Natural Products for the Treatment of Chlamydiaceae Infections
Abstract
:1. Introduction
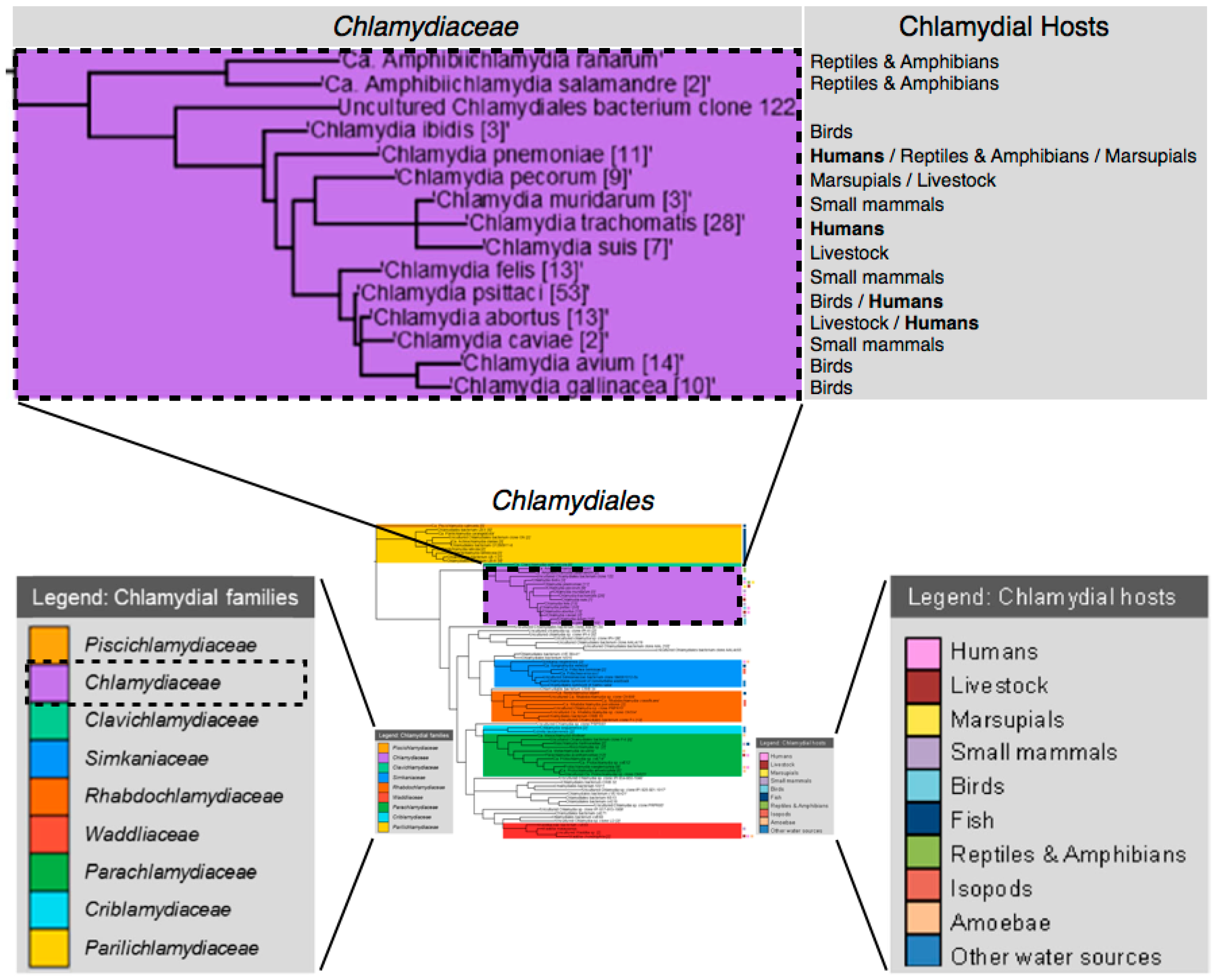
2. Overview of Chlamydiaceae
2.1. Brief History
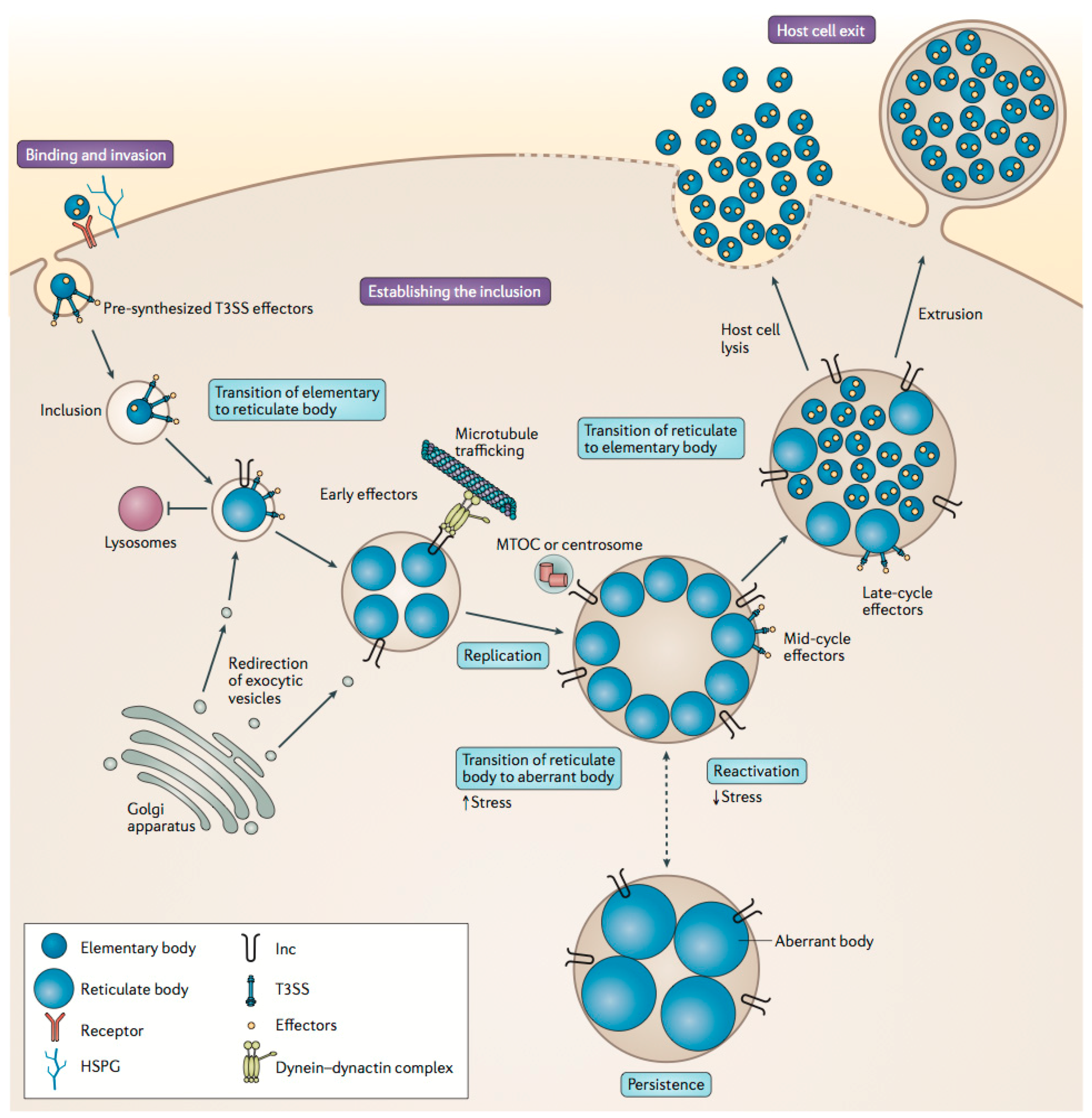
2.2. Chlamydiaceae Infections and Treatment
3. Biomedical Phytochemical Groups and Anti-Infective Action
3.1. Polyphenolic Compounds
3.2. Lipidic Compounds
3.3. Proteinaceous Compounds
3.4. Cellular Metabolites & Probiotics
3.5. Polyherbal Formulations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Taylor-Brown, A.; Vaughan, L.; Greub, G.; Timms, P.; Polkinghorne, A. Twenty years of research into Chlamydia-like organisms: A revolution in our understanding of the biology and pathogenicity of members of the phylum Chlamydiae. Pathog. Dis. 2015, 73, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.R.; Burton, M.J.; Haddad, D.; West, S.; Wright, H. Trachoma. Lancet 2014, 384, 2142–2152. [Google Scholar] [CrossRef]
- Bébéar, C.; de Barbeyrac, B. Genital Chlamydia trachomatis infections. Clin. Microbiol. Infect. 2009, 15, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Grayston, J.T. Infections caused by Chlamydia pneumoniae strain twar. Clin. Infect. Dis. 1992, 15, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Kohlhoff, S.A.; Hammerschlag, M.R. Treatment of chlamydial infections: 2014 update. Expert Opin. Pharmacother. 2015, 16, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.; Lee, E.J.; Timms, P.; Polkinghorne, A. Chlamydia pecorum infections in sheep and cattle: A common and under-recognised infectious disease with significant impact on animal health. Vet. J. 2015, 206, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Tabrizi, S.; Law, M.; Vodstrcil, L.; Chen, M.; Fairley, C.; Guy, R.; Bradshaw, C.; Hocking, J. Azithromycin versus doxycycline for the treatment of genital Chlamydia infection—A meta-analysis of randomised controlled trials. Clin. Infect. Dis. 2014, 59, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.Y.S.; Tabrizi, S.N.; Fairley, C.K.; Vodstrcil, L.A.; Huston, W.M.; Chen, M.; Bradshaw, C.; Hocking, J.S. The efficacy of azithromycin and doxycycline for the treatment of rectal Chlamydia infection: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2015, 70, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Rodolakis, A.; Mohamad, K.Y. Zoonotic potential of chlamydophila. Vet. Microbiol. 2010, 140, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Rodolakis, A.; Laroucau, K. Chlamydiaceae and chlamydial infections in sheep or goats. Vet. Microbiol. 2015, 181, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Horner, P.J. Azithromycin antimicrobial resistance and genital Chlamydia trachomatis infection: Duration of therapy may be the key to improving efficacy. Sex. Transm. Infect. 2012, 88, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.; Schott, F.; Donati, M.; Di Francesco, A.; Hässig, M.; Wanninger, S.; Sidler, X.; Borel, N. Prevalence of chlamydial infections in fattening pigs and their influencing factors. PLoS ONE 2015, 10, e0143576. [Google Scholar] [CrossRef] [PubMed]
- Sandoz, K.M.; Rockey, D.D. Antibiotic resistance in Chlamydiae. Future Microbiol. 2010, 5, 1427–1442. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.C.; Schachter, J.; Moncada, J.; Zhou, Z.; House, J.; Lietman, T.M. Lack of macrolide resistance in Chlamydia trachomatis after mass azithromycin distributions for trachoma. Emerg. Infect. Dis. 2009, 15, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- West, S.; Moncada, J.; Munoz, B.; Mkocha, H.; Storey, P.; Hardick, J.; Gaydos, C.A.; Quinn, T.C.; Schachter, J. Is there evidence for resistance of ocular Chlamydia trachomatis to azithromycin after mass treatment for trachoma control? J. Infect. Dis. 2014, 210, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Vasilevsky, S.; Greub, G.; Nardelli-Haefliger, D.; Baud, D. Genital Chlamydia trachomatis: Understanding the roles of innate and adaptive immunity in vaccine research. Clin. Microbiol. Rev. 2014, 27, 346–370. [Google Scholar] [CrossRef] [PubMed]
- Harkinezhad, T.; Geens, T.; Vanrompay, D. Chlamydophila psittaci infections in birds: A review with emphasis on zoonotic consequences. Vet. Microbiol. 2009, 135, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Saikku, P.; Wang, S.; Kleemola, M.; Brander, E.; Rusanen, E.; Grayston, J. An epidemic of mild pneumonia due to an unusual strain of Chlamydia psittaci. J. Infect. Dis. 1985, 151, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Grayston, J.T.; Kuo, C.-C.; Wang, S.-P.; Altman, J. A new Chlamydia psittaci strain, twar, isolated in acute respiratory tract infections. N. Engl. J. Med. 1986, 315, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.L.; Kuo, C.-C.; Grayston, J.T.; Campbell, L.A. Deoxyribonucleic acid relatedness of Chlamydia sp. Strain twar to Chlamydia trachomatis and Chlamydia psittaci. Int. J. Syst. Evol. Microbiol. 1988, 38, 265–268. [Google Scholar] [CrossRef]
- Grayston, J.; Wang, S.; Kuo, C. Current knowledge of Chlamydia twar, an important cause of pneumonia and other acute respiratory diseases. In Perspectives in Antiinfective Therapy; Springer: Berlin, Germany, 1989; pp. 339–359. [Google Scholar]
- Grayston, J.T.; Kuo, C.-C.; Campbell, L.A.; Wang, S.-P. Chlamydia pneumoniae sp. nov. for Chlamydia sp. Strain twar. Int. J. Syst. Evol. Microbiol. 1989, 39, 88–90. [Google Scholar] [CrossRef]
- Fukushi, H.; Hirai, K. Genetic diversity of avian and mammalian Chlamydia psittaci strains and relation to host origin. J. Bacteriol. 1989, 171, 2850–2855. [Google Scholar] [PubMed]
- Fukushi, H.; Hirai, K. Proposal of Chlamydia pecorum sp. nov. for Chlamydia strains derived from ruminants. Int. J. Syst. Evol. Microbiol. 1992, 42, 306–308. [Google Scholar]
