Application of A Novel Potential Probiotic Lactobacillus paracasei Strain Isolated from Kefir Grains in the Production of Feta-Type Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Kefir Grains Production
2.2. Isolation of LAB Strains
2.3. Bacterial Culture Conditions
2.4. In Vitro Tests Simulating the Human GI Tract
2.4.1. Resistance to Low pH
2.4.2. Resistance to Pepsin and Pancreatin
2.4.3. Tolerance to Bile Salts
2.4.4. Antibiotic Susceptibility Testing
2.5. DNA Extraction from Pure Cultures
2.6. PCR Amplification
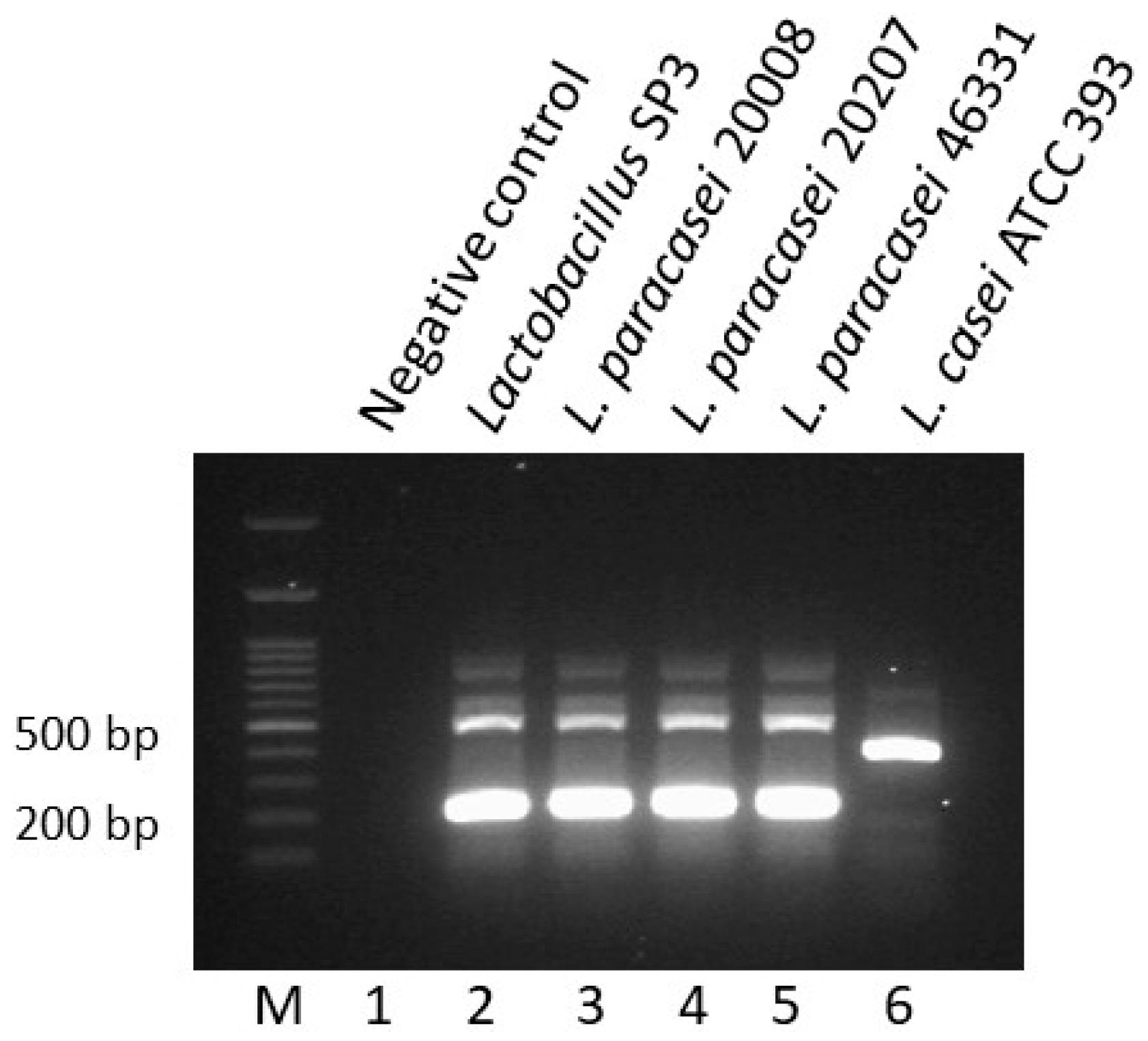
2.7. Species-Specific Multiplex PCR
2.8. Application of L. paracasei SP3 for Feta-Type Cheese Production
2.9. Physicochemical Analysis of Feta-Type Cheese
2.10. Microbiological Analysis of Feta-Type Cheese
2.11. Preliminary Sensory Evaluation
2.12. Statistical Analysis
3. Results and Discussion
3.1. Isolation of LAB Strains and Screening for Probiotic Potential
3.2. Antibiotic Susceptibility
3.3. Molecular Characterization of Lactobacillus Strain SP3
3.4. L. paracasei SP3 as Starter Culture for the Production of Feta-Type Cheese
3.5. Microbiological Analysis of Feta-Type Cheese
3.6. Preliminary Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgements
Conflicts of Interest
References
- Annunziata, A.; Vecchio, R. Consumer perception of functional foods: A conjoint analysis with probiotics. Food Qual. Prefer. 2013, 28, 348–355. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Rzepkowska, A.; Szydłowska, A.; Kołożyn-Krajewska, D. Chapter 3—Trends and Possibilities of the Use of Probiotics in Food Production. In Alternative and Replacement Foods; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 65–94. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Prebiotics and Probiotics in Digestive Health. Clin. Gastroenterol. Hepatol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Bosnea, L.; Alexopoulos, A.; Bezirtzoglou, E. Potential effects of probiotics in cheese and yogurt production: A review. Eng. Life Sci. 2012, 12, 433–440. [Google Scholar] [CrossRef]
- Commane, D.; Hughes, R.; Shortt, C.; Rowland, I. The potential mechanisms involved in the anti-carcinogenic action of probiotics. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2005, 591, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Albano, C.; Morandi, S.; Silvetti, T.; Casiraghi, M.C.; Manini, F.; Brasca, M. Lactic acid bacteria with cholesterol-lowering properties for dairy applications: In vitro and in situ activity. J. Dairy Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Roškar, I.; Švigelj, K.; Štempelj, M.; Volfand, J.; Štabuc, B.; Malovrh, Š.; Rogelj, I. Effects of a probiotic product containing Bifidobacterium animalis subsp. animalis IM386 and Lactobacillus plantarum MP2026 in lactose intolerant individuals: Randomized, placebo-controlled clinical trial. J. Funct. Foods 2017, 35, 1–8. [Google Scholar] [CrossRef]
