Abstract
Human metapneumovirus (hMPV), a member of the Pneumoviridae subfamily, has emerged as a significant etiological agent of acute respiratory tract infections across diverse age groups, particularly affecting infants, the elderly, and immunocompromised individuals. Since its initial identification in 2001, hMPV has been recognized globally for its seasonal circulation pattern, predominantly in late winter and spring. hMPV is a leading etiological agent, accounting for approximately 5% to 10% of hospitalizations among pediatric patients with acute respiratory tract infections. hMPV infection can result in severe bronchiolitis and pneumonia, particularly in young children, with clinical manifestations often indistinguishable from those caused by human RSV. Primary hMPV infection typically occurs during early childhood; however, re-infections are frequent and may occur throughout an individual’s lifetime. hMPV is an enveloped, negative-sense RNA virus transmitted through respiratory droplets and aerosols, with a 3–5-day incubation period. The host immune response is marked by elevated pro-inflammatory cytokines, which contribute to disease severity. Advances in molecular diagnostics, particularly reverse transcription–quantitative polymerase chain reaction (RT-qPCR) and metagenomic next-generation sequencing (mNGS), have improved detection accuracy and efficiency. Despite these advancements, treatment remains largely supportive, as no specific antiviral therapy has yet been approved. Promising developments in vaccine research, including mRNA-based candidates, are currently undergoing clinical evaluation. This review synthesizes current knowledge on hMPV, highlighting its virological, epidemiological, and clinical characteristics, along with diagnostic advancements and emerging therapeutic strategies, while underscoring the critical role of continued research and sustained preventive measures—including vaccines, monoclonal antibodies, and non-pharmaceutical interventions—in mitigating the global burden of hMPV-related disease.
1. Introduction
Human metapneumovirus (hMPV) is a negative-sense, single-stranded RNA virus classified within the Metapneumovirus genus of the Pneumoviridae family. First isolated in the Netherlands in 2001, hMPV has since been identified worldwide as a prominent cause of acute respiratory tract infections [1]. Its genome, approximately 13 kilobases in length, encodes several structural and non-structural proteins essential for viral replication and host interaction [2,3]. The virus is primarily transmitted through respiratory droplets or direct contact with infected individuals, with an incubation period typically ranging from three to six days [4].
hMPV is a globally prevalent respiratory virus primarily affecting children under five years of age [5,6], with documented outbreaks reported across diverse geographical regions including North and South America, Europe, and Asia. While infection rates are highest in infants and young children, older adults—particularly those aged 65 and above—also experience significant morbidity, especially when hospitalized with underlying comorbidities [7]. Surveillance data estimate approximately 473,000 hMPV-associated hospital admissions worldwide among older adults in 2019, highlighting its considerable clinical burden across both pediatric and elderly populations in various healthcare settings [7].
The seasonality of hMPV is well characterized in temperate climates, where incidence peaks during the late winter to early spring months, typically coinciding with respiratory syncytial virus (RSV) activity [4,5]. In tropical and subtropical regions, such as India and Southeast Asia, hMPV circulation is influenced by local climatic conditions, often peaking during or shortly after the monsoon season [8]. These distinct epidemiological patterns underscore the influence of environmental factors on viral transmission dynamics across different global regions. Following a global decline in hMPV circulation during the COVID-19 pandemic due to public health interventions, a post-pandemic resurgence has emerged, often with atypical seasonal patterns. This re-emergence is likely driven by reduced population immunity and co-circulation with other respiratory viruses [5,9,10]. Recent data also indicate altered transmission dynamics, including higher incidence in older children and increased household clustering [5,11].
Clinically, hMPV infections present with a spectrum of respiratory symptoms ranging from mild upper respiratory tract illness to severe lower respiratory tract disease, including bronchiolitis and pneumonia [12,13]. While healthy adults often experience mild symptoms, infants, older adults, and immunocompromised individuals are at higher risk for severe outcomes [7]. Beyond its independent pathogenicity, hMPV is frequently implicated in co-infections with other respiratory viruses, including influenza, RSV, and SARS-CoV-2, which may exacerbate clinical outcomes [14]. Despite its clinical significance, there are currently no licensed antiviral treatments or vaccines available for hMPV, and management remains supportive [15,16]. This underscores the need for ongoing research into the virus’s epidemiology, pathogenesis, and potential treatment strategies.
This review provides a comprehensive overview of the current knowledge on hMPV infection, focusing on its virological characteristics, epidemiological trends, and clinical manifestations, while synthesizing recent data on its global burden, seasonal trends, age-specific risks, and geographic variability. Furthermore, the review critically examines advances in diagnostic methods and emerging therapeutic strategies, including vaccine development, monoclonal antibody therapies, and non-pharmaceutical interventions, aiming to highlight future research priorities and public health strategies to mitigate the impact of hMPV-associated disease worldwide.
2. Pathophysiology
2.1. Structure and Genotypes
hMPV is an encircled, viral agent of approximately 209 nanometer in diameter [2]. The hMPV genome is composed of a negative, single-stranded, and non-segmented RNA sequence of around 13 kilobases in length. The hMPV genome hosts eight genes which encode nine structural proteins, with a discrete amino acid chain identity of 3′-N-P-M-F-M2(-1/-2)-SH-G-L-5′ [2,3]. Each open reading frame (ORF) is surrounded by untranslated regions that play a regulatory role in terminating and reinitiating transcription. This regulatory mechanism leads to a transcriptional gradient, with mRNA abundance progressively decreasing from the 3′ end toward the 5′ end of the viral genome [17].
The N protein, a 43.5 kDa molecular weight nucleoprotein, contributes to the encapsidation of the single stranded RNA genome, forming a nucleoprotein nexus which facilitates the protection of the hMPV genome from degradation by host nucleases [18]. The P protein, is a phosphoprotein of 32.4 kDa and is part of the viral RNA polymerase complex [19]. This protein acts as an essential cofactor, enhancing the structural stability of the viral polymerase L and promoting the efficient assembly of the ribonucleoprotein (RNP) complex required for viral RNA replication [15,16]. It plays a crucial role in assisting the interaction of the L protein with the viral N–RNA protein complex, by binding to both N and L at the same time, ultimately allowing the virus to replicate and transcribe its genome into new viral particles [20,21]. The large polymerase (L) protein (230.6 kDa) contains zinc-binding sites and exhibits multifunctional catalytic activity, playing a key role in viral genome synthesis alongside various cofactors [22]. The RNA genome is closely associated with the viral proteins N, P, L, M2-1, and M2-2, which together constitute the nucleocapsid structure. The nucleocapsid is boarded by the matrix (M) protein, which lines the inner surface of the viral lipid envelope. The M protein is essential for the assembly of the newly formed virus particles and participates in the virion budding process from the host cells, via interacting with various cellular and viral elements [23]. The fusion protein (F), a protein of 58.4 kDa, plays a major role in the invasion of the virus to the host by assisting host cell–virus attachment and the following fusion of viral and cellular membranes, thereby enabling the release of the viral genome into the host cell cytoplasm. The hMPV F glycoprotein is a primary antigenic component and a key focus of the immune system’s neutralizing antibody response [24]. The M2 protein gene contains two open reading frames that enable the expression of the M2-1 (21.2 kDa) and M2-2 (8.1 kDa) proteins. These proteins regulate RNA polymerase activity and are involved in the modulation of the host immune response. In particular, the highly conserved M2-1 protein acts as a zinc-binding transcription anti-terminator and also attaches to mRNA, acting protectively against premature termination [25]. The small hydrophobic (SH) protein (20.9 kDa) modulates the innate immune response by inhibiting the activation of nuclear factor-κB (NF-κB) and interferon (IFN) signaling. It also interferes in the regulation of the fusogenicity of the F protein [26]. The glycoprotein (G) (25.7 kDa) binds to cellular glycosaminoglycans and facilitates viral attachment. It also suppresses the IFN-I response and promotes neutrophil recruitment by enhancing the secretion of chemotactic factors such as C-X-C Motif Chemokine Ligand 2 (CXCL2), C-C Motif Chemokine Ligand 3 (CCL3), CCL4, IL-17, and tumor necrosis factor a (TNF-a) [27] (Figure 1).
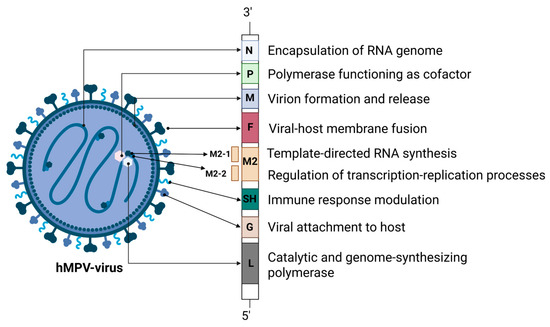
Figure 1.
Overview of hMPV proteins: structure and functional characteristics. hMPV: human metapneumovirus; RNA: ribonucleic acid; SH: small hydrophobic protein. Created in BioRender. L., A. (accessed on 30 April 2025) https://BioRender.com/zj1pa3c.
Whole-genome sequencing has revealed that hMPV comprises two primary genotypes, designated A and B. These genotypes are further classified into sublineages—A1, A2, B1, and B2—based on genetic variability in the viral surface glycoproteins, specifically the attachment (G) and fusion (F) proteins. The subgroup A2 is further subdivided into A2a and A2b. Notably, a study has identified a strain that clusters within genotype A but does not align with either the A1 or A2 subgroups, suggesting the potential emergence of a novel subgroup within the A genotype [28].
2.2. Immunopathology
The hMPV virus is capable of rapidly disseminating throughout the respiratory tract following its initial accumulation in the nasopharyngeal mucosa. hMPV viral particles can display a morphological transition among spherical and filamentous structures and are enveloped by a lipid bilayer membrane adorned with glycoprotein structures measuring around 13 to 17 nanometers [12]. The hMPV genome contains approximately eight genes which encode nine unique proteins that facilitate viral penetration into host cells. Human cells are enveloped by a dense layer of glycoconjugates involved in key homeostatic functions. Pathogens such as hMPV have adapted to target this glycan-rich surface for their initial binding and entry [29]. The wide variety of glycans, characterized by variations in chain length and core structure, creates a heterogeneous environment within the upper and lower respiratory tract, and these glycans can be identified as potential glycoreceptors for hMPV recognition [30].