- Everett, K.D.; Andersen, A.A. Identification of nine species of the Chlamydiaceae using PCR-RFLP. Int. J. Syst. Evol. Microbiol. 1999, 49, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Everett, K.D.; Bush, R.M.; Andersen, A.A. Emended description of the order Chlamydilaes, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Evol. Microbiol. 1999, 49, 415–440. [Google Scholar]
- Everett, K.D. Chlamydia and Chlamydiales: More than meets the eye. Vet. Microbiol. 2000, 75, 109–126. [Google Scholar] [CrossRef]
- Sachse, K.; Laroucau, K.; Riege, K.; Wehner, S.; Dilcher, M.; Creasy, H.H.; Weidmann, M.; Myers, G.; Vorimore, F.; Vicari, N. Evidence for the existence of two new members of the family Chlamydiaceae and proposal of Chlamydia avium sp. nov. and Chlachlamydia gallinacea sp. nov. Syst. Appl. Microbiol. 2014, 37, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 2016, 14, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Beale, A.S.; Upshon, P.A. Characteristics of murine model of genital infection with Chlamydia trachomatis and effects of therapy with tetracyclines, amoxicillin-clavulanic acid, or azithromycin. Antimicrob. Agents Chemother. 1994, 38, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Beeckman, D.S.A.; Vanrompay, D. Zoonotic chlamydophila psittaci infections from a clinical perspective. Clin. Microbiol. Infect. 2009, 15, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, P.; Sachse, K.; Kaltenboeck, B. Chlamydiaceae in cattle: Commensals, trigger organisms, or pathogens? Vet. J. 2011, 189, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Longbottom, D.; Coulter, L. Animal Chlamydioses and zoonotic implications. J. Comp. Pathol. 2003, 128, 217–244. [Google Scholar] [CrossRef] [PubMed]
- Polkinghorne, A.; Hanger, J.; Timms, P. Recent advances in understanding the biology, epidemiology and control of chlamydial infections in koalas. Vet. Microbiol. 2013, 165, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Black, L.; Higgins, D.; Govendir, M. In vitro activity of chloramphenicol, florfenicol and enrofloxacin against Chlamydia pecorum isolated from koalas (Phascolarctos cinereus). Aust. Vet. J. 2015, 93, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.E. Feline chlamydiosis. Clin. Tech. Small Anim. Pract. 2005, 20, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Binet, R.; Bowlin, A.K.; Maurelli, A.T.; Rank, R.G. Impact of azithromycin resistance mutations on the virulence and fitness of Chlamydia caviae in Guinea pigs. Antimicrob. Agents Chemother. 2010, 54, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Borel, N.; Leonard, C.; Slade, J.; Schoborg, R.V. Chlamydial antibiotic resistance and treatment failure in veterinary and human medicine. Curr. Clin. Microbiol. Rep. 2016, 3, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Yeruva, L.; Spencer, N.; Bowlin, A.K.; Wang, Y.; Rank, R.G. Chlamydial infection of the gastrointestinal tract: A reservoir for persistent infection. Pathog. Dis. 2013, 68, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.; Bukhari, S.N.A.; Mohamed, M.A.S.; Wai, L.K.; Mesaik, M.A. The evolving role of natural products from the tropical rainforests as a replenishable source of new drug leads. In Drug Discovery and Development—From Molecules to Medicine; Omboon, V., Suleiman, O., Eds.; InTech: Rijeka, Croatia, 2015. [Google Scholar]
- Potroz, M.G.; Cho, N.-J. Natural products for the treatment of trachoma and Chlamydia trachomatis. Molecules 2015, 20, 4180–4203. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Alvesalo, J.; Vuorela, H.; Tammela, P.; Leinonen, M.; Saikku, P.; Vuorela, P. Inhibitory effect of dietary phenolic compounds on Chlamydia pneumoniae in cell cultures. Biochem. Pharmacol. 2006, 71, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Sajilata, M.; Bajaj, P.R.; Singhal, R. Tea polyphenols as nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2008, 7, 229–254. [Google Scholar] [CrossRef]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta Biomembr. 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Pandey, A.; Kumar, S. Perspective on plant products as antimicrobial agents: A review. Pharmacologia 2013, 4, 469–480. [Google Scholar] [CrossRef]
- Yamazaki, T.; Inoue, M.; Sasaki, N.; Hagiwara, T.; Kishimoto, T.; Shiga, S.; Ogawa, M.; Hara, Y.; Matsumoto, T. In vitro inhibitory effects of tea polyphenols on the proliferation of Chlamydia trachomatis and Chlamydia pneumoniae. Jpn. J. Infect. Dis. 2003, 56, 143–145. [Google Scholar] [PubMed]
- Yamazaki, T.; Kishimoto, T.; Shiga, S.; Sato, K.; Hagiwara, T.; Inoue, M.; Sasaki, N.; Ouchi, K.; Hara, Y. Biosynthesized tea polyphenols inactivate Chlamydia trachomatis in vitro. Antimicrob. Agents Chemother. 2005, 49, 2501–2503. [Google Scholar] [CrossRef] [PubMed]
- Törmäkangas, L.; Vuorela, P.; Saario, E.; Leinonen, M.; Saikku, P.; Vuorela, H. In vivo treatment of acute Chlamydia pneumoniae infection with the flavonoids quercetin and luteolin and an alkyl gallate, octyl gallate, in a mouse model. Biochem. Pharmacol. 2005, 70, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-C.; Huang, T.-C.; Lai, C.-S.; Pan, M.-H. Induction of apoptosis by luteolin through cleavage of BCL-2 family in human leukemia HL-60 cells. Eur. J. Pharmacol. 2005, 509, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Aixia, Y.; Nancai, Y.; Wen, S. Baicalin suppresses expression of Chlamydia protease-like activity factor in HEP-2 cells infected by Chlamydia trachomatis. Fitoterapia 2009, 80, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Salin, O.; Alakurtti, S.; Pohjala, L.; Siiskonen, A.; Maass, V.; Maass, M.; Yli-Kauhaluoma, J.; Vuorela, P. Inhibitory effect of the natural product betulin and its derivatives against the intracellular bacterium Chlamydia pneumoniae. Biochem. Pharmacol. 2010, 80, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Salin, O.; Tormakangas, L.; Leinonen, M.; Saario, E.; Hagstrom, M.; Ketola, R.A.; Saikku, P.; Vuorela, H.; Vuorela, P.M. Corn mint (Mentha arvensis) extract diminishes acute Chlamydia pneumoniae infection in vitro and in vivo. J. Agric. Food Chem. 2011, 59, 12836–12842. [Google Scholar] [CrossRef] [PubMed]
