Human Herpesvirus 6 Activates NF-κB Signalling and CD163-Positive Macrophage Recruitment in Alcohol-Induced Hepatic Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Groups
2.2. Immunohistochemistry Procedure and Evaluation of Staining Results
2.3. Statistical Analysis
3. Results
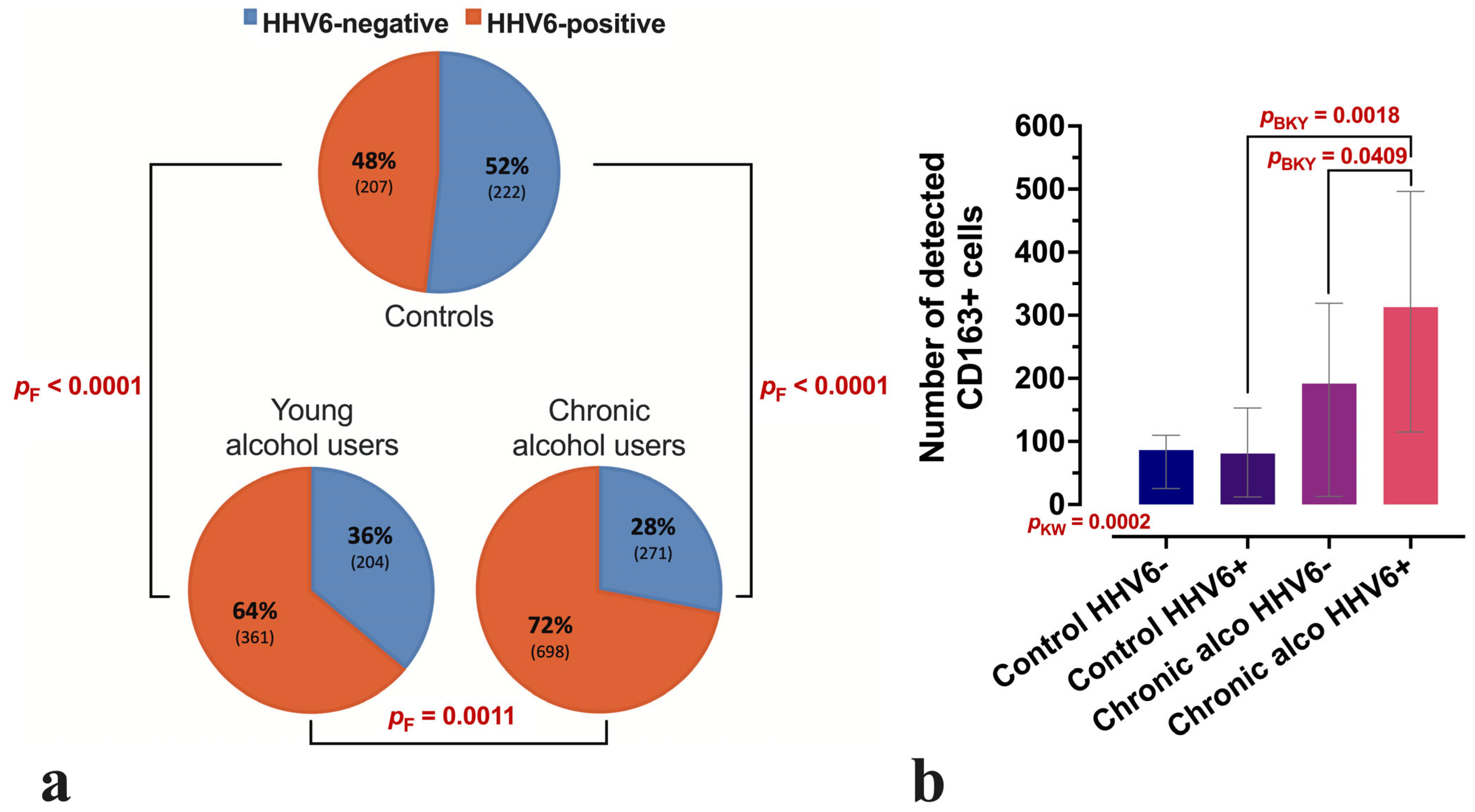
3.1. Characteristics of Study Groups
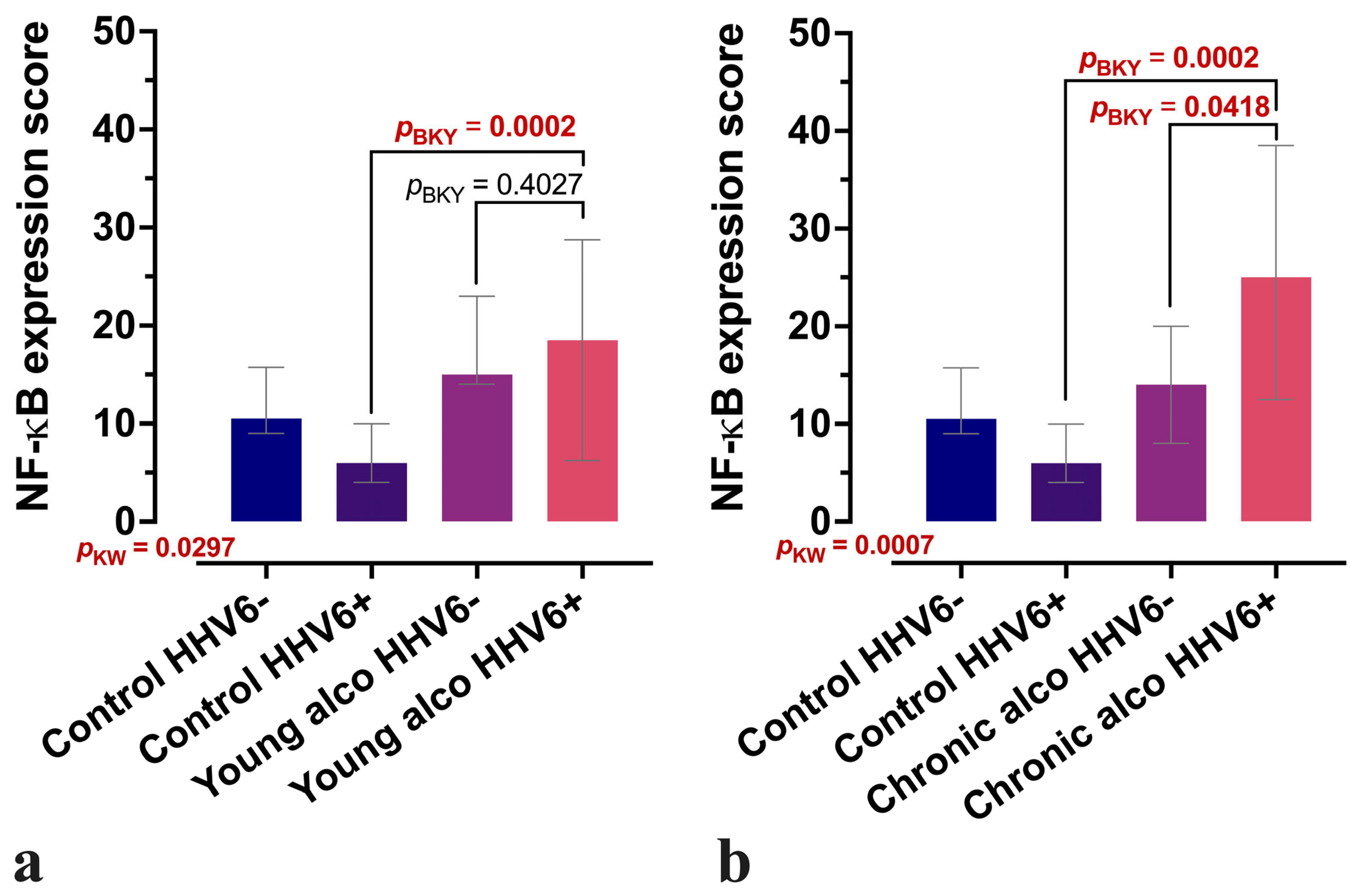
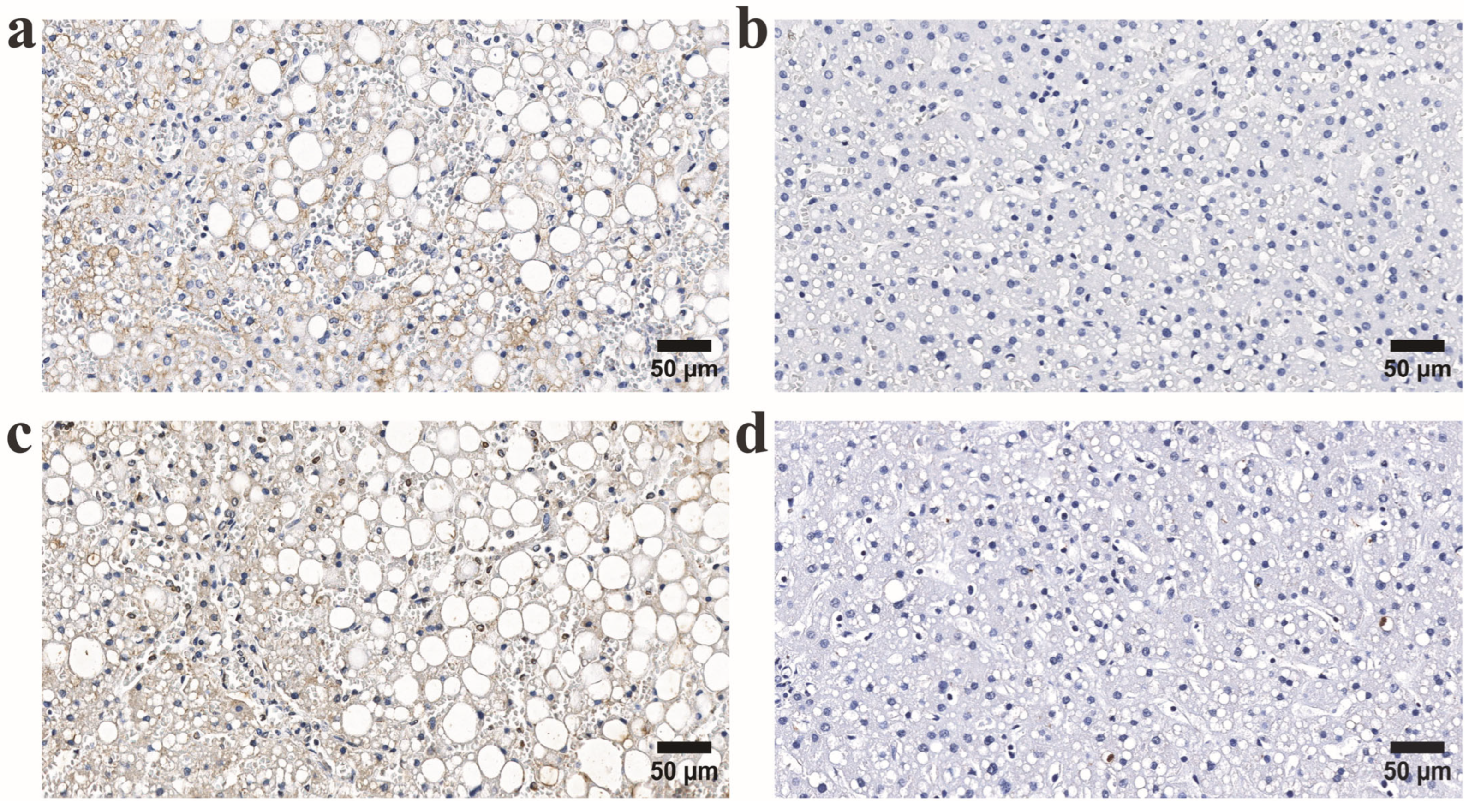
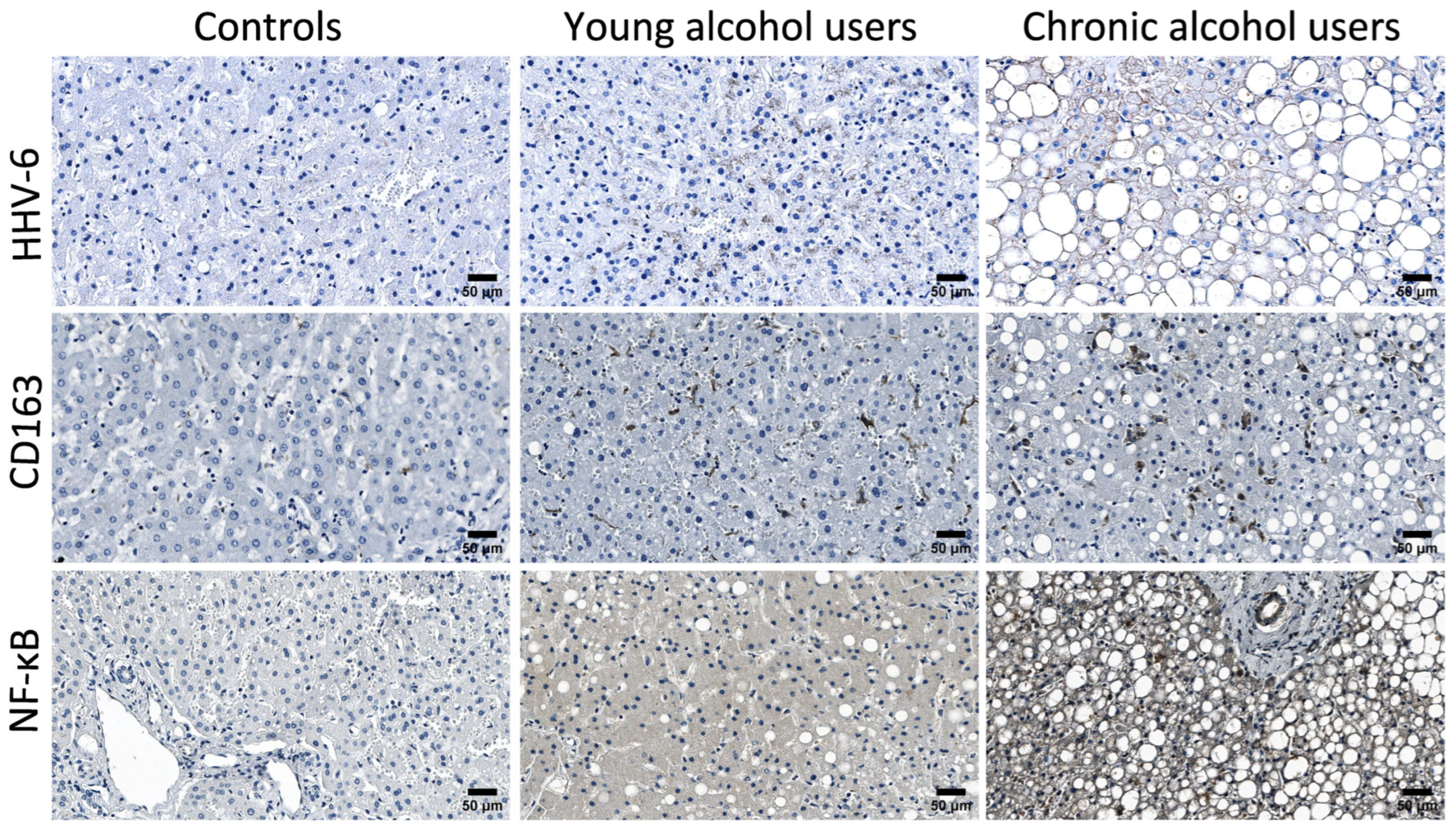
3.2. Comparative Analysis of CD163-Positive Macrophage Density in HHV-6-Positive Liver Lobules of Control and Alcohol-Exposed Individuals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agut, H.; Bonnafous, P.; Gautheret-Dejean, A. Laboratory and Clinical Aspects of Human Herpesvirus 6 Infections. Clin. Microbiol. Rev. 2015, 28, 313–335. [Google Scholar] [CrossRef]
- Agut, H.; Bonnafous, P.; Gautheret-Dejean, A. Human Herpesviruses 6A, 6B, and 7. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Malherbe, D.C.; Messaoudi, I. Transcriptional and Epigenetic Regulation of Monocyte and Macrophage Dysfunction by Chronic Alcohol Consumption. Front. Immunol. 2022, 13, 911951. [Google Scholar] [CrossRef]
