Prevalence and Genetic Characteristics of Avian Chlamydia in Birds in Guangxi, Southwestern China
Abstract
1. Introduction
2. Materials and Methods
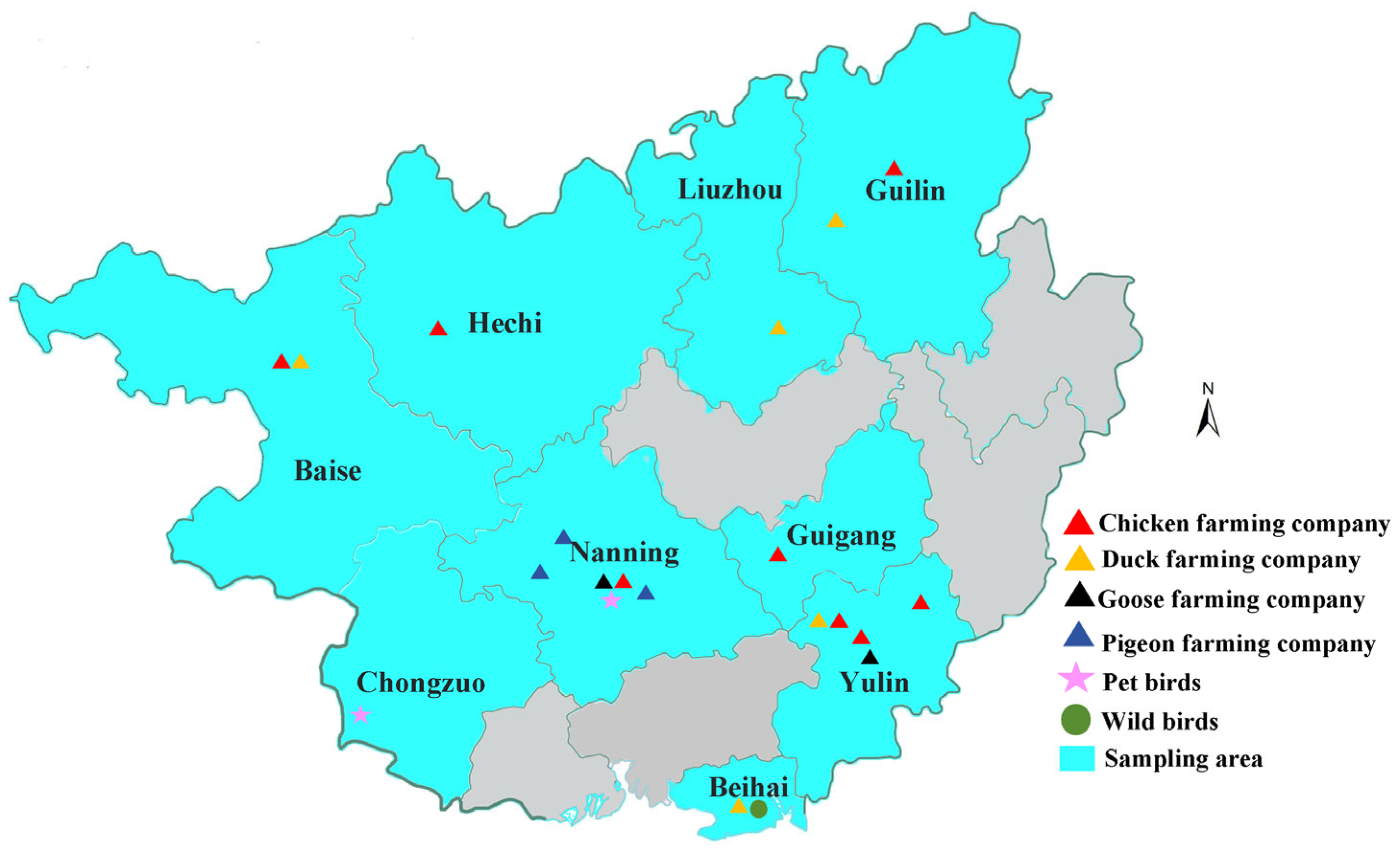
2.1. Sample Collection
2.1.1. Sampling of Poultry Farms
2.1.2. Sampling of Pet Birds
2.1.3. Sampling of Wild Birds
2.2. DNA Extraction and Avian Chlamydia Detection
2.3. Phylogenetic and Sequence Analysis
2.4. Ethics Statement
2.5. Statistical Analysis
3. Results
3.1. Prevalence of Avian Chlamydia in Guangxi
3.2. Genetic Characteristics of the Positive Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sachse, K.; Laroucau, K.; Vanrompay, D. Avian chlamydiosis. Curr. Clin. Microbiol. Rep. 2015, 2, 10–21. [Google Scholar] [CrossRef]
- Kaleta, E.F.; Taday, E.M.A. Avian host range of Chlamydophila spp. Based on isolation, antigen detection and serology. Avian Pathol. 2003, 32, 435–462. [Google Scholar] [CrossRef]
- Knittler, M.R.; Sachse, K. Chlamydia psittaci: Update on an underestimated zoonotic agent. Pathog. Dis. 2014, 73, 1–15. [Google Scholar] [CrossRef]
- Ravichandran, K.; Anbazhagan, S.; Karthik, K.; Angappan, M.; Dhayananth, B. A comprehensive review on avian chlamydiosis: A neglected zoonotic disease. Trop. Anim. Health Prod. 2021, 53, 414. [Google Scholar] [CrossRef]
- Stokes, H.S.; Berg, M.L.; Bennett, A.T.D. A review of chlamydial infections in wild birds. Pathogens 2021, 10, 948. [Google Scholar] [CrossRef]
- Vanrompay, D.; Ducatelle, R.; Haesebrouck, F. Chlamydia psittaci infections: A review with emphasis on avian chlamydiosis. Vet. Microbiol. 1995, 45, 93–119. [Google Scholar] [CrossRef] [PubMed]
- Longbottom, D.; Coulter, L.J. Animal chlamydioses and zoonotic implications. J. Comp. Pathol. 2003, 128, 217–244. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, A.; Wei, X.; Zhang, Z.; Bi, X.; Yuan, X.; Geng, Y.; He, N.; Chen, M.; Xu, X.; et al. Metagenomic next-generation sequencing identified psittacosis among poultry processing workers in Shandong Province, China. Infect. Med. 2022, 1, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Feng, Y.; Zhang, Z.; Wu, S.; Zhong, D.; Liu, C. Prevalence and genotype of Chlamydia psittaci in faecal samples of birds from zoos and pet markets in Kunming, Yunnan, China. J. Zhejiang Univ. B Sci. 2016, 17, 311–316. [Google Scholar] [CrossRef]
- Hogerwerf, L.; Roof, I.; de Jong, M.J.K.; Dijkstra, F.; van der Hoek, W. Animal sources for zoonotic transmission of psittacosis: A systematic review. BMC Infect. Dis. 2020, 20, 192. [Google Scholar] [CrossRef]
- Vanrompay, D.; Butaye, P.; Sayada, C.; Ducatelle, R.; Haesebrouck, F. Characterization of avian Chlamydia psittaci strains using ompl restriction mapping and serovar-specific monoclonal antibodies. Comp. Study 1997, 14, 327–333. [Google Scholar]
- Geens, T.; Desplanques, A.; Van Loock, M.; Bonner, B.M.; Kaleta, E.F.; Magnino, S.; Andersen, A.A.; Everett, K.D.; Vanrompay, D. Sequencing of the chlamydophila psittaci ompA gene reveals a new genotype, E/B, and the need for a rapid discriminatory genotyping method. Microbiol. J. Clin. 2005, 5, 2456–2461. [Google Scholar] [CrossRef]
- Harkinezhad, T.; Geens, T.; Vanrompay, D. Chlamydophila psittaci infections in birds: A review with emphasis on zoonotic consequences. Vet. Microbiol. 2009, 135, 68–77. [Google Scholar]
- Sachse, K.; Laroucau, K.; Hotzel, H.; Schubert, E.; Ehricht, R.; Slickers, P. Genotyping of Chlamydophila psittaci using a new DNA microarray. BMC Microbiol. 2008, 8, 63. [Google Scholar] [CrossRef]