Airway epithelial cells (AECs) are the main sites of hMPV infection, where the virus adheres to the cell surface by binding its glycoprotein to the heparan sulfate molecules present on AECs. The fusion glycoprotein (F) enables hMPV entry into the host cell by binding to integrins on the cell surface, initiating transmembrane penetration of the viral genetic material with the assistance of the accessory glycoprotein (G) [31]. Upon entering the host cell cytoplasm, the viral nucleocapsid undergoes replication. RNA polymerase converts viral negative-sense RNA into positive-sense mRNA, enabling viral protein synthesis. Viral glycoproteins are then processed through the Golgi apparatus and accumulated at the cell membrane for viral particle assembly. Once the protein levels reach a set level, RNA polymerase replicates the genome into positive-sense RNA, which serves as a template for synthesizing new negative-sense genomic RNA to be incorporated into nascent viral particles [32]. The M protein aids in the assembly of viral particles, allowing their exocytosis from the host cell through membrane budding [32]. Upon entry into host cells, hMPV is recognized by pattern recognition receptors such as Toll-like receptors (TLRs) and RIG-I-like receptors (RLRs), initiating innate immune signaling pathways. TLRs, including TLR2, TLR4, TLR3, and TLR7/8, detect viral components either at the cell surface or within endosomal compartments, leading to the activation of adaptor molecules like MYD88 and TRIF. This engagement results in the activation of nuclear factor kappa B (NF-κB) and interferon regulatory factors (IRFs), culminating in the transcription of antiviral genes, including type I interferons (IFN-α and IFN-β) and pro-inflammatory cytokines such as IL-1β, IL-2, IL-4, IL-6, and IL-8 [33]. Concurrently, cytoplasmic RLRs, notably RIG-I and Melanoma Differentiation-Associated Gene 5 (MDA5), identify viral RNA, leading to the activation of mitochondrial antiviral-signaling protein (MAVS). This interaction further stimulates IRF-3 and IRF-7, which translocate to the nucleus to promote the expression of antiviral proteins and cytokines. These coordinated responses limit viral replication and enhance inflammation, underscoring the complex interplay of immune signaling networks during hMPV infection [33,34].
hMPV infection of the airway epithelium triggers a robust innate immune activation characterized by the stimulation of airway epithelial cells, macrophages, and dendritic cells [33,35]. These cells respond by producing a spectrum of proinflammatory cytokines that are implicated in both antiviral defense mechanisms and the promotion of immunopathological effects. Notably, cytokines such as IL-1β, IL-6, IL-8 (CXCL8), TNF-α, Monocyte Chemoattractant Protein-1 (MCP-1), IL-33, and TSLP exhibit markedly upregulated activity in the context of hMPV exposure [36]. In the context of the inflammatory process, monocytes and lymphocytes are recruited to the airway endothelium. Monocyte migration to sites of infection is mediated by MCP-1, a chemokine that plays a key role in promoting airway inflammatory responses [37]. Pro-inflammatory cytokines, and especially IL-8, further stimulate the release of chemokines, which recruit neutrophils to the lung. While these neutrophils play a role in pathogen clearance, their presence also contributes to tissue damage and sustained inflammation [37]. Collectively, these processes contribute to pulmonary inflammation, leading to the manifestation of respiratory symptoms seen in hMPV infections [38].
Along with the secretion of inflammatory cytokines, predominantly with a type 2 profile, hMPV triggers a rapid immune response that involves a significant innate immune component. Cytokines such as TSLP, IL-25, and IL-33 act as potent inducers of type 2 immune responses in innate immune cells, leading to excessive mucus production and the development of lung allergies [39]. The expression of IL-33 and TSLP is significantly increased during hMPV infection, particularly in children, promoting the T-helper 2 (Th2) immune response which exacerbates the progression of lung damage [40]. While the events induced by such cytokines play a critical role in combating viral infections, they also contribute to severe bronchiolitis and persistent pulmonary complications, predominantly in young children, older adults, and immunocompromised individuals [41].
Preclinical studies in animal models demonstrate that in neonates, the immune response to respiratory viral pathogens is skewed toward a type 2 immunological profile. This is characterized by an elevated secretion of epithelial-derived cytokines, including IL-33, IL-25, and TSLP [42]. These cytokines promote the expansion of type 2 innate lymphoid cells (ILC2s) and Th2, as well as the shift of alveolar macrophages toward an M2-like phenotype. Cytotoxic CD8+ T lymphocytes in neonates exhibit a reduced T cell receptor (TCR) affinity, and both CD8+ T cells and natural killer (NK) cells demonstrate impaired activity [43,44]. Additionally, germinal center (GC) responses are attenuated, with decreased differentiation of T follicular helper (Tfh) cells and a consequent reduction in immunoglobulin G (IgG) production by GC B cells. Especially for hMPV infection, preclinical data is quite limited. Evidence from neonatal mice models supports a host defense against hMPV similar to RSV models [45]. In patients under three years of age with hMPV infection, nasal secretion samples exhibited a relative enrichment of proteins linked to Th1 immune responses, with no corresponding increase in markers of Th2 activity. However, this Th1-skewed immune profile was not observed in children with a history of prematurity, suggesting a disruption in the typical Th1/Th2 balance in this subgroup [46]. In contrast, a separate study analyzing protein concentrations in nasal secretions reported that infants infected with hMPV exhibited a reduced interferon-gamma IFN-γ to IL-4 ratio—indicative of a Th2-skewed immune response—when compared to those infected with RSV or influenza virus [47]. A study examining nasal immune responses in infants revealed that full-term infants exhibited strong antiviral immune responses—marked by elevated IFNγ, CCL5/Regulated upon Activation, Normal T Cell Expressed and Secreted (RANTES), IL-10, and a high IFNγ/IL-4 ratio—during RSV and hMPV infections. In contrast, premature infants displayed relatively weakened responses, especially to hMPV, with little or no increase in these inflammatory markers. These results suggest that premature infants have an impaired antiviral defense, particularly against hMPV, which may explain their heightened risk for severe illness [48].
3. Epidemiological Data
hMPV is a globally circulating respiratory pathogen that primarily affects children under the age of five but also poses significant health risks to older adults and immunocompromised individuals. Across diverse geographical regions, surveillance data consistently show the highest hMPV detection rates in infants and young children, particularly those between 6 months and 4 years of age [5,6,49]. While adults generally show lower positivity rates, those over 65 years old are more likely to experience severe disease outcomes, especially when underlying comorbidities are present. Worldwide data concerning the impact of hMPV on older adults reported a pooled hMPV positivity in hospitalized adults aged ≥65 years with acute respiratory tract infection (ARTI), at 2.46% in high-income countries and 2.01% in low-moderate income countries. This corresponds to an estimated 473,000 hMPV-associated hospital admissions globally in 2019. Incidence and hospitalization rates varied by age, region, and setting, with limited mortality data reported [7].
hMPV demonstrates a clear global seasonality, typically peaking in late winter to early spring across many temperate regions [4,5,11]. Pre-pandemic, its activity generally followed that of RSV and was concentrated between January and April [5]. However, during the COVID-19 pandemic (2020–2021), a marked decline in hMPV incidence was observed globally, attributed to social distancing and public health measures [5,11,50]. In the post-pandemic period, circulation resumed but with atypical seasonality. For instance some regions, like parts of China and Korea, experienced delayed or shifted peaks into the summer and autumn months, though many have since returned to their pre-pandemic seasonal patterns [5,10,50,51]. Geographically, hMPV affects both hemispheres with varying intensity: high incidence rates are seen in children under five in North and South America, Europe, and Asia, with notable outbreaks reported in the US, China, Mexico, and Argentina [5,9,52,53]. In tropical and subtropical areas, like India and parts of Southeast Asia, hMPV seasonality is influenced by the local climate, often peaking during or after the monsoon season [54]. Post-pandemic epidemiological trends also show increased incidence in older pediatric age groups and more frequent household clustering, suggesting evolving transmission dynamics (Figure 2) [5,6].
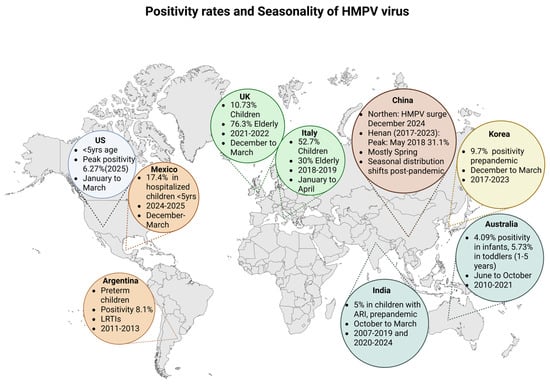
Figure 2.
Positivity rates and seasonality of hMPV virus. US: United States; UK: United Kingdom; LRTIs: Lower respiratory tract infections; ARI: acute respiratory infection. Created in BioRender. L., A. (accessed 30 April 2025) https://BioRender.com/39vha56.
3.1. United States
hMPV remains an endemic virus worldwide, presenting seasonal peaks of disease spread annually. Prior to the pandemic of COVID-19, the seasonal activity of hMPV generally commenced in early January, reaching a peak in late March, and subsided by early June, with a median duration of 21 weeks [5]. Despite a 92% increase in the number of tests performed during and after the pandemic, there was a dramatic 98% drop in hMPV circulation during the period of 2020–2021, likely due to widespread public health measures, which suppressed many respiratory viruses. The 2021–2022 season showed atypical and prolonged circulation (35 weeks) with regional variability, reflecting a period of viral re-emergence and ecological disruption which affected seasonality patterns during and after the pandemic [5]. This non-typical 35-week duration of hMPV activity in the United States was restored to the pre-pandemic seasonal model during the 2022–2023 and 2023–2024 periods. Prior to the COVID-19 pandemic, the circulation of hMPV typically succeeded that of RSV, whose seasonal outbreaks predominantly spanned from October through April. Both viruses commonly exhibited overlapping activity during the winter period [5]. Data from CDC, concerning six seasons prior to the pandemic, demonstrate that the median number of hMPV-positive tests was 16,096.5, with a peak of positive tests during the period of 2017–2018 (4.1% of the PCR tests performed). However, during 2020–2021, the initial COVID-19 period, there was a significant drop in hMPV-positive tests (0.07% of the PCR tests performed) representing the decrease in hMPV circulation during this timeframe (Figure 3).
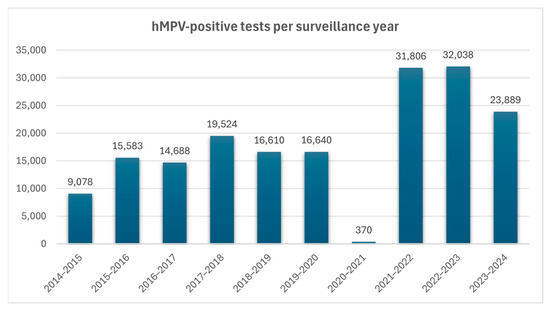
Figure 3.
Numbers of hMPV-positive tests per surveillance year. Data collected from Jobe NB, Rose E, Winn AK, Goldstein L, Schneider ZD, Silk BJ. Human Metapneumovirus Seasonality and Co-Circulation with Respiratory Syncytial Virus—United States, 2014–2024 [5].
The percentage of positive hMPV during the following three seasons reached pre-pandemic levels [5]. During the first 15 weeks of 2025, the number of hMPV-positive test shows a steady increase, reaching a peak of 1216 positive tests during the middle of March of 2025. According to the data from The National Respiratory and Enteric Virus Surveillance System (NRVESS) dashboard, hMPV positivity was estimated at 7.22% during the 15nth week of 2025. This was the highest percentage of positive hMPV PCR tests of this year, possibly following typical seasonal patterns [50] (Figure 4).
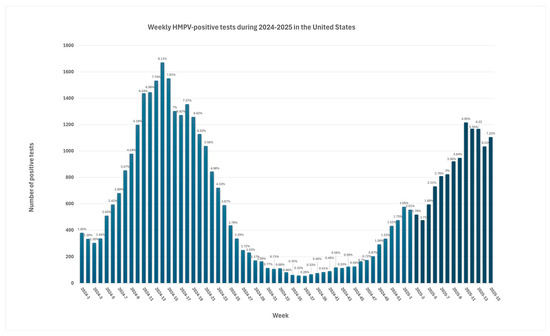
Figure 4.
Weekly hMPV-positive tests during 2024–2025 in the United States. Labels on the chart represent positivity rates [50].
hMPV can affect individuals across the entire age spectrum; however, it is most commonly encountered in children below the age of five years globally [6,8,10,12,49]. Elderly people and immunocompromised patients with hMPV are also susceptible to an acute respiratory illness. Recent data support an estimated hospitalization rate of 231 per 100,000 older adults, equating to about 122,000 cases in 2019 [7]. Although both hMPV and RSV affect pediatric populations, they differ in terms of high-risk groups and clinical management approaches. Hospitalized children with hMPV infection tend to be older compared to those admitted with RSV. In contrast, severe RSV-related illness primarily affects very young infants [6,55]. Bronchiolitis with a need for high-flow oxygen therapy is more commonly associated with RSV. In contrast, hMPV cases more frequently present with pneumonia and have a higher incidence of requiring mechanical ventilation [5]. Data from the CASCADIA Community-Based Cohort—Oregon and Washington for the years 2022–2024 demonstrate an overall incidence of clinically apparent hMPV infection of 7.5 per 100 (95% CI = 6.7–8.4) persons per year. The highest incidence was reported in preschool children aged 2–4 years measuring 19.5 (95% CI = 14.9–25.7) and reaching a peak of 41.3 (95% CI = 29.2–58.4) during January to March. Interestingly further analysis exhibited that hMPV infections tended to cluster within households and different households had different levels of infection risk highlighting the role of close interpersonal contact in the spread of the virus [6].