- Phan, T.L.; Lautenschlager, I.; Razonable, R.R.; Munoz, F.M. HHV-6 in Liver Transplantation: A Literature Review. Liver Int. 2018, 38, 210–223. [Google Scholar] [CrossRef]
- Correia, I.M.; Rodrigues, A.I.; Henriques, A.; Ferreira, D.M.; Cipriano, M.A.; Lima, J. Acute Liver Failure and Human Herpesvirus 6 Infection: Reactivation in Immunoparesis? GE-Port. J. Gastroenterol. 2025, 32, 293–299. [Google Scholar] [CrossRef]
- Hannolainen, L.; Pyöriä, L.; Pratas, D.; Lohi, J.; Skuja, S.; Rasa-Dzelzkaleja, S.; Murovska, M.; Hedman, K.; Jahnukainen, T.; Perdomo, M.F. Reactivation of a Transplant Recipient’s Inherited Human Herpesvirus 6 and Implications to the Graft. J. Infect. Dis. 2025, 231, e267–e276. [Google Scholar] [CrossRef]
- Luedde, T.; Schwabe, R.F. NF-κB in the Liver—Linking Injury, Fibrosis and Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef]
- Nowak, A.J.; Relja, B. The Impact of Acute or Chronic Alcohol Intake on the NF-κB Signaling Pathway in Alcohol-Related Liver Disease. Int. J. Mol. Sci. 2020, 21, 9407. [Google Scholar] [CrossRef]