- Kapp, K.; Hakala, E.; Orav, A.; Pohjala, L.; Vuorela, P.; Püssa, T.; Vuorela, H.; Raal, A. Commercial peppermint (Mentha × piperita L.) teas: Antichlamydial effect and polyphenolic composition. Food Res. Int. 2013, 53, 758–766. [Google Scholar] [CrossRef]
- Hanski, L.; Genina, N.; Uvell, H.; Malinovskaja, K.; Gylfe, Å.; Laaksonen, T.; Kolakovic, R.; Mäkilä, E.; Salonen, J.; Hirvonen, J. Inhibitory activity of the isoflavone biochanin a on intracellular bacteria of genus Chlamydia and initial development of a buccal formulation. PLoS ONE 2014, 9, e115115. [Google Scholar] [CrossRef] [PubMed]
- Salin, O.P.; Pohjala, L.L.; Saikku, P.; Vuorela, H.J.; Leinonen, M.; Vuorela, P.M. Effects of coadministration of natural polyphenols with doxycycline or calcium modulators on acute Chlamydia pneumoniae infection in vitro. J. Antibiot. 2011, 64, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Romano Carratelli, C.; Losacco, A.; Iovene, M.R. Antimicrobial effect of natural polyphenols with or without antibiotics on Chlamydia pneumoniae infection in vitro. Microb. Drug Resist. 2014, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Thormar, H. Antibacterial effects of lipids: Historical review (1881 to 1960). In Lipids and Essential Oils as Antimicrobial Agents; John Wiley & Sons, Ltd.: Philadelphia, PA, USA, 2011; pp. 25–45. [Google Scholar]
- Yoon, B.K.; Jackman, J.A.; Kim, M.C.; Cho, N.-J. Spectrum of membrane morphological responses to antibacterial fatty acids and related surfactants. Langmuir 2015, 31, 10223–10232. [Google Scholar] [CrossRef] [PubMed]
- Bergsson, G.; Arnfinnsson, J.; Karlsson, S.M.; Steingrímsson, Ó.; Thormar, H. In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1998, 42, 2290–2294. [Google Scholar] [PubMed]
- Isaacs, C.E.; Kashyap, S.; Heird, W.C.; Thormar, H. Antiviral and antibacterial lipids in human milk and infant formula feeds. Arch. Dis. Child. 1990, 65, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Lampe, M.; Ballweber, L.; Isaacs, C.; Patton, D.; Stamm, W. Killing of Chlamydia trachomatis by novel antimicrobial lipids adapted from compounds in human breast milk. Antimicrob. Agents Chemother. 1998, 42, 1239–1244. [Google Scholar] [PubMed]
- Skinner, M.; Kiselev, A.; Isaacs, C.; Mietzner, T.; Montelaro, R.; Lampe, M. Evaluation of WLBU2 peptide and 3-O-octyl-sn-glycerol lipid as active ingredients for a topical microbicide formulation targeting Chlamydia trachomatis. Antimicrob. Agents Chemother. 2010, 54, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Yamano, H.; Yamazaki, T.; Sato, K.; Shiga, S.; Hagiwara, T.; Ouchi, K.; Kishimoto, T. In vitro inhibitory effects of hinokitiol on proliferation of Chlamydia trachomatis. Antimicrob. Agents Chemother. 2005, 49, 2519–2521. [Google Scholar] [CrossRef] [PubMed]
- Mandeel, Q.A.; Al-Laith, A.A.A. Ethnomycological aspects of the desert truffle among native bahraini and non-bahraini peoples of the kingdom of bahrain. J. Ethnopharmacol. 2007, 110, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Yasin, B.; Harwig, S.; Lehrer, R.I.; Wagar, E.A. Susceptibility of Chlamydia trachomatis to protegrins and defensins. Infect. Immun. 1996, 64, 709–713. [Google Scholar] [PubMed]
- Lazarev, V.; Parfenova, T.; Gularyan, S.; Misyurina, O.Y.; Akopian, T.; Govorun, V. Induced expression of melittin, an antimicrobial peptide, inhibits infection by Chlamydia trachomatis and Mycoplasmahominis in a HeLa cell line. Int. J. Antimicrob. Agents 2002, 19, 133–137. [Google Scholar] [CrossRef]
- Lazarev, V.; Shkarupeta, M.; Titova, G.; Kostrjukova, E.; Akopian, T.; Govorun, V. Effect of induced expression of an antimicrobial peptide melittin on Chlamydia trachomatis and Mycoplasma hominis infections in vivo. Biochem. Biophys. Res. Commun. 2005, 338, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Ballweber, L.; Jaynes, J.; Stamm, W.; Lampe, M. In vitro microbicidal activities of cecropin peptides D2A21 and D4E1 and gel formulations containing 0.1% to 2% D2A21 against Chlamydia trachomatis. Antimicrob. Agents Chemother. 2002, 46, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, B. Structure and functions of channel-forming peptides: Magainins, cecropins, melittin and alamethicin. J. Membr. Biol. 1997, 156, 197–211. [Google Scholar] [CrossRef]
- Saberwal, G.; Nagaraj, R. Cell-lytic and antibacterial peptides that act by perturbing the barrier function of membranes: Facets of their conformational features, structure-function correlations and membrane-perturbing abilities. Biochim. Biophys. Acta Rev. Biomembr. 1994, 1197, 109–131. [Google Scholar] [CrossRef]
- Boman, H.G. Gene-encoded peptide antibiotics and the concept of innate immunity: An update review. Scand. J. Immunol. 1998, 48, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Chong-Cerrillo, C.; Selsted, M.E.; Peterson, E.; De La Maza, L.M. Susceptibility of human and murine Chlamydia trachomatis serovars to granulocyte-and epithelium-derived antimicrobial peptides. J. Pept. Res. 2003, 61, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Yasin, B.; Pang, M.; Wagar, E. A cumulative experience examining the effect of natural and synthetic antimicrobial peptides vs. Chlamydia trachomatis. J. Pept. Res. 2004, 64, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Donati, M.; Di Leo, K.; Benincasa, M.; Cavrini, F.; Accardo, S.; Moroni, A.; Gennaro, R.; Cevenini, R. Activity of cathelicidin peptides against Chlamydia spp. Antimicrob. Agents Chemother. 2005, 49, 1201–1202. [Google Scholar] [CrossRef] [PubMed]
