CrossRef]
- Coutinho De Oliveira, B.; Duthie, M.S.; Alves Pereira, V.R. Vaccines for leishmaniasis and the implications of their development for American tegumentary leishmaniasis. Hum. Vaccines Immunother. 2019, 1–12. [Google Scholar] [CrossRef]
- Kumar, R.; Engwerda, C. Vaccines to prevent leishmaniasis. Clin. Transl. Immunol. 2014, 3, e13. [Google Scholar] [CrossRef]
- Moafi, M.; Rezvan, H.; Sherkat, R.; Taleban, R. Leishmania vaccines entered in clinical trials: A review of literature. Int. J. Prev. Med. 2019, 10, 95. [Google Scholar] [CrossRef]
- Raman, V.; Reed, S.; Duthie, M.; Fox, C.; Matlashewski, G. Adjuvants for Leishmania vaccines: From models to clinical application. Front. Immunol. 2012, 3, 144. [Google Scholar] [CrossRef]
- Alves-Silva, M.V.; Nico, D.; de Luca, P.M.; Palatnik de-Sousa, C.B. The F1F3 recombinant chimera of Leishmania donovani-nucleoside hydrolase (NH36) and its epitopes induce cross-protection against Leishmania (V.) braziliensis infection in mice. Front. Immunol. 2019, 10, 724. [Google Scholar] [CrossRef] [PubMed]
- Nico, D.; Gomes, D.C.; Palatnik-de-Sousa, I.; Morrot, A.; Palatnik, M.; Palatnik-de-Sousa, C.B. Leishmania donovani nucleoside hydrolase terminal domains in cross-protective immunotherapy against Leishmania amazonensis murine infection. Front. Immunol. 2014, 5, 273. [Google Scholar] [CrossRef] [PubMed]
- Alves-Silva, M.V.; Nico, D.; Morrot, A.; Palatnik, M.; Palatnik-de-Sousa, C.B. A chimera containing CD4+ and CD8+ T-cell epitopes of the Leishmania donovani nucleoside hydrolase (NH36) optimizes cross-protection against Leishmania amazonesis infection. Front. Immunol. 2017, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- De Brito, R.C.F.; Cardoso, J.M.D.O.; Reis, L.E.S.; Vieira, J.F.; Mathias, F.A.S.; Roatt, B.M.; Aguiar-Soares, R.D.D.O.; Ruiz, J.C.; Resende, D.d.M.; Reis, A.B. Peptide vaccines for leishmaniasis. Front. Immunol. 2018, 9, 1043. [Google Scholar] [CrossRef]
- Duthie, M.S.; Raman, V.S.; Piazza, F.M.; Reed, S.G. The development and clinical evaluation of second-generation leishmaniasis vaccines. Vaccine 2012, 30, 134–141. [Google Scholar] [CrossRef]
- Cecílio, P.; Oliveira, F.; Cordeiro-da-Silva, A. Vaccines for human leishmaniasis: Where do we stand and what is still missing. Leishmaniases Reemerging Dis. Rij. Intechopen 2018, 59–93. [Google Scholar]
- Duthie, M.S.; Van Hoeven, N.; MacMillen, Z.; Picone, A.; Mohamath, R.; Erasmus, J.; Hsu, F.-C.; Stinchcomb, D.T.; Reed, S.G. Heterologous immunization with defined RNA and subunit vaccines enhances T cell responses that protect against Leishmania donovani. Front. Immunol. 2018, 9, 2420. [Google Scholar] [CrossRef]
- Valilou, S.F.; Keshavarz-Fathi, M. Chapter 10-Genetic vaccine for cancer. In Vaccines for Cancer Immunotherapy; Rezaei, N., Keshavarz-Fathi, M., Eds.; Academic Press: London, UK, 2018; pp. 129–143. [Google Scholar]
- Kumar, A.; Samant, M. DNA vaccine against visceral leishmaniasis: A promising approach for prevention and control. Parasite Immunol. 2016, 38, 273–281. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, T.; Joshi, J. Immunogenicity and protective efficacy of DNA vaccine against visceral leishmaniasis in BALB/c mice. J. Biomed. Res. 2016, 30, 304–313. [Google Scholar] [CrossRef]
- Louis, L.; Clark, M.; Wise, M.C.; Glennie, N.; Wong, A.; Broderick, K.; Uzonna, J.; Weiner, D.B.; Scott, P. Intradermal synthetic DNA vaccination generates Leishmania-specific T cells in the skin and protection against Leishmania major. Infect. Immun. 2019, 87, IAI-00227. [Google Scholar] [CrossRef]
- Osman, M.; Mistry, A.; Keding, A.; Gabe, R.; Cook, E.; Forrester, S.; Wiggins, R.; Di Marco, S.; Colloca, S.; Siani, L.; et al. A third generation vaccine for human visceral leishmaniasis and post kala azar dermal leishmaniasis: First-in-human trial of ChAd63-KH. PLoS Negl. Trop. Dis. 2017, 11, e0005527. [Google Scholar] [CrossRef] [PubMed]
- Midoux, P.; Pichon, C. Lipid-based mRNA vaccine delivery systems. Expert Rev. Vaccines 2015, 14, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Maruggi, G.; Shan, H.; Li, J. Advances in mRNA vaccines for infectious diseases. Front. Immunol. 2019, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Aline, F.; Bout, D.; Amigorena, S.; Roingeard, P.; Dimier-Poisson, I. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect. Immun. 2004, 72, 4127–4137. [Google Scholar] [CrossRef]