- Sachse, K.; Laroucau, K.; Riege, K.; Wehner, S.; Dilcher, M.; Creasy, H.H.; Weidmann, M.; Myers, G.; Vorimore, F.; Vicari, N.; et al. Evidence for the existence of two new members of the family Chlamydiaceae and proposal of Chlamydia avium sp. Nov. and Chlamydia gallinacea sp. Nov. Syst. Appl. Microbiol. 2014, 37, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, P.; Hou, J.; Xu, G.; Zhang, J.; Lei, Y.; Lou, Z.; Liang, L.; Wen, Y.; Zhou, J. Detection of Chlamydia psittaci and Chlamydia ibidis in the Endangered Crested Ibis (Nipponia nippon). Epidemiol. Infect. 2020, 148, e1. [Google Scholar] [CrossRef]
- Laroucau, K.; Vorimore, F.; Aaziz, R.; Solmonson, L.; Hsia, R.C.; Bavoil, P.M.; Fach, P.; Hölzer, M.; Wuenschmann, A.; Sachse, K. Chlamydia buteonis, a new Chlamydia species isolated from a red-shouldered hawk. Syst. Appl. Microbiol. 2019, 5, 125997. [Google Scholar] [CrossRef]
- Laroucau, K.; Vorimore, F.; Aaziz, R.; Berndt, A.; Schubert, E.; Sachse, K. Isolation of a new chlamydial agent from infected domestic poultry coincided with cases of atypical pneumonia among slaughterhouse workers in France. Infect. Genet. Evol. 2009, 9, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Hulin, V.; Oger, S.; Vorimore, F.; Aaziz, R.; de Barbeyrac, B.; Berruchon, J.; Sachse, K.; Laroucau, K. Host preference and zoonotic potential of Chlamydia psittaci and C. Gallinacea in poultry. Pathog. Dis. 2015, 73, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, J.; Kaltenboeck, B.; Gong, J.; Fan, W.; Wang, C. Chlamydia gallinacea, not C. Psittaci, is the endemic chlamydial species in chicken (Gallus gallus). Sci. Rep. 2016, 6, 19638. [Google Scholar] [CrossRef]
- Stokes, H.S.; Martens, J.M.; Chamings, A.; Walder, K.; Berg, M.L.; Segal, Y.; Bennett, A. Identification of Chlamydia gallinacea in a parrot and in free-range chickens in Australia. Case Rep. 2019, 10, 398–400. [Google Scholar] [CrossRef]
- Ospina-Pinto, M.C.; Alves, B.F.; Soares, H.S.; Jesus Pena, H.F.; Raso, T.F. Chlamydia gallinacea in Brazilian backyard chicken farms. Braz. J. Microbiol. 2024, 55, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Donati, M.; Laroucau, K.; Guerrini, A.; Balboni, A.; Salvatore, D.; Catelli, E.; Lupini, C.; Levi, A.; Di Francesco, A. Chlamydiosis in Backyard Chickens (Gallus gallus) in Italy. Vector-Borne Zoonotic Dis. 2018, 18, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Heijne, M.; van der Goot, J.A.; Fijten, H.; van der Giessen, J.W.; Kuijt, E.; Maassen, C.B.M.; van Roon, A.; Wit, B.; Koets, A.P.; Roest, H.I.J. A cross sectional study on Dutch layer farms to investigate the prevalence and potential risk factors for different Chlamydia species. PLoS ONE 2018, 13, e190774. [Google Scholar] [CrossRef]
- Li, L.; Luther, M.; Macklin, K.; Pugh, D.; Li, J.; Zhang, J.; Roberts, J.; Kaltenboeck, B.; Wang, C. Chlamydia gallinacea: A widespread emerging Chlamydia agent with zoonotic potential in backyard poultry. Epidemiol. Infect. 2017, 145, 2701–2703. [Google Scholar] [CrossRef] [PubMed]
- Stokes, H.S.; Martens, J.M.; Jelocnik, M.; Walder, K.; Segal, Y.; Berg, M.L.; Bennett, A.T.D. Chlamydial diversity and predictors of infection in a wild Australian parrot, the Crimson Rosella (Platycercus elegans). Transbound. Emerg. Dis. 2021, 68, 487–498. [Google Scholar] [CrossRef]
- Stokes, H.S.; Martens, J.M.; Walder, K.; Segal, Y.; Berg, M.L.; Bennett, A.T.D. Species, sex and geographic variation in chlamydial prevalence in abundant wild Australian parrots. Sci. Rep. 2020, 10, 20478. [Google Scholar] [CrossRef]
- Gasparini, J.; Erin, N.; Bertin, C.; Jacquin, L.; Vorimore, F.; Frantz, A.; Lenouvel, P.; Laroucau, K. Impact of urban environment and host phenotype on the epidemiology of Chlamydiaceae in feral pigeons (Columba livia). Environ. Microbiol. 2011, 13, 3186–3193. [Google Scholar] [CrossRef]
- Sachse, K.; Kuehlewind, S.; Ruettger, A.; Schubert, E.; Rohde, G. More than classical Chlamydia psittaci in urban pigeons. Vet. Microbiol. 2012, 157, 476–480. [Google Scholar] [CrossRef]