- Mays, Z.J.S.; Nair, N.U. Synthetic biology in probiotic lactic acid bacteria: At the frontier of living therapeutics. Curr. Opin. Biotechnol. 2018, 53, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Macori, G.; Cotter, P.D. Novel insights into the microbiology of fermented dairy foods. Curr. Opin. Biotechnol. 2018, 49, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A.; Nigam, P. Enhanced probiotic viability and aromatic profile of yogurts produced using wheat bran (Triticum aestivum) as cell immobilization carrier. Process Biochem. 2017, 55, 1–10. [Google Scholar] [CrossRef]
- Innocente, N.; Biasutti, M.; Rita, F.; Brichese, R.; Comi, G.; Iacumin, L. Effect of indigenous Lactobacillus rhamnosus isolated from bovine milk on microbiological characteristics and aromatic profile of traditional yogurt. LWT Food Sci. Technol. 2016, 66, 158–164. [Google Scholar] [CrossRef]
- Schoina, V.; Terpou, A.; Bosnea, L.; Kanellaki, M.; Nigam, P.S. Entrapment of Lactobacillus casei ATCC393 in the viscus matrix of Pistacia terebinthus resin for functional myzithra cheese manufacture. LWT Food Sci. Technol. 2018, 89, 441–448. [Google Scholar] [CrossRef]
- Terpou, A.; Bosnea, L.; Kanellaki, M.; Plessas, S.; Bekatorou, A.; Bezirtzoglou, E.; Koutinas, A.A. Growth Capacity of a Novel Potential Probiotic Lactobacillus paracasei K5 Strain Incorporated in Industrial White Brined Cheese as an Adjunct Culture. J. Food Sci. 2018, 83, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, I.; Plessas, S.; Odatzidou, M.; Alexopoulos, A.; Galanis, A.; Bezirtzoglou, E.; Bekatorou, A. Effect of a novel Lactobacillus paracasei starter on sourdough bread quality. Food Chem. 2019, 271, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Bekatorou, A.; Gallanagh, J.; Nigam, P.; Koutinas, A.A.; Psarianos, C. Evolution of aroma volatiles during storage of sourdough breads made by mixed cultures of Kluyveromyces marxianus and Lactobacillus delbrueckii ssp. bulgaricus or Lactobacillus helveticus. Food Chem. 2008, 107, 883–889. [Google Scholar] [CrossRef]
- Kołożyn-Krajewska, D.; Dolatowski, Z.J. Probiotic meat products and human nutrition. Process Biochem. 2012, 47, 1761–1772. [Google Scholar] [CrossRef]
- Terpou, A.; Nigam, P.S.; Bosnea, L.; Kanellaki, M. Evaluation of Chios mastic gum as antimicrobial agent and matrix forming material targeting probiotic cell encapsulation for functional fermented milk production. LWT 2018. [Google Scholar] [CrossRef]
- Kandylis, P.; Pissaridi, K.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A. Dairy and non-dairy probiotic beverages. Curr. Opin. Food Sci. 2016, 7, 58–63. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Magalhães Júnior, A.I.; Thomaz-Soccol, V.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gangoiti, M.V.; Puertas, A.I.; Hamet, M.F.; Peruzzo, P.J.; Llamas, M.G.; Medrano, M.; Prieto, A.; Dueñas, M.T.; Abraham, A.G. Lactobacillus plantarum CIDCA 8327: An α-glucan producing-strain isolated from kefir grains. Carbohydr. Polym. 