- Aktar, S.; Arii, J.; Tjan, L.H.; Nishimura, M.; Mori, Y. Human Herpesvirus 6A Tegument Protein U14 Induces NF-κB Signaling by Interacting with P65. J. Virol. 2021, 95, e01269-21. [Google Scholar] [CrossRef]
- Hirai, M.; Amaliin, K.; Huang, J.R.; Aktar, S.; Mori, Y.; Arii, J. HHV-6B Ribonucleotide Reductase Sequesters NF-κB Subunit P65 to Inhibit Innate Immune Responses. iScience 2025, 28, 111710. [Google Scholar] [CrossRef]
- Xi, S.; Zheng, X.; Li, X.; Jiang, Y.; Wu, Y.; Gong, J.; Jie, Y.; Li, Z.; Cao, J.; Sha, L.; et al. Activated Hepatic Stellate Cells Induce Infiltration and Formation of CD163+ Macrophages via CCL2/CCR2 Pathway. Front. Med. 2021, 8, 627927. [Google Scholar] [CrossRef]
- Kazankov, K.; Barrera, F.; Mφller, H.J.; Bibby, B.M.; Vilstrup, H.; George, J.; Grφnbæk, H. Soluble CD163, a Macrophage Activation Marker, Is Independently Associated with Fibrosis in Patients with Chronic Viral Hepatitis B and C. Hepatology 2014, 60, 521–530. [Google Scholar] [CrossRef]
- Yap, Y.J.; Wong, P.F.; AbuBakar, S.; Sam, S.S.; Shunmugarajoo, A.; Soh, Y.H.; Misbah, S.; Ab Rahman, A.K. The Clinical Utility of CD163 in Viral Diseases. Clin. Chim. Acta 2023, 541, 117243. