Abstract
Increasingly, Johne’s disease of ruminants and human Crohn’s disease are regarded as the same infectious disease: paratuberculosis. Mycobacterium avium ss. paratuberculosis (MAP) is the cause of Johne’s and is the most commonly linked infectious cause of Crohn’s disease. Humans are broadly exposed to MAP in dairy products and in the environment. MAP has been found within granulomas such as Crohn’s disease and can stimulate autoantibodies in diseases such as type 1 diabetes (T1D) and Hashimoto’s thyroiditis. Moreover, beyond Crohn’s and T1D, MAP is increasingly associated with a host of autoimmune diseases. This article suggests near equivalency between paucibacillary Johne’s disease of ruminant animals and human Crohn’s disease and implicates MAP zoonosis beyond Crohn’s disease to include T1D.
Keywords:
Mycobacterium avium ss. paratuberculosis; MAP; Crohn’s; Johne’s; zoonosis; CARD15; SLC11a1; type 1 diabetes mellitus; T1D; molecular mimicry; ZnT8; HSP65; HERV; TRIGR study; sarcoidosis; Blau syndrome; multiple sclerosis; Hashimoto’s thyroiditis; lupus; Parkinson’s disease; rheumatoid arthritis 1. Introduction
Mycobacterium avium ss. paratuberculosis (MAP) is the cause of paratuberculosis, or Johne’s disease. It is mostly studied in ruminant animals such as cattle, goats and sheep. After one hundred years of controversy, there is a warming to the notion that MAP is also the zoonotic cause of the similar Crohn’s disease of humans [1,2,3,4]. Koch’s postulates are the criteria used to establish a causal relationship between microbe and disease. These postulates state that the microbe must: (1) be found in all cases of the disease, (2) be recovered and maintained in pure culture, (3) be capable of producing the original infection even after several generations in culture and (4) be retrievable from an inoculated animal and cultured again. The basis of the hundred-year controversy is the fact that traditional culturing (and staining) has been largely unsuccessful in identifying MAP in human samples [2,3,4]. Some have argued that these criteria have been met, tying MAP to Crohn’s disease [1,2]. Others contend that the postulates were established for acute infectious diseases and do not equally apply to chronic diseases like paratuberculosis, wherein individuals may become infected but remain in a latent subclinical state without developing a clinical disease, despite a positive culture and/or PCR [5,6,7,8,9,10]. Such latency is also seen in tuberculosis, where the estimated ratio of healthy infected carriers to new TB patients is 219:1 [11]. MAP is very difficult to culture from humans and eludes detection. MAP can exist with a modified cell wall—the component of the bacterium that takes up the characteristic acid stain. MAP can shed its cell wall, becoming a spheroplast or L-form (Figure 1) [12]. The bacterium is then no longer “acid fast” and cannot be detected microscopically in the traditional manner. This morphologic change allows MAP to become spore-like. The spore morphotype capable of surviving heat and other stressors enables MAP to persist in host macrophages and in the environment [13]. Adding to the difficulty of microscopic confirmation of MAP is that MAP, as with leprosy [14] and tuberculosis [15], can persist in a paucibacillary form (low numbers of observed organisms) [10]. Culture-independent methods such as PCR offer a more rapid indication of the presence of MAP than culture [16,17].
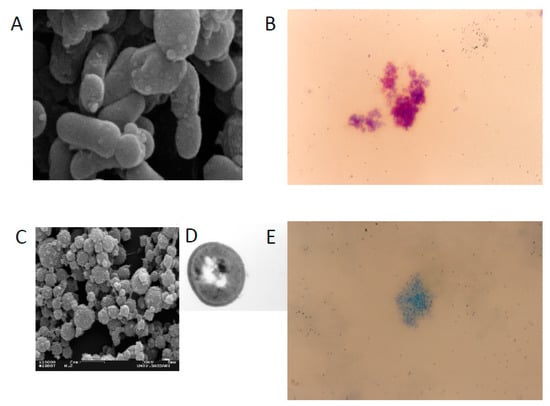
Figure 1.
Scanning electron microscopy and Ziehl Neelsen staining of MAP cells IS900 positives growth in absence of Lysozime (A,B, respectively) with a bacillary shape and wild type cell wall. Scanning, Transmission electron microscopy and Ziehl Neelsen staining of MAP cells IS900 positives growth in presence of Lysozime when the bacteria lost the cell wall that takes up the characteristic acid stain (C–E respectively) with a round shape and cell wall deficient form.
In 2004, Naser was able to culture MAP from the blood of Crohn’s patients [18]. The article was published in The Lancet and was featured on the cover. It read: “We detected viable Mycobacterium avium subspecies paratuberculosis in peripheral blood in a substantial proportion of individuals with Crohn’s disease, adding to the evidence for a role of the organism in the aetiology of this disease.”
This report “resulted in vigorous debate in the literature.” The authors were challenged to reproduce the study in a blind multi-center investigation. They did. Samples were split between four labs: three dedicated labs for MAP and a medical reference lab. All the labs were able to grow MAP except the medical reference lab [19]. This is at the heart of the century-long controversy—it is difficult to detect MAP with older laboratory methods. In 2005, Sechi and associates, in the largest series to date, reported the isolation of MAP from intestinal mucosal biopsies of Crohn’s patients [20]. Of note, MAP has been cultured from the breast milk of patients with Crohn’s disease [21,22]. The linkage of Crohn’s and Johne’s, with contemporary methods, has been validated in testing tissue at both a cellular and molecular level [23].
MAP-associated diseases have been explored due to the identification of shared genetic risks for the specific disease and concomitant mycobacterial infection. Investigations of polymorphisms of the CARD15 (NOD2) [2,24,25], SLC11a1 (NRAMP1) [26,27,28], LRRK2 [29,30], PTPN2/22 [31] and VDR [32] genes have proven fruitful as they impart a permissive state for mycobacterial infection due to the disruption of pathogen recognition and/or phagosome maturation. These genes have been linked to MAP and the following diseases: Crohn’s disease [2,28], Blau syndrome [2], multiple sclerosis [2,33], autoimmune (Hashimoto’s) thyroiditis [34,35,36], Parkinson’s disease [29,37], rheumatoid arthritis [27,31,38], lupus [39] and T1D [32,40].
