Abstract
Polymicrobial infections are of paramount importance because of the potential severity of clinical manifestations, often associated with increased resistance to antimicrobial treatment. The intricate interplay with the host and the immune system, and the impact on microbiome imbalance, are of importance in this context. The equilibrium of microbiota in the human host is critical for preventing potential dysbiosis and the ensuing development of disease. Bacteria and fungi can communicate via signaling molecules, and produce metabolites and toxins capable of modulating the immune response or altering the efficacy of treatment. Most of the bacterial–fungal interactions described to date focus on the human fungal pathogen Candida albicans and different bacteria. In this review, we discuss more than twenty different bacterial–fungal interactions involving several clinically important human pathogens. The interactions, which can be synergistic or antagonistic, both in vitro and in vivo, are addressed with a focus on the quorum-sensing molecules produced, the response of the immune system, and the impact on clinical outcome.
1. Introduction
Identification and subsequent exploitation of the mechanisms that mediate communication between bacteria and fungi, as well as the interplay with the host and the immune system, can provide the basis for the discovery of novel biomarkers facilitating improved diagnostics and more effective treatment approaches. In vivo models are required for clinically relevant understanding of bacterial–fungal interactions (BFI), as currently available in vitro assays may not reflect the implications in the affected host. In some instances, the findings of BFI were even completely discrepant between different experimental settings by revealing antagonistic interactions in vitro and synergistic effects in in vivo models, presenting with increased virulence and pathogenicity in the host [1,2]. Current diagnostic approaches are not designed to routinely assess polymicrobial infections, and may therefore miss the presence of bacterial–fungal co-infections. In instances in which the co-infection results in one-way or mutual inhibitory interactions, treatment with antibiotics or antifungals may therefore promote or unleash growth of the microbiota not covered by the antimicrobial therapy, with possible implications for morbidity and mortality. Studies have shown that mixed cultures of Candida and bacteria occur in about 23% of the reported episodes of candidemia, with a predominance of Staphylococci in these associations [3]. However, no significant differences in the clinical outcome of patients with mixed bloodstream infections were observed in comparison to monomicrobial candidemia. Prior use of antibiotics was associated with decreased prevalence of mixed Candida–bacterial bloodstream infections [3]. The clinical implications of bacterial–fungal interactions are reviewed in [4,5,6,7], and there is evidence that some BFI can promote disease [8]. Associations between Candida and Enterobacter have been detected in all types of clinical specimens investigated and, in most cases, the frequency of these associations was statistically significant. Co-isolation of Enterococcus faecalis and fungi was reported in 22% of patients treated in intensive care units, and other bacteria frequently co-isolated with fungi included the genera Klebsiella and Serratia [7]. Co-infections by Candida and the bacteria Enterobacter, Klebsiella, or Serratia have led to increased rates of hospitalization [3,7]. Another study showed that a significant proportion of candidemias occurred in parallel with bacteremia, although no significant differences in long-term survival were observed between single and co-infections [3]. Kett and colleagues have shown that 38% of patients testing positive for Candida infection had a co-infection with bacteria [9]. Similarly, postmortem blood analysis showed mixed infections by Candida and bacteria in 39% of the cases investigated [10].
Communication between bacteria and fungi is mediated via quorum sensing molecules and proteins [11,12,13]. Identification of these signalling molecules and assessing their interplay with the immune system, e.g., by determining specific cytokine profiles, renders them an attractive target for more specific diagnostics and treatment. Recent studies have identified a set of signaling molecules secreted by human intestinal microbiota that accumulate at detectable concentrations, and have suggested that these molecules could be used as markers of disease (reviewed in [14]). Microbial and, specifically, bacterial–fungal interactions, can also result in the production of molecules potentially affecting host homeostasis. Moreover, recent insights derived from metagenomic and metabolomic analyses indicate that the integration of multifaceted data can provide the basis for improved therapies [15,16]. Krüger and colleagues have described fungal–bacterial interactions with a focus on the mucosal niches and consequences for the human host [17]. In the present review, we focus on describing the spectrum of BFI identified to date, with potential relevance for the human host. The focus is placed on the mechanisms of intermicrobial communication, including the respective metabolites involved and the interplay with the host immune system, addressing the cytokine and chemokine profile of BFI.
2. Bacterial–Fungal Interactions in the Context of the Microbiome
Microbial interactions are an integral part of the highly complex human microbiome. Mapping of the human microbiome has shown a wide diversity of bacteria and fungi occupying specific niches [18,19,20,21]. Whereas most studies have focused primarily on the bacteriome, the realms of fungi and viruses, the mycobiome and virome, have been studied less extensively. The human intestinal mycobiome has a considerably lower diversity compared to the bacteriome. The dominant genera in a healthy individual include Saccharomyces, Malassezia, and Candida [19]. Microbes may have beneficial, neutral, or harmful effects while interacting with the host and the immune system. The microbiota play a key role in host homeostasis, including the regulation of the immune system and production of essential vitamins, amino acids, metabolites, and byproducts necessary for the normal function of many processes [22,23,24,25,26,27]. Intestinal microbiota also protect against colonization by pathogenic microorganisms.
However, once the equilibrium of the microbiota is disturbed, e.g., by a variety of conditions or alterations, including diet, treatment with antibiotics or other drugs, age, stress, chronic inflammation, or various underlying diseases, shifts in the levels and composition will occur, with consequences for the onset and progression of disease [18,26,27,28]. Variations in the intestinal microflora have been helpful in establishing differences between the states of health and disease [16,18,20,29,30,31,32,33,34,35,36,37,38,39].
Experimental approaches addressing the microbiome have been based on the use of completely germ-free (gnotobiotic) mice to assess the impact of an altered microbiome from diseased mice transplanted into these animals [40,41,42]. It was shown that the disease-associated microbiome is commonly adopted by the gnotobiotic mice, facilitating studies on the role of specific microbes and their composition on metabolic and immune processes [43,44,45,46,47]. Such studies in mouse models have contributed to improved understanding of the influence of an imbalanced microbiome on the health status, and have set the stage for studies addressing the complex microbial interactions occurring in the human host. Bacteria and fungi often live in organized structures, termed biofilms, rather than in planktonic state. The formation of either intra-domain (bacterial–bacterial or fungal–fungal) or inter-domain (bacterial–fungal) interactions within biofilms have been implicated in a variety of diseases, such as cystic fibrosis (e.g., interactions between Inquilinus limosus, Dolosigranulum pigrum, Burkholderia cepacia and Pseudomonas aeruginosa), endocarditis, prostatitis, and cancer [48,49]. Bacterial biofilms have been suggested to play a role in the progression of colorectal cancer (CRC) [50], and polymicrobial, i.e., bacterial–fungal co-infections (e.g., interactions between Candida albicans, Aspergillus fumigatus, and P. aeruginosa) were shown to display more deleterious effects in the context of cystic fibrosis compared to single pathogen infections [51,52,53]. Similarly, the fungal genera Candida and Rhodotorula have been linked to atopic diseases, including asthma in infants [54,55]. By contrast, certain bacterial–fungal interactions, such as those of various Candida species and Lactobacilli, which are part of the normal vulvovaginal microflora, were demonstrated to be beneficial for the host by preventing candidiasis at this site [56,57]. Moreover, Candida is also thought to prevent life-threatening urinary tract infections by Escherichia coli [58,59,60].
2.1. Impact of Microbiome Dysbiosis on the Bacterial–Fungal Equilibrium
Dysbiosis is characterized by changes in the amount, composition, distribution, function, and metabolic activity of physiological microbiota. It is associated with loss of biodiversity and overgrowth of pathogenic species [39]. A variety of very diverse diseases have been associated with dysbiosis, including inflammatory bowel disease, obesity, allergy, diabetes, autism, and colorectal cancer, where dysbiosis can either be a causal factor or a secondary effect of the disease [39]. Bacterial dysbiosis, displaying the prevalence of completely different types of bacteria in comparison to healthy lungs, has been described in chronic lung diseases such as asthma, chronic obstructive pulmonary disease (COPD), or cystic fibrosis [18]. In Crohn´s disease (CD), dysbiosis with increased levels of Proteobacteria, Fusobacteria, and the fungal species C. albicans and Candida glabrata, has been described during disease progression [36,61]. Moreover, commensal fungi such as Saccharomyces cerevisiae may also display harmful effects during dysbiosis by inducing damage of the intestinal barrier [62]. Antibiotic or antifungal treatment may have implications for the balance and the interactions between bacteria and fungi. For example, depletion of commensal intestinal fungi may unleash the growth of bacteria with pathogenic potential, leading to an exacerbation of colitis [63]. Colonization with C. albicans in mice treated with antibiotics was shown to increase allergic airway disease [64], but antifungal treatment was also reported to mediate a similar clinical effect [65]. Hence, in various states of dysbiosis, loss of the bacterial–fungal equilibrium and corresponding interactions can play a pathogenetic role in different diseases.
2.2. Microbial Metabolites—the Good and the Bad
Intestinal microbes can communicate with the host via microbial metabolites, which may mediate beneficial or harmful effects [66]. The host benefits from microbiota owing to their production of certain amino acids and vitamins [25,67], and commensal microbes also produce a range of small molecules inhibiting the growth of pathogenic microorganisms. For example, specific intestinal bacteria such as Firmicutes produce short-chain fatty acids (SCFA), including butyrate, acetate, and propionate, through the fermentation of fibers and other polysaccharide compounds, which have an important role in immune development, control of inflammation, and defense against infection [68]. Butyrate is also used as an energy source for intestinal epithelial cells, thereby conferring protection against pathogens [69]. Butyric acid also inhibits the yeast-to-hyphal transition of C. albicans, a key virulence attribute of this opportunistic pathogen [70], and several studies have associated butyrate with protective effects against infectious diseases. However, the net effect of a microbial metabolite can depend on the specific environmental conditions. Butyrate was shown to display protective effects against colorectal cancer (CRC), but a decrease in the butyrate-producing bacteria Firmicutes was described in inflammatory bowel disease (IBD), thus potentially compromising the beneficial effect of this SCFA [71,72]. However, other studies performed in a murine model described butyrate as promoting carcinogenesis by enhancing epithelial cell proliferation [73]. The controversial effects of butyrate are well documented [74]. Other examples of harmful metabolites include alanine and lactate, which are present in the lungs of cystic fibrosis patients, and promote the growth of P. aeruginosa [75]. Furthermore, phenolic compounds, amines, ammonia, acetaldehyde, nitrosamines, and sulphides are microbial metabolites generated by protein fermentation, which have been shown to be toxic for the host (e.g., by impairing metabolic functions or by mediating DNA damage), and also promoting of cancer development [35,38,76,77]. Production of such toxins in the intestinal tract is mediated by quorum sensing communication among different microbial species [28].
3. Communication between Bacteria and Fungi Mediated by Proteins and Small Molecules
Microbial interactions are mediated by several mechanisms that also serve as virulence factors, including quorum sensing, biofilm formation, production of secondary metabolites, and cellular signal transduction [78,79,80,81,82,83]. Such interactions can be quite complex, and occur particularly when different microorganisms share the same niches in the host, resulting in differential effects that can be antagonistic, synergistic, or neutral. Hence, bacteria and fungi can mutually support their growth or exert competitive effects, potentially leading to suppression of one microorganism and dominant growth of the other. Suppressive and inhibitory interactions mediated by different molecules or factors can occur simultaneously, depending on specific stimuli and changes in the microenvironment (Figure 1). Several studies have demonstrated that polymicrobial infections can be more severe and result in considerably higher mortality than infections with single pathogens, as reported, for example, for co-infections with A. fumigatus and P. aeruginosa or with C. albicans and P. aeruginosa in certain clinical settings [1,2,51,52,53,84,85,86]. New therapeutic strategies targeting quorum sensing molecules and bacterial virulence factors are being tested with the aim to deliver more efficient antimicrobial treatment and to prevent development of drug resistance [87,88,89,90,91]. Such approaches were tested specifically against infections with P. aeruginosa and were able to disrupt the cell communication and to reduce the virulence [92,93,94,95]. Other studies have attempted to use antibiotics and antifungals in combination with quorum sensing molecules to treat bacterial–fungal infections [96]. For example, the quorum sensing molecule farnesol, which inhibits filamentation of C. albicans, [97] has been tested in combination with antifungal drugs, and resulted in decreased minimal inhibitory concentration (MIC) values [98].
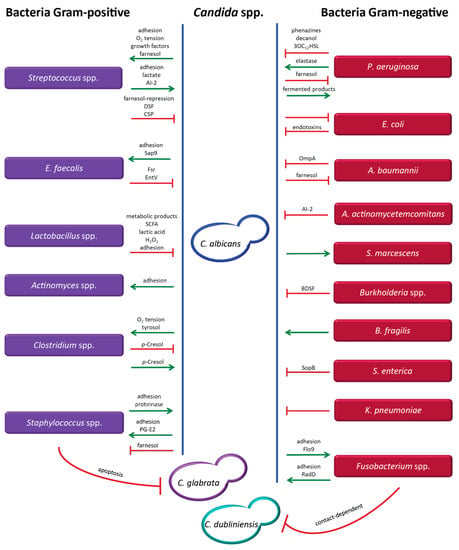
Figure 1.