2017, 170, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Nouska, C.; Karapetsas, A.; Kazakos, S.; Alexopoulos, A.; Mantzourani, I.; Chondrou, P.; Fournomiti, M.; Galanis, A.; Bezirtzoglou, E. Isolation, characterization and evaluation of the probiotic potential of a novel Lactobacillus strain isolated from Feta-type cheese. Food Chem. 2017, 226, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Saxami, G.; Karapetsas, A.; Lamprianidou, E.; Kotsianidis, I.; Chlichlia, A.; Tassou, C.; Zoumpourlis, V.; Galanis, A. Two potential probiotic lactobacillus strains isolated from olive microbiota exhibit adhesion and anti-proliferative effects in cancer cell lines. J. Funct. Foods 2016, 24, 461–471. [Google Scholar] [CrossRef]
- Hernández-Alcántara, A.M.; Wacher, C.; Llamas, M.G.; López, P.; Pérez-Chabela, M.L. Probiotic properties and stress response of thermotolerant lactic acid bacteria isolated from cooked meat products. LWT 2018, 91, 249–257. [Google Scholar] [CrossRef]
- Singh, S.S.; De Mandal, S.; Mathipi, V.; Ghatak, S.; Kumar, N.S. Traditional fermented fish harbors bacteria with potent probiotic and anticancer properties. Biocatal. Agric. Biotechnol. 2018, 15, 283–290. [Google Scholar] [CrossRef]
- Mathur, S.; Singh, R. Antibiotic resistance in food lactic acid bacteria—A review. Int. J. Food Microbiol. 2005, 105, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Gialleli, A.I.; Bosnea, L.; Kanellaki, M.; Koutinas, A.A.; Castro, G.R. Novel cheese production by incorporation of sea buckthorn berries (Hippophae rhamnoides L.) supported probiotic cells. LWT Food Sci. Technol. 2017, 79, 616–624. [Google Scholar] [CrossRef]
- Litopoulou-Tzanetaki, E.; Tzanetakis, N. Microbiological characteristics of Greek traditional cheeses. Small Rumin. Res. 2011, 101, 17–32. [Google Scholar] [CrossRef]
- Plessas, S.; Alexopoulos, A.; Bekatorou, A.; Bezirtzoglou, E. Kefir Immobilized on Corn Grains as Biocatalyst for Lactic Acid Fermentation and Sourdough Bread Making. J. Food Sci. 2012, 77, C1256–C1262. [Google Scholar] [CrossRef] [PubMed]
- Mackie, A. 3—Interaction of food ingredient and nutraceutical delivery systems with the human gastrointestinal tract. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Garti, N., McClements, D.J., Eds.; Woodhead Publishing: Sawston, UK, 2012; pp. 49–70. [Google Scholar] [CrossRef]
- Klijn, N.; Weerkamp, A.H.; De Vos, W.M. Identification of mesophilic lactic acid bacteria by using polymerase chain reaction-amplified variable regions of 16S rRNA and specific DNA probes. Appl. Environ. Microbiol. 1991, 57, 3390–3393. [Google Scholar] [PubMed]
- Ventura, M.; Canchaya, C.; Meylan, V.; Klaenhammer, T.R.; Zink, R. Analysis, Characterization, and Loci of the tuf Genes in Lactobacillus and Bifidobacterium Species and Their Direct Application for Species Identification. Appl. Environ. Microbiol. 2003, 69, 6908–6922. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Bekatorou, A.; Bosnea, L.; Kanellaki, M.; Ganatsios, V.; Koutinas, A.A. Wheat bran as prebiotic cell immobilisation carrier for industrial functional Feta-type cheese making: Chemical, microbial and sensory evaluation. Biocatal. Agric. Biotechnol. 2018, 13, 75–83. [Google Scholar] [CrossRef]