- Schnitzer, J.K.; Berzel, S.; Fajardo-Moser, M.; Remer, K.A.; Moll, H. Fragments of antigen-loaded dendritic cells (DC) and DC-derived exosomes induce protective immunity against Leishmania major. Vaccine 2010, 28, 5785–5793. [Google Scholar] [CrossRef]
- Wahlund, C.J.E.; Güclüler, G.; Hiltbrunner, S.; Veerman, R.E.; Näslund, T.I.; Gabrielsson, S. Exosomes from antigen-pulsed dendritic cells induce stronger antigen-specific immune responses than microvesicles in vivo. Sci. Rep. 2017, 7, 17095. [Google Scholar] [CrossRef]
- Olivier, M.; Fernandez-Prada, C. Leishmania and its exosomal pathway: A novel direction for vaccine development. Future Microbiol. 2019, 14, 559–561. [Google Scholar] [CrossRef]
- Setten, R.L.; Rossi, J.J.; Han, S.-p. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef]
- Seyed, N.; Peters, N.C.; Rafati, S. Translating observations from leishmanization into non-living vaccines: The potential of dendritic cell-based vaccination strategies against Leishmania. Front. Immunol. 2018, 9, 1227. [Google Scholar] [CrossRef]
- Peters, N.C.; Kimblin, N.; Secundino, N.; Kamhawi, S.; Lawyer, P.; Sacks, D.L. Vector transmission of Leishmania abrogates vaccine-induced protective immunity. PLoS Pathog. 2009, 5, e1000484. [Google Scholar] [CrossRef]
- Peters, N.C.; Pagán, A.J.; Lawyer, P.G.; Hand, T.W.; Henrique Roma, E.; Stamper, L.W.; Romano, A.; Sacks, D.L. Chronic parasitic infection maintains high frequencies of short-lived Ly6C+CD4+ effector T cells that are required for protection against re-infection. PLoS Pathog. 2014, 10, e1004538. [Google Scholar] [CrossRef] [PubMed]
- Kimblin, N.; Peters, N.; Debrabant, A.; Secundino, N.; Egen, J.; Lawyer, P.; Fay, M.P.; Kamhawi, S.; Sacks, D. Quantification of the infectious dose of Leishmania major transmitted to the skin by single sand flies. Proc. Natl. Acad. Sci. USA 2008, 105, 10125–10130. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Gomes, F.L.; Roma, E.H.; Carneiro, M.B.H.; Doria, N.A.; Sacks, D.L.; Peters, N.C. Site-dependent recruitment of inflammatory cells determines the effective dose of Leishmania major. Infect. Immun. 2014, 82, 2713. [Google Scholar] [CrossRef] [PubMed]
| Leishmaniasis | Most Common Etiological Agents | Manifestations | Geographical Distribution |
|---|---|---|---|
| Cutaneous (CL) | / | / | 84% CL cases reported in Afghanistan, Algeria, Brazil, Colombia, Iraq, Pakistan, Peru, the Syrian Arab Republic, Tunisia and Yemen (> 90% MCL cases reported in Brazil, Peru and Bolivia) [5,19] |
| Localized (LCL) | L. amazonensis L. major L. aethiopica L. mexicana | Small nodules or papules at the vector’s bite sites that may progress to ulcerated lesions | |
| Diffuse (DCL) | L. amazonensis L. mexicana L. aethiopica | Disseminated nodular and non-ulcerating lesions | |
| Mucocutaneous (MCL) | L. braziliensis L. mexicana L. panamensis L. major 1 L. infantum 1 L. tropica 1 | Metastatic secondary lesions in naso-oral and pharyngeal cavities and tissue destruction | |
| Visceral (VL) | L. donovani L. infantum L. amazonensis 1 | Splenomegaly, hepatomegaly, weight loss, persistent fever and anemia. Post-Kala-azar Dermal Leishmaniasis (PKDL): skin rashes or non-ulcerating cutaneous lesions after apparent resolution of VL disease | Most VL cases reported in Brazil, Ethiopia, India, Nepal, Bangladesh, Kenya, Somalia, South Sudan and Sudan [19] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos Meira, C.; Gedamu, L. Protective or Detrimental? Understanding the Role of Host Immunity in Leishmaniasis. Microorganisms 2019, 7, 695. https://doi.org/10.3390/microorganisms7120695
dos Santos Meira C, Gedamu L. Protective or Detrimental? Understanding the Role of Host Immunity in Leishmaniasis. Microorganisms. 2019; 7(12):695. https://doi.org/10.3390/microorganisms7120695
Chicago/Turabian Styledos Santos Meira, Camila, and Lashitew Gedamu. 2019. "Protective or Detrimental? Understanding the Role of Host Immunity in Leishmaniasis" Microorganisms 7, no. 12: 695. https://doi.org/10.3390/microorganisms7120695
APA Styledos Santos Meira, C., & Gedamu, L. (2019). Protective or Detrimental? Understanding the Role of Host Immunity in Leishmaniasis. Microorganisms, 7(12), 695. https://doi.org/10.3390/microorganisms7120695