3.2. Argentina and Mexico
Between 2011 and 2013, a multicenter study in Argentina assessed severe lower respiratory tract infections (LRTIs) in preterm children under two years of age. Among 664 eligible cases, 8.1% tested positive for hMPV was identified in 2 of 60 near-fatal respiratory failure cases, with no associated deaths reported [52]. Interestingly, the study of Libster et al. reported that hMPV was generally associated with less severe illness in hospitalized children compared to other respiratory pathogens in the study. However, children admitted with hMPV-related LRTI, who were born to asthmatic mothers, exhibited an increased risk of life-threatening disease (LTD) [56]. These results could be in accordance with the fact that hMPV infection can elicit a complex immune response characterized by a predominant T-helper 2 (Th2) cytokine profile, including elevated IL-4, IL-5, and thymic stromal lymphopoietin, which resembles the immune patterns observed in asthma [57,58]. Additionally, hMPV may induce Th17-associated cytokines such as IL-1β, IL-6, and IL-17, contributing to airway inflammation [59]. A study conducted during the 2023–2024 winter season in Mexico investigated the viral etiology of acute respiratory infections in 390 hospitalized children under five years of age. hMPV was identified in 17.4% of cases, making it the third most prevalent virus detected. Among the fourteen cases transferred to the intensive care unit (ICU), hMPV was detected in 28.6%. Among the four reported fatalities, hMPV was co-detected in two cases alongside SARS-CoV-2, highlighting its potential contribution to severe disease outcomes in pediatric populations [53].
3.3. Europe
An analysis of hMPV surveillance data from 2012 to early 2025 revealed consistent seasonal patterns, with weekly positivity rates calculated as the proportion of confirmed hMPV cases among the tested samples. Excluding the COVID-19 pandemic seasons (2020/2021 and 2021/2022), hMPV typically exhibited winter seasonality, peaking between weeks 52 and 3 in 60% of seasons, and between weeks 12 and 17 in 40%. The highest recorded peak occurred in week 49 of the 2021/2022 season (10.33%) following the relaxation of pandemic-related restrictions. The median peak positivity was 5.54% (IQR: 5.07–6.09%). During the 2024/2025 season, the peak activity reached 4.6% in weeks 52 and 1, aligning with historical patterns and classified as moderate. Age-specific trends were also consistent, with children aged 0–4 years showing the highest positivity, peaking at 10.73% in week 52 of 2024—within the historical median for this group (11.11%, IQR: 10.34–12.04) [11]. A retrospective case series conducted at Scarborough General Hospital, UK, analyzed adult patients (≥18 years) with confirmed hMPV infection between 31 August 2022, and 1 September 2023. A total of 38 cases were identified, with the majority (76.3%) occurring in individuals aged over 65 years. Clinical presentation typically included influenza-like symptoms, and laboratory data included elevated C-reactive protein (CRP) and white blood cell count (mainly neutrophilia) and an absence of radiological abnormalities. Most patients were discharged, while five deaths occurred, all in patients over 65 years, 80% of whom had pre-existing comorbidities and concurrent acute conditions [60].
A multicenter study in North and Central Italy analyzed 11,577 respiratory samples collected between 1 December 2018, and 30 April 2019. hMPV was detected in 2% of tested samples (222/11,008), with positivity rates varying between 1.2% and 4%, independent of patient characteristics. hMPV circulation began increasing from week 9 of 2019, peaking in week 16 (12.2%). Pediatric patients accounted for 52.7% of hMPV-positive cases, while 30% were individuals over 65 years. Among hospitalized hMPV cases across the entire age spectrum, 11.3% required intensive care [61].
3.4. China and Other Asian Regions
The Pneumonia Etiology Research for Child Health (PERCH) study included children aged 1 to 59 months who were hospitalized with suspected severe pneumonia, along with age- and season-matched community controls, across seven countries in Africa and Asia. hMPV was more commonly detected in hospitalized cases compared to controls and in the control group, hMPV was more frequently detected within the group with respiratory tract illness. Children aged 6–11 months (8.8%) and 12–23 months (7.4%) had the highest rates of hMPV-positive cases, significantly higher than in the younger (6.1%) and older (5.2%) age groups (p < 0.03). Among the cases testing positive for hMPV, 65.2% were infants under one year of age [49].
3.5. China
China, which covers an extensive geographic area and heterogeneous climatic conditions, demonstrates significant regional differences in the incidence, seasonality, and molecular distribution of respiratory pathogens [62]. At the end of December 2024, a spike in ARTIs during the last weeks of 2024 was reported in northern regions of China following the seasonal detection of respiratory viruses including a significant increase in hMPV cases raising public concerns. Analysis of the F-gene from 28 hMPV strains reveals that the predominant genotype shifts depending on the surveillance year, with the most prevalent genotypes in China being A1, B1, and B2 [9]. Data from 2003 to 2023 report a prevalence of 0.97% to 15.88% across different regions, with the highest prevalence observed in Chongqing city, followed by Henan and Tianjin. hMPV distribution was significantly lower in individuals above the age of 5 years old. Higher prevalence occurred during spring and winter. Regarding seasonal distribution, individuals presenting with ARTIs during the spring season exhibited an approximately ninefold increased risk of hMPV infection compared to those sampled in the summer and autumn months. Summary data indicate the circulation of at least five distinct hMPV lineages (A1, A2b, A2c, B1, and B2) across China, with substantial regional variation in their distribution. Among these, the lineages A2b, B1, and B2 have emerged as the dominant strains nationally [62]. Evidence concerning ARTI patients in Henan between 2017 and 2023 reports a hMPV-positive rate of 6.71%, with children below the age of five presenting the highest detection rate at 7.78%. Seasonal outbreaks were reported mainly in spring during 2018 and 2019, with peak detection rates estimated at 31.11% in May 2018 and 19.57% in May 2019, followed by a remarkable peak of 42.11% in November 2020 that lasted until January 2021. After this period and until March 2023, the positive rates ranged between 0 and 8.7%. The predominant lineages identified were A2c111nt within subtype A and B2 within subtype B [63]. Epidemiological data from Shanghai during 2014–2023, on hospitalized patients with respiratory tract infection, exhibited similar results, with the highest hMPV-positive proportions being in the age group of 6–11 months (5.39%). The seasonality of hMPV spread in this area presented with a typical pattern during the pre-pandemic period. The outbreak seasons included winter and spring with peaks of hMPV-positivity seen from February to April during the period of 2014–2019. This seasonal model disappeared during the COVID-19 pandemic period of 2020–2022. The widespread implementation of non-pharmaceutical interventions (NPIs) significantly disrupted the typical seasonal circulation of hMPV leading to altered peak timing and patterns post-pandemic. The following years were characterized by a reversed seasonality pattern with hMPV outbreaks reported during summer and autumn in the area of Shanghai [10].
In Putian, a city with a distinct subtropical climate located in the southeast of China, hMPV presented a positivity of 9.1% during the 2023–2024 season and was among the most common pathogens participating in co-infections, mostly with Influenza B. Viral distribution exhibited notably higher detection frequencies in infants, toddlers, and preschool-age groups and maintained a consistently high positivity rate year-round, with a pronounced peak in incidence observed during the winter months [51]. Data from Beijing examining the prevalence of various pathogens pre- and post-pandemic support that in 2023, along with other viruses, the count of detected hMPV cases in hospitalized infants was the highest of the past six-year period. Post-pandemic data support a higher incidence of most viral agents including hMPV present during the fourth quarter [64]. In the Hangzhou region during 2022 and 2023, hMPV was among the most commonly detected viruses in children with bronchiolitis under the age of two years with a detection rate of 8.8%. During 2023, there was a shift in the seasonal distribution of hMPV compared to 2022, with a peak in detection observed during November and December. However, the pre-pandemic hMPV distribution mostly occurred during spring [65,66]. In Suzhou, during a period of four years from 2020 to 2024, hMPV was detected at 3.7% among hospitalized children with ARTIs. In this study, the peak hMPV distribution appeared between July to December 2022, after the release of the COVID-19 restriction policy. However, during 2023 and 2024, the monthly numbers of hMPV detected cases decreased by 73.2% and 30.3%, respectively. During the four-year period, the highest percentage of hMPV-positive tests was observed among children below the age of five years old [65]. In central China in the area of Jingzhou, hMPV was among the seven most common pathogens in children below the age of 18 with ARΤIs and remained the third most common pathogen in this patient group during 2022 and 2023. The rate of positive cases reached a peak in April and November 2022 as well as October 2023. hMPV presented as a single as well as a co-infection with Influenza B, Human Rhinovirus, Mycoplasma, RSV, and Adenovirus [67]. According to pre-pandemic data from a 10-year study (2010–2019) in southern China, hMPV was identified in 3.5% of pediatric ARTI cases, predominantly among children under six years of age. Its prevalence peaked primarily during the spring season. RSV was the most frequent co-detected pathogen (5.3%). Phylogenetic analysis showed that the sequenced hMPV strains were distributed across the following sublineages, A2b (1.9%), A2c (31.5%), B1 (50.0%), and B2 (16.7%), with the leading subtype being B1 [66].
3.6. hMPV Trends in Other Asian Countries
Data from a nationwide surveillance study in Korea between 2017 and 2023, concerning individuals under 20 years of age, showed a pre-pandemic detection rate of hMPV virus no less than 9.7%. However, there was a zero detection rate during the pandemic period of 2020–2021. hMPV re-emerged, exhibiting atypical seasonality and unusually elevated detection rates. By 2023, the hMPV seasonal distribution had nearly returned to pre-pandemic patterns, though the peak activity remained delayed by approximately 1–3 months. The mean pre-pandemic age of hMPV prevalence was 2.3 ± 2.0 years of age, while in 2023, the mean age was 3.6 ± 2.5 years of age, presenting a statistically significant increase [68]. The changes in hMPV seasonality and incidence during and after the COVID-19 pandemic were largely driven by widespread NPIs, which reduced virus transmission and healthcare utilization, leading to lower detection rates. After NPIs were lifted, decreased population-level immunity due to the interruption in virus circulation likely contributed to increased susceptibility and atypical, intensified outbreaks of respiratory viruses like hMPV [68].
A ten-year retrospective study in Taiwan, from 2013 to 2023, reported temporal fluctuations in the prevalence of hMPV, with a marked peak in 2021, and an absence of detected cases between 2018 and 2020, as well as in 2022. The A2 lineage was the predominant strain, followed by B2. hMPV infections were detected throughout most of the year in Taiwan, with the highest incidence observed between March and May, reaching a peak in April. Penghu County exhibited the highest detection rate, followed by Changhua County and Hsinchu County, while some regions reported no confirmed cases. The findings indicated that children aged 0 to 4 years accounted for the highest proportion of positive cases. Moreover, a statistically significant difference in virus prevalence was observed between individuals under 4 years of age and those over 60 (p < 0.001) [69].
