Targeted Screening with the Use of Clinical Risk Factors for Detecting Congenital Cytomegalovirus Infection in Newborns: A Prospective Multicenter Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedures
2.2.1. Definition of Maternal and Neonatal Risk Factors
2.2.2. Screening and Diagnostic Testing
2.3. Statistical Analysis
3. Results
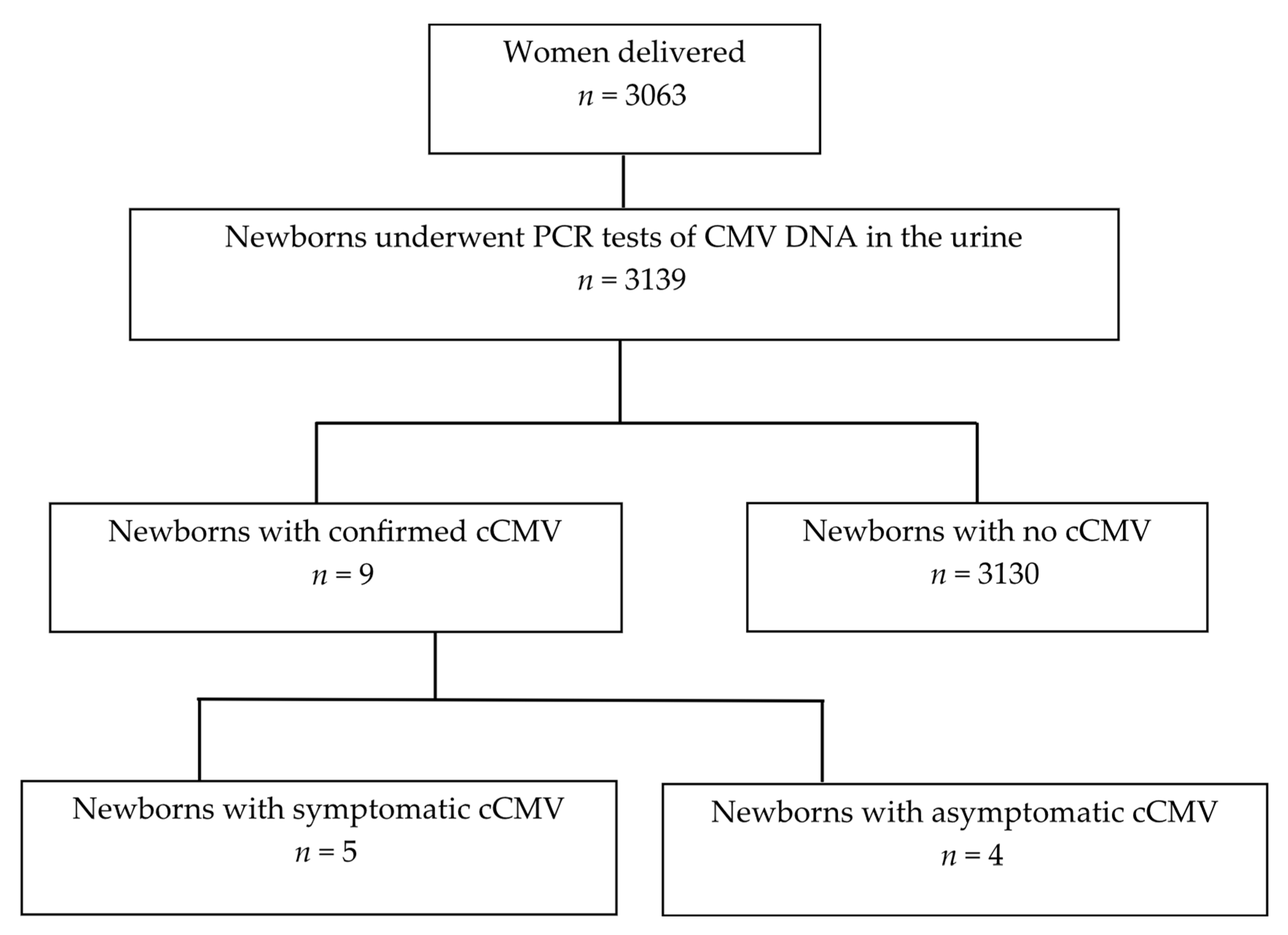
3.1. Study Cohort and Incidence of cCMV
3.2. Clinical Background and Frequency of the Six Risk Factors for cCMV
3.3. Nine Cases with cCMV
3.4. Risk Factors for cCMV and Logistic Regression Analysis
3.5. Diagnostic Performance of Targeted Screening
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CMV | cytomegalovirus |
| cCMV | congenital cytomegalovirus infection |
| AABR | automated auditory brainstem response |
| PD | preterm delivery |
| SNHL | sensorineural hearing loss |
| TPL | threatened miscarriage or preterm labor |
References
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef]
- Lanzieri, T.M.; Dollard, S.C.; Bialek, S.R.; Grosse, S.D. Systematic review of the birth prevalence of congenital cytomegalovirus infection in developing countries. Int. J. Infect. Dis. 2014, 22, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Boppana, S.B.; Ross, S.A.; Fowler, K.B. Congenital cytomegalovirus infection: Clinical outcome. Clin. Infect. Dis. 2013, 57, S178–S181. [Google Scholar] [CrossRef] [PubMed]
- Soper, D.E. Congenital cytomegalovirus infection: An obstetrician’s point of view. Clin. Infect. Dis. 2013, 57, S171–S173. [Google Scholar] [CrossRef]
- Dollard, S.C.; Grosse, S.D.; Ross, D.S. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev. Med. Virol. 2007, 17, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Koyano, S.; Morioka, I.; Oka, A.; Moriuchi, H.; Asano, K.; Ito, Y.; Yoshikawa, T.; Yamada, H.; Suzutani, T.; Inoue, N. Congenital cytomegalovirus in Japan: More than 2-year follow-up of infected newborns. Pediatr. Int. 2018, 60, 57–62. [Google Scholar] [CrossRef]
- Pesch, M.H.; Kuboushek, K.; McKee, M.M.; Thorne, M.C.; Weinberg, J.B. Congenital cytomegalovirus infection. BMJ 2021, 373, n1212. [Google Scholar] [CrossRef]
- Goderis, J.; Keymeulen, A.; Smets, K.; Van Hoecke, H.; De Leenheer, E.; Boudewyns, A.; Desloovere, C.; Kuhweide, R.; Muylle, M.; Royackers, L.; et al. Hearing in children with congenital cytomegalovirus infection: Results of a longitudinal study. J. Pediatr. 2016, 172, 110–115.E2. [Google Scholar] [CrossRef]
- Lazzarotto, T.; Guerra, B.; Gabrielli, L.; Lanari, M.; Landini, M.P. Update on the prevention, diagnosis and management of cytomegalovirus infection during pregnancy. Clin. Microbiol. Infect. 2011, 17, 1285–1293. [Google Scholar] [CrossRef]