Molecules and factors mediating the interaction between different Candida species and a variety of bacteria. Candida species include Candida (C.) albicans, Candida (C.) glabrata and Candida (C.) dubliniensis. Gram-positive bacteria are represented in lilac (Enterococcus (E.) faecalis) and Gram-negative bacteria in red (Pseudomonas (P.) aeruginosa, Escherichia (E.) coli, Acinetobacter (A.) baumannii, Aggregatibacter (A.) actinomycetemcomitans, Serratia (S.) marcescens, Bacteroides (B.) fragilis, Salmonella (S.) enterica, Klebsiella (K.) pneumoniae). Green arrows indicate supportive interactions and red lines represent inhibitory effects. If not indicated above the green arrows and red lines, the molecules mediating the interaction are currently unknown.
The bacterial–fungal interactions described to date mainly involve the interplay of C. albicans with different bacterial species. Exploitation of the growing knowledge of the microbiome offers new insights into the diversity of bacterial and fungal species colonizing the human body, many of which share the same niches. This information, along with pertinent clinical studies, unravels microbial interactions of potential clinical relevance. Here, we describe the mechanisms involved in various bacterial–fungal interactions in the human host, with a focus on quorum sensing molecules and virulence factors.
3.1. Bacterial–Fungal Interactions
3.1.1. Candida Species and Different Bacteria
Candida albicans and Pseudomonas aeruginosa (Figure 1): This bacterial–fungal interaction is one of the most widely studied microbial interplays. Well documented examples of sites revealing an interplay between these pathogens include intravenous catheters, lungs of cystic fibrosis patients, the respiratory tract of ventilated patients, and burn wounds [11]. The interaction between C. albicans and P. aeruginosa, which is mediated by the production of quorum sensing molecules and virulence factors, is rather complex, as synergistic and antagonistic effects can occur simultaneously [2,86,99,100,101,102,103,104]. P. aeruginosa produces phenazines, including pyocyanin as a toxic end product, decanol, and 3-oxo-C12-homoserine lactone (3OC12HSL), which inhibit C. albicans biofilm formation and hyphal development via generation of highly toxic reactive oxygen species (ROS) [2,86,99,100,101,102,103,105,106]. Interestingly, the concentrations of homoserine lactone (HSL) were considerably higher in biofilms than in the presence of these microbes in planktonic state [107]. Additional molecules produced by P. aeruginosa include hemolytic phospholipase C and other virulence factors, such as GacA, LasR, RhlR, and RpoN [108]. The capacity of P. aeruginosa to adhere to the hyphal form of C. albicans is 30 times higher than binding to the yeast form, resulting in condition-dependent killing of hyphae [84,108]. In addition to secreting inhibitory molecules, P. aeruginosa can also increase the virulence of C. albicans by producing the proteolytic enzyme elastase (LasB), thus underscoring the differential effects mediated by P. aeruginosa in this interaction [100]. Conversely, farnesol, a quorum-sensing molecule produced by C. albicans, can downregulate the quorum sensing system of P. aeruginosa by affecting the production of pyocyanin and reducing bacterial motility [109]. To the advantage of P. aeruginosa, generation of fermented products by C. albicans enhances the phenazine secretion by P. aeruginosa, thus promoting colonization of the lungs by the bacterium [110]. More details on this interaction have been described previously [111]. Several studies reported that co-colonization by C. albicans and P. aeruginosa occurs at a statistically significant frequency and results in a decrease of pulmonary function, leading to inferior clinical outcome. Although the mortality rates in some in vivo models including mouse and zebrafish were elevated, possibly as a result of exacerbated inflammatory response, the differential outcomes in various animal models are still a matter of controversy [1,2,51,52,86]. The effects of this interaction may also depend on the specific colonizing strains and the immune status of the host. Thus, more studies are needed to assess the potential importance of this BFI in the clinical setting.
Candida albicans and Streptococcus spp. (Figure 1): The strong adherence and synergistic interaction between different Streptococcus species and C. albicans promoting stable formation of biofilms is well documented, and favors survival and enhanced colonization by these microbes, particularly in the oral cavity and the gastrointestinal tract [112,113]. The adhesins Als1, Als3 and Als5 (agglutinin-like sequence) produced by Candida are important for aggregation and adhesion to bacterial cells [114,115,116], while the binding of Streptococci is mediated by the cell surface polypeptide CshA and the antigen I/II salivary adhesins SspA and SspB [115]. C. albicans induces the growth of various Streptococcus species, including S. oralis, S. gordonii, S. sanguinis, S. mutans, by stimulating the formation of adhesion sites, reducing the oxygen tension, providing growth factors (e.g., polysaccharides) generated by its metabolic activity, and by inducing biofilm formation of Streptococcus spp. via farnesol production [117,118,119]. Importantly, mixed biofilms of C. albicans and Streptococcus display increased resistance to antifungal and antibiotic treatments [120]. Conversely, Streptococcus spp. can promote growth of C. albicans by producing lactate, which can be exploited as a carbon source by the fungus [117,121]. Moreover, Streptococcus species promote adhesion of C. albicans by expressing polysaccharide receptors and polypeptide adhesins. Additionally, Streptococci can stimulate hyphal development via secretion of the quorum sensing molecule AI-2 (autoinducing peptide) and by repression of the C. albicans-derived quorum sensing molecule farnesol, which functions by suppressing hyphal formation at high cell density [122]. Interestingly, a recent study has shown that isolates of C. albicans from patients with recurrent vulvovaginal candidiasis show attenuated hyphal formation in the presence of S. agalactiae [123]. Finally, Streptococcus species including, specifically, S. oralis can also promote dissemination of C. albicans to distal organs by currently unknown mechanisms [113]. However, depending on the environmental conditions, Streptococci can also inhibit hyphal formation through a diffusible signal factor (DSF) and the competence-stimulating peptide (CSP). The factor DSF, a trans-2-decenoic acid, is an intermediate product of unsaturated fatty acid synthesis. This molecule is related to farnesoic acid and farnesol, which are quorum-sensing molecules of C. albicans inhibiting filamentation. However, the mechanism of DSF secretion remains unclear. The molecule CSP is only produced during the early exponential phase of growth, and has been shown to increase biofilm formation, acid tolerance, and production of bacteriocin, a peptide toxin [124,125,126]. Despite the potentially differential effects mediated by the interaction of these microorganisms, the net result is most commonly mutual promotion with strong biofilm formation, suggesting that higher dosages of antimicrobial drugs may be required to control or eradicate the infection.
Candida albicans, Candida glabrata, and Staphylococcus spp. (Figure 1): These microorganisms are responsible for a considerable proportion of hospital infections, and are often co-isolated from urinary tract catheters and in a variety of conditions, including buccal mucositis, cystic fibrosis, keratitis, pneumonia, and wound infections [127,128,129]. The pathogen Staphylococcus aureus is reportedly the third most common bacterial species co-isolated with C. albicans [130]. Adhesion of S. aureus to C. albicans creates a more extensive biofilm, particularly when this bacterium binds to the hyphal form, which displays 30-fold higher adhesion rates compared to the yeast form [84]. Biofilm formation in catheters in in vivo models of S. aureus infections is enhanced by the presence of C. albicans through the attachment of Als3 to S. aureus adhesins [84,131]. Prostaglandin (PG) E2 produced by C. albicans is involved in stimulating growth and biofilm formation of S. aureus in co-culture, and fungal cell wall polysaccharides secreted into the biofilm matrix increase the tolerance of S. aureus to antimicrobial treatment [127,132]. Moreover, C. albicans was shown to promote systemic dissemination of S. aureus to the kidneys in a murine model of oral co-colonization [131]. Conversely, S. aureus supports adhesion of C. albicans to the buccal mucosa via the production of proteinase [133]. However, in the presence of farnesol, either externally added into culture or secreted by C. albicans, the viability and capacity of biofilm formation of S. aureus were reduced, owing to the ability of farnesol to disrupt the cell membrane integrity of the bacterial pathogen [134]. This resulted in increased susceptibility to antibiotic treatment and impaired growth [135,136]. Another Staphylococcus species, S. epidermidis, adheres to both yeast and hyphal forms of C. albicans, and the extracellular matrix produced by S. epidermidis protects the fungus [100]. This association also results in increased resistance to antimicrobial drugs including, for example, fluconazole and vancomycin [100]. In contrast to the largely synergistic interactions outlined above, an antagonistic relationship of S. aureus towards C. glabrata conveyed by an apoptosis-mediated mechanism has recently been described. However, the molecular mediators of this effect have not been identified to date [137].
Candida albicans and Enterococcus faecalis (Figure 1): These microorganisms can be commonly isolated from a variety of clinical samples [138,139], and the bacterium secretes a compound that inhibits hyphal formation of C. albicans via the Fsr quorum sensing system. Two proteases expressed by Fsr, GelE (gelatinase, a metalloprotease II) and SerE (serine protease), play an important role in this process [140]. This inhibitory effect was also observed in an in vivo model using Caenorhabditis elegans as a host organism for the co-infection, where filamentation of C. albicans was inhibited, yet the worm was killed by the infection [138]. The bacteriocin EntV secreted by E. faecalis has been recently identified as the compound inhibiting the yeast-to-hyphae transition of C. albicans, resulting in decreased virulence and biofilm formation [140,141]. This small peptide was also able to degrade mature fungal biofilms and reduce the virulence of C. albicans in a mouse model [141]. In another study, E. faecalis was found to produce a non-hemolytic anti-Candida protein [142]. Adhesion of C. albicans to E. faecalis (and other bacteria, such as Streptococci), is mediated by the cell wall-associated, secreted aspartyl proteinase Sap9, playing an important role in biofilm development [143]. This suggests that factors produced by E. faecalis could be exploited as adjuvants for treatment of Candida infection. Additionally, inhibitors of adhesion and biofilm formation may also attenuate virulence and prevent invasive infection by Candida.
Candida albicans and Lactobacillus spp. (Figure 1): The bacterium displays inhibitory effects against C. albicans. This interaction is relevant in the female reproductive tract, which is populated by Lactobacilli under physiological conditions. The bacteria counteract colonization by C. albicans by reducing adhesion of the fungus to epithelial cells. This is achieved via outcompeting the fungal cells for adhesion sites or by decreasing fungal binding through surlactin, a biosurfactant secreted by the bacteria [8,144]. Lactobacillus species are able to inhibit hyphal formation of C. albicans through soluble metabolic products, short-chain fatty acids (SCFA), H2O2, and lactic acid. Under glucose-limiting conditions, which occur as a result of carbon deprivation during alkalinization, similar to the conditions existing in phagocytic cells, C. albicans can raise the environmental pH by excreting ammonia, thereby inducing hyphal formation [145,146,147].
Candida albicans and Escherichia coli (Figure 1): Murine models have shown that co-infection with C. albicans and E. coli resulted in synergistic lethality. In this case, endotoxins produced by E. coli during the interaction were identified as the key virulence factors contributing to the high mortality [59,60]. In contrast to the observations in murine models, Hall and colleagues reported that, in the human host, the fungus can apparently suppress the growth of E. coli, either directly or indirectly, by dominant interaction during colonization. Under physiological conditions, this effect could possibly inhibit migration of E. coli from the rectum to the vaginal area, thereby offering protection against urinary tract infections caused by E. coli and other bacteria [58]. However, more studies are required to assess the net effects of this interaction in the clinical setting.
Candida albicans and Actinomyces spp. (Figure 1): The fungus is able to adhere to different species of Actinomyces, which are part of the oral bacterial flora, but in vitro studies have shown that the level of aggregation is dependent on the C. albicans strain and the culture medium [148,149,150]. This interaction is mediated by a protein on the Candida surface that interacts with carbohydrate molecules on the surface of Actinomyces. This association results in enhanced cariogenic virulence promoted by increased adhesion, increased biofilm formation, and decreased pH, contributing to oral colonization and oral candidiasis [149,151].
Candida albicans and Acinetobacter baumannii (Figure 1): Association of these microorganisms has been found in clinical isolates from intensive care units [152], and their interaction displays mutually inhibitory effects. While A. baumannii affects hyphal growth of C. albicans and can induce apoptotic cell death via contact-dependent signals mediated by the outer-membrane protein A (OmpA) [153,154], C. albicans responds to the inhibition of filamentation by suppressing the growth of A. baumannii [153]. This effect is conveyed by the secretion of farnesol, which inhibits biofilm formation and reduces the viability of A. baumannii [152,155]. The actual clinical impact is difficult to assess, as clinical studies from different intensive care units (ICUs) have indicated a considerable variation in the rate of invasive infections [156].
Candida albicans and Aggregatibacter actinomycetemcomitans (Figure 1): The bacterium is a Gram-negative opportunistic pathogen causing oral diseases. It produces the quorum-sensing molecule autoinducer-2 (AI-2), inhibiting hyphal structures and biofilm formation of C. albicans in vitro [157]. However, the inhibitory interaction observed in vitro was not confirmed in the clinical setting. Concomitant isolation of these two microorganisms in women using oral contraceptives has been associated with moderate to severe periodontitis, rather supporting a synergistic effect of the interaction [158]. Despite the questionable association of Candida infection with periodontitis, the presence of saliva, which is a strong inducer of hyphal formation, might be responsible for a synergistic effect of the co-infection [157,159]. However, the net effect of this interaction may depend on the amount of bacterial AI-2 produced in saliva-fed biofilms.
Candida albicans and Serratia marcescens (Figure 1): Although the mechanism of interaction has not been identified to date, C. albicans apparently displays a stimulatory effect on the Gram-negative bacterium, enhancing its virulence. This effect has been documented in the peritoneal cavity, and was shown to promote dissemination of the bacterium to several abdominal organs. A similar stimulatory effect was also documented for other bacterial species, including S. aureus and S. faecalis [100,160]. This is of particular importance in immunocompromised patients, in whom disseminated infection may result in severe sepsis.