The epidemiological trends of hMPV in children under ten years were analyzed in Tokyo, Japan, from January 2015 to June 2023. Prior to the COVID-19 pandemic (2015–2019), hMPV activity consistently peaked in spring (March–May) without overlapping with RSV seasons. Following the implementation of social restrictions in April–May 2020, hMPV circulation was undetectable until 2021. In 2022, an atypical seasonal shift occurred, with peak detection from May to November. Normal seasonality resumed in 2023, with a peak in May. Age distribution also shifted, with the most affected age group increasing from 1 year pre-pandemic to 3 years post-pandemic (2021–2023) [70]. A second study conducted in Tokyo between January 2021 and June 2024 reported hMPV positivity rates of 6.6% in children aged 0–9 years and 0.2% in adults over 20 years. Among adults aged 20–59 and those over 60, the hospitalization rates due to hMPV infection were 64.3% and 76.9%, respectively—the highest compared to rhinovirus/enterovirus (RV/EV), RSV, and parainfluenza virus type 3 (PIV3). In the older adult group, 2 fatalities were reported among 13 hospitalizations [71].
According to data from India, the pooled prevalence of hMPV infection among children with acute respiratory illness was estimated at 5% (95% confidence interval: 4–6%) during the time period of 2007–2019, whereas between 2020 and 2024 the prevalence was 4%. Subgroup analysis reported a higher prevalence in children younger than 5 years of age (6%) versus older counterparts (2%). Further analysis revealed highest though not significant prevalence of 7% in the Northeast followed by 6% in both the North and West, 5% in the South and 3% in the East [8]. In Gorakhpur, India, during the period between May 2022 to April 2023, the prevalence of hMPV positivity among children under 5 years of age with ARTI was 1.4%. The peak hMPV detection rates during this timeframe occurred in October (7.21%). Co-infection with other respiratory viruses such as Influenza A and Human Adenovirus was also detected. Eastern Uttar Pradesh, India, experiences a predominantly humid subtropical climate with dry winters, which may significantly influence the transmission dynamics of various respiratory viruses. hMPV cases typically begin to appear in September, peak in October, and gradually decline, with the lowest incidence observed during November and December [54].
The Bangladesh cohort of the PERCH study (2011–2014) reported hMPV detection rates of 14.6% in children with severe pneumonia and 20.9% in community controls. Among pneumonia cases, the highest prevalence was observed in children aged 6–11 months, while in community controls it was highest in those aged 12–23 months. Seasonal peaks occurred during August–November 2012, January–March 2013, and September–November 2014 [49]. Between 2014 and 2018, hMPV was the third most frequently detected viral pathogen in children under 2 years of age with acute lower respiratory infection, with a detection rate of 11.7%, following RV/EV and RSV. hMPV activity predominantly occurred from December to March, subsequent to the RSV seasonal peak, with the highest detection rates observed in children aged 6–11 months and 12–17 months [72]. In a comparative study in severely malnourished Bangladeshi children, involving 360 pneumonia cases and 334 controls, hMPV was identified in 4.5% of cases and 1.5% of controls. hMPV, along with RSV, influenza virus, and HPIV, showed a statistically significant independent association with pneumonia, with an odds ratio of 2.7 (95% CI: 1.3–5.5), indicating a notable link between hMPV infection and the occurrence of pneumonia in pediatric patients [73].
A study conducted in Iran between January 2016 and March 2017 on hospitalized children under 14 years with ARTI reported an hMPV detection rate of 2.64%, with the highest prevalence in children under three years of age. The most frequent hMPV co-infections identified were with adenovirus and influenza A virus [74]. In Pakistan, hMPV was present in 6.1% of children with pneumonia. The highest prevalence was observed in the age group of 1–5 years. The annual hMPV distribution in 2015 and 2016 was 5.3% and 7.7%, respectively [75].
3.7. Australia
In the western Australian region between 2010 and 2021, there were 43,322 hMPV tests in children <5 years with respiratory infection. The hMPV testing rate for infants <12 months were 248 per 1000 child-years while the testing rates in all age groups increased during this timeframe. The positivity rate in children was 4.09% in infants compared to 5.73% in toddlers of 1–5 years of age [76].
4. Transmission and Clinical Manifestations
The modes of transmission and epidemiological features of hMPV closely resemble those of other respiratory viruses such as RSV and influenza. hMPV exhibits a seasonal pattern of circulation, predominantly occurring in late winter and spring in temperate regions, though cases have also been reported during summer and early autumn [5]. The virus is primarily transmitted through respiratory droplets and direct or indirect contact with contaminated secretions, including saliva and aerosols. Transmission may occur via large droplets over short distances or smaller airborne particles capable of remaining suspended for longer periods. Following an incubation period of approximately 3 to 5 days, the virus rapidly infects the respiratory tract after inoculation of the nasopharyngeal mucosa [77]. Notably, both symptomatic and asymptomatic individuals can facilitate transmission, with evidence suggesting a potential for presymptomatic spread underscoring the challenges in early detection and containment [12].
Since its identification in 2001, hMPV has been recognized as a significant causative agent in respiratory tract infections across the entire age spectrum. The clinical manifestations of hMPV infection encompass a broad spectrum, ranging from mild upper respiratory tract infection (URTI) including fever, rhinorrhea, pharyngitis, conjunctivitis, and cough to more severe LRTI, including fever, tachypnea, cough, hypoxia, wheezing, croup, and pneumonia [12,13]. Radiographic evaluation may reveal characteristic findings such as lung hyperinflation, pulmonary infiltrates, and peribronchial thickening. Clinically, the symptoms of hMPV infection often overlap with those observed in RSV infections, including cough, fever, dyspnea, rhinorrhea, shortness of breath, rashes, and hypoxia [4,12].
Similar to other respiratory viruses, hMPV has been identified in pediatric patients presenting with acute otitis media co-existing with either URTI or LRTI [78,79]. The post-viral inflammatory response can compromise Eustachian tube function, leading to obstruction and creating favorable conditions for secondary bacterial infection of the middle ear [13]. Additionally, the detection of hMPV in the nasal secretions of 6% of children diagnosed with acute otitis media supports its potential role as a contributing etiological agent alongside other common pathogens [78].
hMPV not only affects infants and children but is also known to cause acute respiratory illness in immunocompromised individuals, the elderly, and patients with comorbidities such as asthma or malignancies [7]. The involvement of hMPV in the exacerbation of asthma has been reported in multiple studies [80,81]. In adult populations, hMPV has been identified in cases of acute asthma exacerbation requiring hospitalization. Beyond asthma, the virus has also been implicated in various other respiratory illnesses in adults, including pneumonia, influenza-like illness, and chronic obstructive pulmonary disease (COPD) [82]. Although the overall incidence of hMPV infection is generally lower in adults compared to children, its ability to cause disease across all age groups remains a significant clinical consideration.
hMPV is a respiratory pathogen capable of causing a wide spectrum of complications, particularly affecting the respiratory system. Although commonly resulting in mild illness, hMPV has been linked to severe pneumonia in healthy adults and life-threatening acute respiratory distress syndrome (ARDS), often requiring intensive interventions such as extracorporeal membrane oxygenation (ECMO) [83,84]. In infants, hMPV can lead to bronchiolitis obliterans and significant respiratory failure necessitating prolonged ventilation [85]. A considerable number of pediatric patients require pediatric ICU admission and various levels of respiratory support [86]. Co-infection with SARS-CoV-2 has been associated with fatal outcomes in children, underscoring the dangers of concurrent viral infections [87]. Cardiovascular complications include myocarditis and cardiogenic shock in both men and women [88], while neurological manifestations range from seizures and encephalopathy to long-term cognitive and motor impacts [89,90,91]. Rare complications include acute hemorrhagic edema of infancy and rhabdomyolysis with multiorgan dysfunction [92]. These diverse and sometimes fatal outcomes highlight the need for heightened clinical awareness and supportive care strategies in managing hMPV infections [93]. These complications are summarized in Table 1.

Table 1.
Severe clinical manifestations of hMPV infection.
5. Diagnostic Methods
Over the past two decades, a variety of molecular techniques have been developed and refined for the detection of hMPV, each with its own advantages and limitations. Conventional reverse transcription–polymerase chain reaction (RT-PCR) methods, initially widely used, target conserved regions like the F and N genes of hMPV for detection and genotyping [94,95]. While these methods provided a foundation for molecular diagnosis, they have gradually been replaced by more sensitive technologies, such as reverse transcription–quantitative polymerase chain reaction (RT-qPCR). This method is now considered the gold standard due to its higher sensitivity, lower contamination risk, and ability to differentiate hMPV subgroups. Multiplex RT-qPCR assays further enhanced diagnostic capabilities by allowing the simultaneous detection of hMPV and other respiratory pathogens [96].
Emerging isothermal amplification methods like loop-mediated isothermal amplification (LAMP) and recombinase-aided amplification (RAA) have introduced simpler, faster alternatives that operate without complex thermal cycling equipment. These methods are especially valuable in low-resource or point-of-care settings [62,97,98]. Innovations such as clustered regularly interspaced short palindromic repeat (CRISPR) and CRISPR-associated proteins (Cas) (CRISPR-Cas12a) combined with isothermal amplification have further expanded the diagnostic options by offering visual detection capabilities with high specificity and speed [99,100]. Advanced techniques like metagenomic next-generation sequencing (mNGS) have enabled the identification of novel or uncommon pathogens, offering whole-genome resolution and deep insights into viral dynamics [101,102]. Despite their significant investigational and identification value, these techniques remain under evaluation for routine clinical use due to factors such as high cost, complexity, and limited accessibility. Virus isolation, although still considered a gold standard for vaccine development and pathogenesis studies is hindered by hMPV’s slow replication and low cytopathic effect (CPE), making it less practical for routine diagnosis [103,104]. The methods are summarized in Table 2.

Table 2.
hMPV detection methods based on PCR and related technologies.
Advancements in molecular diagnostic techniques have significantly improved the detection and characterization of hMPV, with RT-qPCR emerging as the gold standard [95]. Isothermal amplification methods such as LAMP and RAA, along with CRISPR-based assays, offer rapid, equipment-free alternatives suitable for point-of-care use [97,98,99], while mNGS provides comprehensive genomic insights, facilitating the identification of novel or uncommon strains [100,101]. These innovations hold the potential to improve diagnostic accuracy, enable timely clinical decision-making including the avoidance of unnecessary antibiotic use, and enhance surveillance efforts, particularly in diverse healthcare settings.
6. hMPV and Comorbidities
hMPV has emerged as a significant respiratory pathogen, with its impact on outcomes in individuals with pre-existing comorbidities being under considerable research.
Cystic fibrosis (CF) mortality is largely driven by respiratory complications caused by chronic bacterial infections that lead to airway damage and disease progression, yet the role of viral respiratory infections remains less clear [105]. In a cohort of children with CF, a notably high incidence of hMPV infection was observed, with infection rates of 47.8% and 47.3% among those aged 7–12 and 13–18 years, respectively. hMPV was associated with increased LRTI events in CF children during infection periods; however, these increases, including hospitalization rates, were not statistically significant [106]. In an Australian cohort, the prevalence of hMPV was comparable between individuals with and without CF (2.4% and 3.4%, respectively), with a significantly higher detection rate in pediatric CF patients compared to adults (p = 0.0165). Both hMPV and RSV were more frequently identified in children, irrespective of CF status, aligning with their established role as common etiological agents of viral respiratory infections in pediatric populations. Notably, hMPV was undetected in adult CF patients, and RSV was identified in fewer than 1% of this group [105].