- Revello, M.G.; Fabbri, E.; Furione, M.; Zavattoni, M.; Lilleri, D.; Tassis, B.; Quarenghi, A.; Cena, C.; Arossa, A.; Montanari, L.; et al. Role of prenatal diagnosis and counseling in the management of 735 pregnancies complicated by primary human cytomegalovirus infection: A 20-year experience. J. Clin. Virol. 2011, 50, 303–307. [Google Scholar] [CrossRef]
- Society for Maternal-Fetal Medicine (SMFM); Hughes, B.L.; Gyamfi-Bannerman, C. Diagnosis and antenatal management of congenital cytomegalovirus infection. Am. J. Obstet. Gynecol. 2016, 214, B5–B11. [Google Scholar] [CrossRef]
- Chiopris, G.; Veronese, P.; Cusenza, F.; Procaccianti, M.; Perrone, S.; Daccò, V.; Colombo, C.; Esposito, S. Congenital Cytomegalovirus Infection: Update on Diagnosis and Treatment. Microorganisms 2020, 8, 1516. [Google Scholar] [CrossRef]
- Gantt, S.; Bitnun, A.; Renaud, C.; Kakkar, F.; Vaudry, W. Diagnosis and management of infants with congenital cytomegalovirus infection. Paediatr. Child Health 2017, 22, 72–74. [Google Scholar] [CrossRef]
- Eventov-Friedman, S.; Manor, H.; Bar-Oz, B.; Averbuch, D.; Caplan, O.; Lifshitz, A.; Bdolah-Abram, T.; Wolf, D.G. Saliva Real-Time Polymerase Chain Reaction for Targeted Screening of Congenital Cytomegalovirus Infection. J. Infect. Dis. 2019, 220, 1790–1796. [Google Scholar] [CrossRef]
- Fowler, K.B.; McCollister, F.P.; Sabo, D.L.; Shoup, A.G.; Owen, K.E.; Woodruff, J.L.; Cox, E.; Mohamed, L.S.; Choo, D.I.; Boppana, S.B. A targeted approach for congenital cytomegalovirus screening within newborn hearing screening. Pediatrics 2017, 139, e20162128. [Google Scholar] [CrossRef]
- Diener, M.L.; Zick, C.D.; McVicar, S.B.; Boettger, J.; Park, A.H. Outcomes from a hearing-targeted cytomegalovirus screening program. Pediatrics 2017, 139, e20160789. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, K.; Tairaku, S.; Morioka, I.; Ozaki, K.; Nagamata, S.; Morizane, M.; Deguchi, M.; Ebina, Y.; Minematsu, T.; Yamada, H. Universal screening with use of immunoglobulin G avidity for congenital cytomegalovirus infection. Clin. Infect. Dis. 2017, 65, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, P.-G.; Kourlaba, G.; Kourkouni, E.; Luck, S.; Blázquez-Gamero, D.; Ville, Y.; Lilleri, D.; Dimopoulou, D.; Karalexi, M.; Papaevangelou, V. Maternal type of CMV infection and sequelae in infants with congenital CMV: Systematic review and meta-analysis. J. Clin. Virol. 2020, 129, 104518. [Google Scholar] [CrossRef]