Candida albicans, Candida dubliniensis, and Fusobacterium spp. (Figure 1): The indicated fungi adhere well to several species of Fusobacterium, including, for example, F. nucleatum, F. periodontium, and F. sulci, which are colonizers of the oral mucosa [161,162,163]. The aggregation, resulting in mutual inhibition, is thought to be mediated by bacterial lectins, which may interact with carbohydrates on the cell wall surface of Candida [161,162]. Recently, additional mediators promoting aggregation of these two microorganisms were identified, involving the bacterial membrane protein RadD, and the Candida adhesin-like cell wall mannoprotein Flo9 [164]. Mutual adherence was only observed in the presence of the hyphal form, but not in the yeast form of C. albicans [165]. However, the strong co-aggregation could be inhibited by externally added arginine and mannose, which disrupt the proteins RadD and Flo9, respectively [165]. The level of co-aggregation and the inhibitory effects were shown to be strain-dependent [163]. Moreover, the effect of the bacterial–fungal interaction in vitro may also be influenced by the growth conditions. Recently, the proteins RadD and Flo9 were found to be involved in the inhibition of hyphal formation of C. albicans under specific growth conditions [164]. Growth and filamentation of C. albicans were found to be inhibited by F. nucleatum in a contact-dependent process [164,165]. These studies suggest that the association between Candida and Fusobacterium may permit a long-term commensal state in the oral mucosa [164]. However, more studies are needed to assess the impact of this bacterial–fungal interaction in the host, including studies in in vivo models.
Candida albicans and Burkholderia spp. (Figure 1): The bacterium B. cenocepacia is an opportunistic pathogen found in the respiratory tract. It is mostly acquired from the environment, via hospital devices or by person-to-person spread, and is only rarely carried as a commensal microorganism [166,167]. B. cenocepacia produces a quorum-sensing molecule termed cis-2-dodecenoic acid (BDSF), which inhibits initiation of hyphal formation in C. albicans [168]. This molecule can also inhibit adherence of C. albicans to urinary catheters, as revealed by in vitro models [169].
Candida albicans and Clostridium spp. (Figure 1): These Gram-positive bacteria are obligate anaerobes, and the growth of certain species is promoted by C. albicans under hypoxic conditions [170]. However, the presence of C. albicans can also be exploited by Clostridium difficile to facilitate its growth under aerobic conditions [171]. It has been suggested that C. albicans may use its metabolism to reduce the oxygen tension or produce antioxidants such as tyrosol, which would favor the growth of anaerobic bacteria [172,173]. This indicates that C. albicans may promote the growth of strictly anaerobic bacteria within oxygen-rich environments [174]. Conversely, C. difficile produces p-Cresol, a fermentation product derived from tyrosine, displaying inhibitory effects on hyphal formation of C. albicans. The compound induces hypha-to-yeast transition, and inhibits biofilm formation and virulence of C. albicans [171]. These observations raise the possibility that treatment approaches affecting the aerobic vs. anaerobic environmental conditions may favor the growth of a certain pathogen. Moreover, these studies suggest that patients harboring a C. difficile infection might be less prone to developing a systemic Candida infection. Conversely, however, elimination of the bacterium by appropriate treatment could promote expansion of the fungus.
Candida albicans and Bacteroides fragilis (Figure 1): Current knowledge on the interaction between these microorganisms is restricted to the observation that growth of the Gram-negative bacterium B. fragilis is promoted by C. albicans under aerobic conditions [170].
Candida albicans and Salmonella enterica (Figure 1): The serovar typhimurium of S. enterica has been described as inhibiting growth, hyphal formation, and viability of C. albicans. It has been suggested that a quorum-sensing molecule secreted by the bacterium might be responsible for this effect [175], and recent data indicate that it is apparently mediated by inositol phosphatase (sopB), an effector of the type III secretion system [176].
Candida albicans and Klebsiella pneumoniae (Figure 1): Antagonistic interactions between the Gram-negative bacterium K. pneumoniae and C. albicans have been reported. The bacterium adheres to both yeast and hyphal structures of C. albicans, and inhibits growth of the fungus. However, the specific mechanisms of this interaction have not yet been elucidated [170].
3.1.2. Aspergillus Species and Bacteria
Aspergillus fumigatus and Pseudomonas aeruginosa (Figure 2): Co-localization of A. fumigatus and P. aeruginosa in the lungs of patients with cystic fibrosis has been associated with poorer outcomes when compared to single infections with these pathogens [53,85]. P. aeruginosa has the capacity to inhibit the growth of A. fumigatus [177,178,179,180]. This interaction occurs through the production of quorum-sensing molecules and virulence factors by P. aeruginosa, including, for example, phenazines, decanol, and 3-oxo-C12-homoserine lactone (3OC12HSL), which affect hyphal development through the generation of highly toxic reactive oxygen species (ROS) [179,181,182]. Moreover, the inhibitory effect also involves the phenazine derivatives, pyrrolnitrin and pyocyanin [182], and the LasIR quorum sensing system has been implicated in inhibiting A. fumigatus biofilms [181]. Conversely, A. fumigatus was recently found to be able to inhibit P. aeruginosa in mixed culture, leading to reduced biofilm formation. The compound gliotoxin produced by A. fumigatus was identified as the main agent responsible for the inhibitory effect. In addition, iron regulation also plays a key role in this interaction. A. fumigatus produces siderophores that help the fungus protect itself against iron-chelation by P. aeruginosa [183]. The indicated interactions are mutually antagonistic [180], thus failing to provide a rational explanation for the clinical impact of the co-infection, but P. aeruginosa also has the capability to produce volatile compounds that stimulate the growth of A. fumigatus at a distance rather than by direct contact [184].

Figure 2.
Molecules and factors mediating the interaction between Aspergillus species and bacteria. Aspergillus species include Aspergillus (A.) fumigatus, Aspergillus (A.) nidulans, Aspergillus (A.) niger, Aspergillus (A.) terreus and Aspergillus (A.) flavus. Gram-positive bacteria are represented in lilac (Streptomyces (S.) rapamycinicus) and Gram-negative bacteria in red (Klebsiella (K.) pneumoniae, Pseudomonas (P.) aeruginosa). Green arrows indicate supportive interactions and red lines represent inhibitory effects. If not indicated above the green arrows and red lines, the molecules mediating the interaction are currently unknown.
Aspergillus nidulans and Streptomyces rapamycinicus (Figure 2): The Gram-positive bacteria of the genus Streptomyces are normally encountered in soil. Although infections in humans are rare, Streptomyces can activate genes of secondary fungal metabolism, including those responsible for synthesis of antibiotic and aromatic polyketides [185]. The activation requires physical contact between A. nidulans and S. rapamycinicus [186]. This interaction is thought to reflect a symbiotic relationship, with activation of silent gene clusters in the fungus mediated by chromatin remodelling [185]. Recent findings suggest a new transcription factor, BasR, as a key regulator for the transduction of the bacterial signal [187]. The findings suggest that infections with Streptomyces may change the local environment and alter the microbial composition by activating fungal-derived synthesis of antibiotic compounds.
Aspergillus niger and Salmonella spp. (Figure 2): The interaction between A. niger and Salmonella spp., including all serovars of S. enterica, is mediated by attachment of the bacterial cellulose to the fungal cell wall component chitin on hyphae of A. niger, promoting the formation of multi-layered and branched biofilms [188]. Although the potential clinical consequences of this interaction have not been elucidated, it is conceivable that the enhanced capacity to bind fungi via cellulose production may result in stronger biofilm formation, thus contributing to increased antimicrobial resistance. Cellulose production by pathogenic bacteria may therefore constitute a survival advantage.
Aspergillus spp. and Klebsiella pneumoniae (Figure 2): Recently, studies performed in our laboratory have shown that K. pneumoniae exerts an inhibitory effect on Aspergillus species, including A. fumigatus, A. terreus, A. niger, and A. flavus. Aspergillus spore germination was inhibited, as well as the development of pre-formed hyphal structures. K. pneumoniae also significantly decreased biofilm formation of Aspergillus species [189]. The exact mechanisms and molecules involved in this interaction are currently under investigation. The clinical impact of this bacterial–fungal interaction may be of particular importance in the setting of cystic fibrosis and other lung-associated diseases because both pathogens co-habit in the lungs.
3.1.3. Cryptococcus Species and Bacteria
Cryptococcus spp. and Pseudomonas aeruginosa (Figure 3): The lungs of immunocompromised patients commonly display the concomitant presence of Cryptococcus spp., including in particular C. neoformans, and P. aeruginosa, and the bacterium has the capacity to inhibit the growth of Cryptococcus spp. [190,191]. The inhibitory effect occurs mainly through the production of the metabolite pyocyanin, but also by the production of alkylquinolones such as HHQ (4-hydroxy-2-heptylquinoline) and PQS (3,4-dihydroxy-2-heptylquinoline) [105,106,190,191]. Cell contact is necessary for maximum inhibition of Cryptococcus growth [190]. The clinical impact of this interaction is currently unknown.

Figure 3.
Molecules and factors mediating the interaction between Cryptococcus spp., Cladosporium spp., Rhizopus microsporus, Saccharomyces cerevisiae, Scedosporium aurantiacum, and different bacteria. Gram- positive bacteria are represented in lilac (Bacillus (B.) subtilis) and Gram-negative bacteria are represented in red (Pseudomonas (P.) aeruginosa, Klebsiella (K.) aerogenes). Green arrows indicate supportive interactions and red lines represent inhibitory effects. If not indicated above the green arrows and red lines, the molecules mediating the interaction are currently unknown.
Cryptococcus neoformans and Klebsiella aerogenes (Figure 3): This bacterial species induces melanin production by C. neoformans through secretion of dopamine by the bacterium, thereby leading to enhanced protection of C. neoformans from macrophages [192,193]. Enhanced melanization of Cryptococcus may also confer a protective effect against antifungal treatment.
3.1.4. Interaction of Other Fungal Species with Bacteria
Cladosporium spp. and Bacillus subtilis (Figure 3): Different species of the mould Cladosporium are involved in mediating allergic reactions, particularly in individuals with pre-existent respiratory diseases, and can also cause infections of the skin, sinuses, and lungs [194]. A class of diphenyl ethers with polyhydroxy sidechains has been identified when Cladosporium species and B. subtilis interacted in vitro, and it was suggested that the production of these compounds may be a defensive response of the fungus against growth inhibition mediated by B. subtilis through the secretion of surfactins (antifungal cyclopeptides). Surfactins have been suggested to cause the induction of secondary metabolism in Cladosporium spp. [195]. Activation of secondary metabolism and production of specific metabolites may result in increased fitness and virulence of these fungal species in the host.
Rhizopus microsporus and Burkholderia spp. (Figure 3): Fungi belonging to the genus Rhizopus cause infections known as zygomycosis. The fungus R. microsporus and the bacterium Burkholderia gladioli are plant pathogens but can also cause opportunistic infections in humans [196]. Upon interaction with R. microsporus, B. gladioli produces the compound bongkrekic acid, which acts as a respiratory toxin, but also results in fungal growth inhibition [197,198]. R. microsporus indirectly contributes to production of bongkrekic acid by stimulating bacterial growth [197]. R. microsporus can also establish a symbiotic interaction with B. rhizoxinica. This endosymbiotic bacterium produces an important compound, rhizoxin, which is essential for fungal spore formation, and is also considered as a mediator of antitumor activity [199]. It is crucial, therefore to assess which pathogens can cause infections in humans and, in the presence of polymicrobial interactions, to identify factors capable of affecting concomitant treatment approaches.
Saccharomyces cerevisiae and Acinetobacter spp. (Figure 3): The fungus produces ethanol, which can promote growth of several Acinetobacter species, including A. baumannii, A. haemolyticus, A. johnsonii, and A. radioresistens in vitro [200]. As demonstrated for A. baumannii in a C. elegans in vivo model, these BFI can result in increased bacterial resistance to osmotic stress associated with enhanced pathogenicity and virulence [200]. Improved fitness of these bacteria might be of particular interest in patients with high alcohol consumption [201], and could ultimately affect the responsiveness to antimicrobial treatment.
Scedosporium aurantiacum and Pseudomonas aeruginosa (Figure 3): The filamentous fungus S. aurantiacum is an opportunistic pathogen that can be isolated from the lungs of patients with cystic fibrosis. Interactions between this fungus and P. aeruginosa, one of the most important bacteria in this disease, were shown to be inhibitory for the fungus. The effect does not require biofilm formation involving P. aeruginosa, but metabolites secreted by the bacterium are suspected to be responsible for the inhibitory interaction. Pyocyanin, a molecule commonly secreted by P. aeruginosa, showed no effect against the fungus [202,203] and the mediators of inhibition, and thus the potential clinical impact of the interaction remains obscure.
4. Host Immune Response to Bacterial and Fungal (Co-)Infections
Perturbations of the microbiome and weakening of the host immune system are conditions facilitating the transition of opportunistic microbes from a commensal to a pathogenic state, mediating the initiation of infection. Microbiota can also influence gene expression of mucins and toll-like receptors (TLRs) by the host, and mediate modulation of the immune system and apoptosis [32]. Factors predisposing the human host for invasive fungal infections include i) long-term or repeated exposure to broad-spectrum antibiotics; ii) impairment of epithelial barriers affecting the skin, the gastrointestinal tract, or other mucous membranes, e.g., by chemotherapy, surgery or central venous catheters; and iii) treatment with immunosuppressive agents such as corticosteroids [204].