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, J.; Wang, Z.; Shan, F. The Association between Alcohol Consumption and Herpes Simplex Virus Type 2: A Cross-Sectional Study from National Health and Nutrition Examination Survey 2009–2016. PLoS ONE 2024, 19, e0307702. [Google Scholar] [CrossRef]
- Klepper, A.; Asaki, J.; Kung, A.F.; Vazquez, S.E.; Bodansky, A.; Mitchell, A.; Mann, S.A.; Zorn, K.; Avila-Vargas, I.; Kari, S.; et al. Novel Autoantibody Targets Identified in Patients with Autoimmune Hepatitis (AIH) by PhIP-Seq Reveals Pathogenic Insights. medRxiv 2023. [Google Scholar] [CrossRef]
- Grima, P.; Chiavaroli, R.; Calabrese, P.; Tundo, P.; Grima, P. Severe Hepatitis with Autoimmune Features Following a HHV-6: A Case Report. Cases J. 2008, 1, 110. [Google Scholar] [CrossRef]
- Tabak, F.; Özdemir, F.; Tabak, O.; Erer, B.; Tahan, V.; Ozaras, R. Autoimmune Hepatitis Induced by the Prolonged Hepatitis A Virus Infection. Ann. Hepatol. 2008, 7, 177–179. [Google Scholar] [CrossRef]
- Maya, R.; Gershwin, M.E.; Shoenfeld, Y. Hepatitis B Virus (HBV) and Autoimmune Disease. Clin. Rev. Allergy Immunol. 2008, 34, 85–102. [Google Scholar] [CrossRef]