- Kimberlin, D.W.; Jester, P.M.; Sánchez, P.J.; Ahmed, A.; Arav-Boger, R.; Michaels, M.G.; Ashouri, N.; Englund, J.A.; Estrada, B.; Jacobs, R.F.; et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N. Engl. J. Med. 2015, 372, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, L.; Amir, J.; Attias, J.; Bilavsky, E. Treatment of congenital cytomegalovirus beyond the neonatal period: An observational study. Eur. J. Pediatr. 2020, 179, 807–812. [Google Scholar] [CrossRef]
- Kim, G.A.; Koo, J.W. Validity of bag urine culture for predicting urinary tract infections in febrile infants, a paired comparison of urine collection methods. Korean J. Pediatr. 2015, 58, 183–189. [Google Scholar] [CrossRef]
- Al-Orifi, F.; McGillivray, D.; Tange, S.; Kramer, M.S. Urine culture from bag specimens in young children, Are the risks too high? J. Pediatr. 2000, 137, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Nagano, N.; Imaizumi, T.; Akimoto, T.; Hijikata, M.; Aoki, R.; Seimiya, A.; Okahashi, A.; Kawakami, K.; Komatsu, A.; Kawana, K.; et al. Clinical evaluation of a novel urine collection kit using filter paper in neonates: An observational study. Children 2021, 8, 561. [Google Scholar] [CrossRef]
- Itabashi, K.; Miura, F.; Uehara, R.; Nakamura, Y. New Japanese neonatal anthropometric charts for gestational age at birth. Pediatr. Int. 2014, 56, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Uchida, A.; Tanimura, K.; Morizane, M.; Fujioka, K.; Morioka, I.; Oohashi, M.; Minematsu, T.; Yamada, H. Clinical factors associated with congenital cytomegalovirus infection: A cohort study of pregnant women and newborns. Clin. Infect. Dis. 2020, 71, 2833–2839. [Google Scholar] [CrossRef]
- Imafuku, H.; Yamada, H.; Uchida, A.; Deguchi, M.; Shirakawa, T.; Sasagawa, Y.; Shi, Y.; Fujioka, K.; Morioka, I.; Tanimura, K. Clinical and ultrasound features associated with congenital cytomegalovirus infection as potential predictors for targeted newborn screening in high-risk pregnancies. Sci. Rep. 2020, 10, 19706. [Google Scholar] [CrossRef]
- Morioka, I.; Okahashi, A.; Nagano, N. Screening for Congenital Cytomegalovirus Infection Using Newborn Urine. Jpn. J. Neonatal Screen. 2023, 33, 59–66. [Google Scholar]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Messinger, C.J.; Lipsitch, M.; Bateman, B.T.; He, M.; Huybrechts, K.F.; MacDonald, S.; Mogun, H.; Mott, K.; Hernández-Díaz, S. Association between congenital cytomegalovirus and the prevalence at birth of microcephaly in the United States. JAMA Pediatr. 2020, 174, 1159–1167. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Han, X.; Du, H.; Cao, Y.; Liu, Y.; Wang, W. Association between congenital cytomegalovirus infection and brain injury in neonates: A meta-analysis of cohort studies. Behav. Neurol. 2021, 2021, 9603660. [Google Scholar] [CrossRef]