2. MAP and Human Exposure
According to the USDA (United States Department of Agriculture), the herd-level prevalence of MAP infection in US dairy herds has increased from 21.6% in 1996 to 91.1% in 2007 [41]. MAP is present in pasteurized milk [42,43], infant formula made from pasteurized milk [44], surface water [45,46,47,48], soil [45], cow manure ‘‘lagoons’’ that can leach into surface water, cow manure in both solid and liquid forms that is applied as fertilizer to agricultural land [49,50] and municipal tap water [51]. All of these provide multiple opportunities for MAP exposure. In an Ohio study of domestic tap water, DNA of MAP was found in over 80% of the samples [52]. Filtration and chlorination, normal water treatment processes, kill off MAP competitors, thereby amplifying mycobacteria organisms instead of killing them [53]. Moreover, mycobacteria organisms grow on plastic water bottles [54], on tap water pipes [55] and in biofilms [56]. A study testing 65 samples of infant formula from 18 countries found more than 40% of samples were positive for viable MAP [57].
3. MAP and Crohn’s
Increasingly, Johne’s and Crohn’s are being regarded as the same infectious disease: paratuberculosis [58,59,60,61]. Studies show detection and isolation of MAP in adults with chronic Crohn’s [20], as well as with newly diagnosed pediatric Crohn’s patients [62]. Meta-analyses have shown that a majority of studies associating MAP with Crohn’s demonstrate MAP infection in the Crohn’s patients [63,64]. MAP has been cultured from peripheral blood white cells in a range from 50% to 100% of patients with Crohn’s disease, and less frequently from healthy individuals [65].
As noted, paratuberculosis is a global disease. Extensive testing in India describes an increasing MAP “bio-load” in cattle (43%), buffalo (36%), goats (23%) and sheep (41%). Moreover, in this same geographic area, 30.8% of 28,291 humans (via serum ELISA, blood PCR and stool PCR) tested positive for MAP [61]. Particularly telling of MAP as a zoonotic agent is the resolution of Crohn’s disease with anti-mycobacterial drugs targeting MAP [60]. The combination of clarithromycin, rifabutin and clofazimine, all anti-mycobacterial drugs, has shown efficacy as a primary treatment for Crohn’s disease [66].
An ongoing clinical trial is the RHB-104 study. The treatment is a proprietary combination of these same three anti-mycobacterial drugs for individuals with moderately severe or severe Crohn’s disease. The RHB-104 study is a multi-center, international interventional open clinical trial with 331 participants. Its primary outcome: “Reduction of the total Crohn’s Disease Activity Index (CDAI) score to less than 150” [67]. Results of the RHB-104 study were reported at the 2018 United European Gastroenterology meeting. Adding the anti-MAP therapy to standard therapy provided a “clinically meaningful and statistically significant treatment effect…” The trial data are from the published abstract LB06 [68]. Of note, RHB-104, the same three anti-mycobacterial drugs, is in clinical trial for another MAP-related disease, multiple sclerosis [69].
4. MAP and Diabetes
T1D occurs with the autoimmune destruction of the insulin-producing cells of the pancreas. It is most often seen in childhood or adolescence [70]. T1D has been on the increase since the last half of the 20th century [71]. Autoantibodies to pancreatic protein glutamic acid decarboxylase (GAD) are found in newly diagnosed children with T1D. These autoantibodies are felt to be the result of molecular mimicry whereby a foreign antigen (introduced by a bacterium) provokes an immune response, which then cross-reacts with a similar host protein [72,73,74]. The GAD pancreatic enzyme shares the sequence and conformational structure with the mycobacterial heat shock protein 65 (HSP65) [75]. In one study, all newly diagnosed T1D children had an immune response to mycobacterial HSP65 [76]. Multiple studies have associated T1D with exposure to cow’s milk [77,78,79]. Lastly, the TRIGR study reported no association, but they did not search for MAP presence [80].
In 2006, Dow postulated that MAP may be an environmental trigger for T1D in the genetically at-risk. Three proposals were offered to support the postulate: (1) there are shared genetic susceptibilities to both mycobacterial infection and T1D, (2) MAP is the source of the HSP65 protein, providing epitope homologies between mycobacterial HSP65 and pancreatic glutamic acid decarboxylase (GAD) and (3) epidemiologic findings tie the risk of T1D to early life exposure to cow’s milk [32].
Subsequently, Sechi and associates conducted several studies associating MAP and T1D. They found an association of MAP and T1D patients on their home island of Sardinia [81,82,83]. The island of Sardinia has the second highest incidence of T1D in the world [84]. They reported finding MAP in T1D patients but not in type 2 diabetics [85,86]. They found MAP in T1D children [87,88,89]. They confirmed a genetic risk factor linking mycobacterial infection and T1D [40]. They also identified additional MAP peptides that are homologous with pancreatic proteins [83,90,91] and showed that immune reaction to these MAP peptides cross-react to the classical islet cell antibodies [92]. They demonstrated parallel findings on the Italian mainland [93,94].
Recently, a body of evidence pointed to a role for human endogenous retroviruses (HERVs) in the activation of genes [95]. It is thought that most HERVs are genetically silent. However, assorted environmental stimuli, including infection, may activate HERVs to potentiate certain autoimmune diseases [96]. A recent study demonstrated anti-HERV antibodies correlating with sero-reactivity against MAP in children at risk for T1D [97]. This study showed that an activated HERV gene expressing a specific envelope protein, HERV-W, is associated with T1D in three distinct populations.
Of more than a dozen articles implicating MAP in T1D, only one article failed to do so. That article came from India where MAP was not found in the blood of T1D patients. A few possible explanations offered included the compulsory BCG vaccination against tuberculosis, with the thought that BCG provides cross protection against paratuberculosis as it does with leprosy. Also, the cultural culinary practice of vegetarianism would reduce exposure to MAP, as would the common practice of boiling milk before consumption [98].