- International, A. Official Methods of Analysis of AOAC International; AOAC: Arlington, VA, USA, 2010. [Google Scholar]
- Parker, E.A.; Roy, T.; D’Adamo, C.R.; Wieland, L.S. Probiotics and gastrointestinal conditions: An overview of evidence from the Cochrane Collaboration. Nutrition 2018, 45, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, S. The effect of probiotics and gut microbiota on Th17 cells. Int. Rev. Immunol. 2013, 32, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wu, Z.; Wu, J.; Pan, D.; Zeng, X.; Cheng, K. Effects of Salt Stress on Carbohydrate Metabolism of Lactobacillus plantarum ATCC 14917. Curr. Microbiol. 2016, 73, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Klare, I.; Konstabel, C.; Werner, G.; Huys, G.; Vankerckhoven, V.; Kahlmeter, G.; Hildebrandt, B.; Muller-Bertling, S.; Witte, W.; Goossens, H. Antimicrobial susceptibilities of Lactobacillus, Pediococcus and Lactococcus human isolates and cultures intended for probiotic or nutritional use. J. Antimicrob. Chemother. 2007, 59, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Elisha, B.G.; Courvalin, P. Analysis of genes encoding D-alanine:D-alanine ligase-related enzymes in Leuconostoc mesenteroides and Lactobacillus spp. Gene 1995, 152, 79–83. [Google Scholar] [CrossRef]
- Danielsen, M.; Wind, A. Susceptibility of Lactobacillus spp. to antimicrobial agents. Int. J. Food Microbiol. 2003, 82, 1–11. [Google Scholar] [CrossRef]
- Drago, L.; De Grandi, R.; De Vecchi, E.; Toscano, M. Antibiotic susceptibility profile of a new Lactobacillus kefiri strain. J. Glob. Antimicrob. Resist. 2016, 4, 74–75. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Mattina, R.; De Vecchi, E.; Toscano, M. Phenotypic and genotypic antibiotic resistance in some probiotics proposed for medical use. Int. J. Antimicrob. Agents 2013, 41, 396–397. [Google Scholar] [CrossRef] [PubMed]
- Bozoudi, D.; Kotzamanidis, C.; Hatzikamari, M.; Tzanetakis, N.; Menexes, G.; Litopoulou-Tzanetaki, E. A comparison for acid production, proteolysis, autolysis and inhibitory properties of lactic acid bacteria from fresh and mature Feta PDO Greek cheese, made at three different mountainous areas. Int. J. Food Microbiol. 2015, 200, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Bozoudi, D.; Torriani, S.; Zdragas, A.; Litopoulou-Tzanetaki, E. Assessment of microbial diversity of the dominant microbiota in fresh and mature PDO Feta cheese made at three mountainous areas of Greece. LWT Food Sci. Technol. 2016, 72, 525–533. [Google Scholar] [CrossRef]
- Papadopoulou, O.S.; Argyri, A.A.; Varzakis, E.E.; Tassou, C.C.; Chorianopoulos, N.G. Greek functional Feta cheese: Enhancing quality and safety using a Lactobacillus plantarum strain with probiotic potential. Food Microbiol. 2018, 74, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Kondyli, E.; Pappa, E.C.; Svarnas, C. Ripening changes of the chemical composition, proteolysis, volatile fraction and organoleptic characteristics of a white-brined goat milk cheese. Small Rumin. Res. 2016, 145, 1–6. [Google Scholar] [CrossRef]