- Dietrich, M.L.; Schieffelin, J.S. Congenital cytomegalovirus infection. Ochsner J. 2019, 19, 123–130. [Google Scholar] [CrossRef]
- Grosse, S.D.; Ross, D.S.; Dollard, S.C. Congenital cytomegalovirus infection as a cause of permanent bilateral hearing loss: A quantitative assessment. J. Clin. Virol. 2008, 41, 57–62. [Google Scholar] [CrossRef]
- National CMV Foundation. Newborn Screening. Available online: https://www.nationalcmv.org/congenital-cmv/newborn-screening-(1) (accessed on 24 July 2025).
- Gantt, S.; Dionne, F.; Kozak, F.K.; Goshen, O.; Goldfarb, D.M.; Park, A.H.; Boppana, S.B.; Fowler, K. Cost-effectiveness of universal and targeted newborn screening for congenital cytomegalovirus infection. JAMA Pediatr. 2016, 170, 1173–1180. [Google Scholar] [CrossRef]
- Kharrazi, M.; Hyde, T.; Young, S.; Amin, M.M.; Cannon, M.J.; Dollard, S.C. Use of screening dried blood spots for estimation of prevalence, risk factors, and birth outcomes of congenital cytomegalovirus infection. J. Pediatr. 2010, 157, 191–197. [Google Scholar] [CrossRef]
- Boppana, S.B.; Ross, S.A.; Novak, Z.; Shimamura, M.; Tolan, R.W., Jr.; Palmer, A.L.; Ahmed, A.; Michaels, M.G.; Sánchez, P.J.; Bernstein, D.I.; et al. Dried blood spot realtime polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA 2010, 303, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Leruez-Ville, M.; Vauloup-Fellous, C.; Couderc, S.; Parat, S.; Castel, C.; Avettand-Fenoel, V.; Guilleminot, T.; Grangeot-Keros, L.; Ville, Y.; Grabar, S.; et al. Prospective identification of congenital cytomegalovirus infection in newborns using real-time polymerase chain reaction assays in dried blood spots. Clin. Infect. Dis. 2011, 52, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Boppana, S.B.; Ross, S.A.; Shimamura, M.; Palmer, A.L.; Ahmed, A.; Michaels, M.G.; Sánchez, P.J.; Bernstein, D.I.; Tolan, R.W., Jr.; Novak, Z.; et al. Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. N. Engl. J. Med. 2011, 364, 2111–2118. [Google Scholar] [CrossRef]
- Ross, S.A.; Ahmed, A.; Palmer, A.L.; Michaels, M.G.; Sánchez, P.J.; Bernstein, D.I.; Tolan, R.W., Jr.; Novak, Z.; Chowdhury, N.; Fowler, K.B.; et al. Detection of congenital cytomegalovirus infection by real-time polymerase chain reaction analysis of saliva or urine specimens. J. Infect. Dis. 2014, 210, 1415–1418. [Google Scholar] [CrossRef]