Pediatric oncology patients are particularly vulnerable to infections, primarily due to treatment-induced neutropenia. hMPV ranks among the ten most frequently identified respiratory viruses in this population, detected in 3.3–13.5% of acute respiratory infection cases and in 0.4–44% of febrile neutropenia episodes. hMPV is often the sole pathogen, though co-infections with other viruses or bacteria are common. In some instances, hMPV has been implicated in severe outcomes, including fatal cases, particularly among hematopoietic stem cell transplant recipients [107]. Notably, hMPV was responsible for a documented outbreak in a pediatric oncology unit [108]. A retrospective study of 30 oncology pediatric patients positive for hMPV revealed that 46.7% developed lower respiratory tract infections, while 53.3% had upper respiratory involvement. The median duration of illness was 6.5 days, with 66.6% requiring hospital admission. Notably, none of the patients needed mechanical ventilation, although one case required prolonged inhaled steroid and bronchodilator therapy. All children recovered fully, indicating that despite hMPV infection being able to cause considerable disease in this population it is not typically fatal [109].
The incidence of hMPV in immunocompromised patients, including those with hematological malignancies, can be as high as 40%, with a slightly higher rate observed in adults compared to children [110]. hMPV can cause both upper and lower respiratory tract infections, with symptoms ranging from mild nasal congestion and cough to severe pneumonia, hypotension, and shock if LRTI develops. Risk factors for LRTI include steroid use, low lymphocyte count, and early onset of infection after hematopoietic cell transplantation (HCT) [110,111]. Mortality associated with hMPV infection ranges, depending on the severity of the illness [110]. hMPV was also identified in immunocompromised patients with acute myeloid leukemia (AML) who developed severe respiratory symptoms. Despite treatment with ribavirin, outcomes varied: one patient improved, while others experienced rapid deterioration and death. These cases suggest hMPV as a significant contributor to respiratory complications in neutropenic AML patients, with outcomes potentially worsened by their immunosuppressed state [112].
7. hMPV in the Perinatal Period
Pregnant women represent a distinct population due to the immunomodulatory effects of gestation. Thus, they are at an increased risk for complications from seasonal and pandemic viral infections including hMPV [113,114]. hMPV infection has been accused of causing acute respiratory syndrome in pregnant women [114,115]. Furthermore, a prospective study that included asthmatic and non-asthmatic pregnant women reported that in women with asthma, laboratory-confirmed viral infections (including hMPV) were significantly associated with adverse maternal health outcomes, with 60% of infections correlating with episodes of uncontrolled asthma and an elevated risk of complications [116]. In this study, women with asthma who tested positive for respiratory viruses by PCR (including hMPV) had significantly higher odds of developing preeclampsia or pregnancy-induced hypertension, even after accounting for the established risk factors for preeclampsia [116].
There are a few case reports on hMPV infections during pregnancy, none of which report vertical hMPV transmission. A 24-year-old pregnant woman at 30 weeks’ gestation developed respiratory insufficiency following the onset of fever and urinary tract infection. Despite normal chest X-rays, she progressed to significant hypoxia with bilateral alveolar consolidations identified on a CT scan and tested positive for hMPV. At 36 weeks of gestation, she gave uncomplicated birth to a healthy female neonate [31]. A 40-year-old pregnant woman at 29 weeks’ gestation with a history of asthma was admitted to the ICU due to worsening respiratory symptoms caused by hMPV infection. After receiving ICU care, her condition gradually improved and she was discharged. At nearly 39 weeks of gestation, she underwent uncomplicated vaginal delivery of a 3600 g female neonate, with no clinical evidence of infection in the newborn [117]. A 36-year-old woman at 31 weeks of gestation was admitted for respiratory symptoms secondary to an hMPV infection. Her clinical course was complicated by a secondary lower respiratory tract infection requiring antibiotic therapy. She showed gradual improvement and was discharged on hospital day 8. At 39 weeks of gestation, she underwent an elective repeat cesarean delivery of a healthy 3230 g female neonate [117]. An 18-year-old, late-preterm pregnant woman developed acute respiratory distress syndrome after hMPV infection and required ICU admission, intubation, and primary cesarean delivery due to worsening respiratory function. Her condition further deteriorated postpartum, necessitating extracorporeal membrane oxygenation. She eventually recovered with supportive care. At birth, the neonate required resuscitation; however, by the second minute of life, the infant had been stabilized and showed no signs of infection [114]. A 34-year-old term pregnant woman developed pneumonia due to hMPV shortly after cesarean delivery, presenting with worsening cough, bilateral wheezing, and pleural effusion with left basal consolidation. She was transferred to the ICU for anticipated respiratory decompensation. She showed progressive clinical improvement, leading to discharge on day 6 with stable oxygen saturation on room air [118]. A 37-year-old woman at 7 months of gestation was admitted to the ICU with severe respiratory symptoms and was diagnosed with hMPV pneumonia, confirmed by PCR and supported by CT findings of bilateral ground-glass opacities. Following supportive care and antimicrobial therapy, her respiratory status improved, allowing for de-escalation of oxygen support and discharge in stable condition after six days [118].
Studies have examined the possible effects of viral respiratory infections on both the mother and the fetus, linking them to adverse birth outcomes in the general frame of a systemic inflammatory response [119]. The intrauterine environment may exert a substantial influence on the developing immune system, triggering both innate and adaptive immune responses, potentially mediated by placental inflammation [119]. Maternal hMPV infection has not been found to undergo intrauterine transmission; however, it has been linked to altered birth outcomes [113,120]. The study of Lenahan et al. on the effects of hMPV and other viral infections on birth outcomes in Nepal correlated hMPV febrile illness during pregnancy with an increased risk of SGA in infants (risk ratio 1.7; p = 0.031). In the same study, the median maternal symptom duration was five days. Nearly half (43.6%) of pregnant women infected with hMPV also experienced a viral co-infection, primarily with rhinovirus, and to a lesser extent, coronavirus and parainfluenza [113]. Murphy et al. reported a significantly lower birth weight and length in neonates born from non-asthmatic mothers with at least one PCR-confirmed common cold, including hMPV, during pregnancy [116].
Data from South Africa revealed an increased susceptibility of LRTI hospitalization in hMPV-infected infants under 6 months of age that have been exposed to but are uninfected by maternal HIV infection (HEU), compared to a group of unexposed and uninfected age-matched infants born from HIV-infected mothers [112]. Studies aiming to further investigate this phenomenon found a decrease in hMPV-neutralizing antibody levels in HEU infants, compared to matched HIV-1 unexposed controls. Concurrently, the transfer of hMPV-neutralizing antibodies across the placenta was significantly reduced in women with HIV-1 compared to HIV-negative counterparts. However, lower cord blood titers of hMPV-neutralizing antibodies were not significantly correlated with early hMPV infection in infants under six months of age in this study [121]. The impact of intrauterine programming on the offsprings’ immune system against viral infections such as hMPV has also been examined in animal models. Female adult mice gestated in a hyperthyroxinemic intrauterine environment experienced a more severe hMPV infection, consistent with the increased histopathological pulmonary inflammation after seven days of infection. These results support the notion that gestation under hypothyroxinemia stimulated an extrauterine altered inflammatory response against hMPV infection [122].
8. Treatment and Prevention Strategies—Recent Data
8.1. Putative Antiviral Agents
Currently, the therapeutic options for hMPV infections are predominantly supportive. Symptomatic management includes the administration of antipyretics, such as acetaminophen or ibuprofen, to control fever. Intravenous fluids may be required in cases of dehydration, particularly when oral intake is insufficient [16]. In severe presentations involving respiratory failure, patients—especially those with underlying cardiopulmonary conditions or compromised immune systems—may require supplemental oxygen or mechanical ventilation. While most individuals recover, infection control measures such as droplet precautions are essential to prevent transmission [15,16].
Ribavirin, a nucleoside analog commonly used in the treatment of hepatitis C and other viral infections, is the most frequently administered antiviral agent. However, due to its potential teratogenicity and adverse effects such as hemolytic anemia, its use is generally limited to severe hMPV cases [12]. Recent research on hMPV has identified several promising therapeutic candidates targeting various stages of the viral lifecycle. Computational studies have highlighted the potential of the N protein as an antiviral target, with compounds such as ZINC85629735 (M1) and ZINC85569125 (M3) showing strong binding affinities and favorable stability in molecular dynamics simulations, making them promising drug candidates [123]. Other interventions, such as the use of interferons (IFN-ε and IFN-λ), have been shown to modulate immune responses and reduce viral loads without inducing inflammation, suggesting their therapeutic potential [124,125]. Moreover, FDA-approved drugs like Probenecid have been repurposed for hMPV treatment, exhibiting broad-spectrum antiviral effects [126]. The non-nucleoside inhibitor JNJ-8003 has also shown promise, inhibiting viral replication by targeting the RNA-dependent RNA polymerase complex [127].
Natural glycans such as lacto-N-neotetraose (LNnT) and glycosaminoglycans—especially heparin—have demonstrated strong, dose-dependent inhibition of viral binding to the host cell via interactions with the F protein, highlighting their potential as viral entry inhibitors [30]. Additionally, natural compounds, including Ginkgolic acid (GA), Fumarprotocetraric acid (FUM), and Geraniin (GE), have demonstrated effective antiviral activity, primarily by inhibiting viral entry and replication in the early stages of infection [128,129]. Quercetin, a bioactive flavonoid, exhibited multi-target antiviral effects by interacting with the viral matrix protein and reducing oxidative stress and inflammation, with nanoformulations overcoming its solubility and bioavailability limitations [130]. Researchers identified monoclonal antibodies (mAb 5-1 and mAb 338), with mAb 5-1 demonstrating broad neutralizing activity against both RSV and hMPV by targeting a unique epitope on the viral fusion protein, while mAb 338 effectively reduced viral replication and lung inflammation in hMPV-infected mice, showing both short- and long-term protective effects, thus highlighting their potential therapeutic applications [131,132]. These findings underscore the diverse approaches under investigation for hMPV treatment, from immune modulation to small molecule inhibitors, which hold significant potential for managing this respiratory pathogen. The summarized data is presented in Table 3.

Table 3.
Putative hMPV antiviral agents.
8.2. Research on Immunization Strategies
Although there are currently no licensed vaccines or antiviral treatments for hMPV, several vaccine candidates are undergoing early-stage clinical evaluation. Neutralizing monoclonal antibodies (nMAbs) represent a fundamental component of vaccine-induced immunity and serve as critical therapeutic agents against various infectious agents [133]. Recent advancements in the structural biology of the hMPV F protein, along with prior insights from RSV and other enveloped viruses, have significantly propelled the development of nMAbs targeting hMPV [133]. Multiple strategies are under investigation for the development of effective vaccines against hMPV and related respiratory viruses, including live attenuated, virus-like particle (VLP), mRNA-based, and subunit vaccine platforms. A live attenuated rhMPV vaccine showed limited infectivity and immunogenicity in children leading to trial discontinuation [134]. In contrast, the VLP-based IVX-A12 demonstrated robust and sustained immune responses in older adults [135], while the bivalent Metavac®-RSV LAV induced strong mucosal and systemic immunity in preclinical models [136]. Stabilized prefusion F protein trimers and Gag-fused VLPs elicited potent neutralizing antibody responses, highlighting the role of structure-guided design [137]. Plant-derived adjuvants such as cpcQS-21 showed comparable efficacy to conventional adjuvants, offering a scalable alternative [138]. Additionally, the mRNA-1653 vaccine showed durable humoral responses against hMPV, and a novel dual-cleaved Pre-F protein design enhanced immunogenicity and structural stability [139]. Collectively, these studies underscore the promise of diverse, next-generation platforms in advancing hMPV vaccine development. Current research on the design of hMPV vaccines is summarized in Table 4.

Table 4.
Research on immunization strategies.