- Pisanu, B.; Laroucau, K.; Aaziz, R.; Vorimore, F.; Le Gros, A.; Chapuis, J.; Clergeau, P. Chlamydia avium detection from a ring-necked parakeet (Psittacula krameri) in France. J. Exot. Pet Med. 2018, 27, 68–74. [Google Scholar] [CrossRef]
- Vorimore, F.; Thébault, A.; Poisson, S.; Cléva, D.; Robineau, J.; de Barbeyrac, B.; Durand, B.; Laroucau, K. Chlamydia psittaci in ducks: A hidden health risk for poultry workers. Pathog. Dis. 2014, 73, 1–9. [Google Scholar] [CrossRef]
- Kalmar, I.D.; Dicxk, V.; Dossche, L.; Vanrompay, D. Zoonotic infection with Chlamydia psittaci at an avian refuge centre. Vet. J. 2014, 199, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Laroucau, K.; Aaziz, R.; Meurice, L.; Servas, V.; Chossat, I.; Royer, H.; de Barbeyrac, B.; Vaillant, V.; Moyen, J.L.; Meziani, F.; et al. Outbreak of psittacosis in a group of women exposed to Chlamydia psittaci- infected chickens. Euro Surveill. Bull. Eur. Mal. Transm. 2015, 20, 1. [Google Scholar] [CrossRef]
- Pantchev, A.; Sting, R.; Bauerfeind, R.; Tyczka, J.; Sachse, K. New real-time PCR tests for species-specific detection of Chlamydophila psittaci and Chlamydophila abortus from tissue samples. Vet. J. 2009, 181, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Geens, T.; Dewitte, A.; Boon, N.; Vanrompay, D. Development of a Chlamydophila psittaci species-specific and genotype-specific real-time PCR. Vet. Res. 2005, 36, 787–797. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhong, H. Establishment and Comparison of Three PCR Methods for Avian Chlamydia and Its Molecular Prevalence Investigation in Some Regions of Guangxi During 2021–2023. Master’s Thesis, Guangxi University, Nanning, China, 2024. [Google Scholar]
- Heddema, E.R.; van Hannen, E.J.; Duim, B.; Vandenbroucke-Grauls, C.M.; Pannekoek, Y. Genotyping of Chlamydophila psittaci in human samples. Emerg. Infect. Dis. 2006, 12, 1989–1990. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Sukon, P.; Nam, N.H.; Kittipreeya, P.; Sara-in, A.; Wawilai, P.; Inchuai, R.; Weerakhun, S. Global prevalence of chlamydial infections in birds: A systematic review and meta-analysis. Prev. Vet. Med. 2021, 192, 105370. [Google Scholar] [CrossRef]
- De Meyst, A.; De Clercq, P.; Porrez, J.; Geens, T.; Braeckman, L.; Ouburg, S.; Morré, S.A.; Vanrompay, D. Belgian cross-sectional epidemiological study on zoonotic avian chlamydia spp. in chickens. Microorganisms 2024, 12, 193. [Google Scholar] [CrossRef]
- Solorzano-Morales, A.; Dolz, G. Molecular characterization of Chlamydia species in commercial and backyard poultry farms in Costa Rica. Epidemiol. Infect. 2022, 150, e67. [Google Scholar] [CrossRef]
- Yin, L.; Kalmar, I.D.; Lagae, S.; Vandendriessche, S.; Vanderhaeghen, W.; Butaye, P.; Cox, E.; Vanrompay, D. Emerging Chlamydia psittaci infections in the chicken industry and pathology of Chlamydia psittaci genotype B and D strains in specific pathogen free chickens. Vet. Microbiol. 2013, 162, 740–749. [Google Scholar] [CrossRef]
- Čechová, L.; Halánová, M.; Babinská, I.; Danišová, O.; Bartkovský, M.; Marcinčák, S.; Marcinčáková, D.; Valenčáková, A.; Čisláková, L. Chlamydiosis in farmed chickens in Slovakia and zoonotic risk for humans. Ann. Agric. Env. Med. 2018, 25, 320–325. [Google Scholar] [CrossRef]
- Szymańska-Czerwińska, M.; Mitura, A.; Niemczuk, K.; Zaręba, K.; Jodełko, A.; Pluta, A.; Scharf, S.; Vitek, B.; Aaziz, R.; Vorimore, F.; et al. Dissemination and genetic diversity of chlamydial agents in Polish wildfowl: Isolation and molecular characterisation of avian Chlamydia abortus strains. PLoS ONE 2017, 12, e174599. [Google Scholar] [CrossRef]