- Yamamoto, A.Y.; Mussi-Pinhata, M.M.; Marin, L.J.; Brito, R.M.; Oliveira, P.F.; Coelho, T.B. Is saliva as reliable as urine for detection of cytomegalovirus DNA for neonatal screening of congenital CMV infection? J. Clin. Virol. 2006, 36, 228–230. [Google Scholar] [CrossRef]
- Rawlinson, W.D.; Boppana, S.B.; Fowler, K.B.; Kimberlin, D.W.; Lazzarotto, T.; Alain, S.; Daly, K.; Doutré, S.; Gibson, L.; Giles, M.L.; et al. Congenital cytomegalovirus infection in pregnancy and the neonate: Consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect. Dis. 2017, 17, e177–e188. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.A.; Michaels, M.G.; Ahmed, A.; Palmer, A.L.; Sánchez, P.J.; Bernstein, D.I.; Feja, K.; Stewart, A.; Boppana, S.B.; Fowler, K.B. Contribution of breastfeeding to false-positive saliva polymerase chain reaction for newborn congenital cytomegalovirus screening. J. Infect. Dis. 2018, 217, 1612–1615. [Google Scholar] [CrossRef]
- Vaudry, W.; Rosychuk, R.J.; Lee, B.E.; Cheung, P.Y.; Pang, X.; Preiksaitis, J.K. Congenital cytomegalovirus infection in high-risk Canadian infants: Report of a pilot screening study. Can. J. Infect. Dis. Med. Microbiol. 2010, 21, e12–e19. [Google Scholar] [CrossRef] [PubMed]
- van der Weiden, S.; de Jong, E.P.; Te Pas, A.B.; Middeldorp, J.M.; Vossen, A.C.T.M.; Rijken, M.; Walther, F.J.; Lopriore, E. Is routine TORCH screening and urine CMV culture warranted in small for gestational age neonates? Early Hum. Dev. 2011, 87, 103–107. [Google Scholar] [CrossRef]
- Lorenzoni, F.; Lunardi, S.; Liumbruno, A.; Ferri, G.; Madrigali, V.; Fiorentini, E.; Forli, F.; Berrettini, S.; Boldrini, A.; Ghirri, P. Neonatal screening for congenital cytomegalovirus infection in preterm and small for gestational age infants. J. Matern. Fetal Neonatal Med. 2014, 27, 1589–1593. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, K.; Tairaku, S.; Ebina, Y.; Morioka, I.; Nagamata, S.; Deguchi, K.; Morizane, M.; Deguchi, M.; Minematsu, T.; Yamada, H. Prediction of congenital cytomegalovirus infection in high-risk pregnant women. Clin. Infect. Dis. 2017, 65, 159–165. [Google Scholar] [CrossRef]
- Yamada, H.; Tanimura, K.; Tairaku, S.; Morioka, I.; Deguchi, M.; Morizane, M.; Nagamata, S.; Ozaki, K.; Ebina, Y.; Minematsu, T. Clinical factors associated with congenital cytomegalovirus infection in pregnant women with non-primary infection. J. Infect. Chemother. 2018, 24, 702–706. [Google Scholar] [CrossRef]
- Koyano, S.; Inoue, N.; Oka, A.; Moriuchi, H.; Asano, K.; Ito, Y.; Yamada, H.; Yoshikawa, T.; Suzutani, T.; Japanese Congenital Cytomegalovirus Study Group. Screening for congenital cytomegalovirus infection using newborn urine samples collected on filter paper: Feasibility and outcomes from a multicentre study. BMJ Open 2011, 1, e000118. [Google Scholar] [CrossRef]