Encouraged by the success of novel hRSV vaccines in phase III trials, current hMPV vaccine research emphasizes the structural characterization of viral antigens and their corresponding neutralizing epitopes. A growing repertoire of high-affinity nMAbs against hMPV has been discovered, some demonstrating the cross-neutralization of hRSV. These findings offer valuable perspectives for the development of both prophylactic and therapeutic interventions targeting hMPV and hRSV infections [140]. Such challenges have prompted a shift toward next-generation vaccine strategies, particularly those that are structure-guided and rely on the identification of potent neutralizing antibodies. Recent breakthroughs in vaccine technology, particularly those applied to hRSV, have demonstrated robust immunogenicity and favorable safety profiles in late-stage clinical trials [140,141]. These approaches, grounded in the precision of structural vaccinology and informed by epitope-specific antibody responses, now upgrade hMPV vaccine development.
Despite significant advances in hMPV vaccine development, several challenges remain. Antigenic variation in the hMPV genome, which alters protective epitopes, can undermine vaccine efficacy by failing to provide sufficient immunity against circulating strains [142]. Additionally, while live attenuated vaccines offer effectiveness, they pose safety risks, particularly for immunocompromised individuals, and inactivated vaccines may not generate robust immune responses [134]. Long-lasting immunity is essential for herd immunity, necessitating innovative strategies to extend the duration of vaccine-induced protection. The exploration of novel vaccine platforms, such as mRNA vaccines and nanoparticle-based delivery systems, could improve both safety and efficacy. Collaborative efforts among researchers and manufacturers are crucial to developing univalent or multivalent vaccines that target hMPV alongside other respiratory pathogens.
8.3. Challenges in Outbreak Control in Institutional Settings
Controlling infectious disease outbreaks—and hMPV transmission in particular—in institutional settings such as long-term care facilities (LTCFs) and pediatric long-term care facilities (pLTCFs) presents persistent and multifaceted challenges. A key factor contributing to outbreaks is the failure to consistently implement and adhere to fundamental infection-prevention protocols [143,144]. Despite recommendations from health authorities like the CDC, work restrictions for infected or exposed healthcare personnel are not always enforced, even though staff are frequently implicated in the transmission of viral infections such as hMPV. The presence of symptomatic employees during outbreaks highlights the critical need for the stricter enforcement of exclusion policies and underscores the importance of maintaining rigorous daily infection control practices [143,144].
Additionally, resource limitations in LTCFs may impede the execution of non-pharmaceutical interventions and other infection control strategies. In pediatric settings, the challenges are compounded by unique environmental and clinical factors, including the frequent use of medical devices, developmental behaviors that increase exposure risk, and the presence of communal activities such as schooling [145]. While targeted interventions—such as the employment of specialized infection control personnel and enhanced hygiene protocols—have shown promise in reducing outbreak incidence, the complete prevention of ARIs remains unlikely. Thus, strategies should prioritize minimizing pathogen transmission to prevent hospital transfers and maintain the continuity of essential services. These complexities emphasize the need for ongoing research and policy development to enhance outbreak preparedness and response in institutional care environments [145].
Outbreak control in childcare institutions is particularly complex due to the close contact among young children, who are highly susceptible to and efficient at transmitting infectious agents. Newly enrolled children are at a heightened risk of respiratory infections such as hMPV infection, which can subsequently affect caregivers, families, and the broader community [146,147]. Effective prevention relies on strict adherence to infection control measures such as hand hygiene, immunization compliance, environmental sanitation, and structured exclusion policies. However, asymptomatic shedding often precedes clinical illness, making proactive strategies and continuous education for staff and parents essential. Collaborative efforts with public health authorities and adherence to evidence-based guidelines are crucial for mitigating hMPV transmission and managing outbreaks in these high-risk environments [146].
9. Conclusions
hMPV remains a significant yet underrecognized contributor to acute lower respiratory tract infections, posing a substantial global health burden, especially among vulnerable populations such as young children, older adults, and immunocompromised individuals. Although advancements in diagnostic techniques—including RT-qPCR, mNGS, and immunoassays—have improved detection, clinical management continues to rely primarily on supportive care due to the absence of an approved vaccine or specific antiviral treatment. The virus, although only formally identified in recent decades, likely circulated for years beforehand, and its resurgence underscores the need for continued vigilance and research.
The data presented in this review provide a critical foundation for informing clinical and public health strategies aimed at mitigating the global burden of hMPV. Clinical risk stratification can be assessed by identifying the populations most vulnerable to severe hMPV infection, such as infants, older adults, and immunocompromised individuals. Insights into seasonal and geographic patterns of circulation can support the timing and allocation of diagnostic resources, while the characterization of cytokine responses may guide the development of targeted immunomodulatory therapies. Furthermore, the review’s synthesis of diagnostic advancements can aid clinicians and laboratories in selecting the most appropriate and sensitive detection methods to improve early diagnosis and patient management.
Current surveillance systems for viral respiratory infections, including hMPV, exhibit notable limitations in both timeliness and the integration of critical data streams. The lack of standardized, digitalized, and interoperable frameworks across EU/EEA countries hinders effective response coordination, particularly for emerging or underrecognized pathogens like hMPV. Many national systems still rely on outdated paper-based reporting, contributing to delays and fragmentation in data flow. Real-time diagnostics and the incorporation of electronic health records, mobile health applications, and other digital sources hold considerable promise in bridging these gaps by enabling earlier detection, better risk assessment, and more agile public health interventions [148,149,150]. Expanding molecular and genomic surveillance for hMPV, integrated with epidemiological and environmental data, is essential to improve outbreak prediction and response. Moreover, the COVID-19 pandemic has underscored the urgent need to modernize epidemic intelligence by leveraging big data and AI, ensuring that surveillance outputs are timely, comparable, and actionable at both the national and supranational levels.
Looking ahead, prioritizing the development of effective vaccines, particularly mRNA-based and multivalent platforms, is essential to mitigate the impact of hMPV across diverse population groups. Parallel efforts must focus on the discovery and evaluation of targeted therapeutics, such as monoclonal antibodies and novel small molecules, to expand treatment options. Strengthening surveillance systems, enhancing public awareness, and improving the sensitivity and accessibility of diagnostic tools—especially in resource-limited settings—are crucial to achieving timely intervention and control. Furthermore, large-scale epidemiological and immunological studies are needed to better understand hMPV’s seasonal trends, co-infection dynamics, and geographic distribution. Expanding clinical trials to include high-risk cohorts will be vital to ensure equitable access to emerging therapeutics and vaccines, ultimately reducing the morbidity and mortality associated with this re-emerging respiratory pathogen.
Author Contributions
Conceptualization, A.L., R.S. and A.G.T.; methodology, A.L., R.S. and A.G.T.; data curation, P.I., E.-D.B., A.B., A.K., K.A.T., D.T., M.L. and N.I.; writing—original draft preparation, A.L. and R.S.; writing—review and editing, P.I., E.-D.B., A.B., A.K., K.A.T., D.T., M.L. and N.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- van den Hoogen, B.G.; de Jong, J.C.; Groen, J.; Kuiken, T.; de Groot, R.; Fouchier, R.A.; Osterhaus, A.D. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2001, 7, 719–724. [Google Scholar] [CrossRef] [PubMed]
- van den Hoogen, B.G.; Bestebroer, T.M.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Analysis of the Genomic Sequence of a Human Metapneumovirus. Virology 2002, 295, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Ruigrok, R.W.H.; Crépin, T.; Kolakofsky, D. Nucleoproteins and nucleocapsids of negative-strand RNA viruses. Curr. Opin. Microbiol. 2011, 14, 504–510. [Google Scholar] [CrossRef]
- Porwal, S.; Malviya, R.; Sridhar, S.B.; Shareef, J.; Wadhwa, T. Global impact of hMPV virus: Transmission, pathogenesis, diagnostic and treatment. Diagn. Microbiol. Infect. Dis. 2025, 112, 116809. [Google Scholar] [CrossRef]
- Jobe, N.; Rose, E.; Winn, A.; Goldstein, L.; Schneider, Z.; Silk, B. Human Metapneumovirus Seasonality and Co-Circulation with Respiratory Syncytial Virus—United States, 2014-2024. MMWR. Morb. Mortal. Wkly. Rep. 2025, 74, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Shakya, M.; Chu, H.Y.; Englund, J.A.; Briggs-Hagen, M.; Carone, M.; Kuntz, J.L.; Lockwood, T.; Midgley, C.M.; Schmidt, M.A.; Starita, L.; et al. Epidemiology of Symptomatic Human Metapneumovirus Infection in the CASCADIA Community-Based Cohort—Oregon and Washington, 2022-2024. MMWR Morb Mortal Wkly. Rep. 2025, 74, 188–193. [Google Scholar] [CrossRef]
- Kulkarni, D.; Cong, B.; Ranjini, M.J.K.; Balchandani, G.; Chen, S.; Liang, J.; González Gordon, L.; Sobanjo-ter Meulen, A.; Wang, X.; Li, Y.; et al. The global burden of human metapneumovirus-associated acute respiratory infections in older adults: A systematic review and meta-analysis. Lancet Healthy Longev. 2025, 6, 100679. [Google Scholar] [CrossRef]