- Donati, M.; Di Francesco, A.; Gennaro, R.; Benincasa, M.; Magnino, S.; Pignanelli, S.; Shurdhi, A.; Moroni, A.; Mazzoni, C.; Merialdi, G. Sensitivity of Chlamydia suis to cathelicidin peptides. Vet. Microbiol. 2007, 123, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Donati, M.; Di Francesco, A.; Gennaro, R.; Benincasa, M.; Di Paolo, M.; Shurdhi, A.; Ostanello, F.; Baldelli, R.; Cevenini, R. Increasing effect of a high dose of PG-1 peptide on the infectivity of chlamydophila abortus. FEMS Immunol. Med. Microbiol. 2010, 59, 221–222. [Google Scholar] [CrossRef] [PubMed]
- Bergaoui, I.; Zaïri, A.; Gharsallah, H.; Aouni, M.; Hammami, A.; Hani, K.; Selmi, B. The in vitro evaluation of anti-chlamydial and cytotoxic properties of dermaseptin s4 and derivatives: Peptides from amphibian skin. Med. Chem. Res. 2013, 22, 6096–6104. [Google Scholar] [CrossRef]
- García-Montoya, I.A.; Cendón, T.S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin a multiple bioactive protein: An overview. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Beeckman, D.S.A.; Van Droogenbroeck, C.M.; De Cock, B.J.; Van Oostveldt, P.; Vanrompay, D.C. Effect of ovotransferrin and lactoferrins on chlamydophila psittaci adhesion and invasion in HD11 chicken macrophages. Vet. Res. 2007, 38, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Van Droogenbroeck, C.; Beeckman, D.S.; Harkinezhad, T.; Cox, E.; Vanrompay, D. Evaluation of the prophylactic use of ovotransferrin against chlamydiosis in spf turkeys. Vet. Microbiol. 2008, 132, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Van Droogenbroeck, C.; Dossche, L.; Wauman, T.; Van Lent, S.; Phan, T.T.; Beeckman, D.S.; Vanrompay, D. Use of ovotransferrin as an antimicrobial in turkeys naturally infected with Chlamydia psittaci, Avian metapneumovirus and Ornithobacterium rhinotracheale. Vet. Microbiol. 2011, 153, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Carratelli, C.R.; Rizzo, A.; Paolillo, R.; Catania, M.R.; Catalanotti, P.; Rossano, F. Effect of nitric oxide on the growth of chlamydophila pneumoniae. Can. J. Microbiol. 2005, 51, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Tiso, M.; Schechter, A.N. Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLoS ONE 2015, 10, e0119712. [Google Scholar]
- Pollmann, M.; Nordhoff, M.; Pospischil, A.; Tedin, K.; Wieler, L. Effects of a probiotic strain of enterococcus faecium on the rate of natural Chlamydia infection in swine. Infect. Immun. 2005, 73, 4346–4353. [Google Scholar] [CrossRef] [PubMed]
- Mastromarino, P.; Di Pietro, M.; Schiavoni, G.; Nardis, C.; Gentile, M.; Sessa, R. Effects of vaginal lactobacilli in Chlamydia trachomatis infection. Int. J. Med. Microbiol. 2014, 304, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Luna, Y.; Yu, P.; Fan, H. Lactobacilli inactivate Chlamydia trachomatis through lactic acid but not H2O2. PLoS ONE 2014, 9, e107758. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Fiorentino, M.; Buommino, E.; Donnarumma, G.; Losacco, A.; Bevilacqua, N. Lactobacillus crispatus mediates anti-inflammatory cytokine interleukin-10 induction in response to Chlamydia trachomatis infection in vitro. Int. J. Med. Microbiol. 2015, 305, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Nardini, P.; Palomino, R.A.Ñ.; Parolin, C.; Laghi, L.; Foschi, C.; Cevenini, R.; Vitali, B.; Marangoni, A. Lactobacillus crispatus inhibits the infectivity of Chlamydia trachomatis elementary bodies, in vitro study. Sci. Rep. 2016, 6, 29024. [Google Scholar] [CrossRef] [PubMed]
- Talwar, G.; Garg, S.; Dhar, V.; Chabra, R.; Ganju, A.; Upadhyay, S. Praneem polyherbal cream and pessaries with dual properties of contraception and alleviation of genital infections. Curr. Sci. 1995, 68, 437–440. [Google Scholar]
- Talwar, G.; Raghuvanshi, P.; Mishra, R.; Banerjee, U.; Rattan, A.; Whaley, K.J.; Achilles, S.L.; Zeitlin, L.; Barré-Sinoussi, F.; David, A. Polyherbal formulations with wide spectrum antimicrobial activity against reproductive tract infections and sexually transmitted pathogens. Am. J. Reprod. Immunol. 2000, 43, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Talwar, G. A polyherbal formulation for a wide spectrum of reproductive tract and sexually transmitted infections. In Complementary and Alternative Approaches to Biomedicine; Springer: New York, NY, USA, 2004; pp. 111–119. [Google Scholar]
- Bhengraj, A.; Dar, S.A.; Talwar, G.; Mittal, A. Potential of a novel polyherbal formulation basant for prevention of Chlamydia trachomatis infection. Int. J. Antimicrob. Agents 2008, 32, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Bhengraj, A.; Goyal, A.; Talwar, G.; Mittal, A. Assessment of antichlamydial effects of a novel polyherbal tablet basant. Sex. Transm. Infect. 2009, 85, 561–5621. [Google Scholar] [CrossRef] [PubMed]
- Chait, R.; Vetsigian, K.; Kishony, R. What counters antibiotic resistance in nature? Nat. Chem. Biol. 2012, 8, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Keshavjee, S.; Farmer, P.E. Tuberculosis, drug resistance, and the history of modern medicine. N. Engl. J. Med. 2012, 367, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.A.; Lee, J.; Cho, N.J. Nanomedicine for infectious disease applications: Innovation towards broad-spectrum treatment of viral infections. Small 2016, 12, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
| Chlamydial Species | Standard Treatments | In Vitro and/or Natural Antibiotic Resistance [13] | Asymptomatic Persistence in Host [27] | Infected Tissues [27] |
|---|---|---|---|---|
| C. trachomatis | Humans [5] Doxycycline (TET) or azithromycin (MAC). Erythromycin ethyl-succinate (MAC), levofloxacin (FLQ), or ofloxacin (FLQ) are alternatives. Azithromycin (MAC) with amoxicillin (β-lac) or erythromycin (MAC) advised for pregnant women. | MAC, TET, β-lac, RIF, FLQ, SUL, TRI, LIN, AMI, FOS. | + | Eye, genital, joints, prostate, neonatal lung. |