The interaction between microbial pathogens and the host induces the activation of several virulence factors and adaptation mechanisms [204]. In fungal infections, the virulence factors include morphological transitions (e.g. yeast-to-hyphae), phenotypic switching (e.g., white to opaque state in C. albicans), biofilm formation, increased adhesion capacity, and environmental pH modulation [205].
The first line of defense against fungal pathogens is mediated by the innate immune response. Pattern recognition receptors (PRRs) expressed on the surface of immune cells recognize pathogen-associated molecular patterns (PAMPs) which comprise several cell wall components, such as mannans, mannoproteins, β-glucans, and chitin, as well as fungal-derived RNA and unmethylated DNA [206]. PRRs include toll-like receptors (TLRs) and C-type lectin receptors (CLRs), which are present on macrophages and dendritic cells. Upon ligand binding, the immune response is initiated through a series of signaling cascades, which, in turn, result in fungal internalization via phagocytosis, and production of cytokines and reactive nitrogen and oxygen species (RNS and ROS) [206]. Most of the fungal cell wall components can be recognized by TLR2, TLR4, and TLR9, which trigger the activation of dendritic cells and transcription of proinflammatory cytokines (IL-1β, IL-6, IL-23) [207,208]. Proinflammatory cytokines bind to receptors on Th17 cells [208]. Neutrophils play a key role against bacterial pathogens by producing large amounts of cytotoxic ROS, proteases, and antimicrobial peptides [209,210], but also play an essential role in the defense against fungal infections. In response to chemotactic factors released by the pathogens and the host, neutrophils rapidly migrate to the infection site, and neutropenia is therefore a risk factor for both fungal and bacterial infections associated with adverse clinical outcome [204,211]. However, excessive accumulation of neutrophils in the course of an infection leads to increased tissue damage, underlining the potential pathogenic effect of the immune response [204]. Synergistic associations between different PRRs (e.g., TLR2 and Dectin-1) have also been found to facilitate the PAMP recognition and to enhance downstream responses [204]. However, the immune response is not always effective. Fungi have developed several mechanisms and strategies to escape the attack of the immune system. The escape mechanisms essentially include shielding of PAMPs through the cell wall or capsule, and the formation of biofilms, titan cells, asteroid bodies, or dimorphism (yeast-to-hyphae transition). For example, virulence factors of C. albicans are exclusively expressed at the hyphal stage, and hyphal cells induce low cytokine production compared to yeast cells. Hyphal structures are also important to evade phagocytosis and escape from the immune cells [211].
Bacteria have also developed mechanisms to hide or escape from the immune system. Some of these mechanisms are similar to those used by fungi. Biofilm formation is also an important feature used by bacteria to evade the immune response. Other factors include the secretion of proteins, quorum sensing regulation, production of antigenic exotoxins, pore-forming toxins, and capsular polysaccharides. Different capsular serotypes exist in bacteria and differ in their chemistry and antigenicity [210,212,213]. Capsular polysaccharides minimize or even inhibit the host recognition, either by hiding or modifying the cell surface. Bacteria can also dampen opsonization through the expression of proteins on the cell surface or by their secretion. Recruitment of neutrophils to the infection site can be inhibited, and killing of neutrophils may occur through the secretion of toxins or cytolysins [210]. Moreover, bacterial secretion systems may also be used to inject effector proteins directly into the host cells, including immune cells [214], and bacteria have developed mechanisms to manipulate the inflammatory pathways, induce immune cell death (apoptosis, pyroptosis), and tolerate different pH conditions [212,215]. Escape from the immune system may lead to persistent and chronic infection, bearing the risk of potentially life-threatening reactivation occurring particularly in severely immunocompromised individuals.
5. In Vivo Models of Bacterial and Fungal (Co)-Infections
Bacterial–fungal interactions can display a diverse spectrum of effects, which may not be identical in vivo and in vitro. For example, the interactions between C. albicans and P. aeruginosa observed in in vitro models are mostly antagonistic, but the interaction in the human host displays synergistic effects on the virulence, resulting in higher mortality [1,2,216]. The differential effect may be explained by the host environment and the increased inflammatory response associated with cytokine profiles that are absent in vitro and also differ from single pathogen infections (Table 1). Co-infection with C. albicans and P. aeruginosa revealed significant upregulation of the proinflammatory cytokine IL-6 and a less prominent increase of IL-8, a potent chemoattractant of neutrophils in a zebrafish in vivo model [2]. However, other studies in mice have shown that infection with C. albicans mediates a protective effect against lung tissue damage induced by P. aeruginosa. This effect reportedly occurs by triggering IL-22 production, activation of the IL-17 pathway, and via stimulating the production of antimicrobial peptides by the host [99]. Similarly, co-infection of P. aeruginosa and A. fumigatus has also been described as resulting in poorer outcomes in cystic fibrosis patients, in comparison to infections by single-pathogens [53]. In contrast to the observations in the human host, no additive effect on the inflammatory response was observed in corresponding co-culture experiments in the wax moth Galleria mellonella model. The lack of synergistic inflammatory response in epithelial cells of cystic fibrosis patients may be explained by the saturation of signaling pathways for cytokine production, since both organisms activate the same pathways [180]. Streptococcus species are very important colonizers of the oral mucosa. A co-infection with C. albicans is synergistically pathogenic in a murine model, leading to the formation of hypervirulent mucosal biofilms [113,217], and the inflammatory response has been shown to be dependent on TLR-2 signalling, with specific cytokine and genetic signatures associated with this co-infection [113].

Table 1.
Animal models used for studies of bacterial–fungal interactions and immune response.
Single pathogen infection with S. aureus was shown to be avirulent in a mouse model, whereas co-infection with C. albicans resulted in 100% mortality within 48–72 h post inoculation [218]. Similar observations were also made in a corresponding co-infection model using G. mellonella or C. elegans, where enhanced pathogenicity and increased mortality was observed [219,220]. However, the mortality rate in the mouse model was apparently dependent on the Candida species involved, as co-infections of S. aureus with C. dubliniensis, Candida parapsilosis, or C. glabrata resulted in low or no mortality at all [218]. During these co-infections involving C. albicans and Candida krusei, IL-6 and prostaglandin E2 (PGE2) were found to be significantly elevated, which was not the case in co-infections with other Candida species [218].
Co-infection of C. albicans and E. coli also resulted in 100% mortality in a murine model, compared to only 3% and 20% mortality of single infections by C. albicans and E. coli, respectively [59], and the bacterial endotoxins produced during the co-infection are thought to mediate the synergistic effect on mortality [59].
Surprisingly, in a C. elegans model of co-infection with C. albicans and E. faecalis, the worms lived much longer than upon infection with C. albicans only, and this effect was even more dramatic upon sequential exposure to E. faecalis followed by C. albicans. It is conceivable that priming the host immune system with E. faecalis somehow protected the worm against subsequent exposure to C. albicans, and the effect is thought to be due to the inhibition of C. albicans filamentation, thereby greatly reducing tissue damage in the worm [138,140]. A similar effect was observed in co-infection with C. albicans and A. baumannii in a C. elegans model, where inhibition of C. albicans filamentation by A. baumannii attenuated the pathogenicity of the fungus, leading to reduced lethality [153].
For many of the bacterial–fungal interactions described above, no data are available regarding the interplay with the host and how the immune system responds to such polymicrobial infections compared to the respective single infections. In Table 1, important features of the bacterial–fungal interactions and the interplay with the host immune system are summarized. It is important to emphasize again that bacterial–fungal interactions observed in vitro can greatly differ from the observations made in vivo, either in model animal systems or in the human host. The immune response, including the degree of inflammation, can exert a major effect on factors affecting the pathogenicity and virulence of the individual pathogens involved, and may thus also affect the overall result of the bacterial–fungal interaction.
6. Conclusions
Current data underline the importance of identifying polymicrobial infections involving bacteria and fungi, and taking their possible interactions into consideration as a basis for efficient diagnostics and treatment. Detailed knowledge of clinically relevant bacterial–fungal interactions not yet characterized to date is needed, with particular emphasis on deciphering the ways of communication between multidrug resistant pathogens such as Candida auris or methicillin-resistant Staphylococcus aureus (MRSA). Employment of state-of-the-art technologies, including CRISPR-Cas (clustered regularly interspaced short palindromic repeats) gene editing and mutant libraries, will facilitate the identification of key regulators mediating bacterial–fungal interactions and their interplay with the host immune system. It is necessary to bear in mind, however, that the effects of BFI observed in assays performed in vitro can be variable, depending on the experimental conditions, and the results can be discordant with those obtained in different in vivo models, thus rendering interpretation of the clinical relevance challenging.
Production of certain metabolites during BFI may result in increased fitness and virulence of the microorganisms involved. As described in this review, bacteria such as Streptococcus spp. can promote hyphal development in fungal species. Hyphal structures play a crucial role in the invasion of epithelial cells and organs, thus promoting expansion of the infection. Moreover, hyphae provide better fitness under challenging environmental conditions, mediate increased adhesion properties, and permit strong biofilm development, which is associated with increased antimicrobial resistance. Importantly, dual-species biofilms have shown increased resistance to drug treatment compared to single-species biofilms [219]. Hyphal development, cell adhesion, and biofilm formation often serve as targets for treatment of monoinfections. Since these factors are also affected during many of the bacterial–fungal interactions studied, they may also serve as targets for appropriate treatment strategies in polymicrobial infections. Some authors have argued that specific classes of antifungal drugs, including echinocandins in particular, might prove beneficial for treating or preventing polymicrobial infections by exerting immunomodulatory properties. However, this immune potentiation is apparently non-specific as it also occurs in response to monoinfections [230].
New diagnostic approaches based on the identification and exploitation of novel biomarkers for BFI that will expectedly emanate from ongoing research are required for appropriate management of polymicrobial infections. Diagnostic biomarkers for pathogenetically relevant processes occurring during BFI, including adhesion, development of hyphal structures, and mixed biofilm formation, are crucial for the development of new adjuvant therapy approaches complementing the use of established treatment strategies with antibiotic and antifungal drugs. Therapeutic interference with the communication between bacterial and fungal pathogens, as well as control of exacerbated inflammatory response, could be potential targets for improved control of specific polymicrobial infections. Novel therapeutic approaches targeting quorum sensing and microbial metabolites may be devised to tackle both bacterial and fungal infections [87,88,89,90,91,92,93,94,95] in combination with antibiotic and antifungal drugs to enhance the efficacy of treatment [96,97,98]. New insights acquired in this field will expectedly pave the way for more efficient personalized treatment strategies.
Author Contributions
F.N. and S.S. prepared the figures and wrote the manuscript. K.K. and T.L. provided input and edited the manuscript.
Funding
This work was supported by the European Commission within the FP7 Framework Programme [Fungitect-Grant No 602125] to T.L. and K.K.
Conflicts of Interest
The authors have declared that no competing interests exist.