- Guamis, B.; Trujillo, A.J.; Ferragut, V.; Chiralt, A.; Andres, A.; Fito, P. Ripening control of manchego type cheese salted by brine vacuum impregnation. Int. Dairy J. 1997, 7, 185–192. [Google Scholar] [CrossRef]
- Miloradovic, Z.; Smigic, N.; Djekic, I.; Tomasevic, I.; Kljajevic, N.; Nedeljkovic, A.; Miocinovic, J. The influence of NaCl concentration of brine and different packaging on goat white brined cheese characteristics. Int. Dairy J. 2018, 79, 24–32. [Google Scholar] [CrossRef]
- Michaelidou, A.; Katsiari, M.C.; Kondyli, E.; Voutsinas, L.P.; Alichanidis, E. Effect of a commercial adjunct culture on proteolysis in low-fat Feta-type cheese. Int. Dairy J. 2003, 13, 179–189. [Google Scholar] [CrossRef]
- Gobbetti, M.; Di Cagno, R.; Calasso, M.; Neviani, E.; Fox, P.F.; De Angelis, M. Drivers that establish and assembly the lactic acid bacteria biota in cheeses. Trends Food Sci. Technol. 2018, 78, 244–254. [Google Scholar] [CrossRef]
- Madureira, A.R.; Soares, J.C.; Pintado, M.E.; Gomes, A.M.P.; Freitas, A.C.; Malcata, F.X. Sweet whey cheese matrices inoculated with the probiotic strain Lactobacillus paracasei LAFTI® L26. Dairy Sci. Technol. 2008, 88, 649–665. [Google Scholar] [CrossRef]
- Schoina, V.; Terpou, A.; Angelika-Ioanna, G.; Koutinas, A.; Kanellaki, M.; Bosnea, L. Use of Pistacia terebinthus resin as immobilization support for Lactobacillus casei cells and application in selected dairy products. J. Food Sci. Technol. 2015, 52, 5700–5708. [Google Scholar] [CrossRef] [PubMed]
- Bendali, F.; Madi, N.; Sadoun, D. Beneficial effects of a strain of Lactobacillus paracasei subsp. paracasei in Staphylococcus aureus-induced intestinal and colonic injury. Int. J. Infect. Dis. 2011, 15, e787–e794. [Google Scholar] [CrossRef] [PubMed]
- Capra, M.L.; Tibaldo, M.M.; Vinderola, G.; Reinheimer, J.A.; Quiberoni, A. Technological and probiotic characterisation of Lactobacillus casei/paracasei strains and their phage-resistant mutants. Int. Dairy J. 2014, 37, 39–47. [Google Scholar] [CrossRef]
- Aunsbjerg, S.D.; Honoré, A.H.; Marcussen, J.; Ebrahimi, P.; Vogensen, F.K.; Benfeldt, C.; Skov, T.; Knøchel, S. Contribution of volatiles to the antifungal effect of Lactobacillus paracasei in defined medium and yogurt. Int. J. Food Microbiol. 2015, 194, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Juodeikiene, G.; Bartkiene, E.; Cernauskas, D.; Cizeikiene, D.; Zadeike, D.; Lele, V.; Bartkevics, V. Antifungal activity of lactic acid bacteria and their application for Fusarium mycotoxin reduction in malting wheat grains. LWT 2018, 89, 307–314. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Juodeikiene, G.; Paskevicius, A.; Bartkiene, E. Antimicrobial activity of lactic acid bacteria against pathogenic and spoilage microorganism isolated from food and their control in wheat bread. Food Control 2013, 31, 539–545. [Google Scholar] [CrossRef]
 ) Feta-type cheese produced with rennin enzyme and used as a control sample. FSP3: (