- Debnath, A.; Halder, P.; Achary, T.; Bir, R.; Mondal, A.; Ish, P. Prevalence of human metapneumovirus infection among children suffering from acute respiratory illness in India: A systematic review and meta-analysis. Monaldi Arch. Chest Dis. = Arch. Monaldi Per Le Mal. Del Torace 2025. [Google Scholar] [CrossRef]
- Al-Tawfiq, J.A.; Memish, Z.A. The surge of human metapneumovirus (hMPV) cases in China and global implications. New Microbes New Infect. 2025, 63, 101563. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, L.; Wang, X.; Wu, R.; Zhang, A.; Zhao, B.; Ye, C. Epidemiological and Clinical Characteristics of Human Metapneumovirus-Associated Acute Respiratory Tract Infection Cases in the Pudong New Area, Shanghai, from 2014 to 2023. J. Epidemiol. Glob. Health 2025, 15, 38. [Google Scholar] [CrossRef]
- Sinnathamby, M.A.; Savagar, B.; Rawlinson, C.; Rome, M.; Allen, A.; Watson, C. Human metapneumovirus (hMPV) test-positivity sentinel laboratory trends in England, 2012 to 2025. medRxiv 2025, 2025.2003.2004.25322918. [Google Scholar] [CrossRef]
- Mishra, B.; Mohapatra, D.; Tripathy, M.; Mamidi, P.; Mohapatra, P.R. A Re-emerging Respiratory Virus: Human Metapneumovirus (hMPV). Cureus 2025, 17, e78354. [Google Scholar] [CrossRef]
- Mastrolia, M.V.; Esposito, S. Metapneumovirus Infections and Respiratory Complications. Semin. Respir. Crit. Care Med. 2016, 37, 512–521. [Google Scholar] [CrossRef]
- Babawale, P.I.; Guerrero-Plata, A. Respiratory Viral Coinfections: Insights into Epidemiology, Immune Response, Pathology, and Clinical Outcomes. Pathogens 2024, 13, 316. [Google Scholar] [CrossRef]
- Troncoso-Bravo, T.; Ramírez, M.A.; Loaiza, R.A.; Román-Cárdenas, C.; Papazisis, G.; Garrido, D.; González, P.A.; Bueno, S.M.; Kalergis, A.M. Advancement in the development of mRNA-based vaccines for respiratory viruses. Immunology 2024, 173, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Thomas, M. Human Metapneumovirus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Krüger, N.; Laufer, S.A.; Pillaiyar, T. An overview of progress in human metapneumovirus (hMPV) research: Structure, function, and therapeutic opportunities. Drug Discov. Today 2025, 30, 104364. [Google Scholar] [CrossRef] [PubMed]
- Renner, M.; Bertinelli, M.; Leyrat, C.; Paesen, G.C.; Saraiva de Oliveira, L.F.; Huiskonen, J.T.; Grimes, J.M. Nucleocapsid assembly in pneumoviruses is regulated by conformational switching of the N protein. eLife 2016, 5, e12627. [Google Scholar] [CrossRef]
- Renner, M.; Paesen, G.C.; Grison, C.M.; Granier, S.; Grimes, J.M.; Leyrat, C. Structural dissection of human metapneumovirus phosphoprotein using small angle x-ray scattering. Sci. Rep. 2017, 7, 14865. [Google Scholar] [CrossRef] [PubMed]
- Derdowski, A.; Peters, T.R.; Glover, N.; Qian, R.; Utley, T.J.; Burnett, A.; Williams, J.V.; Spearman, P.; Crowe, J.E. Human metapneumovirus nucleoprotein and phosphoprotein interact and provide the minimal requirements for inclusion body formation. J. Gen. Virol. 2008, 89, 2698–2708. [Google Scholar] [CrossRef]
- Leyrat, C.; Renner, M.; Harlos, K.; Grimes, J.M. Solution and crystallographic structures of the central region of the phosphoprotein from human metapneumovirus. PLoS ONE 2013, 8, e80371. [Google Scholar] [CrossRef]
- El Najjar, F.; Cifuentes-Muñoz, N.; Chen, J.; Zhu, H.; Buchholz, U.J.; Moncman, C.L.; Dutch, R.E. Human metapneumovirus Induces Reorganization of the Actin Cytoskeleton for Direct Cell-to-Cell Spread. PLoS Pathog. 2016, 12, e1005922. [Google Scholar] [CrossRef] [PubMed]
- Leyrat, C.; Renner, M.; Harlos, K.; Huiskonen, J.T.; Grimes, J.M. Structure and Self-Assembly of the Calcium Binding Matrix Protein of Human Metapneumovirus. Structure 2014, 22, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Schowalter Rachel, M.; Smith Stacy, E.; Dutch Rebecca, E. Characterization of Human Metapneumovirus F Protein-Promoted Membrane Fusion: Critical Roles for Proteolytic Processing and Low pH. J. Virol. 2006, 80, 10931–10941. [Google Scholar] [CrossRef] [PubMed]
- Leyrat, C.; Renner, M.; Harlos, K.; Huiskonen, J.T.; Grimes, J.M.J.E. Drastic changes in conformational dynamics of the antiterminator M2-1 regulate transcription efficiency in Pneumovirinae. eLife 2014, 3, e02674. [Google Scholar] [CrossRef]
- Masante, C.; Najjar, F.E.; Chang, A.; Jones, A.; Moncman, C.L.; Dutch, R.E. The Human Metapneumovirus Small Hydrophobic Protein Has Properties Consistent with Those of a Viroporin and Can Modulate Viral Fusogenic Activity. J. Virol. 2014, 88, 6423–6433. [Google Scholar] [CrossRef]
- Cheemarla, N.R.; Guerrero-Plata, A. Human Metapneumovirus Attachment Protein Contributes to Neutrophil Recruitment into the Airways of Infected Mice. Viruses 2017, 9, 310. [Google Scholar] [CrossRef]
- Panda, S.; Mohakud, N.K.; Pena, L.; Kumar, S. Human metapneumovirus: Review of an important respiratory pathogen. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2014, 25, 45–52. [Google Scholar] [CrossRef]
- Thompson, A.; de Vries, R.; Paulson, J. Virus recognition of glycan receptors. Curr. Opin. Virol. 2019, 34, 117–129. [Google Scholar] [CrossRef]
- Van Den Bergh, A.; Bailly, B.; Guillon, P.; von Itzstein, M.; Dirr, L. Novel insights into the host cell glycan binding profile of human metapneumovirus. J. Virol. 2024, 98, e0164123. [Google Scholar] [CrossRef]
- Haas, L.E.M.; de Rijk, N.X.; Thijsen, S.F.T. Human metapneumovirus infections on the ICU: A report of three cases. Ann. Intensive Care 2012, 2, 30. [Google Scholar] [CrossRef]
- Galvez, N.; Andrade Parra, C.; Pacheco, G.; Soto, J.; Stranger, V.; Rivera, T.; Vasquez, A.; Kalergis, A. Host Components That Modulate the Disease Caused by hMPV. Viruses 2021, 13, 519. [Google Scholar] [CrossRef] [PubMed]
- Kolli, D.; Velayutham, T.S.; Casola, A. Host-Viral Interactions: Role of Pattern Recognition Receptors (PRRs) in Human Pneumovirus Infections. Pathogens 2013, 2, 232–263. [Google Scholar] [CrossRef]
- Uche, I.K.; Guerrero-Plata, A. Interferon-Mediated Response to Human Metapneumovirus Infection. Viruses 2018, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.A.; Pacheco, G.A.; Gálvez, N.M.; Soto, J.A.; Bueno, S.M.; Kalergis, A.M.J.V. Innate immune components that regulate the pathogenesis and resolution of hRSV and hMPV infections. Viruses 2020, 12, 637. [Google Scholar] [CrossRef] [PubMed]
- Babawale, P.I.; Guerrero-Plata, A.J.V. Differential Responses of Pediatric and Adult Primary Epithelial Cells to Human Metapneumovirus and Respiratory Syncytial Virus Infection. Viruses 2025, 17, 380. [Google Scholar] [CrossRef]
- Velayutham, T.S.; Ivanciuc, T.; Garofalo, R.P.; Casola, A. Role of human metapneumovirus glycoprotein G in modulation of immune responses. Front. Immunol. 2022, 13, 962925. [Google Scholar] [CrossRef]
- Ni, W.; Wei, X.; Wu, R.J.J.o.P.I.D. Innate Immune Response-Mediated Inflammation in Viral Pneumonia. J. Pediatr. Infect. Dis. 2024, 17, 140–153. [Google Scholar] [CrossRef]
- Sepúlveda-Alfaro, J.; Catalán, E.A.; Vallejos, O.P.; Ramos-Tapia, I.; Madrid-Muñoz, C.; Mendoza-León, M.J.; Suazo, I.D.; Rivera-Asin, E.; Silva, P.H.; Alvarez-Mardones, O.; et al. Human metapneumovirus respiratory infection affects both innate and adaptive intestinal immunity. Front. Immunol. 2024, 15, 1330209. [Google Scholar] [CrossRef]
- Murdaca, G.; Paladin, F.; Tonacci, A.; Borro, M.; Greco, M.; Gerosa, A.; Isola, S.; Allegra, A.; Gangemi, S.J.B. Involvement of Il-33 in the pathogenesis and prognosis of major respiratory viral infections: Future perspectives for personalized therapy. Biomedicines 2022, 10, 715. [Google Scholar] [CrossRef]
- Xiang, W.-q.; Li, L.; Wang, B.-h.; Ali, A.F.; Li, W. Profiles and predictive value of cytokines in children with human metapneumovirus pneumonia. Virol. J. 2022, 19, 214. [Google Scholar] [CrossRef]
- Saravia, J.; You, D.; Shrestha, B.; Jaligama, S.; Siefker, D.; Lee, G.I.; Harding, J.N.; Jones, T.L.; Rovnaghi, C.; Bagga, B.; et al. Respiratory Syncytial Virus Disease Is Mediated by Age-Variable IL-33. PLoS Pathog. 2015, 11, e1005217. [Google Scholar] [CrossRef] [PubMed]
- Lau-Kilby, A.W.; Turfkruyer, M.; Kehl, M.; Yang, L.; Buchholz, U.J.; Hickey, K.; Malloy, A.M.W. Type I IFN ineffectively activates neonatal dendritic cells limiting respiratory antiviral T-cell responses. Mucosal Immunol. 2020, 13, 371–380. [Google Scholar] [CrossRef]
- Eddens, T.; Parks, O.B.; Zhang, Y.; Manni, M.L.; Casanova, J.L.; Ogishi, M.; Williams, J.V. PD-1 signaling in neonates restrains CD8(+) T cell function and protects against respiratory viral immunopathology. Mucosal Immunol. 2024, 17, 476–490. [Google Scholar] [CrossRef]
- Eddens, T.; Parks, O.B.; Williams, J.V. Neonatal Immune Responses to Respiratory Viruses. Front. Immunol. 2022, 13, 863149. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Rajput, C.; Hong, J.Y.; Lei, J.; Hinde, J.L.; Wu, Q.; Bentley, J.K.; Hershenson, M.B. The Innate Cytokines IL-25, IL-33, and TSLP Cooperate in the Induction of Type 2 Innate Lymphoid Cell Expansion and Mucous Metaplasia in Rhinovirus-Infected Immature Mice. J. Immunol. Baltim. Md. 1950 2017, 199, 1308–1318. [Google Scholar] [CrossRef]
- Melendi, G.A.; Laham, F.R.; Monsalvo, A.C.; Casellas, J.M.; Israele, V.; Polack, N.R.; Kleeberger, S.R.; Polack, F.P. Cytokine profiles in the respiratory tract during primary infection with human metapneumovirus, respiratory syncytial virus, or influenza virus in infants. Pediatrics 2007, 120, e410–e415. [Google Scholar] [CrossRef] [PubMed]
- Pancham, K.; Perez, G.F.; Huseni, S.; Jain, A.; Kurdi, B.; Rodriguez-Martinez, C.E.; Preciado, D.; Rose, M.C.; Nino, G. Premature infants have impaired airway antiviral IFNγ responses to human metapneumovirus compared to respiratory syncytial virus. Pediatr. Res. 2015, 78, 389–394. [Google Scholar] [CrossRef]
- Miyakawa, R.; Zhang, H.; Brooks, W.A.; Prosperi, C.; Baggett, H.C.; Feikin, D.R.; Hammitt, L.L.; Howie, S.R.C.; Kotloff, K.L.; Levine, O.S.; et al. Epidemiology of human metapneumovirus among children with severe or very severe pneumonia in high pneumonia burden settings: The Pneumonia Etiology Research for Child Health (PERCH) study experience. Clin. Microbiol. Infect. 2025, 31, 441–450. [Google Scholar] [CrossRef]
- The National Respiratory and Enteric Virus Surveillance System (NREVSS). Available online: https://www.cdc.gov/nrevss/php/dashboard/index.html (accessed on 16 April 2025).