Of interest is the publication that the BCG vaccination of long standing T1D individuals, followed by a booster in six months, resulted in the control of blood sugar (seen after a delay of three years). The effect was durable with normal blood sugars eight years after the vaccination [99,100]. The beneficial effect is postulated to be due to a “reset” of the immune system. An alternative explanation is that BCG vaccination is effective against MAP [101].
5. Discussion
The etiopathology of MAP-related human disease is multifaceted. MAP can initiate a granuloma, stimulate autoantibodies via molecular mimicry and potentiate immune imbalance by activating other stimulants, as found in MAP’s relation with HERVs [95,97].
It is extraordinary that a single pathogen can so significantly impact global animal agriculture, food safety and human disease. This is likely due to MAP’s ability to cause chronic regional intestinal inflammation in so many species [59]. Despite calls to address MAP zoonosis [102,103,104], those calls have been largely unheeded. Moreover, anti-MAP humoral reactivity was recently correlated between serum lipoprotein levels in subjects at T1DM risk (rT1DM) grouped by geographical background and in patients affected by MS or RA [105].
Interestingly, a review of the therapeutic agents for Crohn’s inflammation suggested that previously prescribed anti-inflammatory agents were actually treating MAP [106,107,108], while the strong contemporary anti-inflammatory TNF inhibitors are permissive for both MAP and tuberculosis [109,110,111].
Sufficient evidence points to the fact that until MAP is eliminated from the food chain, it may continue to be said that cows get Crohn’s disease and they are giving us diabetes, multiple sclerosis, sarcoidosis, Blau syndrome, Hashimoto’s thyroiditis, lupus, Parkinson’s disease and rheumatoid arthritis.
Author Contributions
C.T.D. and L.A.S. contributed equally to this manuscript.
Funding
This research was funded by the University of Sassari, finanziamento straordinario una tantum per la ricerca to Leonardo A Sechi, 2019.
Conflicts of Interest
C.T.D. declares that is an investor in Paralab, LLC and MAP/PATH LLC. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Agrawal, G.; Borody, T.J.; Chamberlin, W. ‘Global warming’ to Mycobacterium avium subspecies paratuberculosis. Future Microbiol. 2014, 9, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Sechi, L.A.; Dow, C.T. Mycobacterium avium ss. paratuberculosis Zoonosis–The Hundred Year War–Beyond Crohn’s Disease. Front. Immunol. 2015, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Chamberlin, W.; Borody, W.T.; Naser, S. MAP-associated Crohn’s disease: MAP, Koch’s postulates, causality and Crohn’s disease. Digest. Liver Dis. 2007, 39, 792–794. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.; Thomson, J.M.; El-Omar, E.M.; Hold, G.L. The role of infection in the aetiology of inflammatory bowel disease. J. Gastroenterol. 2010, 45, 266–276. [Google Scholar] [CrossRef]
- Van Kruiningen, H.J.; Chiodini, R.J.; Thayer, W.R.; Coutu, J.A.; Merkal, R.S.; Runnels, P.L. Experimental disease in infant goats induced by a Mycobacterium isolated from a patient with Crohn’s disease. A preliminary report. Dig. Dis. Sci. 1986, 31, 1351–1360. [Google Scholar] [CrossRef]
- Balseiro, A.; Marín, J.G.; Solano, P.; Garrido, J.; Prieto, J. Histopathological Classification of Lesions Observed in Natural Cases of Paratuberculosis in Free-ranging Fallow Deer (Dama dama). J. Comp. Pathol. 2008, 138, 180–188. [Google Scholar] [CrossRef][Green Version]
- Vazquez, P.; Garrido, J.M.; Molina, E.; Geijo, M.V.; Gomez, N.; Pérez, V.; Sevilla, I.A.; Alonso-Hearn, M.; Cortes, A.; Juste, R.A. Latent infections are the most frequent form of paratuberculosis in slaughtered Friesian cattle. Span. J. Agric. Res. 2014, 12, 1049. [Google Scholar] [CrossRef]
- Perez, V.; Marín, J.G.; Badiola, J. Description and classification of different types of lesion associated with natural paratuberculosis infection in sheep. J. Comp. Pathol. 1996, 114, 107–122. [Google Scholar] [CrossRef]
- Corpa, J.; Garrido, J.; Marin, J.G.; Perez, V.; Arenas, J.M.C. Classification of Lesions Observed in Natural Cases of Paratuberculosis in Goats. J. Comp. Pathol. 2000, 122, 255–265. [Google Scholar] [CrossRef]
- González, J.; Geijo, M.; García-Pariente, C.; Verna, A.; Corpa, J.; Reyes, L.; Ferreras, M.; Juste, R.; Marín, J.G.; Perez, V.; et al. Histopathological Classification of Lesions associated with Natural Paratuberculosis Infection in Cattle. J. Comp. Pathol. 2005, 133, 184–196. [Google Scholar] [CrossRef]
- Corbett, E.L.; Watt, C.J.; Walker, N.; Maher, D.; Williams, B.G.; Raviglione, M.C.; Dye, C. The growing burden of tuberculosis: Global trends and interactions with the HIV epidemic. Arch. Intern. Med. 2003, 163, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Hines, M.E.; Styer, E.L. Preliminary characterization of chemically generated Mycobacterium avium subsp. paratuberculosis cell wall deficient forms (spheroplasts). Vet. Microbiol. 2003, 95, 247–258. [Google Scholar] [CrossRef]
