Diversity of Rickettsia in Ticks Collected in Abruzzi and Molise Regions (Central Italy)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Tick Collection and Identification
2.3. Rickettsia DNA Detection and Identification
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.-E.; et al. Update on tick borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clin. Microbiol. Rev. 2005, 18, 719–756. [Google Scholar] [CrossRef] [PubMed]
- Socolovschi, C.; Mediannikov, O.; Raoult, D.; Parola, P. The relationship between spotted fever group Rickettsiae and ixodid ticks. Vet. Res. 2009, 40, 34. [Google Scholar] [CrossRef] [PubMed]
- Socolovschi, C.; Huynh, T.P.; Davoust, B.; Gomez, J.; Raoult, D.; Parola, P. Transovarial and trans-stadial transmission of Rickettsiae africae in Amblyomma variegatum ticks. Clin. Microbiol. Infect. 2009, 15, 317–318. [Google Scholar] [CrossRef]
- Matsumoto, K.; Brouqui, P.; Raoult, D.; Parola, P. Experimental infection models of ticks of the Rhipicephalus sanguineus group with Rickettsia conorii. Vector Borne Zoonotic Dis. 2005, 5, 363–372. [Google Scholar] [CrossRef]
- de Sousa, R.; Nobrega, S.D.; Bacellar, F.; Torgal, J. Mediterranean spotted fever in Portugal: Risk factors for fatal outcome in 105 hospitalized patients. Ann. N. Y. Acad. Sci. 2003, 990, 285–294. [Google Scholar] [CrossRef]
- Duque, V.; Ventura, C.; Seixas, D.; Barai, A.; Mendonça, N.; Martins, J.; da Cunha, S.; Meliço-Silvestre, A. Mediterranean spotted fever and encephalitis: A case report and review of the literature. J. Infect. Chemother. 2012, 18, 105–108. [Google Scholar] [CrossRef]
- Mancini, F.; Rezza, G.; Ciervo, A. Le rickettsiosi in Italia: Diagnosi e sorveglianza. Not. Ist. Super Sanità 2015, 28, 3–8. [Google Scholar]
- Manilla, G. Acari Ixodida. In Fauna d’Italia, 1st ed.; Edizioni Calderini: Bologna, Italy, 1998. [Google Scholar]
- Graziani, C.; Duranti, A.; Morelli, A.; Busani, L.; Pezzoti, P. Zoonosi in Italia Nel Periodo 2009–2013; Rapporti ISTISAN; Istituto Superiore di Sanità: Rome, Italy, 2016; 72p. [Google Scholar]
- Maioli, G.; Pistone, D.; Bonilauri, P.; Pajoro, M.; Barbieri, I.; Mulatti, P.; Vicari, N.; Dottori, M. Etiological agents of rickettsiosis and anaplasmosis in ticks collected in Emilia-Romagna region (Italy) during 2008 and 2009. Exp. Appl. Acarol. 2012, 57, 199–208. [Google Scholar] [CrossRef]
- Scarpulla, M.; Barlozzari, G.; Marcario, A.; Salvato, L.; Blanda, V.; De Liberato, C.; D’Agostini, C.; Torina, A.; Macrì, G. Molecular detection and characterization of spotted fever group rickettsiae in ticks from Central Italy. Ticks Tick Borne Dis. 2016, 7, 1052–1056. [Google Scholar] [CrossRef]