| C. suis | Livestock (Swine) [12] NOTE: The following antibiotic treatments were unable to clear chlamydial infections on a herd level. Therapeutic treatment: aminoglycoside (AMI); β-lactam antibiotic (β-lac); cephalosporin (β-lac); fluoroquinolone (FLQ); or tetracycline (TET). Pro-/metaphylactic herd treatment: amoxicillin (β-lac); chlortetracycline (TET); MDT—chlortetracycline (TET), sulfadimidine (SUL), tylosin (MAC); or MDT—trimethoprim (DRI), sulfadimidine (SUL), sulfathiazole (SUL). | TET, β-lac, RIF, FLQ, SUL. | + | Eye, intestine, lung. |
| C. muridarum | Mice [36] Doxycycline (TET) or azithromycin (MAC). | TET, β-lac, RIF, FLQ. | + | Genital, intestine, liver, lung, kidney, spleen. |
| C. psittaci | Birds & Humans [9,31] Tetracyclines (TET) or macrolides (MAC). Erythromycin (MAC) is advised for children and pregnant women. Livestock (Cattle) [6,32] No evidence pointing to the successful use of antimicrobials to eliminate bovine chlamydial infection. | MAC, β-lac, RIF, SUL, TRI, AMI. | + | Brain, eye, genital, intestine, liver, lung, spleen. |
| C. pneumonia | Humans [5] Doxycycline (TET) or azithromycin (MAC). Clarithromycin (MAC), levofloxacin (FLQ), or moxifloxacin (FLQ) are alternatives. Azithromycin (MAC), clarithromycin (MAC), or erythromycin (MAC) is advised for children and pregnant women. 70%–86% efficacy with erythromycin (MAC), clarithromycin (MAC), azithromycin (MAC), levofloxacin (FLQ), or moxifloxacin (FLQ). | β-lac, RIF, FLQ, SUL. | + | Arteries, brain, joints, lung. |
| C. abortus | Humans [33] Responds well to early treatment with tetracyclines (TET) and erythromycin (MAC). Livestock (Ruminants) [9,10] Oxytetracycline (TET) will reduce the number of abortions and bacterial shedding. Antibiotics are ineffective in clearing the infection. | − | + | Intestine, placenta, spleen, fetal liver. |
| C. pecorum | Livestock (Cattle) [32] No evidence pointing to the successful use of antimicrobials to eliminate bovine chlamydial infection. Marsupials (Koalas) [34,35] Chloramphenicol (CHL) or enrofloxacin (FLQ) produce mixed results. Macrolides (MAC) and tetracyclines (TET) induce inappetence, emaciation, and death in koalas. | − | + | Bladder, brain, eye, intestine, lymph, joints, prostate. |
| C. felis | Cats [37] Doxycycline (TET) and topical tetracycline (TET). MDT: Amoxicillin/clavulanic acid (β-lac) may be a safe alternative for kittens. | − | − | Eye, genital, joints, lung. |
| C. caviae | Guinea Pigs [38] Doxycycline (TET) or azithromycin (MAC). | β-lac, RIF, FLQ, SUL. | − | Bladder, eye, genital, lung. |
| C. avium | Birds Tetracyclines (TET) or macrolides (MAC)—based on treatment for C. psittaci. | − | + | − |
| C. gallinacea | Birds Tetracyclines (TET) or macrolides (MAC)—based on treatment for C. psittaci. | − | + | − |
| Antimicrobial Agent | Chlamydial Species | Study Design | Effects | Reference |
|---|---|---|---|---|
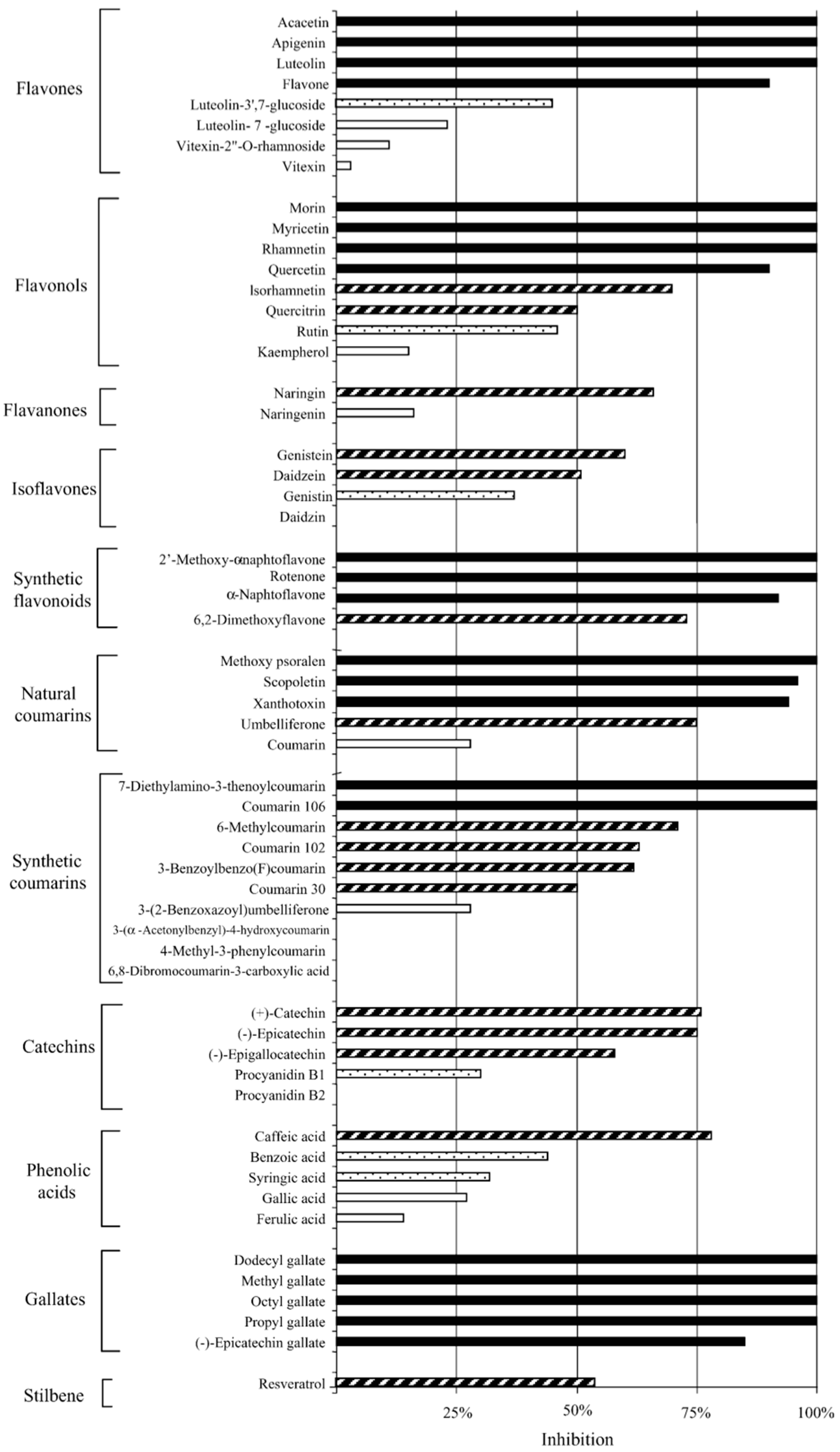
| Flavones (×8), Flavonols (×8), Flavonones (×2), Isoflavones (×4), Synthetic flavonoids (×4), Natural coumarins (×5), Synthetic courmarins (×10), Catechins (×5), Phenolic acids (×5), Gallates (×5), Stilbene (×1) | C. pneumoniae (K7) | In vitro Pre-inoculation: incubated with cells for 24 h prior to EB inoculation. Post-inoculation: administered at 0 h post inoculation (p.i.). | From 57 compounds, at 50 μM: 21 were highly active, 16 active, 6 moderately active, and 14 inactive. 10 compounds achieved an MIC of 50 μM or less with luteolin being 8.8 μM and dodecyl gallate being 18 μM. Gallates was the most active group. Some compounds accumulated inside cells or in cell membranes and cause inhibition when present only prior to infection. Compound structural variations, either free from sugar moieties, or with greater hydrophobicity, were found to be more active. All compounds were non-toxic to the host cells. | [43] |
| Polyphenon 70S: Epigallocatechin (18.3%), Epicatechin (8.6%), Epigallocatechin gallate (35.9%), Epicatechin gallate (11.2%), Gallocatechin gallate (3.5%) | C. trachomatis (D), (L2) C. Pneumoniae (AR-39), (AC-43) | In vitro Pre-treatment: incubated with EBs for 30, 60, or 90 min prior to inoculation. Post-inoculation: administered at 0 h p.i. | Polyphenon 70S, post-incubation, 100% inhibition of chlamydial inclusions, at 0.5 mg/mL, with toxicity to host cells at 0.25 mg/mL. Pre-incubation, 100% inhibition, at 0.4–1.6 mg/mL, with no toxicity to host cells. | [47] |
| Catechin, Epicatechin, Epigallocatechin, Epicatechin gallate, Epigallocatechin gallate | C. pneumoniae (AR-39) | In vitro Pre-treatment: incubated with EBs for 90 min prior to inoculation. | Inhibition was observed for concentrations from 0.4 to >6.4 mg/mL. Most active compounds were epigallocatechin gallate and epicatechin gallate, followed by epicatechin. Catechin and epigallocatechin exhibited intermediate activity. Epicatechin was the least toxic. | [48] |
| Luteolin, Octyl gallate, Quercetin | C. pneumoniae (K7) | In vivo Pre- & Post-inoculation: administered daily for 3 days prior to inoculation; administered daily for 12 days p.i. (mice) | Luteolin suppressed inflammation in lung tissue, C. pneumoniae-specific antibodies, and the presence of chlamydia in lung tissue. Octyl gallate had no significant effect on infection. Quercetin increased the inflammatory responses and the chlamydial load in the lungs. | [49] |
| Baicalin | C. trachomatis (D) | In vitro Post-inoculation: administered at 24 h p.i. | Blocks the infection of Hep-2 cells. Down-regulates the production of the chlamydia-secreted protein (CPAF). CPAF degradation of host transcription factors RFX5 may allow chlamydia to escape efficient immune detection. Baicalin may assist the host immune system to detect the chlamydial infection. | [51] |