- Lamont, E.A.; Bannantine, J.P.; Armien, A.; Ariyakumar, D.S.; Sreevatsan, S. Identification and Characterization of a Spore-Like Morphotype in Chronically Starved Mycobacterium avium Subsp. Paratuberculosis Cultures. PLoS ONE 2012, 7, e30648. [Google Scholar] [CrossRef] [PubMed]
- Gaschignard, J.; Grant, A.V.; Thuc, N.V.; Orlova, M.; Cobat, A.; Huong, N.T.; Ba, N.N.; Thai, V.H.; Abel, L.; Schurr, E.; et al. Pauci- and Multibacillary Leprosy: Two Distinct, Genetically Neglected Diseases. PLoS Negl. Trop. Dis. 2016, 10, e0004345.26984977. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.J.; Starke, J.R.; Revell, P.A. Laboratory Diagnosis of Mycobacterium tuberculosis Infection and Disease in Children. J. Clin. Microbiol. 2016, 54, 1434–1441. [Google Scholar] [CrossRef]
- Hanna, S.E.; Connor, C.J.; Wang, H.H. Real-time polymerase chain reaction for the food microbiologist: Technologies, applications, and limitations. J. Food Sci. 2005, 70, 49–53. [Google Scholar] [CrossRef]
- Sung, J.S.; Chang, Y.-F.; Huang, C.; Zhu, J.; Huang, L.; Han, S.Y.; Shin, K.-S.; Stehman, S.; Shin, S.J.; Torres, A. Development of a Polymerase Chain Reaction Test to Confirm Mycobacterium Avium Subsp. Paratuberculosis in Culture. J. Veter Diagn. Investig. 2004, 16, 116–120. [Google Scholar] [CrossRef]
- Naser, S.; Ghobrial, G.; Romero, C.; Valentine, J. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn’s disease. Lancet 2004, 364, 1039–1044. [Google Scholar] [CrossRef]
- Naser, S.A.; Collins, M.T.; Crawford, J.T.; Valentine, J.F. Culture of Mycobacterium avium subspecies paratuberculosis (MAP) from the Blood of Patients with Crohn’s disease: A Follow-Up Blind Multi Center Investigation. Open Inflamm. J. 2009, 2, 22–23. [Google Scholar] [CrossRef]
- Sechi, L.A.; Scanu, A.M.; Molicotti, P.; Cannas, S.; Mura, M.; Dettori, G.; Fadda, G.; Zanetti, S. Detection and Isolation of Mycobacterium avium subspecies paratuberculosis from intestinal mucosal biopsies of patients with and without Crohn’s disease in Sardinia. Am. J. Gastroenterol. 2005, 100, 1529–1536. [Google Scholar] [CrossRef]
- Naser, S.A.; Schwartz, D.; Shafran, I. Isolation of Mycobacterium avium subsp paratuberculosis from breast milk of Crohn’s disease patients. Am. J. Gastroenterol. 2000, 95, 1094–1095. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.L.; San-Pedro, A.; Culebras, E.; Cíes, R.; Taxonera, C.; Lana, R.; Urcelay, E.; De La Torre, F.; Picazo, J.J.; Díaz-Rubio, M. High prevalence of viable Mycobacterium avium subspecies paratuberculosis in Crohn’s disease. World J. Gastroenterol. 2010, 16, 4558–4563. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.; Madsen-Bouterse, S. Crohn’s disease and Mycobacterium avium subsp. paratuberculosis: The need for a study is long overdue. Vet. Immunol. Immunopathol. 2012, 145, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dow, C.T.; Ellingson, J.L.E. Detection of Mycobacterium avium ss. Paratuberculosis in Blau Syndrome Tissues. Autoimmune Dis. 2010, 2010, 1–5. [Google Scholar] [CrossRef]
- Sechi, L.A.; Gazouli, M.; Ikonomopoulos, J.; Lukas, J.C.; Scanu, A.M.; Ahmed, N.; Fadda, G.; Zanetti, S. Mycobacterium avium subsp. paratuberculosis, genetic susceptibility to Crohn’s disease, and Sardinians: The way ahead. J. Clin. Microbiol. 2005, 43, 5275–5277. [Google Scholar] [CrossRef]
- Dow, C.T.M. paratuberculosis Heat Shock Protein 65 and Human Diseases: Bridging Infection and Autoimmunity. Autoimmune Dis. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, F.F.; Dai, Z.W.; Wang, B.; Ye, D.Q. Association between rheumatoid arthritis and genetic variants of natural resistance-associated macrophage protein 1 gene: A meta-analysis. Int. J. Rheum Dis. 2018, 21, 1651–1658. [Google Scholar] [CrossRef]
- Sechi, L.A.; Gazouli, M.; Sieswerda, L.E.; Molicotti, P.; Ahmed, N.; Ikonomopoulos, J.; Scanu, A.M.; Paccagnini, D.; Zanetti, S. Relationship between Crohn’s disease, infection withMycobacterium aviumsubspeciesparatuberculosisandSLC11A1gene polymorphisms in Sardinian patients. World J. Gastroenterol. 2006, 12, 7161–7164. [Google Scholar] [CrossRef]
- Dow, C.T.M. paratuberculosis and Parkinson’s disease—Is this a trigger. Med. Hypotheses 2014, 83, 709–712. [Google Scholar] [CrossRef]
- Härtlova, A.; Herbst, S.; Peltier, J.; Rodgers, A.; Bilkei-Gorzo, O.; Fearns, A.; Dill, B.D.; Lee, H.; Flynn, R.; Cowley, S.A.; et al. LRRK2 is a negative regulator of Mycobacterium tuberculosis phagosome maturation in macrophages. EMBO J. 2018, 37, e98694. [Google Scholar] [CrossRef]
