Antimicrobial Activity of Several Cineole-Rich Western Australian Eucalyptus Essential Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction of Essential Oils
2.3. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis of Essential Oils
2.4. Microorganisms
2.5. Evaluation of Antimicrobial Activity
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ogunwande, I.A.; Olawore, N.O.; Adeleke, K.A.; Konig, W.A. Chemical composition of the essential oils from the leaves of three Eucalyptus species growing in Nigeria. J. Essent. Oil Res. 2003, 15, 297–301. [Google Scholar] [CrossRef]
- Turnbull, J.W.; Booth, T.H. Eucalypts in cultivation: An overview. In Eucalyptus: The Genus Eucalyptus; Coppen, J.J.W., Ed.; Taylor and Francis: London, UK, 2002; pp. 52–74. [Google Scholar]
- Pino, J.A.; Marbot, R.; Quert, R.; Garcia, H. Study of essential oils of Eucalyptus resinifera Smith, E. tereticornis Smith and Corymbia maculata (Hook.) K. D. Hill & L. A. S. Johnson, grown in Cuba. Flavour Frag. J. 2002, 17, 1–4. [Google Scholar] [CrossRef]
- Brophy, J.J.; Southwell, I.A. Eucalyptus Chemistry. In Eucalyptus: The Genus Eucalyptus; Coppen, J.J.W., Ed.; Taylor and Francis: London, UK, 2002; pp. 102–160. [Google Scholar]
- Beerling, J.; Meakins, S.; Small, L. Eucalyptus oil products: Formulations and legislation. In Eucalyptus: The Genus Eucalyptus; Coppen, J.J.W., Ed.; Taylor and Francis: London, UK, 2002; pp. 345–364. [Google Scholar]
- Coppen, J.J.W.; Hone, G.A. Eucalyptus Oils: A Review of Production and Markets; Natural Resources Institute: Kent, UK, 1992. [Google Scholar]
- González-Burgos, E.; Liaudanskas, M.; Viškelis, J.; Žvikas, V.; Janulis, V.; Gómez-Serranillos, M. Antioxidant activity, neuroprotective properties and bioactive constituents analysis of varying polarity extracts from Eucalyptus globulus leaves. J. Food Drug Anal. 2018, 26, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Abebe, W.; Sousa, S.M.; Duarte, V.G.; Machado, M.I.; Matos, F. Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. J. Ethnopharmacol. 2003, 89, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kokubo, R.; Sakaino, M. Antimicrobial activities of eucalyptus leaf extracts and flavonoids from Eucalyptus maculata. Lett. Appl. Microbiol. 2004, 39, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Sidana, J.; Rohilla, R.K.; Roy, N.; Barrow, R.A.; Foley, W.J.; Singh, I.P. Antibacterial sideroxylonals and loxophlebal A from Eucalyptus loxophleba foliage. Fitoterapia 2010, 81, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Djenane, D.; Yanguela, J.; Amrouche, T.; Boubrit, S.; Boussad, N.; Roncales, P. Chemical composition and antimicrobial effects of essential oils of Eucalyptus globulus, Myrtus communis and Satureja hortensis against Escherichia coli O157:H7 and Staphylococcus aureus in minced beef. Food Sci. Technol. Int. 2011, 17, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Oyedeji, A.O.; Ekundayo, O.; Olawore, O.N.; Adeniyi, B.A.; Koenig, W.A. Antimicrobial activity of the essential oils of five Eucalyptus species growing in Nigeria. Fitoterapia 1999, 70, 526–528. [Google Scholar] [CrossRef]
- Gilles, M.; Zhao, J.; An, M.; Agboola, S. Chemical composition and antimicrobial properties of essential oils of three Australian Eucalyptus species. Food Chem. 2010, 119, 731–737. [Google Scholar] [CrossRef]
- Worth, H.; Schacher, C.; Dethlefsen, U. Concomitant therapy with Cineole (Eucalyptole) reduces exacerbations in COPD: A placebo-controlled double-blind trial. Respir. Res. 2009, 10. [Google Scholar] [CrossRef]
- Worth, H.; Dethlefsen, U. Patients with asthma benefit from concomitant therapy with cineole: A placebo-controlled, double-blind trial. J. Asthma 2012, 49, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Bernardo, C.; Sonoda, L.; Hayashi, F.; Romito, G.; De Lima, L.; Lotufo, R.; Pannuti, C. Subgingival ultrasonic instrumentation of residual pockets irrigated with essential oils: A randomized controlled trial. J. Clin. Periodontol. 2011, 38, 637–643. [Google Scholar] [CrossRef]
- Barker, S.C.; Altman, P.M. An ex vivo, assessor blind, randomised, parallel group, comparative efficacy trial of the ovicidal activity of three pediculicides after a single application—Melaleuca oil and lavender oil, eucalyptus oil and lemon tea tree oil, and a “suffocation” pediculicide. BMC Dermatol. 2011, 11, 14. [Google Scholar]
- Clinical and Laboratory Standards Institute: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; CLSI document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008.