References
- Neely, A.N.; Law, E.J.; Holder, I.A. Increased susceptibility to lethal Candida infections in burned mice preinfected with Pseudomonas aeruginosa or pretreated with proteolytic enzymes. Infect. Immun. 1986, 52, 200–204. [Google Scholar] [PubMed]
- Bergeron, A.C.; Seman, B.G.; Hammond, J.H.; Archambault, L.S.; Hogan, D.A.; Wheeler, R.T. Candida and Pseudomonas interact to enhance virulence of mucosal infection in transparent zebrafish. Infect. Immun. 2017. [Google Scholar] [CrossRef]
- Kim, S.H.; Yoon, Y.K.; Kim, M.J.; Sohn, J.W. Risk factors for and clinical implications of mixed Candida/bacterial bloodstream infections. Clin. Microbiol. Infect. 2013, 19, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Wargo, M.J.; Hogan, D.A. Fungal--bacterial interactions: a mixed bag of mingling microbes. Curr. Opin. Microbiol. 2006, 9, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.M.; Jabra-Rizk, M.A.; O’May, G.A.; Costerton, J.W.; Shirtliff, M.E. Polymicrobial interactions: impact on pathogenesis and human disease. Clin. Microbiol. Rev. 2012, 25, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.I.; Strausbaugh, L.D.; Dongari-Bagtzoglou, A. Fungal-bacterial interactions and their relevance to oral health: linking the clinic and the bench. Front. Cell. Infect. Microbiol. 2014, 4, 101. [Google Scholar] [CrossRef] [PubMed]
- Hermann, C.; Hermann, J.; Munzel, U.; Ruchel, R. Bacterial flora accompanying Candida yeasts in clinical specimens. Mycoses 1999, 42, 619–627. [Google Scholar] [CrossRef]
- Morales, D.K.; Hogan, D.A. Candida albicans interactions with bacteria in the context of human health and disease. Plos Pathog. 2010, 6, 1000886. [Google Scholar] [CrossRef]
- Kett, D.H.; Azoulay, E.; Echeverria, P.M.; Vincent, J.L. Candida bloodstream infections in intensive care units: analysis of the extended prevalence of infection in intensive care unit study. Crit. Care. Med. 2011, 39, 665–670. [Google Scholar] [CrossRef]
- Thorn, J.L.; Gilchrist, K.B.; Sobonya, R.E.; Gaur, N.K.; Lipke, P.N.; Klotz, S.A. Postmortem Candidemia: Marker of disseminated disease. J. Clin. Pathol. 2010, 63, 337–340. [Google Scholar] [CrossRef]
- De Sordi, L.; Muhlschlegel, F.A. Quorum sensing and fungal-bacterial interactions in Candida albicans: A communicative network regulating microbial coexistence and virulence. Fems Yeast Res. 2009, 9, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Dixon, E.F.; Hall, R.A. Noisy neighbourhoods: quorum sensing in fungal-polymicrobial infections. Cell Microbiol. 2015, 17, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.M.; Dourado, M.N.; Araujo, W.L. Microbial interactions: ecology in a molecular perspective. Braz. J. Microbiol. 2016, 47, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A. Microbiome: Focus on Causation and Mechanism. Cell 2018, 174, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.; Vrbanac, A.; Taylor, B.C.; Aksenov, A.; Callewaert, C.; Debelius, J.; Gonzalez, A.; Kosciolek, T.; McCall, L.I.; McDonald, D.; et al. Best practices for analysing microbiomes. Nat. Rev. Microbiol. 2018, 16, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Kruger, W.; Vielreicher, S.; Kapitan, M.; Jacobsen, I.D.; Niemiec, M.J. Fungal-Bacterial Interactions in Health and Disease. Pathogens 2019, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Marsland, B.J.; Gollwitzer, E.S. Host-microorganism interactions in lung diseases. Nat. Rev. Immunol. 2014, 14, 827–835. [Google Scholar] [CrossRef]
- Nash, A.K.; Auchtung, T.A.; Wong, M.C.; Smith, D.P.; Gesell, J.R.; Ross, M.C.; Stewart, C.J.; Metcalf, G.A.; Muzny, D.M.; Gibbs, R.A.; et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 2017, 5, 153. [Google Scholar] [CrossRef]
- Witherden, E.A.; Shoaie, S.; Hall, R.A.; Moyes, D.L. The Human Mucosal Mycobiome and Fungal Community Interactions. J. Fungi (Basel) 2017, 3, 56. [Google Scholar] [CrossRef]
- Jo, J.H.; Kennedy, E.A.; Kong, H.H. Topographical and physiological differences of the skin mycobiome in health and disease. Virulence 2017, 8, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut microbiota: the neglected endocrine organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Caballero, S.; Pamer, E.G. Microbiota-mediated inflammation and antimicrobial defense in the intestine. Annu. Rev. Immunol. 2015, 33, 227–256. [Google Scholar] [CrossRef]
- Selber-Hnatiw, S.; Rukundo, B.; Ahmadi, M.; Akoubi, H.; Al-Bizri, H.; Aliu, A.F.; Ambeaghen, T.U.; Avetisyan, L.; Bahar, I.; Baird, A. Human Gut Microbiota: Toward an Ecology of Disease. Front. Microbiol. 2017, 8, 1265. [Google Scholar] [CrossRef]
- Tojo, R.; Suarez, A.; Clemente, M.G.; de los Reyes-Gavilan, C.G.; Margolles, A.; Gueimonde, M.; Ruas-Madiedo, P. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J. Gastroenterol. 2014, 20, 15163–15176. [Google Scholar] [CrossRef]
- Candela, M.; Turroni, S.; Biagi, E.; Carbonero, F.; Rampelli, S.; Fiorentini, C.; Brigidi, P. Inflammation and colorectal cancer, when microbiota-host mutualism breaks. World J. Gastroenterol. 2014, 20, 908–922. [Google Scholar] [CrossRef]
- Galloway-Pena, J.; Brumlow, C.; Shelburne, S. Impact of the Microbiota on Bacterial Infections during Cancer Treatment. Trends Microbiol. 2017, 25, 992–1004. [Google Scholar] [CrossRef]
- Heisel, T.; Montassier, E.; Johnson, A.; Al-Ghalith, G.; Lin, Y.W.; Wei, L.N.; Knights, D.; Gale, C.A. High-Fat Diet Changes Fungal Microbiomes and Interkingdom Relationships in the Murine Gut. mSphere 2017, 2. [Google Scholar] [CrossRef]
- Lof, M.; Janus, M.M.; Krom, B.P. Metabolic Interactions between Bacteria and Fungi in Commensal Oral Biofilms. J. Fungi (Basel) 2017, 3, 40. [Google Scholar] [CrossRef]
- Raskov, H.; Burcharth, J.; Pommergaard, H.C. Linking Gut Microbiota to Colorectal Cancer. J. Cancer 2017, 8, 3378–3395. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, M.C.; Fodor, A.; Jobin, C. Carcinogenesis and therapeutics: the microbiota perspective. Nat. Microbiol. 2017, 2, 17008. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Daillere, R.; Roberti, M.P.; Routy, B.; Kroemer, G. Anticancer effects of the microbiome and its products. Nat. Rev. Microbiol. 2017, 15, 465–478. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef]
- Liguori, G.; Lamas, B.; Richard, M.L.; Brandi, G.; da Costa, G.; Hoffmann, T.W.; Di Simone, M.P.; Calabrese, C.; Poggioli, G.; Langella, P.; et al. Fungal Dysbiosis in Mucosa-associated Microbiota of Crohn’s Disease Patients. J. Crohns. Colitis. 2016, 10, 296–305. [Google Scholar] [CrossRef]
- Pope, J.L.; Tomkovich, S.; Yang, Y.; Jobin, C. Microbiota as a mediator of cancer progression and therapy. Transl. Res. 2017, 179, 139–154. [Google Scholar] [CrossRef]
- Gagniere, J.; Raisch, J.; Veziant, J.; Barnich, N.; Bonnet, R.; Buc, E.; Bringer, M.A.; Pezet, D.; Bonnet, M. Gut microbiota imbalance and colorectal cancer. World J. Gastroenterol. 2016, 22, 501–518. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Williams, S.C. Gnotobiotics. Proc. Natl. Acad. Sci. USA 2014, 111, 1661. [Google Scholar] [CrossRef]
- Gordon, H.A.; Pesti, L. The gnotobiotic animal as a tool in the study of host microbial relationships. Bacteriol. Rev. 1971, 35, 390–429. [Google Scholar] [PubMed]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Backhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Cash, H.L.; Whitham, C.V.; Behrendt, C.L.; Hooper, L.V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 2006, 313, 1126–1130. [Google Scholar] [CrossRef]
- Umesaki, Y. Use of gnotobiotic mice to identify and characterize key microbes responsible for the development of the intestinal immune system. Proc. Jpn. Acad. Ser. B. 2014, 90, 313–332. [Google Scholar] [CrossRef]
- Burmolle, M.; Ren, D.; Bjarnsholt, T.; Sorensen, S.J. Interactions in multispecies biofilms: do they actually matter? Trends Microbiol. 2014, 22, 84–91. [Google Scholar] [CrossRef]
- de Vos, W.M. Microbial biofilms and the human intestinal microbiome. Npj Biofilms Microbiomes 2015, 1, 15005. [Google Scholar] [CrossRef]
- Raskov, H.; Kragh, K.N.; Bjarnsholt, T.; Alamili, M.; Gogenur, I. Bacterial biofilm formation inside colonic crypts may accelerate colorectal carcinogenesis. Clin. Transl. Med. 2018, 7, 018–0209. [Google Scholar] [CrossRef]
- Leclair, L.W.; Hogan, D.A. Mixed bacterial-fungal infections in the, C.F. respiratory tract. Med. Mycol. 2010, 48, 521522. [Google Scholar] [CrossRef]
- Chotirmall, S.H.; O’Donoghue, E.; Bennett, K.; Gunaratnam, C.; O’Neill, S.J.; McElvaney, N.G. Sputum Candida albicans presages, F.E.V(1) decline and hospital-treated exacerbations in cystic fibrosis. Chest 2010, 138, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Reece, E.; Segurado, R.; Jackson, A.; McClean, S.; Renwick, J.; Greally, P. Co-colonisation with Aspergillus fumigatus and Pseudomonas aeruginosa is associated with poorer health in cystic fibrosis patients: an Irish registry analysis. BMC Pulm. Med. 2017, 17, 70. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef]
- Sobel, J.D. Vulvovaginal candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef]
- van de Wijgert, J.H.; Borgdorff, H.; Verhelst, R.; Crucitti, T.; Francis, S.; Verstraelen, H.; Jespers, V. The vaginal microbiota: what have we learned after a decade of molecular characterization? PLoS ONE 2014, 9, e105998. [Google Scholar] [CrossRef]
- Hall, R.A.; Noverr, M.C. Fungal interactions with the human host: exploring the spectrum of symbiosis. Curr. Opin. Microbiol. 2017, 40, 58–64. [Google Scholar] [CrossRef]
- Burd, R.S.; Raymond, C.S.; Dunn, D.L. Endotoxin promotes synergistic lethality during concurrent Escherichia coli and Candida albicans infection. J. Surg. Res. 1992, 52, 537–542. [Google Scholar] [CrossRef]
- Ikeda, T.; Suegara, N.; Abe, S.; Yamaguchi, H. Efficacy of antibacterial drugs in mice with complex infection by Candida albicans and Escherichia coli. J. Antibiot. 1999, 52, 552–558. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Standaert-Vitse, A.; Sendid, B.; Joossens, M.; Francois, N.; Vandewalle-El Khoury, P.; Branche, J.; Van Kruiningen, H.; Jouault, T.; Rutgeerts, P.; Gower-Rousseau, C.; et al. Candida albicans colonization and, A.S.CA in familial Crohn’s disease. Am. J. Gastroenterol. 2009, 104, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Chiaro, T.R.; Soto, R.; Zac Stephens, W.; Kubinak, J.L.; Petersen, C.; Gogokhia, L.; Bell, R.; Delgado, J.C.; Cox, J.; Voth, W.; et al. A member of the gut mycobiota modulates host purine metabolism exacerbating colitis in mice. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhang, F.; Yang, X.; Wu, N.; Jiang, W.; Li, X.; Liu, Y. Changes in the composition of intestinal fungi and their role in mice with dextran sulfate sodium-induced colitis. Sci. Rep. 2015, 5, 10416. [Google Scholar] [CrossRef] [PubMed]
- Noverr, M.C.; Noggle, R.M.; Toews, G.B.; Huffnagle, G.B. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect. Immun. 2004, 72, 4996–5003. [Google Scholar] [CrossRef]
- Wheeler, M.L.; Limon, J.J.; Bar, A.S.; Leal, C.A.; Gargus, M.; Tang, J.; Brown, J.; Funari, V.A.; Wang, H.L.; Crother, T.R.; et al. Immunological Consequences of Intestinal Fungal Dysbiosis. Cell Host Microbe. 2016, 19, 865–873. [Google Scholar] [CrossRef]
- Sommer, F.; Backhed, F. The gut microbiota--masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Butcher, J.; Mack, D.; Stintzi, A. Functional impacts of the intestinal microbiome in the pathogenesis of inflammatory bowel disease. Inflamm. Bowel Dis. 2015, 21, 139–153. [Google Scholar] [CrossRef]
- Sam, Q.H.; Chang, M.W.; Chai, L.Y. The Fungal Mycobiome and Its Interaction with Gut Bacteria in the Host. Int. J. Mol. Sci. 2017, 18, 330. [Google Scholar] [CrossRef]