- Selmi, M.; Ballardini, M.; Salvato, L.; Ricci, E. Rickettsia spp. in Dermacentor marginatus ticks: Analysis of the host–vector–pathogen interactions in a northern Mediterranean area. Exp. Appl. Acarol. 2017, 72, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Chisu, V.; Leulmi, H.; Masala, G.; Piredda, M.; Foxi, C.; Parola, P. Detection of Rickettsia hoogstraalii, Rickettsia helvetica, Rickettsia massiliae, Rickettsia slovaca and Rickettsia aeschlimannii in ticks from Sardinia, Italy. Ticks Tick Borne Dis. 2017, 8, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Barroso, D.; Vescio, M.F.; Bella, A.; Ciervo, A.; Busani, L.; Rizzo, C.; Rezza, G.; Pezzotti, P. Mediterranean spotted fever rickettsiosis in Italy, 2001–2015: Spatio-temporal distribution based on hospitalization records. Ticks Tick Borne Dis. 2019, 10, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, M.; Cammà, C.; Curini, V.; Dall’Acqua, F.; Di Sabatino, D.; Pascucci, I. Molecular survey of tick-borne pathogens in wild boars from Central Italy. In Proceedings of the 15th Biodefence Conference, Munich, Germany, 26–29 April 2016. [Google Scholar]
- Pascucci, I.; Di Domenico, M.; Angelico, G.; Curini, V.; Cammà, C.; Sozio, G. Ecology of tick-borne pathogens: Investigation on rodents and ticks in protected areas in Central Italy. In Proceedings of the 20th International Conference of European Society for Vector Ecology E Sove, Lisbon, Portugal, 2–7 October 2016. [Google Scholar]
- Kato, C.Y.; Chung, I.H.; Robinson, L.K.; Austin, A.L.; Dasch, G.A.; Massung, R.F. Assessment of real-time PCR assay for detection of Rickettsia spp. and Rickettsia rickettsii in banked clinical samples. J. Clin. Microbiol. 2013, 51, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Santibáñez, S.; Portillo, A.; Santibáñez, P.; Palomar, A.M.; Oteo, J.A. Usefulness of rickettsial PCR assays for the molecular diagnosis of human rickettsioses. Enferm. Infecc. Microbiol. Clin. 2013, 31, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Bertelloni, F.; Turchi, B.; Filogari, D.; Cerri, D. Molecular survey of tick-borne pathogens in Ixodid ticks collected from hunted wild animals in Tuscany, Italy. Asian Pac. J. Trop. Med. 2015, 8, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Vescio, M.F.; Toma, L.; Di Luca, M.; Severini, F.; Cacciò, S.M.; Mariano, C.; Nicolai, G.; Laghezza Masci, V.; Fausto, A.M.; et al. Detection of tick-borne pathogens in ticks collected in the suburban area of Monte Romano, Lazio Region, Central Italy. Ann. Ist Super Sanita 2019, 55, 143–150. [Google Scholar] [CrossRef]