- You, J.; Wu, Y.; Zhang, X.; Wang, X.; Gong, J.; Zhao, Z.; Zhang, J.; Zhang, J.; Sun, Z.; Li, J.; et al. Efficient fecal-oral and possible vertical, but not respiratory, transmission of emerging Chlamydia gallinacea in broilers. Vet. Microbiol. 2019, 230, 90–94. [Google Scholar] [CrossRef]
- Zhang, F.; Li, S.; Yang, J.; Pang, W.; Yang, L.; He, C. Isolation of Chlamydophila psittaci from Laying Hens in China. Avian Dis. 2008, 1, 74–78. [Google Scholar] [CrossRef]
- Hou, L.; Jia, J.; Qin, X.; Fang, M.; Liang, S.; Deng, J.; Pan, B.; Zhang, X.; Wang, B.; Mao, C.; et al. Prevalence and genotypes of Chlamydia psittaci in birds and related workers in three cities of China. PLoS ONE 2024, 19, e308532. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, K.; Hsieh, M.; Chang, P.; Shien, J.; Ou, S. Prevalence and genotyping of Chlamydia psittaci from domestic waterfowl, companion birds, and wild birds in Taiwan. Vector-Borne Zoonotic Dis. 2019, 19, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Chen, T.; Liao, L.; Wang, Z.; Xiao, J.; Lu, J.; Song, C.; Qin, J.; Chen, F.; Chang, Y.F.; et al. A parrot-type Chlamydia psittaci strain is in association with egg production drop in laying ducks. Transbound. Emerg. Dis. 2019, 66, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Le Gall-Ladevèze, C.; Vollot, B.; Hirschinger, J.; Lèbre, L.; Aaziz, R.; Laroucau, K.; Guérin, J.; Paul, M.; Cappelle, J.; Le Loc’h, G. Limited transmission of avian influenza viruses, avulaviruses, coronaviruses and Chlamydia sp. at the interface between wild birds and a free-range duck farm. Vet. Res. 2025, 56, 36. [Google Scholar] [CrossRef]
- Abdu, A.M.; Osman, K.M.; Ali, H.A. Detection and PCR-Based diagnosis of Chlamydia psittaci isolated from some avian species in Egypt. Ann. Rom. Soc. Cell Biol. 2021, 25, 5009–5022. [Google Scholar]
- Cong, W.; Huang, S.Y.; Zhang, X.Y.; Zhou, D.H.; Xu, M.J.; Zhao, Q.; Song, H.Q.; Zhu, X.Q.; Qian, A.D. Seroprevalence of Chlamydia psittaci infection in market-sold adult chickens, ducks and pigeons in north-western China. J. Med. Microbiol. 2013, 62, 1211–1214. [Google Scholar] [CrossRef]
- Li, M.; Yang, B.; Yin, Z.; Wang, W.; Zhao, Q.; Jiang, J. A seroepidemiological survey of Toxoplasma gondii and Chlamydia infection in chickens, ducks, and geese in Jilin province, northeastern China. Vector-Borne Zoonotic Dis. 2020, 20, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Soon, X.Q.; Gartrell, B.; Gedye, K. Presence and shedding of Chlamydia psittaci in waterfowl in a rehabilitation facility and in the wild in New Zealand. N. Z. Vet. J. 2021, 69, 240–246. [Google Scholar] [CrossRef]
- Laroucau, K.; de Barbeyrac, B.; Vorimore, F.; Clerc, M.; Bertin, C.; Harkinezhad, T.; Verminnen, K.; Obeniche, F.; Capek, I.; Bébéar, C.; et al. Chlamydial infections in duck farms associated with human cases of psittacosis in France. Vet. Microbiol. 2009, 135, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.Q. Seroprevalence of Chlamydophila infection in chickens, ducks, geese and pigeons in Southern China. Afr. J. Microbiol. Res. 2011, 5, 4240–4242. [Google Scholar] [CrossRef]
- Dickx, V.; Kalmar, I.D.; Tavernier, P.; Vanrompay, D. Prevalence and genotype distribution of Chlamydia psittaci in feral Canada geese (Branta canadensis) in Belgium. Vector-Borne Zoonotic Dis. 2013, 13, 382–384. [Google Scholar] [CrossRef]
- Szymańska-Czerwińska, M.; Mitura, A.; Zaręba, K.; Schnee, C.; Koncicki, A.; Niemczuk, K. Poultry in Poland as Chlamydiaceae carrier. J. Vet. Res. 2017, 61, 411–419. [Google Scholar] [CrossRef]