- Clinical and Laboratory Standards Institute: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 9th ed.; CLSI document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012.
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 95th ed.; CRC Press LLC: Boca Raton, FL, USA, 2014. [Google Scholar]
- Elaissi, A.; Medini, H.; Khouja, M.L.; Simmonds, M.; Lynene, F.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F. Variation in volatile leaf oils of five eucalyptus species harvested from Jbel Abderrahman Arboreta (Tunisia). Chem. Biodivers. 2011, 8, 352–361. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Sporer, F.; Reichling, J.; Wink, M. Antibacterial activity of essential oils from Eucalyptus and of selected components against multidrug-resistant bacterial pathogens. Pharm. Biol. 2011, 49, 893–899. [Google Scholar] [CrossRef]
- Wildy, D.T.; Pate, J.S.; Bartle, J.R. Variations in composition and yield of leaf oils from alley-farmed oil mallees (Eucalyptus spp.) at a range of contrasting sites in the Western Australian wheatbelt. For. Ecol. Manag. 2000, 134, 205–217. [Google Scholar] [CrossRef]
- Bignell, C.M.; Dunlop, P.J.; Brophy, J.J. Volatile leaf oils of some south-western and southern Australian species of the genus Eucalyptus (series I). Part XVIII. A. Subgenus monocalyptus. B. Subgenus symphyomyrtus: (i) section guilfoyleanae; (ii) section bisectaria, series accedentes, series occidentales, series levispermae, series loxophlebae, series macrocarpae, series orbifoliae, series calycogonae; (iii) section dumaria, series incrassatae and series ovulares. Flavour Frag. J. 1997, 12, 423–432. [Google Scholar]
- Barton, A.F.M. The oil mallee project: A multifaceted industrial ecology case study. J. Ind. Ecol. 1999, 3, 161–176. [Google Scholar] [CrossRef]
- Nicolle, D.; Byrne, M.; Whalen, M. A taxonomic revision and morphological variation within Eucalyptus series Subulatae subseries Oleaginae (Myrtaceae), including the oil mallee complex, of south-western Australia. Aust. Syst. Bot. 2005, 18, 525–553. [Google Scholar] [CrossRef]
- Barton, A.F.M.; Tjandra, J.; Nicholas, P.G. Chemical evaluation of volatile oils in Eucalyptus species. J. Agric. Food Chem. 1989, 37, 1253–1257. [Google Scholar] [CrossRef]
- Smale, P.E.; Nelson, M.A.; Porter, N.G.; Hay, A.J. Essential oil of Eucalyptus olida L. Johnson et K. Hill 1: Variability of yield and composition in foliage from a seedling population. J. Essent. Oil Res. 2000, 12, 569–574. [Google Scholar] [CrossRef]