- Sharp, R.C.; Beg, S.A.; Naser, S.A. Polymorphisms in Protein Tyrosine Phosphatase Non-receptor Type 2 and 22 (PTPN2/22) Are Linked to Hyper-Proliferative T-Cells and Susceptibility to Mycobacteria in Rheumatoid Arthritis. Front. Microbiol. 2018, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Dow, C.T. Paratuberculosis and Type I diabetes: Is this the trigger? Med. Hypotheses. 2006, 67, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Cossu, D.; Masala, S.; Cocco, E.; Paccagnini, D.; Tranquilli, S.; Frau, J.; Marrosu, M.G.; Sechi, L.A. Association of Mycobacterium avium subsp. paratuberculosis and SLC11A1 polymorphisms in Sardinian multiple sclerosis patients. J. Infect. Dev. Ctries. 2013, 7, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Liu, B.; Wang, J.; Pan, H.; Qi, A.; Zhang, S.; Wu, J.; Yang, P.; Wang, B. Novel missense mutation in PTPN22 in a Chinese pedigree with Hashimoto’s thyroiditis. BMC Endocr. Disord. 2018, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, M.; Lisi, S.; Sisto, M.; Cucci, L.; Dow, C.T. Molecular identification of Mycobacterium avium subspecies paratuberculosis in an Italian patient with Hashimoto’s thyroiditis and Melkersson-Rosenthal syndrome. J. Med. Microbiol. 2010, 59, 137–139. [Google Scholar] [CrossRef]
- Sisto, M.; Cucci, L.; D’Amore, M.; Dow, T.C.; Mitolo, V.; Lisi, S. Proposing a relationship between Mycobacterium avium subspecies paratuberculosis infection and Hashimoto’s thyroiditis. Scand. J. Infect. Dis. 2010, 42, 787–790. [Google Scholar] [CrossRef]
- Arru, G.; Caggiu, E.; Paulus, K.; Sechi, G.P.; Mameli, G.; Sechi, L.A. Is there a role for Mycobacterium avium subspecies paratuberculosis in Parkinson’s disease? J. Neuroimmunol. 2016, 293, 86–90. [Google Scholar] [CrossRef]
- Bo, M.; Erre, G.L.; Niegowska, M.; Piras, M.; Taras, L.; Longu, M.G.; Passiu, G.; Sechi, L.A. Interferon regulatory factor 5 is a potential target of autoimmune response triggered by Epstein-barr virus and Mycobacterium avium subsp. paratuberculosis in rheumatoid arthritis, investigating a mechanism of molecular mimicry. Clin. Exp. Rheumatol. 2018, 36, 376–381. [Google Scholar]
- Dow, C.T. Detection of M. paratuberculosis Bacteremia in a Child with Lupus Erythematosus and Sjogren’s Syndrome. Autoimmun. Infect. Dis. 2016, 2. [Google Scholar] [CrossRef]
- Paccagnini, D.; Sieswerda, L.; Rosu, V.; Masala, S.; Pacifico, A.; Gazouli, M.; Ikonomopoulos, J.; Ahmed, N.; Zanetti, S.; Sechi, L.A. Linking Chronic Infection and Autoimmune Diseases: Mycobacterium avium Subspecies paratuberculosis, SLC11A1 Polymorphisms and Type-1 Diabetes Mellitus. PLoS ONE 2009, 4, e7109. [Google Scholar] [CrossRef]
- Lombard, J.E.; Gardner, I.A.; Jafarzadeh, S.R.; Fossler, C.P.; Harris, B.; Capsel, B.R. Herd-level prevalence of Mycobacterium avium subsp. paratuberculosis infection in United States dairy herds in 2007. Prev. Vet. Med. 2013, 108, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Millar, D.; Ford, J.; Sanderson, J.; Withey, S.; Tizard, M.; Doran, T.; Hermon-Taylor, J. IS900 PCR to detect Mycobacterium paratuberculosis in retail supplies of whole pasteurized cows’ milk in England and Wales. Appl. Environ. Microbiol. 1996, 62, 3446–3452. [Google Scholar] [PubMed]
- Ellingson, J.L.; Anderson, J.L.; Koziczkowski, J.J.; Radcliff, R.P.; Sloan, S.J.; Allen, S.E. Detection of viable Mycobacterium avium subsp. paratuberculosis in retail pasteurized whole milk by two culture methods and PCR. J. Food Prot. 2005, 68, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Hruska, K.; Bartos, M.; Kralik, P.; Pavlik, I. Mycobacterium avium subsp. paratuberculosis in powdered infant milk: Paratuberculosis in cattle – the public health problem to be solved. Vet. Med. Czech. 2005, 50, 327–335. [Google Scholar] [CrossRef]
- Pickup, R.W.; Rhodes, G.; Arnott, S.; Sidi-Boumedine, K.; Bull, T.J.; Weightman, A. Mycobacterium avium subsp. paratuberculosis in the catchment area and water of the River Taff in South Wales, United Kingdom, and its potential relationship to clustering of Crohn’s disease cases in the city of Cardiff. Appl. Environ. Microbiol. 2003, 71, 2130–2139. [Google Scholar] [CrossRef]
- Whan, L.; Ball, H.J.; Grant, I.R.; Rowe, M.T. Occurrence of Mycobacterium avium subsp. paratuberculosis in untreated water in Northern Ireland. Appl. Environ. Microbiol. 2006, 71, 7107–7112. [Google Scholar] [CrossRef]
- Pickup, R.W.; Rhodes, G.; Bull, T.J.; Arnott, S.; Sidi-Boumedine, K.; Hurley, M.; Hermon-Taylor, J. Mycobacterium avium subsp. paratuberculosis in Lake Catchments, in River Water Abstracted for Domestic Use, and in Effluent from Domestic Sewage Treatment Works: Diverse Opportunities for Environmental Cycling and Human Exposure. Appl. Environ. Microbiol. 2006, 72, 4067–4077. [Google Scholar] [CrossRef]
- Richardson, H.; Rhodes, G.; Henrys, P.; Sedda, L.; Weightman, A.J.; Pickup, R.W. Presence of Mycobacterium avium Subspecies paratuberculosis Monitored Over Varying Temporal and Spatial Scales in River Catchments: Persistent Routes for Human Exposure. Microorganisms 2019, 7, 136. [Google Scholar] [CrossRef]