- Qin, X.; Huang, J.; Yang, Z.; Sun, X.; Wang, W.; Gong, E.; Cao, Z.; Lin, J.; Qiu, Y.; Wen, B.; et al. Severe community-acquired pneumonia caused by Chlamydia psittaci genotype E/B strain circulating among geese in Lishui city, Zhejiang province, China. Emerg. Microbes Infect. 2022, 11, 2715–2723. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, H.; Cao, H.; Ji, J.; Zhang, R.; Li, W.; Guo, H.; Chen, L.; Ma, C.; Cui, M.; et al. Human-to-human transmission of Chlamydia psittaci in China, 2020: An epidemiological and aetiological investigation. Lancet Microbe 2022, 3, e512–e520. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, N.; Ma, C.; Zhang, X.; Zhao, Q.; Ni, H. Epidemiological investigation and genotype of Chlamydia exposure in pigeons in three provinces in northern China. Vector-Borne Zoonotic Dis. 2018, 18, 181–184. [Google Scholar] [CrossRef]
- Zhang, R.; Fu, H.; Luo, C.; Huang, Z.; Pei, R.; Di, Y.; Zhu, C.; Peng, J.; Hu, H.; Chen, S.; et al. Chlamydia psittaci detected at a live poultry wholesale market in central China. BMC Infect. Dis. 2024, 24, 585. [Google Scholar] [CrossRef] [PubMed]
- Teske, L.; Ryll, M.; Rubbenstroth, D.; Hanel, I.; Hartmann, M.; Kreienbrock, L.; Rautenschlein, S. Epidemiological investigations on the possible risk of distribution of zoonotic bacteria through apparently healthy homing pigeons. Avian Pathol. 2013, 42, 397–407. [Google Scholar] [CrossRef]
- Mattmann, P.; Marti, H.; Borel, N.; Jelocnik, M.; Albini, S.; Vogler, B.R. Chlamydiaceae in wild, feral and domestic pigeons in Switzerland and insight into population dynamics by Chlamydia psittaci multilocus sequence typing. PLoS ONE 2019, 14, e226088. [Google Scholar] [CrossRef] [PubMed]
- AL-sultani, S. Molecular detection and genotyping of chlamydia psittaci in domestic pigeons and human contacts in Baghdad city. Kerbala J. Vet. Med. Sci. 2025, 1, 165–171. [Google Scholar]
- Chen, W.; Teng, C.; Shih, C.; Huang, W.; Jiang, Y.; Chang, H.; Jeng, C.; Lai, Y.; Guo, J.; Wang, P.; et al. Investigation of lethal concurrent outbreak of chlamydiosis and pigeon circovirus in a zoo. Animals 2021, 11, 1654. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, T.; Pestka, D.; Choszcz, D. The prevalence and genetic characterization of Chlamydia psittaci from domestic and feral pigeons in Poland and the correlation between infection rate and incidence of pigeon circovirus. Poult. Sci. 2014, 93, 3009–3016. [Google Scholar] [CrossRef] [PubMed]
- Kik, M.; Heijne, M.; IJzer, J.; Grinwis, G.; Pannekoek, Y.; Gröne, A. Fatal Chlamydia avium Infection in Captive Picazuro Pigeons, the Netherlands. Emerg. Infect. Dis. 2020, 26, 2520–2522. [Google Scholar] [CrossRef]
- Floriano, A.M.; Rigamonti, S.; Comandatore, F.; Scaltriti, E.; Longbottom, D.; Livingstone, M.; Laroucau, K.; Gaffuri, A.; Pongolini, S.; Magnino, S.; et al. Complete genome sequence of Chlamydia avium PV 4360/2, isolated from a feral pigeon in Italy. Microbiol. Resour. Announc. 2020, 9, 10–1128. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, O.M.; Kang, S.I.; Yeo, Y.G.; Jeong, J.Y.; Kwon, Y.K.; Kang, M.S. Prevalence of asymptomatic infections of Chlamydia psittaci in psittacine birds in Korea. Zoonoses Public Health 2023, 70, 451–458. [Google Scholar] [CrossRef]
- Kabeya, H.; Sato, S.; Maruyama, S. Prevalence and characterization of Chlamydia DNA in zoo animals in Japan. Microbiol. Immunol. 2015, 59, 507–515. [Google Scholar] [CrossRef]
- De Meyst, A.; Aaziz, R.; Pex, J.; Braeckman, L.; Livingstone, M.; Longbottom, D.; Laroucau, K.; Vanrompay, D. Prevalence of new and established avian chlamydial species in humans and their psittacine pet birds in Belgium. Microorganisms 2022, 10, 1758. [Google Scholar] [CrossRef]
- Ko, J.C.K.; Choi, Y.W.Y.; Poon, E.S.K.; Wyre, N.; Go, J.L.L.; Poon, L.L.M.; Sin, S.Y.W. Prevalence and genotypes of Chlamydia psittaci in pet birds of Hong Kong. PLoS ONE 2024, 19, e306528. [Google Scholar] [CrossRef]