- Lahlou, M. Methods to study the phytochemistry and bioactivity of essential oils. Phytother. Res. 2004, 18, 435–448. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Cimanga, K.; Kambu, K.; Tona, L.; Apers, S.; De Bruyne, T.; Hermans, N.; Totte, J.; Pieters, L.; Vlietinck, A.J. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J. Ethnopharmacol. 2002, 79, 213–220. [Google Scholar] [CrossRef]
- Hines, B.; Byrne, M. Genetic differentiation between mallee and tree forms in the Eucalyptus loxophleba complex. Heredity 2001, 87, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wang, J.; Shao, X.; Xu, F.; Wang, H. Antifungal modes of action of tea tree oil and its two characteristic components against Botrytis cinerea. J. Appl. Microbiol. 2015, 119, 1253–1262. [Google Scholar] [CrossRef]
- Marino, M.; Bersani, C.; Comi, G. Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceae and Compositae. Int. J. Food Microbiol. 2001, 67, 187–195. [Google Scholar] [CrossRef]
- Knezevic, P.; Aleksic, V.; Simin, N.; Svircev, E.; Petrovic, A.; Mimica-Dukic, N. Antimicrobial activity of Eucalyptus camaldulensis essential oils and their interactions with conventional antimicrobial agents against multi-drug resistant Acinetobacter baumannii. J. Ethnopharmacol. 2016, 178, 125–136. [Google Scholar] [CrossRef]
- Luís, A.; Duarte, A.; Gominho, J.; Domingues, F.; Duarte, A.P. Chemical composition, antioxidant, antibacterial and anti-quorum sensing activities of Eucalyptus globulus and Eucalyptus radiata essential oils. Ind. Crops Prod. 2016, 79, 274–282. [Google Scholar] [CrossRef]
- Scott, C.C.L.; Makula, R.A.; Finnerty, W.R. Isolation and characterisation of membranes from a hydrocarbon-oxidizing Acinetobacter sp. J. Bacteriol. 1976, 127, 469–480. [Google Scholar]
- Zgurskaya, H.I.; Löpez, C.A.; Gnanakaran, S. Permeability barrier of Gram-negative cell envelopes and approaches to bypass it. ACS Infect. Dis. 2015, 1, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Juergens, U.R. Anti-inflammatory properties of the monoterpene 1.8-cineole: Current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014, 64, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.U.; Ali, A.; Khan, I.A. Plant based products: Use and development as repellents against mosquitoes: A review. Fitoterapia 2014, 95, 65–74. [Google Scholar] [CrossRef] [PubMed]
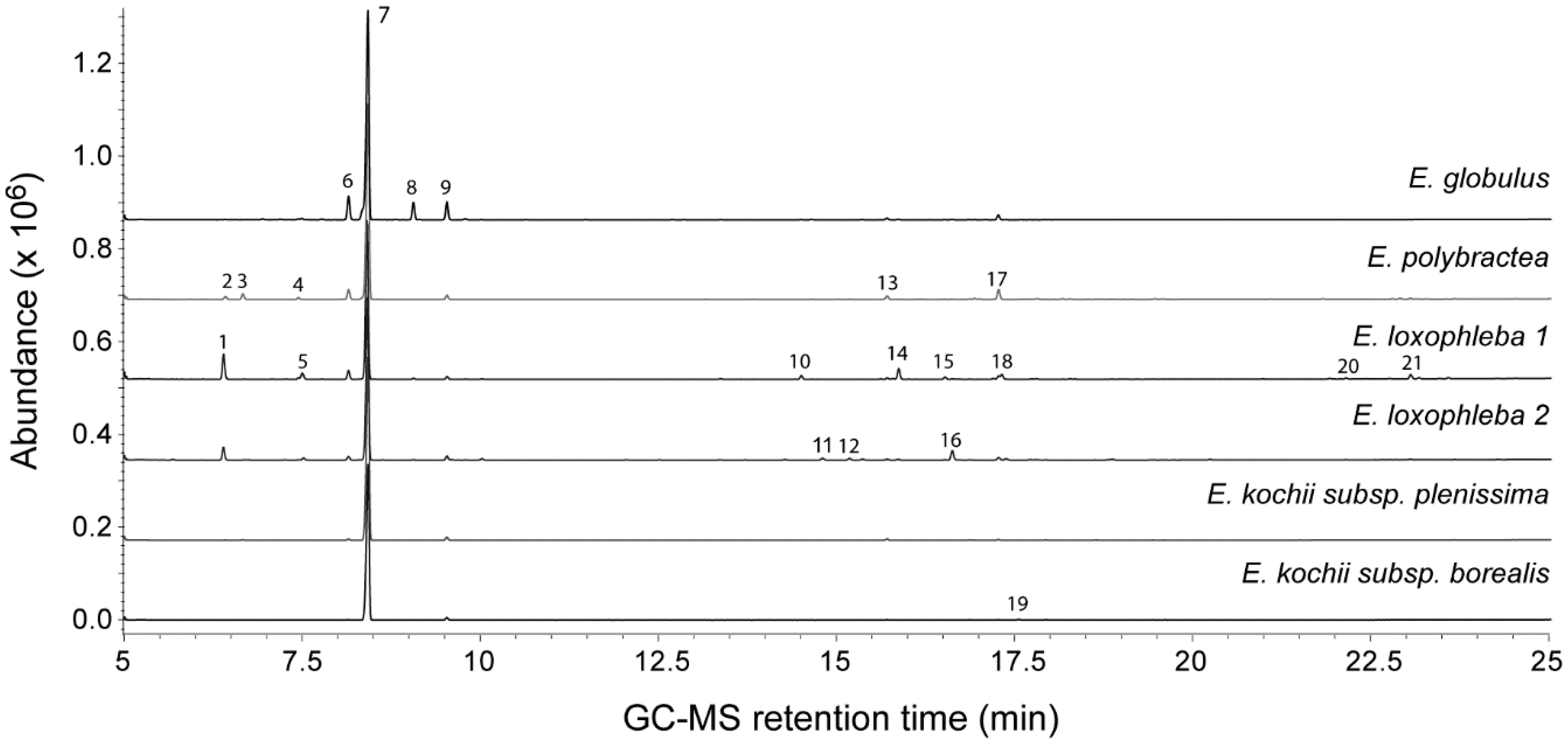
| Compound | RI (DB-wax) | RI (Rtx-5) | Eucalyptus globulus | Eucalyptus polybractea | Eucalyptus loxophleba 1 | E. loxophleba 2 | Eucalyptus kochii Subsp. plenissima plenissima | E. kochii subsp. borealis |