| Betulin, Betulin derivatives (×32) | C. pneumoniae (CWL-029) | In vitro Post-inoculation: administered at 0 h p.i. | At a concentration of 1 μM, three derivatives showed >80% growth inhibition, and 15 compounds 20%–80% growth inhibition. Betulin dioxime exhibited an MIC of 1 μM, and achieved 50% inhibition at 290 nM. Compounds were well tolerated by host cells. | [52] |
| Corn mint extract (Mentha arvensis): Rosmarinic acid (5.2%), Linarin (6.0%), Acacetin-acetylglucoside-rhamnoglycoside (2.5%) Pure compounds: Rosmarinic acid, Linarin, Acacetin | C. pneumoniae (CWL-029), (K7) | In vitro Post-inoculation: administered at 0 h p.i. In vivo Pre- & Post-inoculation: administered daily for 3 days prior to inoculation; administered daily for 10 days p.i. (mice) | For corn mint extract, at 256 μg/mL, 73% inhibition of chlamydial inclusions was achieved for strain CWL-029, and 90% for strain K7, with ~78% host cell viability. Pure compound, inhibition at 100 μg/mL for strain CWL-029: linarin 100%, acacetin 97%, and rosmarinic acid 73%, with ~99% host cell viability. Pure compound, inhibition at 100 μg/mL for strain K7: linarin 62%, acacetin 81%, and rosmarinic acid 74%. In vivo, corn mint extract in nutritionally relevant dosages resulted in reduced inflammatory responses to chlamydial infection. | [53] |
| Peppermint tea extracts (Mentha × piperita L.): Eriocitrin, 12-Hydroxyjasmonate sulfate, Luteolin-O-rutinoside, Rosmarinic acid, Salvianolic acid B, Trace polyphenols, Trace plant acids | C. pneumoniae (K7) | In vitro Post-inoculation: administered at 0 h p.i. | Seven tea extracts were shown to be active against C. pneumoniae. At 250 μg/mL, from 20.7% to 69.5% inhibition. Higher content of luteolin and apigenin glycosides showed high activity. Host cell viability after the 72 h exposure to tea extracts ranged from 82.4% to 99.4%. | [54] |
| Isoflavones: Biochanin A, Formononetin, Genistein, Daidzein, Genistin, Daidzin | C. trachomatis (K), (L2) C. Pneumoniae (K7) | In vitro Post-inoculation: administered at 0 h p.i. | Biochanin A at 50 μM, complete inhibition of C. pneumoniae. Biochanin A, IC50: C. trachomatis—62 μM; C. pneumoniae—12 μM. No harmful effects on host cell viability. Biochanin A methylated hydroxyl group provides improved the antichlamydial activity. Biochanin A does not affect C. pneumoniae in its extracellular (EB) form. Oromucosal buccal dosage forms improve dissolution of biochanin A and allow for permeation of porcine buccal tissue. | [55] |
| Polyphenols: Quercetin, Luteolin, Rhamnetin, Octyl gallate Coadministrants: Doxycycline, Verapamil (Ca2+), Isradipine (Ca2+), Thapsigargin (Ca2+) | C. pneumoniae (CWL-029) | In vitro Post-inoculation: administered at 0 h p.i. | Quercetin, luteolin, rhamnetin and octyl gallate did not improve the antichlamydial effect of doxycycline. Some coadministration combinations of Ca2+ modulators with phenolic compounds resulted in potentiation of the antichlamydial effect of phenolic compounds. More antagonistic combinations were found than synergic or additive combinations. | [56] |
| Polyphenols: Resveratrol, Quercetin Coadministrants: Clarithromycin, Ofloxacin | C. pneumoniae (CWL-029) | In vitro Pre-inoculation: incubated with cells for 24 h prior to inoculation. | Resveratrol at 40 μM and quercetin at 20 μM exhibited significant growth inhibition in presence of clarithromycin or ofloxacin compared to controls. Immunomodulatory effects via strong inhibition of the IL-23 levels with coadministration of resveratrol or quercetin and ofloxacin or clarithromycin. | [57] |
| Antimicrobial Agent | Chlamydial Species | Study Design | Effects | Reference |
|---|---|---|---|---|
| Caprylic acid, Capric acid, Lauric acid, Myristic acid, Palmitoleic acid, Oleic acid, 1-monoglyceride of each fatty acid | C. trachomatis (K) | In vitro Pre-treatment: incubated with EBs for 1, 5, 10, or 120 min prior to inoculation. | Lauric acid, capric acid, and monocaprin caused >10,000-fold reduction in the infectivity titer. Monocaprin was the most active, with >100,000-fold inactivation of C. trachomatis at a concentration of 5 mM for 5 min. Results indicate that bacteria are killed by disrupting membranes of chlamydial elementary bodies. | [60] |
| 1-O-octyl-, 2-O-octyl-, 1-O-heptyl-, 1-O-hexyl-, 2-O-hexyl-sn-glycerol | C. trachomatis (D), (F) | In vitro Pre-treatment: incubated with EBs for 0, 30, 60, 90, or 120 min prior to inoculation. | 2-O-octyl-sn-glycerol, at 7.5 mM, completely prevented growth of C. trachomatis after 120 min of contact with the organism. The lipids were shown to have disrupted the chlamydial inner membrane, allowing leakage of the cytoplasmic contents from the cell. | [62] |
| Lipidic compound: 3-O-octyl-sn-glycerol Coadministrant: Antimicrobial peptide (WLBU2) | C. trachomatis (D), (E), (L2) | In vitro Pre-treatment: incubated with EBs for 5 or 120 min prior to inoculation. | 3-O-octyl-sn-glycerol, at 6.25 mM, was 100% inhibitory after 5 min of exposure. Coadministration with WLBU2 produces significantly increased activity. 3-O-octyl-sn-glycerol could be used at up to 30 mM without causing toxicity. | [63] |
| Hinokitiol | C. trachomatis (D) | In vitro Pre-treatment: incubated with EBs for 1 h prior to inoculation. Post-inoculation: administered at 0 h p.i. | Hinokitiol, was shown to have an MIC and minimum lethal concentration (MLC) of 32 μg/mL. High concentrations of hinokitiol have been shown to be cytotoxic. | [64] |
| Antimicrobial Agent | Chlamydial Species | Study Design | Effects | Reference |
|---|---|---|---|---|
| Aqueous protein extract from mycorrhizal fungi (Terfezia claveryi) | C. trachomatis | In vivo Clinical treatment: administered to infected patients. (humans) | Sterilized aqueous T. claveryi extracts of were found to be effective, although slower acting than conventional antibiotic treatment. Partially purified proteins extracted from the aqueous T. claveryi extract were more effective. | [65] |