- Yang, J.; Ling, Y.; Yuan, J.; Pang, W.; He, C. Isolation and characterization of peacock Chlamydophila psittaci infection in China. Avian Dis. 2011, 55, 76–81. [Google Scholar] [CrossRef]
- Origlia, J.A.; Cadario, M.E.; Frutos, M.C.; Lopez, N.F.; Corva, S.; Unzaga, M.F.; Piscopo, M.V.; Cuffini, C.; Petruccelli, M.A. Detection and molecular characterization of Chlamydia psittaci and Chlamydia abortus in psittacine pet birds in Buenos Aires province, Argentina. Rev. Argent. Microbiol. 2019, 51, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Kasimov, V.; Dong, Y.; Shao, R.; Brunton, A.; Anstey, S.I.; Hall, C.; Chalmers, G.; Conroy, G.; Booth, R.; Timms, P.; et al. Emerging and well-characterized chlamydial infections detected in a wide range of wild Australian birds. Transbound. Emerg. Dis. 2022, 69, e3154–e3170. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; An, I.; Oem, J.; Wang, S.; Kim, Y.; Shin, J.; Woo, C.; Kim, Y.; Jo, S.; Son, K.; et al. Molecular prevalence and genotyping of Chlamydia spp. in wild birds from South Korea. J. Vet. Med. Sci. 2017, 79, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Stalder, S.; Marti, H.; Borel, N.; Mattmann, P.; Vogler, B.; Wolfrum, N.; Albini, S. Detection of Chlamydiaceae in Swiss wild birds sampled at a bird rehabilitation centre. Vet. Rec. Open 2020, 7, e000437. [Google Scholar] [CrossRef]

| Positive Sample | Source | Host | Location | GenBank No. |
|---|---|---|---|---|
| DF3 | Chicken farm-DF-A | Breeder chicken | Guilin | PV211947 |
| DF17 | Chicken farm-DF-A | Breeder chicken | Guilin | PV211948 |
| DF31 | Chicken farm-DF-B | Breeder chicken | Guilin | PV211949 |
| DL29 | Chicken farm-DL-A | Breeder chicken | Hechi | PV211950 |
| DL48 | Chicken farm-DL-B | Breeder chicken | Hechi | PV211951 |
| GF7 | Chicken farm-GF-A | Breeder chicken | Guigang | PV211959 |
| GF18 | Chicken farm-GF-B | Breeder chicken | Guigang | PV211960 |
| ZS5 | Chicken farm-ZS-A | Breeder chicken | Yulin | PV212007 |
| ZS43 | Chicken farm-ZS-B | Breeder chicken | Yulin | PV212008 |
| GLCJ19 | Chicken farm-GLC-A | Bioiler chcken | Yulin | PV211961 |
| GLCJ52 | Chicken farm-GLC-B | Bioiler chicken | Yulin | PV211962 |
| BSTLJ49 | Chicken farm-TL-A | Bioiler chicken | Baise | PV211943 |
| BSTLJ51 | Chicken farm-TL-B | Broiler chiken | Baise | PV211944 |
| NBB5 | Chicken farm-NBB-A | Layer chicken | Nanning | PV211976 |
| NBB32 | Chicken farm-NBB-B | Layer chicken | Nanning | PV211977 |
| NBB45 | Chicken farm-NBB-B | Layer chicken | Nanning | PV211978 |
| SH10 | Chicken farm-SH-A | Layer chicken | Yulin | PV212004 |
| SH22 | Chicken farm-SH-A | Layer chicken | Yulin | PV212005 |
| SH39 | Chicken farm-SH-B | Layer chicken | Yulin | PV212006 |
| GLLYY1 | Duck farm-LY-A | Breeder mallard duck | Guilin | PV211963 |
| GLLYY2 | Duck farm-LY-B | Breeder mallard duck | Guilin | PV211965 |
| GLLYY3 | Duck farm-LY-C | Breeder cherry valley duck | Guilin | PV211966 |
| BSTLY12 | Duck farm-TL-B | Meat duck | Baise | PV211945 |
| BSTLY9 | Duck farm-TL-A | Meat duck | Baise | PV211946 |
| GLCY41 | Duck farm-GLC-B | Meat duck | Yulin | PV211964 |
| LZY3 | Duck farm-LZ-A | Meat duck | Liuzhou | PV211973 |
| LZY37 | Duck farm-LZ-B | Meat duck | Liuzhou | PV211974 |
| LZY38 | Duck farm-LZ-C | Meat duck | Liuzhou | PV211975 |
| HPDY1 | Duck farm-HP-A | Layer duck | Beihai | PV211967 |
| HPDY2 | Duck farm-HP-B | Layer duck | Beihai | PV211968 |
| HPDY3 | Duck farm-HP-D | Layer duck | Beihai | PV211969 |
| LDE5 | Goose farm-LYLE-A | Meat goose | Nanning | PV211970 |
| LDE7 | Goose farm-LYLE-B | Meat goose | Nanning | PV211971 |
| LDE11 | Goose farm-LYLE-C | Meat goose | Nanning | PV211972 |
| NNE4 | Goose farm-NNST-B | Meat goose | Nanning | PV211979 |