- Nguyen, G.C. Editorial: bugs and drugs: insights into the pathogenesis of inflammatory bowel disease. Am. J. Gastroenterol. 2011, 106, 2143–2145. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.D.; Atay, C.; Heringer, J.; Romrig, F.K.; Schwitalla, S.; Aydin, B.; Ziegler, P.K.; Varga, J.; Reindl, W.; Pommerenke, C.; et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature 2014, 514, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Belcheva, A.; Irrazabal, T.; Robertson, S.J.; Streutker, C.; Maughan, H.; Rubino, S.; Moriyama, E.H.; Copeland, J.K.; Surendra, A.; Kumar, S.; et al. Gut microbial metabolism drives transformation of, M.S.H2-deficient colon epithelial cells. Cell 2014, 158, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Lupton, J.R. Microbial degradation products influence colon cancer risk: the butyrate controversy. J. Nutr. 2004, 134, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.L.; Aye, L.M.; Whiteley, M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J. Bacteriol. 2007, 189, 8079–8087. [Google Scholar] [CrossRef] [PubMed]
- Gainza-Cirauqui, M.L.; Nieminen, M.T.; Novak Frazer, L.; Aguirre-Urizar, J.M.; Moragues, M.D.; Rautemaa, R. Production of carcinogenic acetaldehyde by Candida albicans from patients with potentially malignant oral mucosal disorders. J. Oral Pathol. Med. 2013, 42, 243–249. [Google Scholar] [CrossRef]
- Meurman, J.H.; Uittamo, J. Oral micro-organisms in the etiology of cancer. Acta. Odontol. Scand. 2008, 66, 321–326. [Google Scholar] [CrossRef]
- Whiteley, M.; Diggle, S.P.; Greenberg, E.P. Progress in and promise of bacterial quorum sensing research. Nature 2017, 551, 313–320. [Google Scholar] [CrossRef]
- Rio, R.V.M. Don’t Bite the Hand that Feeds You. Cell Host Microbe. 2017, 21, 552–554. [Google Scholar] [CrossRef]
- Polke, M.; Jacobsen, I.D. Quorum sensing by farnesol revisited. Curr. Genet. 2017, 63, 791–797. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A. Classic Spotlight: Quorum Sensing and the Multicellular Life of Unicellular Organisms. J. Bacteriol. 2016, 198, 601. [Google Scholar] [CrossRef] [PubMed]
- Hofer, U. Biofilms: Turning tides for quorum sensing. Nat. Rev. Microbiol. 2016, 14, 64. [Google Scholar] [CrossRef] [PubMed]
- Pammi, M.; Liang, R.; Hicks, J.; Mistretta, T.A.; Versalovic, J. Biofilm extracellular, D.N.A enhances mixed species biofilms of Staphylococcus epidermidis and Candida albicans. BMC Microbiol. 2013, 13, 1471–2180. [Google Scholar] [CrossRef]
- Smith, K.; Rajendran, R.; Kerr, S.; Lappin, D.F.; Mackay, W.G.; Williams, C.; Ramage, G. Aspergillus fumigatus enhances elastase production in Pseudomonas aeruginosa co-cultures. Med. Mycol. 2015, 53, 645–655. [Google Scholar] [CrossRef]
- Mear, J.B.; Kipnis, E.; Faure, E.; Dessein, R.; Schurtz, G.; Faure, K.; Guery, B. Candida albicans and Pseudomonas aeruginosa interactions: more than an opportunistic criminal association? Med. Mal. Infect. 2013, 43, 146–151. [Google Scholar] [CrossRef]
- Tan, C.H.; Koh, K.S.; Xie, C.; Zhang, J.; Tan, X.H.; Lee, G.P.; Zhou, Y.; Ng, W.J.; Rice, S.A.; Kjelleberg, S. Community quorum sensing signalling and quenching: microbial granular biofilm assembly. Npj Biofilms Microbiomes 2015, 1, 15006. [Google Scholar] [CrossRef]
- Dong, Y.H.; Xu, J.L.; Li, X.Z.; Zhang, L.H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 2000, 97, 3526–3531. [Google Scholar] [CrossRef]
- Defoirdt, T. Quorum-Sensing Systems as Targets for Antivirulence Therapy. Trends Microbiol. 2017. [Google Scholar] [CrossRef]
- Abraham, W.R. Going beyond the Control of Quorum-Sensing to Combat Biofilm Infections. Antibiotics (Basel) 2016, 5, 3. [Google Scholar] [CrossRef]
- Thompson, J.A.; Oliveira, R.A.; Djukovic, A.; Ubeda, C.; Xavier, K.B. Manipulation of the quorum sensing signal, A.I.-2 affects the antibiotic-treated gut microbiota. Cell Rep. 2015, 10, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, R. Is Quorum Sensing Interference a Viable Alternative to Treat Pseudomonas aeruginosa Infections? Front. Microbiol. 2016, 7, 1454. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wei, Y.; Xia, B.; Jin, Y.; Liu, C.; Pan, X.; Shi, J.; Zhu, F.; Li, J.; Qian, L.; et al. Identification of a small molecule that simultaneously suppresses virulence and antibiotic resistance of Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 19141. [Google Scholar] [CrossRef] [PubMed]
- Muimhneacháin, E.Ó.; Reen, F.J.; O’Gara, F.; McGlacken, G.P. Analogues of Pseudomonas aeruginosa signalling molecules to tackle infections. Org. Biomol. Chem. 2018, 16, 169–179. [Google Scholar] [CrossRef]
- Smith, A.C.; Rice, A.; Sutton, B.; Gabrilska, R.; Wessel, A.K.; Whiteley, M.; Rumbaugh, K.P. Albumin Inhibits Pseudomonas aeruginosa Quorum Sensing and Alters Polymicrobial Interactions. Infect. Immun. 2017, 85, 116. [Google Scholar] [CrossRef]
- Imperi, F.; Leoni, L.; Visca, P. Antivirulence activity of azithromycin in Pseudomonas aeruginosa. Front. Microbiol. 2014, 5, 178. [Google Scholar] [CrossRef]
- Ramage, G.; Saville, S.P.; Wickes, B.L.; Lopez-Ribot, J.L. Inhibition of Candida albicans Biofilm Formation by Farnesol, a Quorum-Sensing Molecule. Appl. Environ. Microbiol. 2002, 68, 5459–5463. [Google Scholar] [CrossRef]
- Cordeiro, R.A.; Teixeira, C.E.; Brilhante, R.S.; Castelo-Branco, D.S.; Paiva, M.A.; Giffoni Leite, J.J.; Lima, D.T.; Monteiro, A.J.; Sidrim, J.J.; Rocha, M.F. Minimum inhibitory concentrations of amphotericin B, azoles and caspofungin against Candida species are reduced by farnesol. Med. Mycol. 2013, 51, 53–59. [Google Scholar] [CrossRef]
- Mear, J.B.; Gosset, P.; Kipnis, E.; Faure, E.; Dessein, R.; Jawhara, S.; Fradin, C.; Faure, K.; Poulain, D.; Sendid, B.; et al. Candida albicans airway exposure primes the lung innate immune response against Pseudomonas aeruginosa infection through innate lymphoid cell recruitment and interleukin-22-associated mucosal response. Infect. Immun. 2014, 82, 306–315. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Hogan, D.A.; Mylonakis, E. Medically important bacterial-fungal interactions. Nat. Rev. Microbiol. 2010, 8, 340–349. [Google Scholar] [CrossRef]
- Gibson, J.; Sood, A.; Hogan, D.A. Pseudomonas aeruginosa-Candida albicans interactions: localization and fungal toxicity of a phenazine derivative. Appl. Env. Microbiol. 2009, 75, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.A.; Vik, A.; Kolter, R. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol. Microbiol. 2004, 54, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.A. Talking to themselves: autoregulation and quorum sensing in fungi. Eukaryot. Cell. 2006, 5, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Purschke, F.G.; Hiller, E.; Trick, I.; Rupp, S. Flexible survival strategies of Pseudomonas aeruginosa in biofilms result in increased fitness compared with Candida albicans. Mol. Cell Proteomics. 2012, 11, 1652–1669. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.R. Suppression of fungal growth exhibited by Pseudomonas aeruginosa. J. Clin. Microbiol. 1994, 32, 525–527. [Google Scholar]
- Kerr, J.R.; Taylor, G.W.; Rutman, A.; Hoiby, N.; Cole, P.J.; Wilson, R. Pseudomonas aeruginosa pyocyanin and 1-hydroxyphenazine inhibit fungal growth. J. Clin. Pathol. 1999, 52, 385–387. [Google Scholar] [CrossRef]
- Charlton, T.S.; de Nys, R.; Netting, A.; Kumar, N.; Hentzer, M.; Givskov, M.; Kjelleberg, S. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography-mass spectrometry: application to a model bacterial biofilm. Env. Microbiol. 2000, 2, 530–541. [Google Scholar] [CrossRef]
- Hogan, D.A.; Kolter, R. Pseudomonas-Candida interactions: an ecological role for virulence factors. Science 2002, 296, 2229–2232. [Google Scholar] [CrossRef]
- Cugini, C.; Calfee, M.W.; Farrow, J.M., 3rd; Morales, D.K.; Pesci, E.C.; Hogan, D.A. Farnesol, a common sesquiterpene, inhibits, P.Q.S production in Pseudomonas aeruginosa. Mol. Microbiol. 2007, 65, 896–906. [Google Scholar] [CrossRef]
- Chen, A.I.; Dolben, E.F.; Okegbe, C.; Harty, C.E.; Golub, Y.; Thao, S.; Ha, D.G.; Willger, S.D.; O’Toole, G.A.; Harwood, C.S.; et al. Candida albicans ethanol stimulates Pseudomonas aeruginosa WspR-controlled biofilm formation as part of a cyclic relationship involving phenazines. PloS Pathog. 2014, 10, e1004480. [Google Scholar] [CrossRef]
- Fourie, R.; Pohl, C.H. Beyond Antagonism: The Interaction Between Candida Species and Pseudomonas aeruginosa. J. Fungi (Basel) 2019, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, I.M.; Del Bel Cury, A.A.; Jenkinson, H.F.; Nobbs, A.H. Interactions between Streptococcus oralis, Actinomyces oris, and Candida albicans in the development of multispecies oral microbial biofilms on salivary pellicle. Mol. Oral Microbiol. 2017, 32, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Sobue, T.; Thompson, A.; Xie, Z.; Poon, K.; Ricker, A.; Cervantes, J.; Diaz, P.I.; Dongari-Bagtzoglou, A. Streptococcal co-infection augments Candida pathogenicity by amplifying the mucosal inflammatory response. Cell Microbiol. 2014, 16, 214–231. [Google Scholar] [CrossRef]
- Holmes, A.R.; Gopal, P.K.; Jenkinson, H.F. Adherence of Candida albicans to a cell surface polysaccharide receptor on Streptococcus gordonii. Infect. Immun. 1995, 63, 1827–1834. [Google Scholar]
- Holmes, A.R.; McNab, R.; Jenkinson, H.F. Candida albicans binding to the oral bacterium Streptococcus gordonii involves multiple adhesin-receptor interactions. Infect. Immun. 1996, 64, 4680–4685. [Google Scholar]
- Nobbs, A.H.; Vickerman, M.M.; Jenkinson, H.F. Heterologous expression of Candida albicans cell wall-associated adhesins in Saccharomyces cerevisiae Reveals differential specificities in adherence and biofilm formation and in binding oral Streptococcus gordonii. Eukaryot. Cell 2010, 9, 1622–1634. [Google Scholar] [CrossRef]
- Jenkinson, H.F.; Lala, H.C.; Shepherd, M.G. Coaggregation of Streptococcus sanguis and other streptococci with Candida albicans. Infect. Immun. 1990, 58, 1429–1436. [Google Scholar]
- O’Sullivan, J.M.; Jenkinson, H.F.; Cannon, R.D. Adhesion of Candida albicans to oral streptococci is promoted by selective adsorption of salivary proteins to the streptococcal cell surface. Microbiology 2000, 146, 41–48. [Google Scholar] [CrossRef]
- Kim, D.; Sengupta, A.; Niepa, T.H.; Lee, B.H.; Weljie, A.; Freitas-Blanco, V.S.; Murata, R.M.; Stebe, K.J.; Lee, D.; Koo, H. Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci. Rep. 2017, 7, 41332. [Google Scholar] [CrossRef]
- Montelongo-Jauregui, D.; Saville, S.P.; Lopez-Ribot, J.L. Contributions of Candida albicans Dimorphism, Adhesive Interactions, and Extracellular Matrix to the Formation of Dual-Species Biofilms with Streptococcus gordonii. Mbio 2019, 10, e01179-19. [Google Scholar] [CrossRef]
- Holmes, A.R.; van der Wielen, P.; Cannon, R.D.; Ruske, D.; Dawes, P. Candida albicans binds to saliva proteins selectively adsorbed to silicone. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Bamford, C.V.; d’Mello, A.; Nobbs, A.H.; Dutton, L.C.; Vickerman, M.M.; Jenkinson, H.F. Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect. Immun. 2009, 77, 3696–3704. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Fu, F.; Kong, W.N.; Xuan, Q.K.; Wen, D.H.; Chen, X.Q.; He, Y.M.; He, L.H.; Guo, J.; Zhou, A.P.; et al. Streptococcus agalactiae Inhibits Candida albicans Hyphal Development and Diminishes Host Vaginal Mucosal, T.H.17 Response. Front. Microbiol. 2018, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Vilchez, R.; Lemme, A.; Ballhausen, B.; Thiel, V.; Schulz, S.; Jansen, R.; Sztajer, H.; Wagner-Döbler, I. Streptococcus mutans inhibits Candida albicans hyphal formation by the fatty acid signaling molecule trans-2-decenoic acid (SDSF). Chembiochem 2010, 11, 1552–1562. [Google Scholar] [CrossRef]
- Jarosz, L.M.; Deng, D.M.; van der Mei, H.C.; Crielaard, W.; Krom, B.P. Streptococcus mutans competence-stimulating peptide inhibits Candida albicans hypha formation. Eukaryot. Cell 2009, 8, 1658–1664. [Google Scholar] [CrossRef]
- Ahn, S.J.; Wen, Z.T.; Burne, R.A. Multilevel control of competence development and stress tolerance in Streptococcus mutans, U.A.159. Infect. Immun. 2006, 74, 1631–1642. [Google Scholar] [CrossRef]