- Grewal, S.K.; Rajeev, S.; Sreevatsan, S.; Michel, F.C., Jr. Persistence of Mycobacterium avium subsp. paratuberculosis and other zoonotic pathogens during simulated composting, manure packing, and liquid storage of dairy manure. Appl. Environ. Microbiol. 2003, 72, 565–574. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Murru, N.; Coppola, R.; Aponte, M. Persistence of bacterial indicators and zoonotic pathogens in contaminated cattle wastes. BMC Microbiol. 2016, 16, 87. [Google Scholar]
- Collins, M.T. International Handbook of Foodborne Pathogens; Marianne, D.M., Jeffrey, W., Eds.; Bier CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Beumer, A.; King, D.; Donohue, M.; Mistry, J.; Covert, T.; Pfaller, S. Detection of Mycobacterium avium subsp. paratuberculosis in drinking water and biofilms by quantitative PCR. Appl. Environ. Microb. 2010, 76, 7367–7370. [Google Scholar] [CrossRef]
- Falkinham, J.O., III. Factors influencing the chlorine susceptibility of Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum. Appl. Environ. Microbiol. 2003, 69, 5685–5689. [Google Scholar] [CrossRef]
- Tatchou-Nyamsi-Konig, J.A.; Dailloux, M.; Block, J.C. Survival of Mycobacterium avium attached to polyethylene terephtalate (PET) water bottles. J. Appl. Microbiol. 2009, 106, 825–832. [Google Scholar] [CrossRef]
- Falkinham, J.O., III; Norton, C.D.; LeChevallier, M.W. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other Mycobacteria in drinking water distribution systems. Appl. Environ. Microbiol. 2001, 67, 1225–1231. [Google Scholar] [CrossRef]
- Vaerewijck, M.J.; Huys, G.; Palomino, J.C.; Swings, J.; Portaels, F. Mycobacteria in drinking water distribution systems: Ecology and significance for human health. FEMS Microbiol. Rev. 2005, 29, 911–934. [Google Scholar] [CrossRef]
- Grant, I.; Foddai, A.; Kunkel, B.; Collins, M.T. Detection of Viable Mycobacterium avium subsp. paratuberculosis (MAP) in Infant Formula. In Proceedings of the 12th International Colloquium on Paratuberculosis, Parma, Italy, 22–26 June 2014. [Google Scholar]
- Davis, W.C.; Park, K.T. Progress Towards Control of a Mycobacterial Pathogen, Mycobacterium avium subsp. paratuberculosis, the Causative Agent of Johne’s Disease in Cattle and Humans. J. Food Hyg. Saf. 2018, 33, 221–228. [Google Scholar] [CrossRef]
- Balseiro, A.; Perez, V.; Juste, R.A. Chronic regional intestinal inflammatory disease: A trans-species slow infection? Comp. Immunol. Microbiol. Infect. Dis. 2019, 62, 88–100. [Google Scholar] [CrossRef]
- Davis, W.C.; Kuenstner, J.T.; Singh, S.V. Resolution of Crohn’s (Johne’s) disease with antibiotics: What are the next steps? Expert Rev. Gastroenterol. Hepatol. 2017, 21, 1–4. [Google Scholar] [CrossRef][Green Version]
- Chaubey, K.K.; Singh, S.V.; Gupta, S.; Singh, M.; Sohal, J.S.; Kumar, N.; Singh, M.K.; Bhatia, A.K.; Dhama, K. Mycobacterium avium subspecies paratuberculosis—An important food borne pathogen of high public health significance with special reference to India: An update. Vet. Q. 2017, 37, 282–299. [Google Scholar] [CrossRef]
- Kirkwood, C.D.; Wagner, J.; Boniface, K.; Vaughan, J.; Michalski, W.P.; Catto-Smith, A.G.; Cameron, D.J.; Bishop, R.F. Mycobacterium avium subspecies paratuberculosis in children with early-onset Crohn’s disease. Inflamm. Bowel. Dis. 2009, 15, 1643–1655. [Google Scholar] [CrossRef]
- Feller, M.; Huwiler, K.; Stephan, R.; Altpeter, E.; Shang, A.; Furrer, H.; Pfyffer, G.E.; Jemmi, T.; Baumgartner, A.; Egger, M. Mycobacterium avium subspecies paratuberculosis and Crohn’s disease: A systematic review and meta-analysis. Lancet Infect. Dis. 2007, 7, 607–613. [Google Scholar] [CrossRef]
- Abubakar, I.; Myhill, D.; Aliyu, S.H.; Hunter, P.R. Detection of Mycobacterium avium subspecies paratuberculosis from patients with Crohn’s disease using nucleic acid-based techniques: A systematic review and meta-analysis. Inflamm. Bowel. Dis. 2008, 14, 401–410. [Google Scholar] [CrossRef]
- McNees, A.L.; Markesich, D.; Zayyani, N.R.; Graham, D.Y. Mycobacterium paratuberculosis as a cause of Crohn’s disease. Expert. Rev. Gastroenterol. Hepatol. 2015, 9, 1523–1534. [Google Scholar] [CrossRef]
- Chamberlin, W.; Borody, T.J.; Campbell, J. Primary treatment of Crohn’s disease: Combined antibiotics taking center stage. Expert Rev. Clin. Immunol. 2011, 7, 751–760. [Google Scholar] [CrossRef]
- Open Label Efficacy and Safety of Anti-MAP (Mycobacterium avium subsp. paratuberculosis) Therapy in Adult Crohn’s Disease (MAPUS2). Available online: https://clinicaltrials.gov/ct2/show/NCT03009396 (accessed on 24 May 2019).
- Late Breaking Abstracts. Available online: https://journals.sagepub.com/doi/full/10.1177/2050640618812015#_i15 (accessed on 24 May 2019).
- Proof of Concept Study of RHB-104 as Add-On Therapy to Interferon Beta-1a in Relapsing Remitting Multiple Sclerosis (RRMS) (CEASE-MS). Available online: https://clinicaltrials.gov/ct2/show/NCT01717664 (accessed on 24 May 2019).