- Wozniak, H.; Vionnet, J.L.; Schibler, M.; Paula Heidegger, C.; Pugin, J.; Spahr, L.; Cereghetti, S. Human Herpesvirus 6 Reactivation: A Rare Case of Acute Liver Failure and Literature Review. Arch. Clin. Med. Case Rep. 2023, 7, 1–4. [Google Scholar] [CrossRef]
- Härmä, M.; Höckerstedt, K.; Lautenschlager, I. Human Herpesvirus-6 and Acute Liver Failure1. Transplantation 2003, 76, 536–539. [Google Scholar] [CrossRef]
- Cao, H.; Wang, M.; Cheng, A.; Tian, B.; Yang, Q.; Ou, X.; Sun, D.; He, Y.; Wu, Z.; Zhao, X.; et al. The Functions of Herpesvi-rus Shuttling Proteins in the Virus Lifecycle. Front. Microbiol. 2025, 16, 1515241. [Google Scholar] [CrossRef] [PubMed]
- Cianci, R.; Caldarelli, M.; Brani, P.; Bosi, A.; Ponti, A.; Giaroni, C.; Baj, A. Role of the Virome in Vaccine-Induced Immuniza-tion. Vaccines 2025, 13, 895. [Google Scholar] [CrossRef]
- Nikitina, E.; Larionova, I.; Choinzonov, E.; Kzhyshkowska, J. Monocytes and Macrophages as Viral Targets and Reservoirs. Int. J. Mol. Sci. 2018, 19, 2821. [Google Scholar] [CrossRef] [PubMed]
| Group | n | Female | Male | Medan Age (IQR) | Blood Ethanol (‰) |
|---|---|---|---|---|---|
| Controls | 11 | 1 | 10 | 32 (22–36) | 0 |
| Age-matched alcohol users | 15 | 2 | 13 | 31 (26–34) | 0.59–4.61 |