| NNE12 | Goose farm-NNST-C | Meat goose | Nanning | PV211980 |
| NNG1 | Pigeon farm-HP-A | Meat pigeon | Nanning | PV211981 |
| NNG2 | Pigeon farm-HP-B | Meat pigeon | Nanning | PV211982 |
| NNG3 | Pigeon farm-LT-E | Meat pigeon | Nanning | PV211983 |
| NNG4 | Pigeon farm-XR-B | Meat pigeon | Nanning | PV211984 |
| AC5 | Pet market | Quail (Galliformes) | Chongzuo | PV211935 |
| AC9 | Pet market | Quail (Galliformes) | Chongzuo | PV211936 |
| AC22 | Pet market | Quail (Galliformes) | Chongzuo | PV211937 |
| PX3 | Pet market | Blue-breasted Quail (Galliformes) | Chongzuo | PV211985 |
| PX4 | Pet market | Budgerigar (Psittacidae) | Chongzuo | PV211986 |
| PX5 | Pet market | Budgerigar (Psittacidae) | Chongzuo | PV211987 |
| PX7 | Pet market | White Java Sparrow (Passeriformes) | Chongzuo | PV211988 |
| PX15 | Pet market | Blue-breasted Quail (Galliformes) | Chongzuo | PV211989 |
| PX17 | Pet market | Zebra Finch (Passeriformes) | Chongzuo | PV211990 |
| PX21 | Pet market | Antipodes Green Parakeet (Psittacidae) | Chongzuo | PV211991 |
| PX24 | Pet market | Budgerigar (Psittacidae) | Chongzuo | PV211992 |
| PX25 | Pet market | Budgerigar (Psittacidae) | Chongzuo | PV211993 |
| PX30 | Pet market | Budgerigar (Psittacidae) | Chongzuo | PV211994 |
| PX34 | Pet market | White Java Sparrow (Passeriformes) | Chongzuo | PV211995 |
| PX35 | Pet market | Budgerigar (Psittacidae) | Chongzuo | PV211996 |
| PX38 | Pet market | Antipodes Green Parakeet (Psittacidae) | Chongzuo | PV211997 |
| PX55 | Pet market | Budgerigar (Psittacidae) | Chongzuo | PV211998 |
| PX43 | Pet market | Zebra Finch (Passeriformes) | Chongzuo | PV211998 |
| PX59 | Pet market | Zebra Finch (Passeriformes) | Chongzuo | PV212000 |
| PX60 | Pet market | Budgerigar (Psittacidae) | Chongzuo | PV212001 |
| PX61 | Pet market | Zebra Finch (Passeriformes) | Chongzuo | PV212002 |
| PX66 | Pet market | Zebra Finch (Passeriformes) | Chongzuo | PV212003 |
| DWY9 | Zoo | Mandarin Duck (Anseriformes) | Nanning | PV211952 |
| DWY12 | Zoo | Swan (Anseriformes) | Nanning | PV211953 |
| DWY15 | Zoo | Swan (Anseriformes) | Nanning | PV211954 |
| DWY21 | Zoo | Pelican (Pelecaniformes) | Nanning | PV211955 |
| DWY42 | Zoo | Swan (Anseriformes) | Nanning | PV211956 |
| DWY46 | Zoo | Silver Pheasant (Galliformes) | Nanning | PV211957 |
| DWY47 | Zoo | Peafowl (Galliformes) | Nanning | PV211938 |
| BH2 | Pet hospital | Oriental Turtle-dove (Columbiformes) | Beihai | PV211939 |
| BH3 | Pet hospital | Besra (Falconiformes) | Beihai | PV211940 |
| BH5 | Pet hospital | Oriental Turtle-Dove (Columbiformes) | Beihai | PV211941 |
| BH6 | Pet hospital | Oriental Turtle-Dove (Columbiformes) | Beihai | PV211942 |
| BH14 | Pet hospital | Red Turtle Dove (Columbiformes) | Beihai | PV211938 |
| Source | Farm | Positivity Rate (95% CI, Positive Samples/Total Samples) | |
|---|---|---|---|
| Chicken b | Breeder chicken | 20.95% (95% CI, 16.70–25.94%, 62/296) | 25.05% (95% CI, 21.25–29.23%, 128/511) |
| Broiler chicken | 11% (95% CI, 6.25–18.63%, 11/100) | ||
| Layer chicken | 47.83% (95% CI, 38.92–56.88%, 55/115) | ||
| Duck c | Breeder duck | 7.67% (95% CI, 5.16–11.24%, 23/300) | 14.14% (95% CI, 11.57–17.26%, 82/580) |
| Meat duck | 13.89% (95% CI, 9.59–19.70%, 25/180) | ||
| Layer duck | 34% (95% CI, 25.46–43.72%, 34/100) | ||
| Goose bc | Breeder goose | 18.12% (95% CI, 12.93–24.82%, 29/160) | 18.12% (95% CI, 12.93–24.82%, 29/160) |
| Pigeon a | Meat pigeon | 62.30% (95% CI, 55.37–68.69%, 152/244) | 62.30% (95% CI, 55.37–68.69%, 152/244) |
| Total | 26.16% (95% CI, 23.98–28.45%, 391/1495) | ||
| Source | Order | Species | Positivity Rate (95% CI, Positive Samples/Total Samples) | |
|---|---|---|---|---|
| Zoo | Anseriformes b | Swan | 18.75% (95% CI: 10.00–31.23%, 9/48) | 19.61% (95% CI, 11.85–31.56%) 10/51) |