- Atkinson, M.A. The Pathogenesis and Natural History of Type 1 Diabetes. Cold Spring Harb. Perspect. Med. 2012, 2, a007641. [Google Scholar] [CrossRef]
- Gale, E.A. The rise of childhood type 1 diabetes in the 20th century. Diabetes 2002, 51, 3353–3361. [Google Scholar] [CrossRef]
- The 64 K question in diabetes. Lancet 1990, 336, 597–598. [CrossRef]
- Baekkeskov, S.; Nielsen, J.H.; Marner, B.; Bilde, T.; Ludvigsson, J.; Lernmark, Å. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature 1982, 298, 167–169. [Google Scholar] [CrossRef]
- Jones, D.B.; Hunter, N.R.; Duff, G.W. Heat-shock protein 65 as a beta cell antigen of insulin-dependent diabetes. Lancet 1990, 336, 583. [Google Scholar] [CrossRef]
- Naser, S.A.; Thanigachalam, S.; Dow, C.T.; Collins, M.T. Exploring the role of Mycobacterium avium subspecies paratuberculosis in the pathogenesis of type 1 diabetes mellitus: A pilot study. Gut Pathog. 2013, 5, 14. [Google Scholar] [CrossRef]
- Scheinin, T.; Minh, N.-N.T.; Tuomi, T.; Miettinen, A.; Kontiainen, S. Islet cell and glutamic acid decarboxylase antibodies and heat-shock protein 65 responses in children with newly diagnosed insulin-dependent diabetes mellitus. Immunol. Lett. 1996, 49, 123–126. [Google Scholar] [CrossRef]
- Kennedy, D. International Efforts at Paratuberculosis Control. Veter Clin. N. Am. Food Anim. Pr. 2011, 27, 647–654. [Google Scholar] [CrossRef]
- Bakker, D.; Willemsen, P.; Van Zijderveld, F. Paratuberculosis recognized as a problem at last: A review. Veter Q. 2000, 22, 200–204. [Google Scholar] [CrossRef]
- Gerstein, H.C. Cow’s milk exposure and type I diabetes mellitus. A critical overview of the clinical literature. Diabetes Care 1994, 17, 13–19. [Google Scholar] [CrossRef]
- Writing Group for the TRIGR Study Group; Knip, M.; Åkerblom, H.K.; Al Taji, E.; Becker, D.; Bruining, J. Effect of Hydrolyzed Infant Formula vs Conventional Formula on Risk of Type 1 Diabetes: The TRIGR Randomized Clinical Trial. JAMA 2018, 319, 38–48. [Google Scholar]
- Sechi, L.A.; Rosu, V.; Pacifico, A.; Fadda, G.; Ahmed, N.; Zanetti, S. Humoral immune responses of type 1 diabetes patients to Mycobacterium avium subsp. paratuberculosis lend support to the infectious trigger hypothesis. Clin. Vaccine Immunol. 2008, 15, 320–326. [Google Scholar] [CrossRef]
- Sechi, L.A.; Paccagnini, D.; Salza, S.; Pacifico, A.; Ahmed, N.; Zanetti, S. Mycobacterium avium subspecies paratuberculosis bacteremia in type 1 diabetes mellitus: An infectious trigger? Clin. Infect. Dis. 2008, 46, 148–149. [Google Scholar] [CrossRef]
- Cossu, A.; Rosu, V.; Paccagnini, D.; Cossu, D.; Pacifico, A.; Sechi, L.A. MAP3738c and MptD are specific tags of Mycobacterium avium subsp. paratuberculosis infection in type I diabetes mellitus. Clin. Immunol. 2011, 141, 49–57. [Google Scholar] [CrossRef]
- Songini, M.; Mannu, C.; Targhetta, C.; Bruno, G. Type 1 diabetes in Sardinia: Facts and hypotheses in the context of worldwide epidemiological data. Acta Diabetol. 2017, 54, 9–17. [Google Scholar] [CrossRef]
- Rosu, V.; Ahmed, N.; Paccagnini, D.; Pacifico, A.; Zanetti, S.; Sechi, L.A. Mycobacterium avium subspecies paratuberculosis is not associated with Type-2 Diabetes Mellitus. Ann. Clin. Microbiol. Antimicrob. 2008, 7, 9. [Google Scholar] [CrossRef]
- Rosu, V.; Ahmed, N.; Paccagnini, D.; Gerlach, G.; Fadda, G.; Hasnain, S.E.; Zanetti, S.; Sechi, L.A. Specific Immunoassays Confirm Association of Mycobacterium avium Subsp. paratuberculosis with Type-1 but Not Type-2 Diabetes Mellitus. PLoS ONE 2009, 4, e4386. [Google Scholar] [CrossRef]
- Bitti, M.L.M.; Masala, S.; Capasso, F.; Rapini, N.; Piccinini, S.; Angelini, F.; Pierantozzi, A.; Lidano, R.; Pietrosanti, S.; Paccagnini, D.; et al. Mycobacterium avium subsp. paratuberculosis in an Italian Cohort of Type 1 Diabetes Pediatric Patients. Clin. Dev. Immunol. 2012, 2012, 1–5. [Google Scholar] [CrossRef][Green Version]
- Cossu, A.; Ferrannini, E.; Fallahi, P.; Antonelli, A.; Sechi, L.A. Antibodies recognizing specific Mycobacterium avium subsp. paratuberculosis’s MAP3738c protein in type 1 diabetes mellitus children are associated with serum Th1 (CXCL10) chemokine. Cytokine 2013, 61, 337–339. [Google Scholar] [CrossRef]
- Masala, S.; Zedda, M.A.; Cossu, D.; Ripoli, C.; Palermo, M.; Sechi, L.A. Zinc Transporter 8 and MAP3865c Homologous Epitopes are Recognized at T1D Onset in Sardinian Children. PLoS ONE 2013, 8, e63371. [Google Scholar] [CrossRef]
- Masala, S.; Paccagnini, D.; Cossu, D.; Brezar, V.; Pacifico, A.; Ahmed, N.; Mallone, R.; Sechi, L.A. Antibodies Recognizing Mycobacterium avium paratuberculosis Epitopes Cross-React with the Beta-Cell Antigen ZnT8 in Sardinian Type 1 Diabetic Patients. PLoS ONE 2011, 6, 26931. [Google Scholar] [CrossRef]
- Scotto, M.; Afonso, G.; Larger, E.; Raverdy, C.; Lemonnier, F.A.; Carel, J.C.; Dubois-Laforgue, D.; Baz, B.; Levy, D.; Gautier, J.F.; et al. Zinc transporter (ZnT)8(186–194) is an immunodominant CD8+ T cell epitope in HLA-A2+ type 1 diabetic patients. Diabetologia 2012, 55, 2026–2031. [Google Scholar] [CrossRef]