- Vauloup-Fellous, C.; Picone, O.; Cordier, A.-G.; Parent-du-Châtelet, I.; Senat, M.-V.; Frydman, R.; Grangeot-Keros, L. Does hygiene counseling have an impact on the rate of CMV primary infection during pregnancy? Results of a 3-year prospective study in a French hospital. J. Clin. Virol. 2009, 46, S49–S53. [Google Scholar] [CrossRef] [PubMed]
- Revello, M.G.; Tibaldi, C.; Masuelli, G.; Frisina, V.; Sacchi, A.; Furione, M.; Arossa, A.; Spinillo, A.; Klersy, C.; Ceccarelli, M.; et al. Prevention of primary cytomegalovirus infection in pregnancy. EBioMedicine 2015, 2, 1205–1210. [Google Scholar] [CrossRef]
- Billette de Villemeur, A.; Tattevin, P.; Salmi, L.-R. Hygiene promotion might be better than serological screening to deal with cytomegalovirus infection during pregnancy: A methodological appraisal and decision analysis. BMC Infect. Dis. 2020, 20, 418. [Google Scholar] [CrossRef]
| Total n = 3139 | cCMV n = 9 | Non-cCMV n = 3130 | p Value | |
|---|---|---|---|---|
| Clinical background | ||||
| Maternal age, years | 32 (15–51) | 28 (18–39) | 32 (15–51) | 0.021 |
| Gestational weeks at delivery | 38 (22–42) | 38 (33–40) | 38 (22–42) | 0.419 |
| Primipara | 1524 (48.6%) | 4 (44.4%) | 1520 (48.6%) | 1 |
| Multiple pregnancy | 153 (4.9%) | 0 (0%) | 153 (4.9%) | 1 |
| Body mass index, kg/m2 | 21.8 (13.6–45.7) | 21.7 (17.1–44.6) | 21.8 (13.6–45.7) | 0.793 |
| PD < 37 weeks | 392 (12.5%) | 2 (22.2%) | 390 (12.5%) | 0.312 |
| Risk factors for cCMV | ||||
| Fever/flu-like symptoms | 198 (6.3%) | 3 (33.3%) | 195 (6.2%) | 0.0156 |
| Hospitalization < 34 for TPL | 335 (10.7%) | 2 (22.2%) | 333 (10.6%) | 0.248 |
| PD < 34 | 170 (5.4%) | 1 (11.1%) | 169 (5.4%) | 0.395 |
| Fetal ultrasound abnormalities | 88 (%) | 3 (33.3%) | 85 (2.7%) | 0.0016 |
| SGA | 332 (2.8%) | 4 (44.4%) | 328 (10.5%) | 0.0037 |
| AABR refer | 56 (1.8%) | 5 (55.6%) | 51 (1.6%) | <0.0001 |
| Case | Age (Year) | Gravidity/Parity | BMI Before Pregnancy (kg/m2) | Gestational Week at Delivery | Birth Weight (g) | Sex | Fever/ Flu-like Symptoms (GW) | Hospitalization < 34 for TPL (GW) | Fetal Ultrasound Abnormalities (GW) | PD < 34 (GW) | SGA | AABR Refer | Symptoms of Newborns with cCMV | Antiviral Therapy | Development/Sequelae (Age) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 | 1/0 | 24.2 | 37 | 2674 | Male | No | No | Ventriculomegaly (37) | No | No | Presence | Ventriculomegaly, hearing abnormality | Valganciclovir | Hearing loss (3 y and 4 mo) |