- Kiewra, D.; Lonc, E. Epidemiological consequences of host specificity of ticks (Ixodida). Ann. Parasitol. 2012, 58, 181–187. [Google Scholar]
- Arthur, D.R. The host relationships of Ixodes hexagonus leach in Britain. Parasitology 1953, 43, 227–238. [Google Scholar] [CrossRef]
- Zemtsova, G.E.; Apanaskevich, D.A.; Reeves, W.K.; Hahn, M.; Snellgrove, A.; Levin, M.L. Phylogeography of Rhipicephalus sanguineus sensu lato and its relationships with climatic factors. Exp. Appl. Acarol. 2016, 69, 191–203. [Google Scholar] [CrossRef]
- Selmi, M.; Tomassone, L.; Ceballos, L.A.; Crisci, A.; Ragagli, C.; Pintore, M.D.; Mignone, W.; Pautasso, A.; Ballardini, M.; Casalone, C.; et al. Analysis of the environmental and host-related factors affecting the distribution of the tick Dermacentor marginatus. Exp. Appl. Acarol. 2018, 75, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Chisu, V.; Foxi, C.; Masala, G. First molecular detection of the human pathogen Rickettsia raoultii and other spotted fever group Rickettsiae in Ixodid ticks from wild and domestic mammals. Parasitol. Res. 2018, 117, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Satta, G.; Chisu, V.; Cabras, P.; Fois, F.; Masala, G. Pathogens and symbionts in ticks: A survey on tick species distribution and presence of tick-transmitted micro-organisms in Sardinia, Italy. J. Med. Microbiol. 2011, 60, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, G.; Mancini, F.; Caddeo, A.; Ciervo, A.; Babudieri, S.; Maida, I.; Fiori, M.L.; Rezza, G.; Mura, M.S. Rickettsia monacensis as cause of Mediterranean spotted fever-like illness, Italy. Emerg. Infect. Dis. 2012, 18, 702–704. [Google Scholar] [CrossRef] [PubMed]
- Neet, C.R. Population dynamics and management of Sus scrofa in western Switzerland: A statistical modeling approach. Ibex JME 1995, 3, 188–191. [Google Scholar]
- Apollonio, M.; Andersen, R.; Putman, R. European Ungulates and Their Management in the 21st Century; Cambridge University Press: Cambridge, UK, 2010; p. 604. [Google Scholar]
- Socolovschi, C.; Gaudart, J.; Bitam, I.; Huynh, T.P.; Raoult, D.; Parola, P. Why are there so few Rickettsia conorii conorii-infected Rhipicephalus sanguineus ticks in the wild? PLoS Negl. Trop. Dis. 2012, 6, e1697. [Google Scholar] [CrossRef]