) Feta-type cheese produced with rennin enzyme and used as a control sample. FSP3: ( ) Feta-type cheese produced with L. paracasei SP3 as starter culture.
) Feta-type cheese produced with L. paracasei SP3 as starter culture.
 ) Feta-type cheese produced with rennin enzyme and used as a control sample. FSP3: (
) Feta-type cheese produced with rennin enzyme and used as a control sample. FSP3: ( ) Feta-type cheese produced with L. paracasei SP3 as starter culture.
) Feta-type cheese produced with L. paracasei SP3 as starter culture.
| Final Counts (log cfu/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolated Lactobacillus Strains | ||||||||||||
| Time (h) | SP3 | SP16 | SP18 | SP10 | SP21 | SP22 | SP27 | SP30 | SP33 | SP36 | L. plantarum ATCC 14971 | |
| Resistance to low pH | 0 | 8.9 ± 0.10 | 9.0 ± 0.23 | 8.8 ± 0.13 * | 8.6 ± 0.20 | 8.9 ± 0.17 | 8.3 ± 0.24 * | 8.1 ± 0.24 * | 8.2 ± 0.15 | 8.5 ± 0.25 * | 8.4 ± 0.18 * | 9.1 ± 0.21 |
| pH = 2 | 2 | 7.1 ± 0.13 * | 3.2 ± 0.12 * | 2.5 ± 0.41 * | 1.7 ± 0.19 * | 1.3 ± 0.23 * | 1.8 ± 0.11 * | 0 | 0 | 0 | 0 | 7.9 ± 0.15 |
| pH = 3 | 2 | 7.6 ± 0.11 | 6.9 ± 0.12 * | 7.3 ± 0.26 * | 6.5 ± 0.07 * | 6.9 ± 0.08 * | 8.0 ± 0.13 | 8.6 ± 0.23 * | 7.8 ± 0.11 | 6.9 ± 0.23 * | 6.8 ± 0.15 * | 7.8 ± 0.05 |
| pH = 4 | 2 | 8.5 ± 0.14 * | 8.3 ± 0.19 | 7.7 ± 0.12 * | 7.9 ± 0.11 | 7.8 ± 0.11 * | 8.5 ± 0.21 * | 7.6 ± 0.11 * | 7.2 ± 0.17 * | 7.3 ± 0.08 * | 7.2 ± 0.09 * | 8.1 ± 0.10 |
| Pepsin | 0 | 7.4 ± 0.15 | 7.6 ± 0.23 | 7.1 ± 0.29 | 7.2 ± 0.13 | 7.3 ± 0.15 | 7.2 ± 0.29 | 7.1 ± 0.18 | 7.3 ± 0.21 | 7.2 ± 0.13 | 7.5 ± 0.11 | 7.3 ± 0.05 |
| 3 | 6.6 ± 0.14 | 6.6 ± 0.06 | 4.7 ± 0.11 * | 5.1 ± 0.18 * | 4.9 ± 0.15 * | 5.2 ± 0.25 * | 5.6 ± 0.22 * | 3.3 ± 0.19 * | 3.8 ± 0.23 * | 4.5 ± 0.23 * | 6.7 ± 0.15 | |
| Pancreatin | 0 | 8.8 ± 0.11 * | 8.3 ± 0.17 | 8.0 ± 0.26 | 8.4 ± 0.18 | 8.0 ± 0.17 | 8.1 ± 0.14 | 8.2 ± 0.21 | 8.3 ± 0.13 | 8.2 ± 0.15 | 8.5 ± 0.17 * | 8.2 ± 0.10 |
| 4 | 7.3 ± 0.05 | 5.1 ± 0.19 | 4.2 ± 0.07 | 5.6 ± 0.15 | 5.3 ± 0.15 | 4.5 ± 0.15 | 5.0 ± 0.19 | 5.4 ± 0.28 | 5.2 ± 0.11 | 5.2 ± 0.11 | 7.5 ± 0.10 | |
| Bile salts | 0 | 8.8 ± 0.08 | 8.6 ± 0.27 | 8.6 ± 0.12 | 8.5 ± 0.10 | 8.5 ± 0.11 | 8.7 ± 0.29 | 8.5 ± 0.13 | 8.5 ± 0.21 | 8.9 ± 0.11 | 8.4 ± 0.19 | 8.7 ± 0.20 |
| 4 | 8.5 ± 0.05 * | 7.0 ± 0.31 * | 6.4 ± 0.27 * | 7.1 ± 0.11 * | 7.2 ± 0.19 * | 6.4 ± 0.27 * | 6.3 ± 0.17 * | 7.1 ± 0.27 * | 6.2 ± 0.09 * | 5.5 ± 0.21 * | 8.0 ± 0.15 | |
| Agent | SP3 | SP16 | SP18 | SP10 | SP21 | SP22 | SP27 | SP30 | SP33 | SP36 | L. plantarum ATCC 14917 | Cut-Off a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (MIC μg/mL) | ||||||||||||
| Amoxycillin | 2.28 ± 0.27 | 1.58 ± 0.11 * | 3.7 ± 0.11 | 3.84 ± 0.31 * | 4.41 ± 0.79 * | 2.76 ± 0.91 | 3.10 ± 0.17 | 4.15 ± 0.23 * | 3.75 ± 0.29 * | 3.84 ± 0.59 * | 2.86 ± 0.78 | n.r. b,c |
| Amoxycillin + Clavulanic acid | 1.57 ± 0.31 | 1.14 ± 0.15 * | 1.76 ± 0.15 * | 1.14 ± 0.13 * | 0.49 ± 0.05 * | 1.44 ± 0.19 * | 1.95 ± 0.11 * | 1.25 ± 0.51 * | 0.84 ± 0.11 * | 1.24 ± 0.29 * | 2.67 ± 0.15 | n.r. b,c |