| Mandarin Duck | 33.33 (95% CI, 3.10–79.30%, 1/3) | |||
| Ciconiiformes b | White Stork | 0% (95% CI, 0–35%, 0/7) | 7.14% (95% CI, 1.26–31.47%, 1/14) | |
| Flamingo | 14.29% (95% CI, 3–51%, 1/7) | |||
| Galliformes a | Peafowl | 55.56% (95% CI, 26.57–81.23%, 5/9) | 63.64% (95% CI, 35.48–84.83%, 7/11) | |
| Silver pheasant | 100% (95% CI, 34.23–100%, 2/2) | |||
| Gruiformes ab | White Crane | 50% (95% CI, 9.46–90.54%, 1/2) | 22.22% (95% CI, 6.29–54.78%, 2/9) | |
| Red-crowned Crane | 14.29% (95% CI, 2.575–51.33%, 1/7) | |||
| Pelecaniformes ab | Pelican | 33.33% (95% CI, 7.29–80.14%, 1/3) | 33.33% (95% CI 7.29–80.14%, 1/3) | |
| Subtotal | 23.86% (95% CI, 16.17–33.74%, 21/88) | |||
| Pet market | Galliformes b | Blue-breasted Quail | 71.43% (95% CI, 35.90–91.81%, 5/7) | 32.26% (95% CI, 21.95–44.64%, 20/62) |
| Quail | 27.27% (95% CI, 17.28–40.23%, 15/55) | |||
| Passeriformes a | Zebra finch | 77.77% (95% CI, 45.24–93.69%, 7/9) | 76.19% (95% CI, 54.93–89.34%, 16/21) | |
| White Java sparrow | 75% (95% CI, 46.76–91.13%, 9/12) | |||
| Psittacidae a | Budgerigar | 58.90% (95% CI, 43.43–72.905, 23/39) | 61.40% (95% CI, 48.42–72.96%, 35/57) | |
| Antipodes Green Parakeet | 66.67% (95% CI, 43.76–83.73%, 12/18) | |||
| Subtotal | 50.71% (95% CI, 41.76–60.26%, 71/140) | |||
| Total | 40.35% (95% CI, 34.20–46.83%, 92/228) | |||
| Source | Order | Species | Positivity Rate (95% CI, Positive Samples/Total Samples) | |
|---|---|---|---|---|
| Pet hospital | Accipitriformes | Cinereous Vulture | 50% (95% CI, 9.46–90.53%, 1/2) | 50% (95% CI, 9.45–90.55% 1/2) |
| Caprimulgiformes | Savannah Nightjar | 0 (95% CI, 0–79.37%, 0/1) | 0 (0/1) | |
| Columbiformes | Red Turtle Dove | 66.67% (95% CI, 29.45–98.68%, 4/6) | 58.33% (95% CI, 31.90–80.12%, 7/12) | |
| Oriental Turtle-dove | 66.67% (95% CI, 20.68–98.68%, 2/3) | |||
| Spotted Dove | 33.33% (95% CI, 6.49–78.68%, 1/3) | |||
| Falconiformes | Besra | 50% (95% CI, 9.46–90.53%, 1/2) | 33.33% (95% CI, 6.14–79.24%, 1/3) | |
| Peregrine Falcon | 0 (95% CI, 0–79.37%, 0/1) | |||
| Pelecaniformes | Egret | 0 (95% CI, 0–79.37%, 0/1) | 0 (95% CI, 0–65.76%, 0/2) | |
| Great Cormorant | 0 (95% CI, 0–79.37%, 0/1) | 0 (95% CI, 0–79.37%, 0/1) | ||
| Strigiformes | Eastern Grass-owl | 0 (95% CI, 0–79.37%, 0/1) | 0 (95% CI, 0–79.37%, 0/1) | |
| Total | 42.86 (95% CI, 24.52–61.83%, 9/21) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, J.-M.; Zhong, H.-T.; Deng, Y.-Y.; Yang, J.-W.; Chen, M.-C.; Liang, Y.-J.; Chen, K.-W.; Yang, J.-T.; Wei, T.-C.; Wei, P.; et al. Prevalence and Genetic Characteristics of Avian Chlamydia in Birds in Guangxi, Southwestern China. Microorganisms 2025, 13, 2220. https://doi.org/10.3390/microorganisms13092220
Long J-M, Zhong H-T, Deng Y-Y, Yang J-W, Chen M-C, Liang Y-J, Chen K-W, Yang J-T, Wei T-C, Wei P, et al. Prevalence and Genetic Characteristics of Avian Chlamydia in Birds in Guangxi, Southwestern China. Microorganisms. 2025; 13(9):2220. https://doi.org/10.3390/microorganisms13092220
Chicago/Turabian StyleLong, Jian-Ming, Hai-Tao Zhong, Ya-Yu Deng, Jun-Wei Yang, Mei-Chi Chen, Yan-Jiao Liang, Ke-Wei Chen, Jing-Ting Yang, Tian-Chao Wei, Ping Wei, and et al. 2025. "Prevalence and Genetic Characteristics of Avian Chlamydia in Birds in Guangxi, Southwestern China" Microorganisms 13, no. 9: 2220. https://doi.org/10.3390/microorganisms13092220
APA StyleLong, J.-M., Zhong, H.-T., Deng, Y.-Y., Yang, J.-W., Chen, M.-C., Liang, Y.-J., Chen, K.-W., Yang, J.-T., Wei, T.-C., Wei, P., & Huang, J.-N. (2025). Prevalence and Genetic Characteristics of Avian Chlamydia in Birds in Guangxi, Southwestern China. Microorganisms, 13(9), 2220. https://doi.org/10.3390/microorganisms13092220