- Mediannikov, O.; Matsumoto, K.; Samoylenko, I.; Drancourt, M.; Roux, V.; Rydkina, E.; Davoust, B.; Tarasevich, I.; Brouqui, P.; Fournier, P.E. Rickettsia raoultii sp. nov., a spotted fever group Rickettsia associated with Dermacentor ticks in Europe and Russia. Int. J. Syst. Evol. Microbiol. 2008, 58, 1635–1639. [Google Scholar] [CrossRef]
- Toma, L.; Mancini, F.; Di Luca, M.; Cecere, J.G.; Bianchi, R.; Khoury, C.; Quarchioni, E.; Manzia, F.; Rezza, G.; Ciervo, A. Detection of Microbial Agents in Ticks Collected from Migratory Birds in Central Italy. Vector-Borne Zoonotic Dis. 2014, 14, 199–205. [Google Scholar] [CrossRef]
- Wallménius, K.; Barboutis, C.; Fransson, T.; Jaenson, T.G.; Lindgren, P.E.; Nyström, F.; Olsen, B.; Salaneck, E.; Nilsson, K. Spotted fever Rickettsia species in Hyalomma and Ixodes ticks infesting migratory birds in the European Mediterranean area. Parasit. Vectors 2014, 10, 318. [Google Scholar] [CrossRef]
- Pascucci, I.; Di Domenico, M.; Capobianco Dondona, G.; Di Gennaro, A.; Polci, A.; Capobianco Dondona, A.; Mancuso, E.; Cammà, C.; Savini, G.; Cecere, J.; et al. Assessing the role of migratory birds in the introduction of ticks and tick-borne pathogens from African countries: An Italian experience. Ticks Tick Borne Dis. 2019, 10, 101272. [Google Scholar] [CrossRef]
- Beati, L.; Meskini, M.; Thiers, B.; Raoult, D. Rickettsia aeschlimannii sp. nov., a new spotted fever group rickettsia associated with Hyalomma marginatum ticks. Int. J. Syst. Bacteriol. 1997, 47, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Tosoni, A.; Mirijello, A.; Ciervo, A.; Mancini, F.; Rezza, G.; Damiano, F.; Cauda, R.; Gasbarrini, A.; Addolorato, G. Human Rickettsia aeschlimannii infection: First case with acute hepatitis and review of the literature. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2630–2633. [Google Scholar] [PubMed]
- Angelakis, E.; Mediannikov, O.; Parola, P.; Raoult, D. Rickettsia felis: The complex journey of an emergent human pathogen. Trends Parasitol. 2016, 32, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Duh, D.; Punda-Polić, V.; Trilar, T.; Petrovec, M.; Bradarić, N.; Avsic-Zupanc, T. Molecular identification of Rickettsia felis-like bacteria in Haemaphysalis sulcata ticks collected from domestic animals in southern Croatia. Ann. N. Y. Acad. Sci. 2006, 1078, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Duh, D.; Punda-Polic, V.; Avsic-Zupanc, T.; Bouyer, D.; Walker, D.H.; Popov, V.L.; Jelovsek, M.; Gracner, M.; Trilar, T.; Bradaric, N.; et al. Rickettsia hoogstraalii sp. nov., isolated from hard- and soft-bodied ticks. Int. J. Syst. Evol. Microbiol. 