| Ampicillin | 0.38 ± 0.07 | 0.99 ± 0.05 * | 1.26 ± 0.09 * | 2.08 ± 0.19 * | 1.76 ± 0.21 * | 1.76 ± 0.09 * | 1.29 ± 0.23 * | 1.47 ± 0.09 * | 0.76 ± 0.08 * | 0.76 ± 0.31 * | 0.58 ± 0.33 | 4 b |
| Clindamycin | 0.73 ± 0.05 | 1.14 ± 0.26 * | 1.54 ± 0.20 * | 0.96 ± 0.08 | 2.29 ± 0.21 * | 1.33 ± 0.21 * | 1.12 ± 0.10 * | 1.51 ± 0.15 * | 1.95 ± 0.21 * | 0.97 ± 0.13 * | 0.67 ± 0.20 | 1 b |
| Erythromycin | 0.34 ± 0.11 * | 0.43 ± 0.09 * | 1.08 ± 0.11 | 1.79 ± 0.15 * | 1.89 ± 0.09 * | 1.81 ± 0.15 * | 0.35 ± 0.05 * | 1.23 ± 0.19 | 1.74 ± 0.19 * | 1.41 ± 0.10 | 1.00 ± 0.87 | 1 b |
| Gentamycin | 5.18 ± 0.12 * | 6.57 ± 1.15 * | 5.73 ± 0.31 * | 8.41 ± 0.71 * | 7.29 ± 0.31 * | 9.15 ± 0.47 * | 8.71 ± 1.11 * | 5.21 ± 0.79 * | 6.43 ± 0.31 * | 6.08 ± 1.71 * | 3.33 ± 1.15 * | 32 b |
| Metronidazole | 103.4 ± 22.7 * | 144.1 ± 21.9 * | 175.2 ± 30.4 * | 148.1 ± 3.08 * | 77.9 ± 10.23 * | 104.4 ± 18.3 * | 200.1 ± 21.8 * | 153.1 ± 21.39 * | 148.3 ± 20.15 * | 138.1 ± 19.2 * | >256 | n.r. b,c |
| Tetracyclinne | 4.13 ± 0.10 * | 5.29 ± 0.39 * | 9.55 ± 0.81 * | 5.36 ± 0.71 * | 6.23 ± 0.58 * | 4.48 ± 0.93 * | 5.12 ± 0.76 * | 6.53 ± 0.87 * | 10.89 ± 0.95 * | 14.08 ±1.39 | 13.3 ± 4.62 | 4 b |
| Tigecycline | 0.35 ± 0.05 | 0.47 ± 0.08 | 0.41 ± 0.15 | 0.49 ± 0.08 | 0.64 ± 0.07 * | 0.59 ± 0.11 * | 0.62 ± 0.13 * | 0.59 ± 0.11 * | 0.61 ± 0.08 * | 0.44 ± 0.05 | 0.33 ± 0.14 | n.r. b,c |
| Vancomycin | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | >256 | n.r. b,c |
| Cheese | Ripening Period (Days) | Lactose (g/100 g of Cheese) | Glucose (g/100 g of Cheese) | Galactose (g/100 g of Cheese) | Ethanol (g/100 g of Cheese) | pH | Acidity (g of Lactic Acid/100 g of Cheese) | Moisture (%, wt/wt) | Total N in DM (%) |
|---|---|---|---|---|---|---|---|---|---|
| No starter culture | 0 | 3.85 ± 0.10 | 0.19 ± 0.03 | 0.26± 0.05 | 0.03 ± 0.01 | 6.50 ± 0.10 | 0.13 ± 0.02 | 60.0 ± 1.5 | |
| 1 | 3.78 ± 0.15 | 0.06 ± 0.02 | 0.15± 0.02 | 0.04 ± 0.01 | 6.48 ± 0.05 | 0.14 ± 0.02 | 51.5 ± 2.0 | ||
| 5 | 3.50 ± 0.05 | Tr1 | Tr | 0.12 ± 0.01 | 6.44 ± 0.10 | 0.18 ± 0.02 | 45.1 ± 2.5 | ||
| 14 | 2.24 ± 0.15 | Tr | Tr | 0.15 ± 0.01 | 5.75 ± 0.10 | 0.53 ± 0.03 | 56.0 ± 2.5 | ||
| 30 | 1.84 ± 0.10 | Tr | Tr | 0.17 ± 0.02 | 5.60 ± 0.10 | 0.39 ± 0.01 | 54.5 ± 1.0 | ||
| 45 | 1.20 ± 0.05 | Tr | Tr | 0.11 ± 0.01 | 5.62 ± 0.10 | 0.30 ± 0.02 | 54.9 ± 1.0 | ||
| 70 | 0.78 ± 0.05 | Tr | Tr | 0.08 ± 0.01 | 5.48 ± 0.10 | 0.20 ± 0.01 | 54.1 ± 1.5 | 4.89 ± 0.10 | |
| L. paracasei SP3 | 0 | 1.95 ± 0.05 * | 0.32 ± 0.04 | 0.27± 0.04 | 0.03 ± 0.01 | 6.18 ± 0.10 | 0.21 ± 0.02 * | 60.5 ± 2.3 | |
| 1 | 1.58 ± 0.05 * | 0.12 ± 0.03 | 0.19± 0.03 | 0.03 ± 0.01 | 5.32 ± 0.10 * | 0.42 ± 0.05 * | 50.1 ± 2.1 | ||
| 5 | 1.32 ± 0.07 * | 0.06 ± 0.02 * | 0.06± 0.02 * | 0.04 ± 0.01 * | 4.95 ± 0.05 * | 0.62 ± 0.05 * | 55.0 ± 1.1 * | ||
| 14 | 0.65 ± 0.07 * | Tr | Tr | 0.08 ± 0.01 * | 4.78 ± 0.10 * | 0.80 ± 0.07 * | 50.1 ± 1.5 * | ||
| 30 | 0.32 ± 0.08 * | Tr | Tr | 0.10 ± 0.01 * | 4.80 ± 0.10 * | 0.91 ± 0.09 * | 51.9 ± 1.0 * | ||
| 45 | 0.15 ± 0.10 * | Tr | Tr | 0.09 ± 0.02 * | 4.72 ± 0.10 * | 0.89 ± 0.05 * | 50.5 ± 3.7 * | ||