| 2 | 29 | 1/0 | 17.1 | 39 | 2291 | Male | No | Presence (24–26) | Ventriculomegaly (39) | No | Presence | Presence | SGA, hypoxic-ischemic encephalopathy, bilateral internal carotid artery occlusion | No | Cerebral palsy (3 y and 1 mo) |
| 3 | 31 | 3/2 | 24.2 | 38 | 3252 | Female | Presence (12) | No | No | No | No | No | Asymptomatic | No | Normal (2 y and 10 mo) |
| 4 | 28 | 3/1 | 20.2 | 38 | 2274 | Male | No | No | No | No | Presence | No | SGA, white matter abnormality of the brain (MRI) | Valganciclovir | Normal (2 y and 8 mo) |
| 5 | 25 | 2/1 | 20.3 | 35 | 2362 | Male | Presence (32, 35) | No | No | No | No | Presence | Hearing abnormality | Valganciclovir | Normal (2 y and 6 mo) |
| 6 | 23 | 3/1 | 28.4 | 40 | 3580 | Female | Presence (13) | No | No | No | No | No | Asymptomatic | No | Normal (1 y and 0 mo) |
| 7 | 39 | 2/0 | 21.7 | 33 | 1657 | Female | No | Presence (31–33) | No | Presence (33) | Present | No | SGA, asymptomatic | No | Normal (2 y and 2 mo) |
| 8 | 25 | 1/0 | 44.6 | 40 | 2920 | Female | No | No | No | No | No | Presence | Asymptomatic | No | Normal (1 y and 0 mo) |
| 9 | 31 | 5/2 | 19.8 | 37 | 1702 | Male | No | No | Ventriculomegaly, intracranial calcifications, hepatosplenomegaly, echogenic bowel (23) | No | Presence | Presence | SGA, ventriculomegaly, hearing abnormality | Valganciclovir | Hearing loss (11 mo) |
| Univariate Analysis | Forward Stepwise Multivariate Regression | |||||||
|---|---|---|---|---|---|---|---|---|
| Risk Factors for cCMV | cCMV n = 9 | Non-cCMV n = 3130 | OR | 95% CI | p Value | OR | 95% CI | p Value |
| Fever/flu-like symptoms | 3 (33.3%) | 195 (6.2%) | 7.5 | 1.9–30.3 | 0.0045 | 12.3 | 2.4–63.3 | 0.0026 |
| Hospitalization < 34 for TPL | 2 (22.2%) | 333 (10.6%) | 2.4 | 0.50–11.6 | 0.28 | |||
| PD < 34 | 1 (11.1%) | 169 (5.4%) | 2.2 | 0.27–17.6 | 0.46 | |||
| Fetal ultrasound abnormalities | 3 (33.3%) | 85 (2.7%) | 28.7 | 7.6–108.6 | <0.0001 | 11.9 | 3.2–100.8 | 0.0011 |
| SGA | 4 (44.4%) | 328 (10.5%) | 6.8 | 1.8–25.6 | 0.0043 | 4.2 | 0.7–24.3 | 0.1087 |
| AABR refer | 5 (55.6%) | 51 (1.6%) | 75.5 | 19.7–289.2 | <0.0001 | 62.2 | 13.2–292.8 | <0.0001 |
| Risk Factors for cCMV | Sensitivity (95% CI) | Specificity (95% CI) | Positive Predictive Value (95% CI) | Negative Predictive Value (95% CI) | Accuracy % (95% CI) | Youden Index |
|---|---|---|---|---|---|---|
| Fever/flu-like symptoms | 0.333 (0.075–0.701) | 0.938 (0.929–0.946) | 0.015 (0.003–0.044) | 0.998 (0.996–0.999) | 0.936 (0.927–0.944) | 0.271 |