| Peptides: Human defensin HNP-2, Porcine protegrin PG-1 | C. trachomatis (D), (H1), (L2) | In vitro Pre-treatment: incubated with EBs for 2 h prior to inoculation. | Both HNP-2 and PG-1 inhibited chlamydial infection, but HNP-2 was the most potent. PG-1-treated EBs exhibited morphological changes, membrane damage, and loss of cytoplasmic contents. | [66] |
| Peptide: Melittin | C. trachomatis (E) | In vitro Pre-treatment: incubated with EBs for 24 h prior to inoculation. | C. trachomatis inhibition after the introduction of recombinant plasmid vectors expressing the melittin gene. Main mechanism is its direct cytotoxic effect. Secondary mechanism is lowering the transmembrane potential of a transfected cell, which disturbs chlamydial adhesion to the cell. | [67] |
| Peptide: Melittin | C. trachomatis (D) | In vivo Pre- & Post-inoculation: administered 1 day prior to inoculation; administered at 14 days p.i. | Vaginal administration and induction of melittin gene transcription with doxycycline inhibited subsequent infection in mice. Half of the mice were free from infection within 3–4 weeks. | [68] |
| Peptides: Cecropin D2A21, Cecropin D4E1 | C. trachomatis (D), (F) | In vitro Pre-treatment: incubated with EBs for 0, 5, 30, 60, 90, or 120 min prior to inoculation. Post-inoculation: administered at 0 h p.i. | D2A21, was shown to have a minimum cidal concentration (MCC) of 5 μM (18.32 μg/mL), and D4E1, an MCC of 7.5 μM (21.69 μg/mL). A 2% D2A21 gel formulation had an MCC of 0.2 mM (0.7 mg/mL). D2A21 incubation for 90 min caused chlamydial EB membranes to rupture causing the leaking of cytoplasm. | [69] |
| Peptide: WLBU2 Coadministrant: 3-O-octyl-sn-glycerol (3-OG) | C. trachomatis (D), (E), (L2) | In vitro Pre-treatment: incubated with EBs for 5 or 120 min prior to inoculation. | WLBU2, at 50 μM, was 89% inhibitory after 5 min of exposure, and 100% after 120 min. Coadministration with 3-OG produces significantly increased activity. WLBU2 could be used at up to 60 μM without causing toxicity. | [63] |
| Peptides: Protegrin-1, RTD-1, Cryptdin-4, Indolicidin | C. trachomatis (E), (L2), (MoPn) | In vitro Pre-treatment: incubated with EBs for 2 h prior to inoculation. | Protegrin-1 was found to have the strongest antichlamydial activity. Protegrin-1 inhibited the infectivity of the L2 serovar by 50% at a concentration (inhibitory concentration: IC50) of 6 μg/mL. Interaction between specific peptides and the various isolates tested appears to be complex and remarkably specific. Protegrins may have a broader antimicrobial activity than defensins. | [73] |
| Peptides: Full-length β-sheet (×13), Truncated protegrins (×7), PG-1 disulfide variants (×7), α-Helical peptides (×12), Circular peptides (×6) | C. trachomatis (D), (E), (L2) | In vitro Pre-treatment: incubated with EBs for 2 h prior to inoculation. | β-Sheet protegrins and α-helical peptides were equally active. Enantiomers were as active as native structures. Moderate-sized circular mini-defensins were less effective against C. trachomatis. Moderate-sized cationic peptides may be useful in microbicide preparations designed to prevent chlamydial infection. | [74] |
| Cathelicidin peptides: SMAP-29 (sheep), LL-37 (humans), BMAP-27 (cattle), BMAP-28 (cattle), BAC-7 (cattle), PG-1 (pigs) | C. trachomatis (A, D, E, H, I, LGV2) C. pneumoniae (IOL-207, CM-1) C. felis C. abortus C. psittaci C. pecorum | In vitro Pre-treatment: incubated with EBs for 2 h prior to inoculation. | SMAP-29 was most active, C. trachomatis inhibition by >50% at 10 μg/mL, with BMAP-27, BMAP-28, and BAC-7, >50% at 80 μg/mL. SMAP-29 also active against C. pneumoniae and C. felis. C. pneumoniae strains were less susceptible to peptides than C. trachomatis. Most animal chlamydiae were not sensitive to cathelicidins at concentrations of around 10–80 μg/mL. PG-1 at 80 μg/mL resulted in an increase in the number of inclusions in some animal chlamydial species. | [75] |
| Cathelicidin peptides: SMAP-29 (sheep), LL-37 (humans), BMAP-27 (cattle), BMAP-28 (cattle), BAC- 7 (cattle), PG-1 (pigs) | C. suis (MS04), (MS06 1–8) | In vitro Pre-treatment: incubated with EBs for 2 h prior to inoculation. | SMAP-29 was the most effective, six of the nine isolates, inhibition by >50% at 10 μg/mL (~3 μM). BAC-7 and BMAP-27, six of the nine isolates, inhibition by >50% at 80 μg/mL (~25 μM). LL-37 and PG-1 did not show any antichlamydial activity at 80 μg/mL. | [76] |
| Cathelicidin peptides: PG-1 (pigs) | C. abortus (S26/3) | In vitro Pre-treatment: incubated with EBs for 2 h prior to inoculation. | PG-1-pretreated cells, resulted in a ×8 increase in the number of inclusions. PG-1 treatment after chlamydial infection had no increase in infectivity. Experiments demonstrated that PG-1 pretreatment facilitates the entry of C. abortus into host cells. | [77] |
| Dermaseptin peptides: S4 D4D20S4 K4K20S4 S4 (5–28) S4 (1–12) | C. trachomatis (E) | In vitro Pre-treatment: incubated with EBs for 1 h prior to inoculation. Pre-inoculation: incubated with cells for 1 h prior to inoculation. Co-inoculation: inoculated cells simultaneously with EBs. Post-inoculation: administered at 0 h p.i. | S4, 81% inhibition after 48 h at 5 μg/mL. K4K20S4, 96% inhibition after 48 h at 5 μg/mL. 50% cytotoxic concentrations were determined to be higher than 25 μg/mL for each peptide, except for S4 at 10 μg/mL. Increasing the number of peptide positive charges reduced cytotoxicity. | [78] |
| Transferrins: Ovotransferrin, Human lactoferrin, Bovine lactoferrin | C. psittaci (D) | In vitro Pre-treatment: incubated with EBs for 1 h prior to inoculation. Post-inoculation: administered at 3 h p.i. | Ovotransferrin, pre-incubation, at 0.5–5 mg/mL, prior to infecting BGM cells significantly lowered infection rates. Ovotransferrin was more effective than human and bovine lactoferrin in inhibiting bacterial irreversible attachment and cell entry. | [80] |