- Kong, E.F.; Tsui, C.; Kucharikova, S.; Andes, D.; Van Dijck, P.; Jabra-Rizk, M.A. Commensal Protection of Staphylococcus aureus against Antimicrobials by Candida albicans Biofilm Matrix. Mbio 2016, 7. [Google Scholar] [CrossRef]
- Pate, J.C.; Jones, D.B.; Wilhelmus, K.R. Prevalence and spectrum of bacterial co-infection during fungal keratitis. Br. J. Ophthalmol. 2006, 90, 289–292. [Google Scholar] [CrossRef]
- Gupta, N.; Haque, A.; Mukhopadhyay, G.; Narayan, R.P.; Prasad, R. Interactions between bacteria and Candida in the burn wound. Burns 2005, 31, 375–378. [Google Scholar] [CrossRef]
- Klotz, S.A.; Chasin, B.S.; Powell, B.; Gaur, N.K.; Lipke, P.N. Polymicrobial bloodstream infections involving Candida species: analysis of patients and review of the literature. Diagn. Microbiol. Infect. Dis. 2007, 59, 401–406. [Google Scholar] [CrossRef]
- Schlecht, L.M.; Peters, B.M.; Krom, B.P.; Freiberg, J.A.; Hansch, G.M.; Filler, S.G.; Jabra-Rizk, M.A.; Shirtliff, M.E. Systemic Staphylococcus aureus infection mediated by Candida albicans hyphal invasion of mucosal tissue. Microbiology 2015, 161, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Krause, J.; Geginat, G.; Tammer, I. Prostaglandin E2 from Candida albicans Stimulates the Growth of Staphylococcus aureus in Mixed Biofilms. PLoS ONE 2015, 10, e0135404. [Google Scholar] [CrossRef] [PubMed]
- Shirtliff, M.E.; Peters, B.M.; Jabra-Rizk, M.A. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol. Lett. 2009, 299, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.F.; Tsui, C.; Kucharikova, S.; Van Dijck, P.; Jabra-Rizk, M.A. Modulation of Staphylococcus aureus Response to Antimicrobials by the Candida albicans Quorum Sensing Molecule Farnesol. Antimicrob. Agents Chemother. 2017, 61, e01573-17. [Google Scholar] [CrossRef]
- Inoue, Y.; Shiraishi, A.; Hada, T.; Hirose, K.; Hamashima, H.; Shimada, J. The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action. FEMS Microbiol. Lett. 2004, 237, 325–331. [Google Scholar] [CrossRef]
- Jabra-Rizk, M.A.; Meiller, T.F.; James, C.E.; Shirtliff, M.E. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob. Agents Chemother. 2006, 50, 1463–1469. [Google Scholar] [CrossRef]
- Camarillo-Marquez, O.; Cordova-Alcantara, I.M.; Hernandez-Rodriguez, C.H.; Garcia-Perez, B.E.; Martinez-Rivera, M.A.; Rodríguez-Tovar, A.V. Antagonistic Interaction of Staphylococcus aureus Toward Candida glabrata During in vitro Biofilm Formation Is Caused by an Apoptotic Mechanism. Front. Microbiol. 2018, 9, 2031. [Google Scholar] [CrossRef]
- Garsin, D.A.; Lorenz, M.C. Candida albicans and Enterococcus faecalis in the gut: synergy in commensalism? Gut Microbes 2013, 4, 409–415. [Google Scholar] [CrossRef]
- Kovac, J.; Kovac, D.; Slobodnikova, L.; Kotulova, D. Enterococcus faecalis and Candida albicans in the dental root canal and periapical infections. Bratisl. Lek. Listy. 2013, 114, 716–720. [Google Scholar]
- Cruz, M.R.; Graham, C.E.; Gagliano, B.C.; Lorenz, M.C.; Garsin, D.A. Enterococcus faecalis inhibits hyphal morphogenesis and virulence of Candida albicans. Infect. Immun. 2013, 81, 189–200. [Google Scholar] [CrossRef]
- Graham, C.E.; Cruz, M.R.; Garsin, D.A.; Lorenz, M.C. Enterococcus faecalis bacteriocin EntV inhibits hyphal morphogenesis, biofilm formation, and virulence of Candida albicans. Proc. Natl. Acad. Sci. USA 2017, 114, 4507–4512. [Google Scholar] [CrossRef] [PubMed]
- Shekh, R.M.; Roy, U. Biochemical characterization of an anti-Candida factor produced by Enterococcus faecalis. BMC Microbiol. 2012, 12, 1471–2180. [Google Scholar] [CrossRef] [PubMed]
- Dutton, L.C.; Jenkinson, H.F.; Lamont, R.J.; Nobbs, A.H. Role of Candida albicans secreted aspartyl protease Sap9 in interkingdom biofilm formation. Pathog. Dis. 2016, 74, 14. [Google Scholar] [CrossRef] [PubMed]
- Boris, S.; Barbes, C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect. 2000, 2, 543–546. [Google Scholar] [CrossRef]
- Noverr, M.C.; Huffnagle, G.B. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect. Immun. 2004, 72, 6206–6210. [Google Scholar] [CrossRef] [PubMed]
- Vylkova, S.; Carman, A.J.; Danhof, H.A.; Collette, J.R.; Zhou, H.; Lorenz, M.C. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. Mbio 2011, 2, e00055-11. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Yang, E.; Yan, L.; Li, T.; Zhuang, H. Antimicrobial Compounds Produced by Vaginal Lactobacillus crispatus Are Able to Strongly Inhibit Candida albicans Growth, Hyphal Formation and Regulate Virulence-related Gene Expressions. Front. Microbiol. 2017, 8, 564. [Google Scholar] [CrossRef]
- Grimaudo, N.J.; Nesbitt, W.E.; Clark, W.B. Coaggregation of Candida albicans with oral Actinomyces species. Oral Microbiol. Immunol. 1996, 11, 59–61. [Google Scholar] [CrossRef]
- Arzmi, M.H.; Dashper, S.; Catmull, D.; Cirillo, N.; Reynolds, E.C.; McCullough, M. Coaggregation of Candida albicans, Actinomyces naeslundii and Streptococcus mutans is Candida albicans strain dependent. FEMS Yeast Res. 2015, 15, 7. [Google Scholar] [CrossRef]
- Arzmi, M.H.; Alnuaimi, A.D.; Dashper, S.; Cirillo, N.; Reynolds, E.C.; McCullough, M. Polymicrobial biofilm formation by Candida albicans, Actinomyces naeslundii, and Streptococcus mutans is Candida albicans strain and medium dependent. Med. Mycol. 2016, 54, 856–864. [Google Scholar] [CrossRef]
- Deng, L.; Li, W.; He, Y.; Wu, J.; Ren, B.; Zou, L. Cross-kingdom interaction of Candida albicans and Actinomyces viscosus elevated cariogenic virulence. Arch. Oral Biol. 2019, 100, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Tampakakis, E.; Fuchs, B.B.; Eliopoulos, G.M.; Moellering, R.C., Jr.; Mylonakis, E. Prokaryote-eukaryote interactions identified by using Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2008, 105, 14585–14590. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, J.A.; Tomaras, A.P.; Actis, L.A. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect. Immun. 2009, 77, 3150–3160. [Google Scholar] [CrossRef]
- Kostoulias, X.; Murray, G.L.; Cerqueira, G.M.; Kong, J.B.; Bantun, F.; Mylonakis, E.; Khoo, C.A.; Peleg, A.Y. Impact of a Cross-Kingdom Signaling Molecule of Candida albicans on Acinetobacter baumannii Physiology. Antimicrob. Agents Chemother. 2015, 60, 161–167. [Google Scholar] [CrossRef]
- Liu, C.Y.; Liao, C.H.; Chen, Y.C.; Chang, S.C. Changing epidemiology of nosocomial bloodstream infections in 11 teaching hospitals in Taiwan between 1993 and 2006. J. Microbiol. Immunol. Infect. 2010, 43, 416–429. [Google Scholar] [CrossRef]
- Bachtiar, E.W.; Bachtiar, B.M.; Jarosz, L.M.; Amir, L.R.; Sunarto, H.; Ganin, H.; Meijler, M.M.; Krom, B.P. AI-2 of Aggregatibacter actinomycetemcomitans inhibits Candida albicans biofilm formation. Front. Cell Infect. Microbiol. 2014, 4, 94. [Google Scholar] [CrossRef]
- Brusca, M.I.; Rosa, A.; Albaina, O.; Moragues, M.D.; Verdugo, F.; Pontón, J. The impact of oral contraceptives on women’s periodontal health and the subgingival occurrence of aggressive periodontopathogens and Candida species. J. Periodontol. 2010, 81, 1010–1018. [Google Scholar] [CrossRef]
- Rickard, A.H.; Campagna, S.R.; Kolenbrander, P.E. Autoinducer-2 is produced in saliva-fed flow conditions relevant to natural oral biofilms. J. Appl. Microbiol. 2008, 105, 2096–2103. [Google Scholar] [CrossRef]
- Carlson, E. Enhancement by Candida albicans of Staphylococcus aureus, Serratia marcescens, and Streptococcus faecalis in the establishment of infection in mice. Infect. Immun. 1983, 39, 193–197. [Google Scholar]
- Bagg, J.; Silverwood, R.W. Coagglutination reactions between Candida albicans and oral bacteria. J. Med. Microbiol. 1986, 22, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Grimaudo, N.J.; Nesbitt, W.E. Coaggregation of Candida albicans with oral Fusobacterium species. Oral Microbiol. Immunol. 1997, 12, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Jabra-Rizk, M.A.; Falkler, W.A., Jr.; Merz, W.G.; Kelley, J.I.; Baqui, A.A.; Meiller, T.F. Coaggregation of Candida dubliniensis with Fusobacterium nucleatum. J. Clin. Microbiol. 1999, 37, 1464–1468. [Google Scholar] [PubMed]
- Bor, B.; Cen, L.; Agnello, M.; Shi, W.; He, X. Morphological and physiological changes induced by contact-dependent interaction between Candida albicans and Fusobacterium nucleatum. Sci. Rep. 2016, 6, 27956. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Cen, L.; Kaplan, C.; Zhou, X.; Lux, R.; Shi, W.; He, X. Cellular Components Mediating Coadherence of Candida albicans and Fusobacterium nucleatum. J. Dent. Res. 2015, 94, 1432–1438. [Google Scholar] [CrossRef]
- Baldwin, A.; Mahenthiralingam, E.; Drevinek, P.; Vandamme, P.; Govan, J.R.; Waine, D.J.; LiPuma, J.J.; Chiarini, L.; Dalmastri, C.; Henry, D.A.; et al. Environmental Burkholderia cepacia complex isolates in human infections. Emerg. Infect. Dis. 2007, 13, 458–461. [Google Scholar] [CrossRef]
- Sousa, S.A.; Ramos, C.G.; Leitao, J.H. Burkholderia cepacia Complex: Emerging Multihost Pathogens Equipped with a Wide Range of Virulence Factors and Determinants. Int. J. Microbiol. 2011, 10, 3. [Google Scholar] [CrossRef]
- Boon, C.; Deng, Y.; Wang, L.H.; He, Y.; Xu, J.L.; Fan, Y.; Pan, S.Q.; Zhang, L.H. A novel, D.S.F-like signal from Burkholderia cenocepacia interferes with Candida albicans morphological transition. ISME J. 2008, 2, 27–36. [Google Scholar] [CrossRef]
- Tian, J.; Weng, L.X.; Zhang, Y.Q.; Wang, L.H. BDSF inhibits Candida albicans adherence to urinary catheters. Microb. Pathog. 2013, 64, 33–38. [Google Scholar] [CrossRef]
- Fox, E.P.; Cowley, E.S.; Nobile, C.J.; Hartooni, N.; Newman, D.K.; Johnson, A.D. Anaerobic bacteria grow within Candida albicans biofilms and induce biofilm formation in suspension cultures. Curr. Biol. 2014, 24, 2411–2416. [Google Scholar] [CrossRef]
- van Leeuwen, P.T.; van der Peet, J.M.; Bikker, F.J.; Hoogenkamp, M.A.; Oliveira Paiva, A.M.; Kostidis, S.; Mayboroda, O.A.; Smits, W.K.; Krom, B.P. Interspecies Interactions between Clostridium difficile and Candida albicans. Msphere 2016, 1, e00187-16. [Google Scholar] [CrossRef] [PubMed]
- Somerville, G.A.; Proctor, R.A. Cultivation conditions and the diffusion of oxygen into culture media: the rationale for the flask-to-medium ratio in microbiology. BMC Microbiol. 2013, 13, 1471–2180. [Google Scholar] [CrossRef] [PubMed]
- Dione, N.; Khelaifia, S.; Lagier, J.C.; Raoult, D. The aerobic activity of metronidazole against anaerobic bacteria. Int. J. Antimicrob. Agents 2015, 45, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Janus, M.M.; Crielaard, W.; Volgenant, C.M.; van der Veen, M.H.; Brandt, B.W.; Krom, B.P. Candida albicans alters the bacterial microbiome of early in vitro oral biofilms. J. Oral. Microbiol. 2017, 9, 1270613. [Google Scholar] [CrossRef] [PubMed]
- Tampakakis, E.; Peleg, A.Y.; Mylonakis, E. Interaction of Candida albicans with an intestinal pathogen, Salmonella enterica serovar Typhimurium. Eukaryot. Cell. 2009, 8, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Mylonakis, E. Killing of Candida albicans filaments by Salmonella enterica serovar Typhimurium is mediated by sopB effectors, parts of a type, I.I.I secretion system. Eukaryot. Cell. 2011, 10, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Briard, B.; Mislin, G.L.A.; Latge, J.P.; Beauvais, A. Interactions between Aspergillus fumigatus and Pulmonary Bacteria: Current State of the Field, New Data, and Future Perspective. J. Fungi (Basel) 2019, 5, 48. [Google Scholar] [CrossRef]
- Sass, G.; Nazik, H.; Penner, J.; Shah, H.; Ansari, S.R.; Clemons, K.V.; Groleau, M.C.; Dietl, A.M.; Visca; Haas, H.; et al. Aspergillus-Pseudomonas interaction, relevant to competition in airways. Med Mycol 2019, 57, S228–S232. [Google Scholar] [CrossRef]
- Sass, G.; Nazik, H.; Penner, J.; Shah, H.; Ansari, S.R.; Clemons, K.V.; Groleau, M.C.; Dietl, A.M.; Visca, P.; Haas, H.; et al. Studies of Pseudomonas aeruginosa Mutants Indicate Pyoverdine as the Central Factor in Inhibition of Aspergillus fumigatus Biofilm. J. Bacteriol. 2017, 200, e00345-17. [Google Scholar] [CrossRef]