|---|---|---|---|---|---|---|---|---|
| 4-Methyl-2-pentyl acetate (1) | 1109 (1110 a) | – | – | – | 9.86 | 5.53 | – | – |
| β–Pinene (2) * | 1110 (1116 b) | 974 (981 b) | – | 1.07 | – | – | – | – |
| Sabinene (3) | 1123 (1123 b) | – | – | 1.98 | – | – | – | – |
| β-Myrcene (4) * | 1164 (1160 c) | 992 (992 b) | – | 0.59 | 0.57 | – | – | – |
| α-Phellandrene (5) * | 1166 (1166 b) | 1003 (1007 b) | 0.43 | – | 2.75 | – | – | – |
| Limonene (6) * | 1201 (1201 b) | – | 7.52 | 3.67 | 3.52 | 1.58 | 0.53 | – |
| 1,8-Cineole (7) * | 1214 (1213 b) | 1032 (1030 b) | 77.02 | 82.95 | 66.93 | 78.78 | 96.55 | 97.32 |
| γ-Terpinene (8) * | 1248 (1238 b) | 1058 (1074 b) | 5.34 | – | 0.37 | – | – | – |
| p-Cymene (9) * | 1272 (1261 b) | 1026 (1027 b) | 5.53 | 1.50 | 1.11 | 1.77 | 1.39 | 1.34 |
| α-Gurjunene (10) | 1541 (1536 d) | 1409 (1412 e) | – | – | 1.48 | – | – | – |
| 3-Pinanone (11) | 1558 (1576 a) | 1173 (1163 a) | – | – | – | 0.89 | – | – |
| Pinocarvone (12) * | 1578 (1565 a) | 1162 (1160 a) | – | – | – | 0.79 | – | – |
| Terpinen-4-ol (13) * | 1609 (1618 d) | 1177 (1176 e) | 0.56 | 1.39 | 0.52 | 0.39 | 0.61 | 0.12 |
| Aromadendrene (14) * | 1619 (1625 d) | 1440 (1446 e) | – | – | 4.37 | 0.3 | – | – |
| allo-Aromadendrene (15) * | 1659 (1667 d) | 1462 (1466 e) | – | – | 0.94 | – | – | – |
| trans-Pinocarveol (16) | 1664 (1675 d) | 1137 (1127 f) | – | – | – | 4.66 | – | – |
| α-Terpineol (17) * | 1702 (1709 a) | 1190 (1189 a) | 1.49 | 3.67 | 1.45 | 1.22 | 0.33 | 0.11 |
| Ledene (18) * | 1708 (1706 d) | 1498 (1504 e) | – | – | 1.87 | – | – | __ |
| Verbenone (19) | 1722 (1728 d) | 1210 (1228 a) | – | – | – | – | – | 0.36 |
| epi-Globulol (20) | 2025 (2039 d) | 1561 (1566 e) | – | – | 0.44 | – | – | – |
| Globulol (21) | 2091 (2103 d) | 1585 (1595 e) | – | – | 1.90 | 0.3 | – | – |
| Total identified compounds | 97.89 | 96.82 | 98.08 | 96.21 | 99.41 | 99.25 |
| Eucalyptus Oils | Staphylococcus aureus ATCC 29213 | Escherichia coli ATCC 25922 | ||
|---|---|---|---|---|
| 25 µL | 50 µL | 25 µL | 50 µL | |
| E. globulus | 13.0 ± 1.0 | 15.3 ± 0.6 | 11.3 ± 0.6 | 15.0 ± 0.0 |
| E. loxophleba 1 | 15.3 ± 0.6 | 16.7 ± 0.6 | 14.7 ± 0.6 | 16.7 ± 0.6 |
| E. loxophleba 2 | 13.0 ± 0.6 | 15.0 ± 0.0 | 12.3 ± 0.6 | 15.3 ± 0.6 |
| E. polybractea | 28.0 ± 0.0 | 29.5 ± 0.7 | 14.0 ± 1.0 | 16.7 ± 0.6 |
| E. kochii subsp. plenissima | 13.0 ± 0.0 | 15.7 ± 0.6 | 13.0 ± 0.0 | 13.0 ± 0.0 |
| E. kochii subsp. borealis | 12.7 ± 0.6 | 14.7 ± 0.6 | 11.3 ± 0.6 | 13.0 ± 0.0 |
| 1,8 Cineole | 11.0 ± 0.0 | 12.7 ± 0.6 | 13.3 ± 0.6 | 14.3 ± 0.6 |