| Transferrin: Ovotransferrin | C. psittaci (D) | In vivo Pre-inoculation: one dose administered pre-inoculation. Pre- & Post-inoculation: one dose administered pre-inoculation; administered daily for 12 days p.i. (turkeys) | A single pre-infection dose of 10 mg or a daily dose of 5 mg did not prevent turkeys from becoming infected with C. psittaci. Treatment significantly reduced the severity of infection. | [81] |
| Transferrin: Ovotransferrin | C. psittaci (D), (F), (E/B) | In vivo Prophylaxis: from 2 weeks old, administered daily for 12 days. (turkeys) | A daily 5 mg dose for 12 days prevented any symptoms of C. psittaci infection in turkeys. Respiratory disease occurred at 9 weeks although, overall treatment was associated with 46% reduction of mortality. | [82] |
| Antimicrobial Agent | Chlamydial Species | Study Design | Effects | Reference |
|---|---|---|---|---|
| Nitric oxide | C. pneumoniae | In vitro Pre-treatment: incubated with EBs for 2 h prior to inoculation. Co-inoculation: inoculated cells simultaneously with EBs. | Increases in nitric oxide (NO) concentration resulted in chlamydial inhibition in a dose-dependent manner. Immune control of chlamydial infections may trigger NO production. | [83] |
| Enterococcus faecium | C. suis | In vivo Prophylaxis: from 24 days after mating, administered daily for 13 weeks for sows & 8 weeks for piglets. (pigs) | Swine consuming E. faecium for 13 weeks before and 8 weeks after giving birth, reduced the rate of infected piglets from 85% to 60%. The appearance of infection was also delayed. | [85] |
| L. brevis, L. salivarius | C. trachomatis (L2) | In vitro Pre-treatment: incubated with EBs for 1 h prior to inoculation. Co-inoculation: inoculated cells simultaneously with EBs for 1 h. Post-inoculation: administered at 0 h p.i. | L. brevis was significantly more effective than L. salivarius. Both lactobacilli had an adverse effect on chlamydial EBs, on chlamydial adsorption to epithelial cells, and on intracellular phases of chlamydial replication. L. brevis inhibited HSV-2-induced C. trachomatis persistence. | [86] |
| L. crispatus (×2), L. gasseri, L. jensenii | C. trachomatis (D), (L2) | In vitro Pre-treatment: incubated with EBs for 1 h prior to inoculation. | Lactobacillus-conditioned media from each of the lactobacillus strains exhibited similar inhibitory activity. Acidic pH due to lactic acid production was attributed to chlamydial inhibition. Levels of H2O2 present did not produce chlamydial inhibition. | [87] |
| L. crispatus | C. trachomatis (D) | In vitro Pre-inoculation: incubated with cells for 6 h prior to inoculation. | L. crispatus inhibits the adhesion of chlamydial cells to human epithelial cells or macrophages, and inhibited C. trachomatis infectivity. Modulation of inflammatory cytokines, IL-6, IL-8, and TNF-α, and anti-inflammatory cytokine, IL-10, was observed. | [88] |
| Bacteria L. crispatus (×8), L. gasseri (×6), L. vaginalis (×3) Cellular Metabolite Lactic acid | C. trachomatis (D) | In vitro Pre-treatment: incubated with EBs for 7, 15, or 60 min prior to inoculation. | L. crispatus exhibited highest efficacy although all lactobacilli exerted a strong inhibitory effect. Activity corresponds to increased cellular metabolites and a resulting lower pH. Both lactic acid and acidic conditions were necessary for inhibition. Lactobacilli supernatants exhibited greater inhibition than only lactic acid. | [89] |
| Antimicrobial Agent | Chlamydial Species | Study Design | Effects | References |
|---|---|---|---|---|
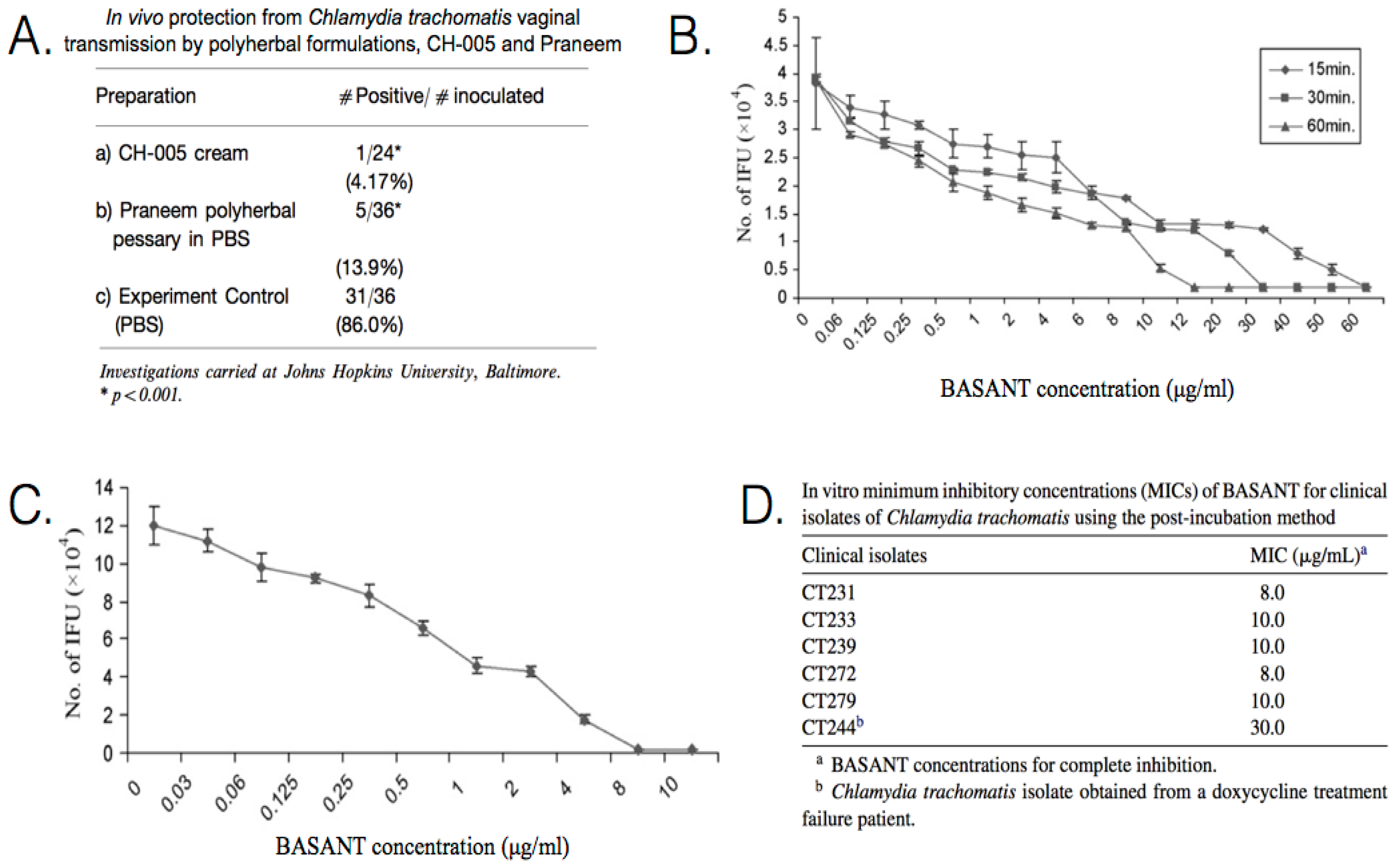
| Praneem: S. mukerossi saponins, A. indica seed extract, Quinine hydrochloride | C. trachomatis (D) | In vivo Pre-inoculation: one dose administered prior to inoculation. (mice) Clinical treatment: administered daily for 7 days. (human) | Application of 5 mL of cream for 8 days, resulted in C. trachomatis being cleared from the cervicovaginal region of patients. Topical application is effective in blocking chlamydial vaginal transmission, with a transmission rate of only ~14%. Toxicity studies indicate a lack of side effects, such as skin irritation or sensitization. | [90,91,92] |
| CH-005: S. mukerossi saponins, M. citrata oil, Natural polycationic polymer | C. trachomatis (D) | In vivo Pre-inoculation: one dose administered prior to inoculation. (mice) | Topical application is effective in blocking chlamydial vaginal transmission with a transmission rate of only ~4%. | [91] |
| BASANT: S. mukerossi saponins, A. vera, P. emblica, curcumin | C. trachomatis (D) | In vitro Pre-treatment: incubated with EBs for 15, 30, or 60 min prior to inoculation. Post-inoculation: administered at 2 h p.i. | In vitro pre-incubation exposure, 100% inhibition was achieved in 15 min at 65 μg/mL, 30 min at 35 μg/mL, and 60 min at 15 μg/mL. In vitro post-incubation exposure, the MIC was determined to be ~9 μg/mL. There are no known side effects of BASANT, which is equally effective as a cream or tablet. | [93,94] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, M.A.; Potroz, M.G.; Teh, S.-W.; Cho, N.-J. Natural Products for the Treatment of Chlamydiaceae Infections. Microorganisms 2016, 4, 39. https://doi.org/10.3390/microorganisms4040039
Brown MA, Potroz MG, Teh S-W, Cho N-J. Natural Products for the Treatment of Chlamydiaceae Infections. Microorganisms. 2016; 4(4):39. https://doi.org/10.3390/microorganisms4040039
Chicago/Turabian StyleBrown, Mika A., Michael G. Potroz, Seoh-Wei Teh, and Nam-Joon Cho. 2016. "Natural Products for the Treatment of Chlamydiaceae Infections" Microorganisms 4, no. 4: 39. https://doi.org/10.3390/microorganisms4040039
APA StyleBrown, M. A., Potroz, M. G., Teh, S.-W., & Cho, N.-J. (2016). Natural Products for the Treatment of Chlamydiaceae Infections. Microorganisms, 4(4), 39. https://doi.org/10.3390/microorganisms4040039