| Hospitalization < 34 for TPL | 0.222 (0.028–0.600) | 0.894 (0.882–0.904) | 0.006 (0.001–0.021) | 0.998 (0.995–0.999) | 0.892 (0.880–0.902) | 0.116 |
| PD < 34 | 0.111 (0.003–0.482) | 0.946 (0.938–0.954) | 0.006 (0.000–0.032) | 0.997 (0.995–0.999) | 0.944 (0.935–0.951) | 0.057 |
| Fetal ultrasound abnormalities | 0.333 (0.075–0.701) | 0.974 (0.967–0.979) | 0.034 (0.007–0.096) | 0.998 (0.996–0.999) | 0.972 (0.965–0.977) | 0.307 |
| SGA | 0.444 (0.137–0.788) | 0.895 (0.884–0.906) | 0.012 (0.003–0.031) | 0.998 (0.996–0.999) | 0.894 (0.883–0.904) | 0.339 |
| AABR refer | 0.556 (0.212–0.863) | 0.984 (0.979–0.988) | 0.089 (0.030–0.196) | 0.999 (0.997–1.000) | 0.982 (0.977–0.987) | 0.54 |
| Any of the above six risks | 1.000 (0.555–1.000) | 0.707 (0.691–0.723) | 0.010 (0.004–0.018) | 1.000 (0.997–1.000) | 0.708 (0.692–0.724) | 0.707 |
| Any of the four risks (fever/flu-like symptoms, fetal ultrasound abnormalities, SGA, AABR refer) | 1.000 (0.555–1.000) | 0.812 (0.798–0.826) | 0.015 (0.007–0.028) | 1.000 (0.998–1.000) | 0.813 (0.799–0.826) | 0.812 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kataoka, S.; Kaneko, M.; Yang, L.; Ota, H.; Seki, M.; Kobamatsu, A.; Nakayama, D.; Furuta, Y.; Tanuma, F.; Fukushi, Y.; et al. Targeted Screening with the Use of Clinical Risk Factors for Detecting Congenital Cytomegalovirus Infection in Newborns: A Prospective Multicenter Cohort Study. Microorganisms 2025, 13, 2197. https://doi.org/10.3390/microorganisms13092197
Kataoka S, Kaneko M, Yang L, Ota H, Seki M, Kobamatsu A, Nakayama D, Furuta Y, Tanuma F, Fukushi Y, et al. Targeted Screening with the Use of Clinical Risk Factors for Detecting Congenital Cytomegalovirus Infection in Newborns: A Prospective Multicenter Cohort Study. Microorganisms. 2025; 13(9):2197. https://doi.org/10.3390/microorganisms13092197
Chicago/Turabian StyleKataoka, Soromon, Masatoki Kaneko, Li Yang, Hajime Ota, Moeka Seki, Aya Kobamatsu, Daiki Nakayama, Yu Furuta, Fumie Tanuma, Yoshiyuki Fukushi, and et al. 2025. "Targeted Screening with the Use of Clinical Risk Factors for Detecting Congenital Cytomegalovirus Infection in Newborns: A Prospective Multicenter Cohort Study" Microorganisms 13, no. 9: 2197. https://doi.org/10.3390/microorganisms13092197
APA StyleKataoka, S., Kaneko, M., Yang, L., Ota, H., Seki, M., Kobamatsu, A., Nakayama, D., Furuta, Y., Tanuma, F., Fukushi, Y., Wada, S., Haseyama, K., & Yamada, H. (2025). Targeted Screening with the Use of Clinical Risk Factors for Detecting Congenital Cytomegalovirus Infection in Newborns: A Prospective Multicenter Cohort Study. Microorganisms, 13(9), 2197. https://doi.org/10.3390/microorganisms13092197