| Trimethoprim 5 µg | 27.7 ± 0.6 | 26.3 ± 0.6 | ||
| Essential Oil | Parameter a | S. aureus ATCC 29213 | S. aureus MRSA NCTC 10442 | Enterococcus faecalis ATCC 29212 | E. faecalis VRE ATCC 51299 | Staphylococcus epidermidis NCTC 11047 | Candida albicans ATCC 90028 | Salmonella Typhimurium ATCC 13311 | E. coli ATCC 25922 | Pseudomonas aeruginosa ATCC 27853 | Acinetobacter baumannii NCTC 7844 | Geometric Mean of the MIC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. globulus | MIC | 4 | 2 | >8 | 4 | 4 | >8 | 0.5 | 8 | 8 | 2 | 4.3 |
| MBC/MFC | 4 | >8 | >8 | >8 | >8 | >8 | 0.5 | 8 | 8 | 2 | ||
| E. loxophleba 1 | MIC | >8 | 8 | >8 | 2 | >8 | >8 | 8 | 8 | 4 | 2 | 7.5 |
| MBC/MFC | >8 | >8 | >8 | 8 | >8 | >8 | 8 | 8 | 4 | 4 | ||
| E. loxophleba 2 | MIC | 4 | 4 | >8 | 4 | 8 | 8 | 2 | 8 | >8 | 0.25 | 4.6 |
| MBC/MFC | >8 | 4 | >8 | 4 | >8 | >8 | 4 | 8 | >8 | 0.25 | ||
| E. polybractea | MIC | 8 | 4 | 8 | 2 | 2 | 8 | 8 | 4 | 4 | 2 | 4.3 |
| MBC/MFC | >8 | 8 | 8 | >8 | >8 | >8 | 8 | 4 | 8 | 2 | ||
| E. kochii subsp. | MIC | 2 | 4 | >8 | >8 | >8 | 8 | 2 | 2 | >8 | 2 | 5.6 |
| plenissima | MBC/MFC | 8 | 4 | >8 | >8 | >8 | >8 | 4 | 2 | >8 | 4 | |
| E. kochii subsp. | MIC | 2 | 4 | >8 | 4 | 8 | 8 | 4 | 2 | >8 | 1 | 4.6 |
| borealis | MBC/MFC | 4 | 8 | >8 | 4 | >8 | >8 | 4 | 2 | >8 | 2 | |
| 1,8 Cineole | MIC | >8 | >8 | >8 | 8 | >8 | >8 | 2 | 1 | >8 | 1 | 7.0 |
| MBC/MFC | >8 | >8 | >8 | >8 | >8 | >8 | 4 | 4 | >8 | 1 | ||
| Geometric mean | MIC | 5.4 | 4.9 | 14.5 | 4.4 | 8.0 | 10.8 | 2.7 | 3.6 | 9.7 | 1.2 | |
| MBC/MFC | 9.7 | 8.8 | 14.5 | 9.7 | >8.0 | >8.0 | 3.6 | 4.4 | 9.8 | 1.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldoghaim, F.S.; Flematti, G.R.; Hammer, K.A. Antimicrobial Activity of Several Cineole-Rich Western Australian Eucalyptus Essential Oils. Microorganisms 2018, 6, 122. https://doi.org/10.3390/microorganisms6040122
Aldoghaim FS, Flematti GR, Hammer KA. Antimicrobial Activity of Several Cineole-Rich Western Australian Eucalyptus Essential Oils. Microorganisms. 2018; 6(4):122. https://doi.org/10.3390/microorganisms6040122
Chicago/Turabian StyleAldoghaim, Fahad S., Gavin R. Flematti, and Katherine A. Hammer. 2018. "Antimicrobial Activity of Several Cineole-Rich Western Australian Eucalyptus Essential Oils" Microorganisms 6, no. 4: 122. https://doi.org/10.3390/microorganisms6040122
APA StyleAldoghaim, F. S., Flematti, G. R., & Hammer, K. A. (2018). Antimicrobial Activity of Several Cineole-Rich Western Australian Eucalyptus Essential Oils. Microorganisms, 6(4), 122. https://doi.org/10.3390/microorganisms6040122