| 70 | Tr | Tr | Tr | 0.04 ± 0.01 * | 4.62 ± 0.05 * | 0.90 ± 0.05 * | 50.5 ± 1.8 * | 6.22 ± 0.09 * |
| Cheese Type | Ripening and Storage Period (d) | Total Aerobic Count (log cfu/g) | Lactococci (log cfu/g) | Lactobacilli (log cfu/g) | Yeasts & Fungi (log cfu/g) | Coliforms (log cfu/g) |
|---|---|---|---|---|---|---|
| No starter culture | 0 | 5.55 ± 0.15 | 5.78 ± 0.20 | 6.17 ± 0.35 | 5.05 ± 0.20 | 4.65 ± 0.15 |
| 1 | 6.25 ± 0.25 | 6.95 ± 0.25 | 6.50 ± 0.19 | 6.01 ± 0.18 | 5.21 ± 0.20 | |
| 4 | 8.95 ± 0.35 | 6.54 ± 0.18 | 6.95 ± 0.24 | 7.15 ± 0.30 | 5.12 ± 0.35 | |
| 15 | 9.55 ± 0.30 | 7.32 ± 0.21 | 7.75 ± 0.29 | 7.21 ± 0.28 | 4.58 ± 0.30 | |
| 30 | 8.75 ± 0.30 | 7.11 ± 0.32 | 7.21 ± 0.32 | 6.55 ± 0.25 | 5.10 ± 0.29 | |
| 45 | 8.20 ± 0.48 | 6.99 ± 0.28 | 7.11 ± 0.28 | 6.12 ± 0.31 | 4.69 ± 0.25 | |
| 70 | 7.95 ± 0.34 | 6.58 ± 0.24 | 7.05 ± 0.30 | 5.25 ± 0.32 | 4.37 ± 0.20 | |
| Lactobacillus paracasei SP3 | 0 | 6.12 ± 0.39 | 5.82 ± 0.34 | 7.93 ± 0.28 * | 5.10 ± 0.19 | 4.90 ± 0.19 |
| 1 | 6.85 ± 0.33 | 6.21 ± 0.37 * | 8.12 ± 0.35 * | 6.10 ± 0.24 | 5.12 ± 0.31 | |
| 4 | 8.93 ± 0.29 | 6.88 ± 0.26 | 8.75 ± 0.28 * | 6.65 ± 0.26 | 4.42 ± 0.30 | |
| 15 | 9.58 ± 0.38 | 6.67 ± 0.33 * | 9.02 ± 0.22 * | 5.85 ± 0.24 * | 4.05 ± 0.25 | |
| 30 | 9.05 ± 0.34 | 5.12 ± 0.34 * | 8.86 ± 0.38 * | 4.95 ± 0.24 * | 3.56 ± 0.41 * | |
| 45 | 8.59 ± 0.36 | 5.12 ± 0.29 * | 8.43 ± 0.24 * | 3.77 ± 0.35 * | 3.07 ± 0.35 * | |
| 70 | 8.42 ± 0.32 | 5.02 ± 0.35 * | 8.18 ± 0.32 * | 3.19 ± 0.39 * | 2.15 ± 0.34 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantzourani, I.; Terpou, A.; Alexopoulos, A.; Chondrou, P.; Galanis, A.; Bekatorou, A.; Bezirtzoglou, E.; Koutinas, A.A.; Plessas, S. Application of A Novel Potential Probiotic Lactobacillus paracasei Strain Isolated from Kefir Grains in the Production of Feta-Type Cheese. Microorganisms 2018, 6, 121. https://doi.org/10.3390/microorganisms6040121
Mantzourani I, Terpou A, Alexopoulos A, Chondrou P, Galanis A, Bekatorou A, Bezirtzoglou E, Koutinas AA, Plessas S. Application of A Novel Potential Probiotic Lactobacillus paracasei Strain Isolated from Kefir Grains in the Production of Feta-Type Cheese. Microorganisms. 2018; 6(4):121. https://doi.org/10.3390/microorganisms6040121
Chicago/Turabian StyleMantzourani, Ioanna, Antonia Terpou, Athanasios Alexopoulos, Pelagia Chondrou, Alex Galanis, Argyro Bekatorou, Eugenia Bezirtzoglou, Athanasios A Koutinas, and Stavros Plessas. 2018. "Application of A Novel Potential Probiotic Lactobacillus paracasei Strain Isolated from Kefir Grains in the Production of Feta-Type Cheese" Microorganisms 6, no. 4: 121. https://doi.org/10.3390/microorganisms6040121
APA StyleMantzourani, I., Terpou, A., Alexopoulos, A., Chondrou, P., Galanis, A., Bekatorou, A., Bezirtzoglou, E., Koutinas, A. A., & Plessas, S. (2018). Application of A Novel Potential Probiotic Lactobacillus paracasei Strain Isolated from Kefir Grains in the Production of Feta-Type Cheese. Microorganisms, 6(4), 121. https://doi.org/10.3390/microorganisms6040121