2010, 60, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Raele, D.A.; Galante, D.; Pugliese, N.; Salandra, G.; Cafiero, M.A. Spotted fever group rickettsiae associated with ixodid ticks in wild environment in Southern Italy. Microbiologyopen 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Radzijevskaja, J.; Kaminskienė, E.; Lipatova, I.; Mardosaitė-Busaitienė, D.; Balčiauskas, L.; Stanko, M.; Paulauskas, A. Prevalence and diversity of Rickettsia species in ectoparasites collected from small rodents in Lithuania. Parasites Vectors 2018, 28, 375. [Google Scholar] [CrossRef]
- Merhej, V.; Angelakis, E.; Socolovschi, C.; Raoult, D. Genotyping, evolution and epidemiological findings of Rickettsia species. Infect. Genet. Evol. 2014, 25, 122–137. [Google Scholar] [CrossRef]
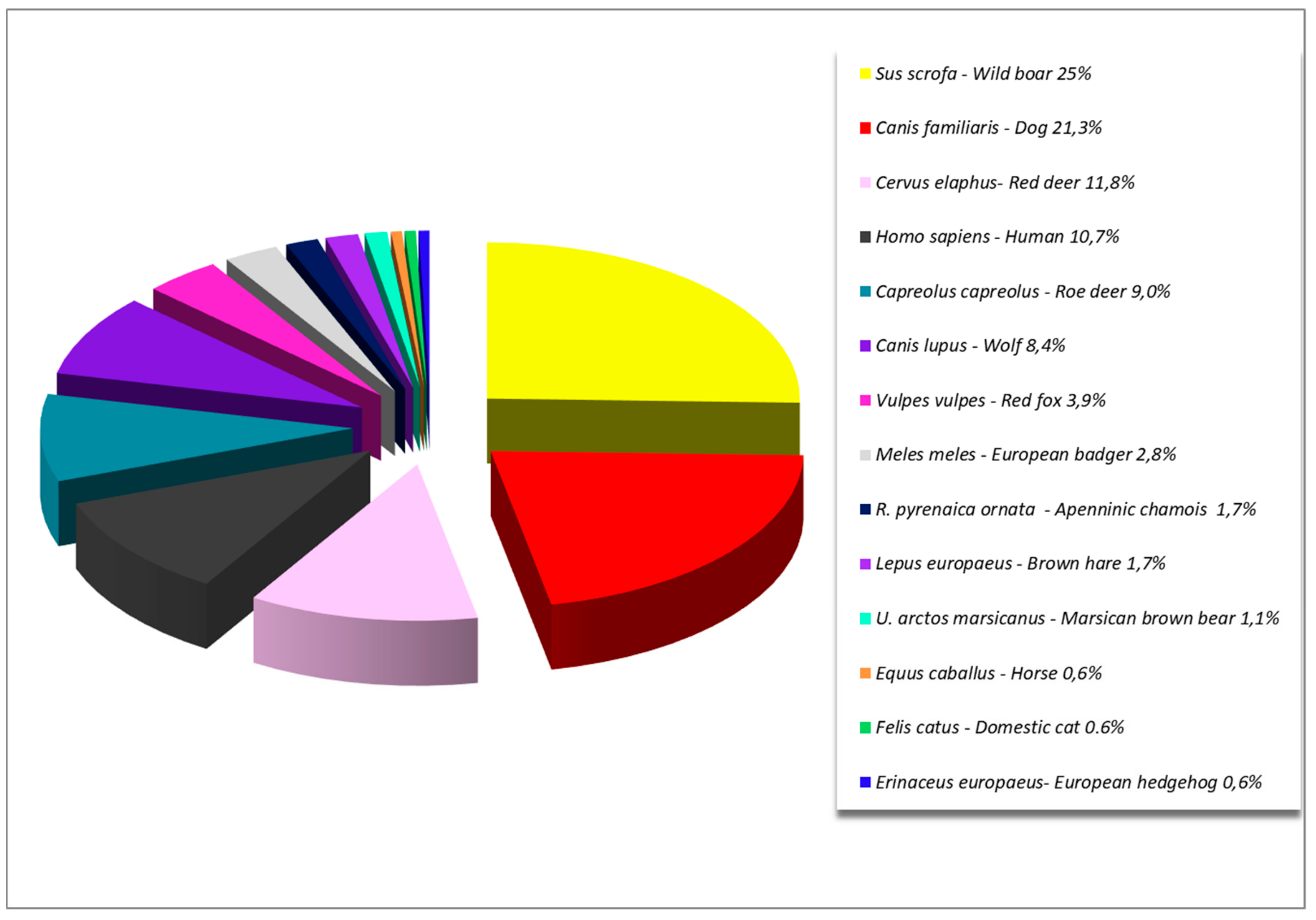
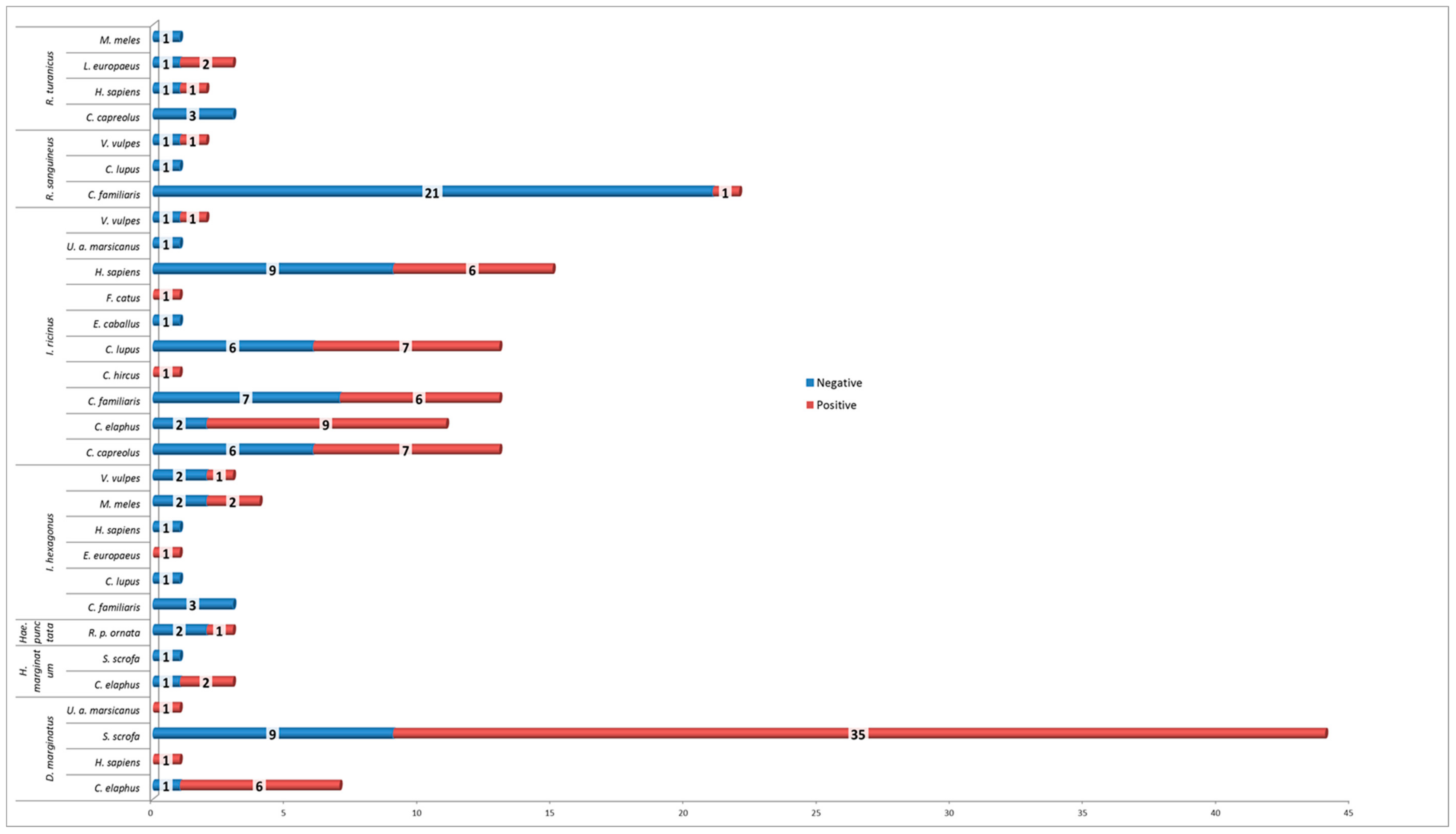
| Table | Negative | Positive | Total | Tick Species Frequency | Minimum Infection Rate |
|---|---|---|---|---|---|
| I. ricinus | 33 | 38 | 71 | 39.9% | 53.5% |
| D. marginatus | 10 | 43 | 53 | 29.8% | 81.1% |
| R. sanguineus | 23 | 2 | 25 | 14.0% | 8.0% |
| I. hexagonus | 9 | 4 | 13 | 7.3% | 30.8% |
| R. turanicus | 6 | 3 | 9 | 5.1% | 33.3% |
| H. marginatum | 2 | 2 | 4 | 2.2% | 50.0% |
| Hae. punctata | 2 | 1 | 3 | 1.7% | 33.3% |
| Total | 85 | 93 | 178 | 52.25% |
| Rickettsia species | Host | Ticks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D. marginatus | H. marginatum | Hae. punctata | I. hexagonus | I. ricinus | R. sanguineus s.l. | R. turanicus | Total | % | Total MIR | ||
| R. conorii | L. europaeus | / | / | / | / | / | / | 1 | / | / | / |
| R. conorii Total | / | / | / | / | / | / | 1 | 1 | 1.1% | 0.6% | |
| R. felis | E. europaeus | / | / | / | 1 | / | / | / | / | / | / |
| V. vulpes | / | / | / | 1 | / | / | / | / | / | / | |
| R. felis Total | / | / | / | 2 | / | / | / | 2 | 2.2% | 1.1% | |
| R. haeschlimanni | C. elaphus | / | 2 | / | / | / | / | / | / | / | / |
| R. haeschlimanni Total | / | / | 2 | / | / | / | / | / | 2 | 2.2% | 1.1% |
| R.helvetica | H. sapiens | / | / | / | / | 1 | / | / | / | / | / |
| V. vulpes | / | / | / | / | 1 | / | / | / | / | / | |
| R. helvetica Total | / | / | / | / | / | 2 | / | / | 2 | 2.2% | 1.1% |
| R. massiliae | H. sapiens | / | / | / | / | / | / | 1 | / | / | / |
| L. europaeus | / | / | / | / | / | / | 1 | / | / | / | |
| V. vulpes | / | / | / | / | / | 1 | / | / | / | / | |
| R. massiliae Total | / | / | / | / | / | / | 1 | 2 | 3 | 3.2% | 1.7% |