- Niegowska, M.; Paccagnini, D.; Mannu, C.; Targhetta, C.; Songini, M.; Sechi, L.A. Recognition of ZnT8, Proinsulin, and Homologous MAP Peptides in Sardinian Children at Risk of T1D Precedes Detection of Classical Islet Antibodies. J. Diabetes Res. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Masala, S.; Cossu, D.; Piccinini, S.; Rapini, N.; Massimi, A.; Porzio, O.; Pietrosanti, S.; Lidano, R.; Bitti, M.L.M.; Sechi, L.A. Recognition of zinc transporter 8 and MAP3865c homologous epitopes by new-onset type 1 diabetes children from continental Italy. Acta Diabetol. 2014, 51, 577–585. [Google Scholar] [CrossRef]
- Masala, S.; Cossu, D.; Piccinini, S.; Rapini, N.; Mameli, G.; Bitti, M.L.M.; Sechi, L.A. Proinsulin and MAP3865c homologous epitopes are a target of antibody response in new-onset type 1 diabetes children from continental Italy. Pediatr. Diabetes 2015, 16, 189–195. [Google Scholar] [CrossRef]
- Greenig, M. HERVs, immunity, and autoimmunity: Understanding the connection. PeerJ 2019, 7, e6711. [Google Scholar] [CrossRef]
- Levet, S.; Medina, J.; Joanou, J.; Demolder, A.; Queruel, N.; Réant, K.; Normand, M.; Seffals, M.; Dimier, J.; Germi, R.; et al. An ancestral retroviral protein identified as a therapeutic target in type-1 diabetes. JCI Insight 2017, 2, 94387. [Google Scholar] [CrossRef]
- Niegowska, M.; Wajda-Cuszlag, M.; Stępień-Ptak, G.; Trojanek, J.; Michałkiewicz, J.; Szalecki, M.; Sechi, L.A. Anti-HERV-WEnv antibodies are correlated with seroreactivity against Mycobacterium avium subsp. paratuberculosis in children and youths at T1D risk. Sci. Rep. 2019, 9, 6282. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.S.; Doddam, S.N.; Agrawal, S.; Hasnain, S.E.; Sechi, L.A.; Kumar, A.; Ahmed, N. Mycobacterium avium subsp. paratuberculosis is not discerned in diabetes mellitus patients in Hyderabad, India. Int. J. Med. Microbiol. 2014, 304, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Kühtreiber, W.M.; Tran, L.; Kim, T.; Dybala, M.; Nguyen, B.; Plager, S.; Huang, D.; Janes, S.; DeFusco, A.; Baum, D.; et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: The value of induced aerobic glycolysis with BCG vaccinations. Npj Vaccines 2018, 3, 23. [Google Scholar] [CrossRef]
- Kühtreiber, W.M.; Faustman, D.L. BCG Therapy for Type 1 Diabetes: Restoration of Balanced Immunity and Metabolism. Trends Endocrinol. Metab. 2019, 30, 80–92. [Google Scholar] [CrossRef]
- Dow, C.T. BCG, Autoimmune Diabetes and M. Paratuberculosis. J. Diabetes Metab. Disord. 2018, 5, 24. [Google Scholar]
- Davis, W.C. On deaf ears, Mycobacterium avium paratuberculosis in pathogenesis Crohn’s and other diseases. World J. Gastroenterol. 2015, 21, 13411–13417. [Google Scholar] [CrossRef]
- Hermon-Taylor, J. Treatment with drugs active against Mycobacterium avium subspecies paratuberculosis can heal Crohn’s disease: More evidence for a neglected public health tragedy. Dig. Liver Dis. 2002, 34, 9–12. [Google Scholar] [CrossRef]
- Kuenstner, J.T.; Naser, S.; Chamberlin, W.; Borody, T.; Graham, D.Y.; McNees, A. The Consensus from the Mycobacterium avium ssp. paratuberculosis (MAP) Conference 2017. Front. Public Health 2017, 5, 208. [Google Scholar] [CrossRef]
- Bo, A.; Niegowska, M.; Erre Manchia, P.A.; Sechi, L.A.; Bo, M.; Arru, G.; Erre, G.L. Association between Lipoprotein Levels and Humoral Reactivity to Mycobacterium avium subsp. paratuberculosis in Multiple Sclerosis, Type 1 Diabetes Mellitus and Rheumatoid Arthritis. Microorganisms 2019, 7, 423. [Google Scholar] [CrossRef]
- Greenstein, R.J.; Su, L.; Juste, R.A.; Brown, S.T. On the Action of Cyclosporine A, Rapamycin and Tacrolimus on M. avium Including Subspecies paratuberculosis. PLoS ONE 2008, 3, e2496. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greenstein, R.; Su, L.; Haroutunian, V.; Shahidi, A.; Brown, S. On the action of methotrexate and 6-Mercaptopurine on M. avium subspecies paratuberculosis. Inflamm. Bowel Dis. 2007, 13, 650. [Google Scholar]
- Greenstein, R.J.; Su, L.; Shahidi, A.; Brown, S.T. On the Action of 5-Amino-Salicylic Acid and Sulfapyridine on M. avium including Subspecies paratuberculosis. PLoS ONE 2007, 2, e516. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qasem, A.; Naser, A.E.; Naser, S.A. The alternate effects of anti-TNFα therapeutics and their role in mycobacterial granulomatous infection in Crohn’s disease. Expert Rev. Anti-Infect. Ther. 2017, 15, 637–643. [Google Scholar] [CrossRef]
- Cao, B.L.; Qasem, A.; Sharp, R.C.; Abdelli, L.S.; Naser, S.A. Systematic review and meta-analysis on the association of tuberculosis in Crohn’s disease patients treated with tumor necrosis factor-α inhibitors (Anti-TNFα). World J. Gastroenterol. 2018, 24, 2764–2775. [Google Scholar] [CrossRef] [PubMed]
- Qasem, A.; Naser, S.A. TNFα inhibitors exacerbate Mycobacterium paratuberculosis infection in tissue culture: A rationale for poor response of patients with Crohn’s disease to current approved therapy. BMJ Open Gastroenterol. 2018, 5, e000216. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).