- Reece, E.; Doyle, S.; Greally, P.; Renwick, J.; McClean, S. Aspergillus fumigatus Inhibits Pseudomonas aeruginosa in Co-culture: Implications of a Mutually Antagonistic Relationship on Virulence and Inflammation in the, C.F. Airway. Front. Microbiol. 2018, 9, 1205. [Google Scholar] [CrossRef]
- Mowat, E.; Rajendran, R.; Williams, C.; McCulloch, E.; Jones, B.; Lang, S.; Ramage, G. Pseudomonas aeruginosa and their small diffusible extracellular molecules inhibit Aspergillus fumigatus biofilm formation. FEMS Microbiol. Lett. 2010, 313, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.; Penner, J.C.; Moss, R.B.; Haagensen, J.A.; Clemons, K.V.; Spormann, A.M.; Nazik, H.; Cohen, K.; Banaei, N.; Carolino, E.; et al. Inhibition of Aspergillus fumigatus and Its Biofilm by Pseudomonas aeruginosa Is Dependent on the Source, Phenotype and Growth Conditions of the Bacterium. PLoS ONE 2015, 10, e0134692. [Google Scholar] [CrossRef] [PubMed]
- Sass, G.; Ansari, S.R.; Dietl, A.M.; Deziel, E.; Haas, H.; Stevens, D.A. Intermicrobial interaction: Aspergillus fumigatus siderophores protect against competition by Pseudomonas aeruginosa. PLoS ONE 2019, 14, e0216085. [Google Scholar] [CrossRef] [PubMed]
- Briard, B.; Heddergott, C.; Latge, J.P. Volatile Compounds Emitted by Pseudomonas aeruginosa Stimulate Growth of the Fungal Pathogen Aspergillus fumigatus. Mbio 2016, 7, e00219. [Google Scholar] [CrossRef]
- Nutzmann, H.W.; Reyes-Dominguez, Y.; Scherlach, K.; Schroeckh, V.; Horn, F.; Gacek, A.; Schümann, J.; Hertweck, C.; Strauss, J.; Brakhage, A.A. Bacteria-induced natural product formation in the fungus Aspergillus nidulans requires Saga/Ada-mediated histone acetylation. Proc. Natl. Acad. Sci. USA 2011, 108, 14282–14287. [Google Scholar] [CrossRef] [PubMed]
- Schroeckh, V.; Scherlach, K.; Nutzmann, H.W.; Shelest, E.; Schmidt-Heck, W.; Schuemann, J.; Martin, K.; Hertweck, C.; Brakhage, A.A. Intimate bacterial-fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 2009, 106, 14558–14563. [Google Scholar] [CrossRef]
- Fischer, J.; Muller, S.Y.; Netzker, T.; Jager, N.; Gacek-Matthews, A.; Scherlach, K.; Stroe, M.C.; García-Altares, M.; Pezzini, F.; Schoeler, H.; et al. Chromatin mapping identifies BasR, a key regulator of bacteria-triggered production of fungal secondary metabolites. Elife 2018, 12, 40969. [Google Scholar] [CrossRef]
- Brandl, M.T.; Carter, M.Q.; Parker, C.T.; Chapman, M.R.; Huynh, S.; Zhou, Y. Salmonella biofilm formation on Aspergillus niger involves cellulose--chitin interactions. PLoS ONE 2011, 6, e25553. [Google Scholar] [CrossRef]
- Nogueira, M.F.; Pereira, L.; Jenull, S.; Kuchler, K.; Lion, T. Klebsiella pneumoniae prevents spore germination and hyphal development of Aspergillus species. Sci. Rep. 2019, 9, 018–36524. [Google Scholar] [CrossRef]
- Rella, A.; Yang, M.W.; Gruber, J.; Montagna, M.T.; Luberto, C.; Zhang, Y.M.; Del Poeta, M. Pseudomonas aeruginosa inhibits the growth of Cryptococcus species. Mycopathologia 2012, 173, 451–461. [Google Scholar] [CrossRef]
- Teoh-Chan, H.; Chau, P.Y.; Ng, M.H.; Wong, P.C. Inhibition of Cryptococcus neoformans by Pseudomonas aeruginosa. J. Med. Microbiol. 1975, 8, 77–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frases, S.; Chaskes, S.; Dadachova, E.; Casadevall, A. Induction by Klebsiella aerogenes of a melanin-like pigment in Cryptococcus neoformans. Appl. Env. Microbiol. 2006, 72, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Frases, S.; Salazar, A.; Dadachova, E.; Casadevall, A. Cryptococcus neoformans can utilize the bacterial melanin precursor homogentisic acid for fungal melanogenesis. Appl. Env. Microbiol. 2007, 73, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Rick, E.M.; Woolnough, K.; Pashley, C.H.; Wardlaw, A.J. Allergic Fungal Airway Disease. J. Investig. Allergol. Clin. Immunol. 2016, 26, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Pan, C.; Wang, K.; Chen, X.; Wu, X.; Chen, C.A.; Wu, B. Synthetic multispecies microbial communities reveals shifts in secondary metabolism and facilitates cryptic natural product discovery. Env. Microbiol. 2017, 19, 3606–3618. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.T.; Thomas, M.S. Iron Acquisition Mechanisms and Their Role in the Virulence of Burkholderia Species. Front. Cell Infect. Microbiol. 2017, 7, 460. [Google Scholar] [CrossRef]
- Ross, C.; Opel, V.; Scherlach, K.; Hertweck, C. Biosynthesis of antifungal and antibacterial polyketides by Burkholderia gladioli in coculture with Rhizopus microsporus. Mycoses 2014, 3, 48–55. [Google Scholar] [CrossRef]
- Moebius, N.; Ross, C.; Scherlach, K.; Rohm, B.; Roth, M.; Hertweck, C. Biosynthesis of the respiratory toxin bongkrekic acid in the pathogenic bacterium Burkholderia gladioli. Chem. Biol. 2012, 19, 1164–1174. [Google Scholar] [CrossRef]
- Lackner, G.; Moebius, N.; Partida-Martinez, L.P.; Boland, S.; Hertweck, C. Evolution of an endofungal lifestyle: Deductions from the Burkholderia rhizoxinica genome. BMC Genomics 2011, 12, 1471–2164. [Google Scholar] [CrossRef]
- Smith, M.G.; Des Etages, S.G.; Snyder, M. Microbial synergy via an ethanol-triggered pathway. Mol. Cell Biol. 2004, 24, 3874–3884. [Google Scholar] [CrossRef]
- Gandhi, J.A.; Ekhar, V.V.; Asplund, M.B.; Abdulkareem, A.F.; Ahmadi, M.; Coelho, C.; Martinez, L.R. Alcohol enhances Acinetobacter baumannii-associated pneumonia and systemic dissemination by impairing neutrophil antimicrobial activity in a murine model of infection. PLoS ONE 2014, 9, e95707. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Pethani, B.P.; Kumar, S.; Kim, M.; Sunna, A.; Kautto, L.; Penesyan, A.; Paulsen, I.T.; Nevalainen, H. Pseudomonas aeruginosa inhibits the growth of Scedosporium aurantiacum, an opportunistic fungal pathogen isolated from the lungs of cystic fibrosis patients. Front. Microbiol. 2015, 6, 866. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Patel, S.; Meyer, W.; Chapman, B.; Yu, H.; Byth, K.; Middleton, P.G.; Nevalainen, H.; Sorrell, T.C. Pseudomonas aeruginosa Inhibits the Growth of Scedosporium and Lomentospora In Vitro. Mycopathologia 2018, 183, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Chin, V.K.; Lee, T.Y.; Rusliza, B.; Chong, P.P. Dissecting Candida albicans Infection from the Perspective of C. albicans Virulence and Omics Approaches on Host-Pathogen Interaction: A Review. Int. J. Mol. Sci. 2016, 17, 1643. [Google Scholar] [CrossRef]
- Patin, E.C.; Thompson, A.; Orr, S.J. Pattern recognition receptors in fungal immunity. Semin. Cell Dev. Biol. 2018, 8, 30541–30544. [Google Scholar] [CrossRef]
- Espinosa, V.; Rivera, A. Cytokines and the regulation of fungus-specific, C.D.4 T cell differentiation. Cytokine 2012, 58, 100–106. [Google Scholar] [CrossRef]
- Pichard, D.C.; Freeman, A.F.; Cowen, E.W. Primary immunodeficiency update: Part, I.I.. Syndromes associated with mucocutaneous candidiasis and noninfectious cutaneous manifestations. J. Am. Acad. Dermatol. 2015, 73, 367–381. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, 980. [Google Scholar] [CrossRef]
- Lewis, M.L.; Surewaard, B.G.J. Neutrophil evasion strategies by Streptococcus pneumoniae and Staphylococcus aureus. Cell Tissue Res. 2018, 371, 489–503. [Google Scholar] [CrossRef]
- Hernandez-Chavez, M.J.; Perez-Garcia, L.A.; Nino-Vega, G.A.; Mora-Montes, H.M. Fungal Strategies to Evade the Host Immune Recognition. J. Fungi (Basel) 2017, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.H.; Deo, P.; Naderer, T. Macrophage cell death in microbial infections. Cell Microbiol. 2016, 18, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, B.F.; Muthukrishnan, G.; Masters, E.; Ninomiya, M.; Lee, C.C.; Schwarz, E.M. Staphylococcus aureus Evasion of Host Immunity in the Setting of Prosthetic Joint Infection: Biofilm and Beyond. Curr. Rev. Musculoskelet Med. 2018, 9, 018–9501. [Google Scholar] [CrossRef] [PubMed]
- Byndloss, M.X.; Tsolis, R.M. Chronic Bacterial Pathogens: Mechanisms of Persistence. Microbiol. Spectr. 2016, 4, 0020–2015. [Google Scholar] [CrossRef]
- Bernal-Bayard, J.; Ramos-Morales, F. Molecular Mechanisms Used by Salmonella to Evade the Immune System. Curr. Issues Mol. Biol. 2018, 25, 133–168. [Google Scholar] [CrossRef] [PubMed]
- Roux, D.; Gaudry, S.; Dreyfuss, D.; El-Benna, J.; de Prost, N.; Denamur, E.; Saumon, G.; Ricard, J.D. Candida albicans impairs macrophage function and facilitates Pseudomonas aeruginosa pneumonia in rat. Crit. Care Med. 2009, 37, 1062–1067. [Google Scholar] [CrossRef]
- Xu, H.; Sobue, T.; Bertolini, M.; Thompson, A.; Vickerman, M.; Nobile, C.J.; Dongari-Bagtzoglou, A. S. oralis activates the Efg1 filamentation pathway in C. albicans to promote cross-kingdom interactions and mucosal biofilms. Virulence 2017, 8, 1602–1617. [Google Scholar] [CrossRef]
- Nash, E.E.; Peters, B.M.; Fidel, P.L.; Noverr, M.C. Morphology-Independent Virulence of Candida Species during Polymicrobial Intra-abdominal Infections with Staphylococcus aureus. Infect. Immun. 2015, 84, 90–98. [Google Scholar] [CrossRef]
- Kean, R.; Rajendran, R.; Haggarty, J.; Townsend, E.M.; Short, B.; Burgess, K.E.; Lang, S.; Millington, O.; Mackay, W.G.; Williams, C.; et al. Candida albicans Mycofilms Support Staphylococcus aureus Colonization and Enhances Miconazole Resistance in Dual-Species Interactions. Front. Microbiol. 2017, 8, 258. [Google Scholar] [CrossRef]
- Holt, J.E.; Houston, A.; Adams, C.; Edwards, S.; Kjellerup, B.V. Role of extracellular polymeric substances in polymicrobial biofilm infections of Staphylococcus epidermidis and Candida albicans modelled in the nematode Caenorhabditis elegans. Pathog Dis. 2017, 75. [Google Scholar] [CrossRef]
- Ader, F.; Jawhara, S.; Nseir, S.; Kipnis, E.; Faure, K.; Vuotto, F.; Chemani, C.; Sendid, B.; Poulain, D.; Guery, B. Short term Candida albicans colonization reduces Pseudomonas aeruginosa-related lung injury and bacterial burden in a murine model. Crit. Care 2011, 15, R150. [Google Scholar] [CrossRef]
- Lopez-Medina, E.; Fan, D.; Coughlin, L.A.; Ho, E.X.; Lamont, I.L.; Reimmann, C.; Hooper, L.V.; Koh, A.Y. Candida albicans Inhibits Pseudomonas aeruginosa Virulence through Suppression of Pyochelin and Pyoverdine Biosynthesis. Plos Pathog. 2015, 11, e1005129. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.I.; Xie, Z.; Sobue, T.; Thompson, A.; Biyikoglu, B.; Ricker, A.; Ikonomou, L.; Dongari-Bagtzoglou, A. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect. Immun. 2012, 80, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Nash, E.E.; Peters, B.M.; Palmer, G.E.; Fidel, P.L.; Noverr, M.C. Morphogenesis is not required for Candida albicans-Staphylococcus aureus intra-abdominal infection-mediated dissemination and lethal sepsis. Infect. Immun. 2014, 82, 3426–3435. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ermolaeva, M.A.; Schumacher, B. Insights from the worm: the C. elegans model for innate immunity. Semin. Immunol. 2014, 26, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Fehrmann, C.; Jurk, K.; Bertling, A.; Seidel, G.; Fegeler, W.; Kehrel, B.E.; Peters, G.; Becker, K.; Heilmann, C. Role for the fibrinogen-binding proteins coagulase and Efb in the Staphylococcus aureus-Candida interaction. Int. J. Med. Microbiol. 2013, 303, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.; Salva, S.; Aguero, G.; Alvarez, S. Immunomodulatory and protective effect of probiotic Lactobacillus casei against Candida albicans infection in malnourished mice. Microbiol. Immunol. 2011, 55, 434–445. [Google Scholar] [CrossRef]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef]
- Bork, P.; Beckmann, G. The, C.U.B domain. A widespread module in developmentally regulated proteins. J. Mol. Biol. 1993, 231, 539–545. [Google Scholar] [CrossRef]
- Arvanitis, M.; Mylonakis, E. Characteristics, Clinical Relevance, and the Role of Echinocandins in Fungal-Bacterial Interactions. Clin. Infect. Dis. 2015, 61, S630–634. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).