| R. monacensis | C. capreolus | / | / | / | / | 7 | / | / | / | / | / |
| C. elaphus | / | / | / | / | 8 | / | / | / | / | / | |
| C. familiaris | / | / | / | / | 6 | 1 | / | / | / | / | |
| C. lupus | / | / | / | / | 7 | / | / | / | / | / | |
| F. catus | / | / | / | / | 1 | / | / | / | / | / | |
| H. sapiens | / | / | / | / | 5 | / | / | / | / | / | |
| R. p. ornata | / | / | 1 | / | / | / | / | / | / | / | |
| U. a. marsicanus | 1 | / | / | / | / | / | / | / | / | / | |
| C. hircus | / | / | / | / | 1 | / | / | / | / | / | |
| R. monacensis Total | / | 1 | / | 1 | / | 35 | 1 | / | 38 | 40.9% | 21.3% |
| R. raoultii/R. slovaca | C. elaphus | / | / | / | / | 1 | / | / | / | / | / |
| M. meles | / | / | / | 1 | / | / | / | / | / | / | |
| S. scrofa | 1 | / | / | / | / | / | / | / | / | / | |
| R. raoultii/R. slovaca Total | / | 1 | / | / | 1 | 1 | / | / | 3 | 3.2% | 1.7% |
| R. slovaca | C. elaphus | 6 | / | / | / | / | / | / | / | / | / |
| H. sapiens | 1 | / | / | / | / | / | / | / | / | / | |
| S. scrofa | 34 | / | / | / | / | / | / | / | / | / | |
| R. slovaca Total | / | 41 | / | / | / | / | / | / | 41 | 44.1% | 23.0% |
| R. raoultii | M. meles | / | / | / | 1 | / | / | / | / | / | / |
| R. raoultii Total | / | / | / | 1 | / | / | / | 1 | 1,1% | 0.6% | |
| Total | / | 43 | 2 | 1 | 4 | 38 | 2 | 3 | 93 | 100% | 52.2% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascucci, I.; Di Domenico, M.; Curini, V.; Cocco, A.; Averaimo, D.; D’Alterio, N.; Cammà, C. Diversity of Rickettsia in Ticks Collected in Abruzzi and Molise Regions (Central Italy). Microorganisms 2019, 7, 696. https://doi.org/10.3390/microorganisms7120696
Pascucci I, Di Domenico M, Curini V, Cocco A, Averaimo D, D’Alterio N, Cammà C. Diversity of Rickettsia in Ticks Collected in Abruzzi and Molise Regions (Central Italy). Microorganisms. 2019; 7(12):696. https://doi.org/10.3390/microorganisms7120696
Chicago/Turabian StylePascucci, Ilaria, Marco Di Domenico, Valentina Curini, Antonio Cocco, Daniela Averaimo, Nicola D’Alterio, and Cesare Cammà. 2019. "Diversity of Rickettsia in Ticks Collected in Abruzzi and Molise Regions (Central Italy)" Microorganisms 7, no. 12: 696. https://doi.org/10.3390/microorganisms7120696
APA StylePascucci, I., Di Domenico, M., Curini, V., Cocco, A., Averaimo, D., D’Alterio, N., & Cammà, C. (2019). Diversity of Rickettsia in Ticks Collected in Abruzzi and Molise Regions (Central Italy). Microorganisms, 7(12), 696. https://doi.org/10.3390/microorganisms7120696