| Chronic alcohol users | 28 | 8 | 20 | 50 (41–60) | 1.07–8.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upane, A.; Svirskis, S.; Groma, V.; Skuja, S. Human Herpesvirus 6 Activates NF-κB Signalling and CD163-Positive Macrophage Recruitment in Alcohol-Induced Hepatic Injury. Microorganisms 2025, 13, 2204. https://doi.org/10.3390/microorganisms13092204
Upane A, Svirskis S, Groma V, Skuja S. Human Herpesvirus 6 Activates NF-κB Signalling and CD163-Positive Macrophage Recruitment in Alcohol-Induced Hepatic Injury. Microorganisms. 2025; 13(9):2204. https://doi.org/10.3390/microorganisms13092204
Chicago/Turabian StyleUpane, Anda, Simons Svirskis, Valerija Groma, and Sandra Skuja. 2025. "Human Herpesvirus 6 Activates NF-κB Signalling and CD163-Positive Macrophage Recruitment in Alcohol-Induced Hepatic Injury" Microorganisms 13, no. 9: 2204. https://doi.org/10.3390/microorganisms13092204
APA StyleUpane, A., Svirskis, S., Groma, V., & Skuja, S. (2025). Human Herpesvirus 6 Activates NF-κB Signalling and CD163-Positive Macrophage Recruitment in Alcohol-Induced Hepatic Injury. Microorganisms, 13(9), 2204. https://doi.org/10.3390/microorganisms13092204