- Zhu, J.; Wu, S.; Chen, Y.; Zheng, L. Prevalence and distribution of respiratory pathogens in pediatric acute respiratory infections in Putian, China. BMC Infect. Dis. 2025, 25, 278. [Google Scholar] [CrossRef]
- Ofman, G.; Pradarelli, B.; Caballero, M.T.; Bianchi, A.; Grimaldi, L.A.; Sancilio, A.; Duenas, K.; Rodriguez, A.; Ferrero, F.; Ferretti, A.; et al. Respiratory Failure and Death in Vulnerable Premature Children With Lower Respiratory Tract Illness. J. Infect. Dis. 2020, 222, 1129–1137. [Google Scholar] [CrossRef]
- Leija-Martínez, J.J.; Cadena-Mota, S.; González-Ortiz, A.M.; Muñoz-Escalante, J.C.; Mata-Moreno, G.; Hernández-Sánchez, P.G.; Vega-Morúa, M.; Noyola, D.E. Respiratory Syncytial Virus and Other Respiratory Viruses in Hospitalized Infants During the 2023-2024 Winter Season in Mexico. Viruses 2024, 16, 1917. [Google Scholar] [CrossRef]
- Deval, H.; Kumar, N.; Srivastava, M.; Potdar, V.; Mehta, A.; Verma, A.; Singh, R.; Kavathekar, A.; Kant, R.; Murhekar, M. Human metapneumovirus (hMPV): An associated etiology of severe acute respiratory infection in children of Eastern Uttar Pradesh, India. Access Microbiol. 2024, 6, 000829.v4. [Google Scholar] [CrossRef] [PubMed]
- Illan Montero, J.; Berger, A.; Levy, J.; Busson, L.; Hainaut, M.; Goetghebuer, T. Retrospective comparison of respiratory syncytial virus and metapneumovirus clinical presentation in hospitalized children. Pediatr. Pulmonol. 2023, 58, 222–229. [Google Scholar] [CrossRef]
- Libster, R.; Esteban, I.; Bianchi, A.; Grimaldi, L.A.; Dueñas, K.; Sancillo, A.; Rodriguez, A.; Ferrero, F.; Stein, K.; Acosta, P.L.; et al. Role for Maternal Asthma in Severe Human Metapneumovirus Lung Disease Susceptibility in Children. J. Infect. Dis. 2021, 223, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lund, C.; Nervik, I.; Loevenich, S.; Døllner, H.; Anthonsen, M.W.; Johnsen, I.B. Characterization of signaling pathways regulating the expression of pro-inflammatory long form thymic stromal lymphopoietin upon human metapneumovirus infection. Sci. Rep. 2018, 8, 883. [Google Scholar] [CrossRef] [PubMed]
- Ballegeer, M.; Saelens, X. Cell-Mediated Responses to Human Metapneumovirus Infection. Viruses 2020, 12, 542. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, T.; Zhao, X.; Dong, S.; Zhu, J.; Peng, D.; Zhong, J.; Li, T.; Chen, X. Skewed balance of regulatory T cell and inflammatory T cell in IL-17 defect with human metapneumovirus infection. Cell. Immunol. 2018, 331, 161–167. [Google Scholar] [CrossRef]
- Khan, A.; Khanna, V.; Majumdar, K. Demographics, Clinical Presentation and Outcome of Metapneumovirus Infection in Adults: A Case Series Analysis at Scarborough General Hospital, United Kingdom. Cureus 2024, 16, e73292. [Google Scholar] [CrossRef]
- Pierangeli, A.; Piralla, A.; Uceda Renteria, S.; Giacomel, G.; Lunghi, G.; Pagani, E.; Giacobazzi, E.; Vian, E.; Biscaro, V.; Piccirilli, G.; et al. Multicenter epidemiological investigation and genetic characterization of respiratory syncytial virus and metapneumovirus infections in the pre-pandemic 2018-2019 season in northern and central Italy. Clin. Exp. Med. 2023, 23, 2725–2737. [Google Scholar] [CrossRef]
- Feng, Y.; He, T.; Zhang, B.; Yuan, H.; Zhou, Y. Epidemiology and diagnosis technologies of human metapneumovirus in China: A mini review. Virol. J. 2024, 21, 59. [Google Scholar] [CrossRef]
- Zhibo, X.; Zhen, Z.; Jin, X.; Naiying, M.; Aili, C.; Wenhui, W.; Yage, W.; Zhibo, Z.; Baicheng, X.; Haoran, W.; et al. Seasonal and Genetic Characteristics of Human Metapneumovirus Circulating—Henan Province, China, 2017–2023. China CDC Wkly. 2024, 6, 450–456. [Google Scholar] [CrossRef]
- Han, T.; Wang, Y.; Zhang, D.; Li, Y.; Zhang, L.; Yan, J.; Li, C.; Yang, S.; Guo, L.; Yan, H. Changes in infant respiratory pathogens pre-, during, and post-COVID-19 non-pharmacological interventions in Beijing. Ital. J. Pediatr. 2025, 51, 8. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Y.; Xu, L.; Jiang, W.; Hao, C. Analysis of respiratory pathogen detection in hospitalized children with acute respiratory tract infections after ending the zero COVID policy. Sci. Rep. 2024, 14, 31784. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, S.; Zhang, K.; Li, Y.; Chen, D.; Tan, Y.; Liang, L.; Liu, M.; Liang, J.; An, S.; et al. Epidemiology, genetic characteristics, and association with meteorological factors of human metapneumovirus infection in children in southern China: A 10-year retrospective study. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2023, 137, 40–47. [Google Scholar] [CrossRef]
- Liu, S.; Lei, Y.; Chen, X.; Wen, Z.; Mei, B. Epidemiological characteristics of respiratory pathogens infections among children after the removal of non-pharmaceutical interventions in central China. Virol. J. 2024, 21, 303. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Rhee, J.E.; Kang, D.; Choi, E.H.; Lee, N.J.; Woo, S.; Lee, J.; Lee, S.W.; Kim, E.J.; Yun, K.W. Epidemiology of Respiratory Viruses in Korean Children Before and After the COVID-19 Pandemic: A Prospective Study From National Surveillance System. J. Korean Med. Sci. 2024, 39, e171. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.L.; Chiu, T.Y.; Tsai, K.L.; Li, C.H.; Yang, J.Y.; Liu, M.T.; Wu, F.T. Epidemiology of human metapneumovirus in Taiwan from 2013 to 2023. Arch. Virol. 2024, 169, 229. [Google Scholar] [CrossRef]
- Nagasawa, M.; Udagawa, T.; Okada, M.; Nakagawa, R.; Yokoyama, H.; Kato, T.; Furuya, M.; Sakaguchi, H. COVID-19 pandemic-altered epidemiology of respiratory syncytial virus and human metapneumovirus infections in young children. GHM Open 2024, 4, 47–49. [Google Scholar] [CrossRef]
- Nagasawa, M.; Udagawa, T.; Kato, T.; Tanaka, I.; Yamamoto, R.; Sakaguchi, H.; Sekikawa, Y. Observational Study on the Clinical Reality of Community-Acquired Respiratory Virus Infections in Adults and Older Individuals. Pathogens 2024, 13, 983. [Google Scholar] [CrossRef]
- Reller, M.E.; Mehta, K.; McCollum, E.D.; Ahmed, S.; Anderson, J.; Roy, A.D.; Chowdhury, N.H.; Saha, S.; Moulton, L.H.; Santosham, M.; et al. Viral Acute Lower Respiratory Tract Infections (ALRI) in Rural Bangladeshi Children Prior to the COVID-19 Pandemic. Influenza Other Respir. Viruses 2024, 18, e70062. [Google Scholar] [CrossRef]
- Chowdhury, F.; Bin Shahid, A.S.M.S.; Ghosh, P.K.; Rahman, M.; Hassan, M.Z.; Akhtar, Z.; Muneer, S.M.-E.; Shahrin, L.; Ahmed, T.; Chisti, M.J. Viral etiology of pneumonia among severely malnourished under-five children in an urban hospital, Bangladesh. PLoS ONE 2020, 15, e0228329. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.; Austin, K.; Avansino, J.R.; Badillo, A.; Calkins, C.M.; Crady, R.C.; Durham, M.M.; Fuller, M.K.; Rana, A.; Reeder, R.W.; et al. Does Delayed Diagnosis of Hirschsprung Disease Impact Post-operative and Functional Outcomes? A Multi-Center Review From the Pediatric Colorectal and Pelvic Learning Consortium. J. Pediatr. Surg. 2024, 59, 1250–1255. [Google Scholar] [CrossRef]
- Nisar, M.I.; Kerai, S.; Shahid, S.; Qazi, M.F.; Rehman, S.; Aziz, F.; Jehan, F. Predictors of Respiratory Syncytial Virus, Influenza Virus, and Human Metapneumovirus Carriage in Children Under 5 Years With WHO-Defined Fast-Breathing Pneumonia in Pakistan. Influenza Other Respir. Viruses 2024, 18, e13285. [Google Scholar] [CrossRef]
- Taye, B.W.; Sarna, M.; Le, H.; Levy, A.; Minney-Smith, C.; Richmond, P.; Menzies, R.; Blyth, C.C.; Moore, H.C. Respiratory Viral Testing Rate Patterns in Young Children Attending Tertiary Care Across Western Australia: A Population-Based Birth Cohort Study. Influenza Other Respir. Viruses 2024, 18, e70005. [Google Scholar] [CrossRef]
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne transmission of respiratory viruses. Science 2021, 373, abd9149. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.V.; Tollefson, S.J.; Nair, S.; Chonmaitree, T. Association of human metapneumovirus with acute otitis media. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 1189–1193. [Google Scholar] [CrossRef]
- Williams, J.V.; Harris, P.A.; Tollefson, S.J.; Halburnt-Rush, L.L.; Pingsterhaus, J.M.; Edwards, K.M.; Wright, P.F.; Crowe, J.E., Jr. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N. Engl. J. Med. 2004, 350, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Rudd, P.A.; Thomas, B.J.; Zaid, A.; MacDonald, M.; Kan-O, K.; Rolph, M.S.; Soorneedi, A.R.; Bardin, P.G.; Mahalingam, S. Role of human metapneumovirus and respiratory syncytial virus in asthma exacerbations: Where are we now? Clin. Sci. 2017, 131, 1713–1721. [Google Scholar] [CrossRef]
- Furuta, T.; Hasegawa, S.; Mizutani, M.; Iwai, T.; Ohbuchi, N.; Kawano, S.; Tashiro, N.; Uchida, M.; Hasegawa, M.; Motoyama, M.; et al. Burden of Human Metapneumovirus and Respiratory Syncytial Virus Infections in Asthmatic Children. Pediatr. Infect. Dis. J. 2018, 37, 1107–1111. [Google Scholar] [CrossRef]
- Ilvan, A.; Aslan, G.; Serin, M.S.; Calıkoğlu, M.; Yılmaz, F.M.; Tezcan, S.; Taş, D.; Ayrık, C.; Uygungül, E.; Sezer, O.; et al. Investigation of the presence of human metapneumovirus in patients with chronic obstructive pulmonary disease and asthma and its relationship with the attacks. Mikrobiyol Bul. 2013, 47, 636–649. [Google Scholar] [CrossRef]
- Costa-Filho, R.C.; Saddy, F.; Costa, J.L.F.; Tavares, L.R.; Castro Faria Neto, H.C. The Silent Threat of Human Metapneumovirus: Clinical Challenges and Diagnostic Insights from a Severe Pneumonia Case. Microorganisms 2025, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Basta, M.N. Severe Acute Respiratory Distress Syndrome in an Adult Patient with Human Metapneumovirus Infection Successfully Managed With Veno-Venous Extracorporeal Membrane Oxygenation. Semin. Cardiothorac. Vasc. Anesthesia 2024, 29, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Yeşilbaş, O.; Şevketoğlu, E.; Kıhtır, H.S.; Talip Petmezci, M.; Bato, E.; Balkaya, S.; Hatipoğlu, N.; Kuşkucu, M.A.; Palabıyık, F.; Çakır, E. A case of bronchiolitis obliterans secondary to human metapneumovirus bronchiolitis. Mikrobiyol. Bul. 2016, 50, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.; Wang, W.; Jaggi, P.; Dvorchik, I.; Ramilo, O.; Koranyi, K.; Mejias, A. Human metapneumovirus infections are associated with severe morbidity in hospitalized children of all ages. Epidemiol. Infect. 2013, 141, 2213–2223. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Safamanesh, S.; Ghasemzadeh-Moghaddam, H.; Ghafouri, M.; Mohajerzadeh-Heydari, M.S.; Namdar-Ahmadabad, H.; Azimian, A. Report of death in children with SARS-CoV-2 and human metapneumovirus (hMPV) coinfection: Is hMPV the trigger? J. Med. Virol. 2021, 93, 579–581. [Google Scholar] [CrossRef]
- Hennawi, H.A.; Khan, A.; Shpilman, A.; Mazzoni, J.A. Acute myocarditis secondary to human metapneumovirus: A case series and mini-review. Glob. Cardiol. Sci. Pract. 2024, 2024, e202452. [Google Scholar] [CrossRef]
- Mori, A.; Kawano, Y.; Hara, S.; Numoto, S.; Kurahashi, H.; Okumura, A. A nationwide survey of human metapneumovirus-associated encephalitis/encephalopathy in Japan. Brain Dev. 2023, 45, 197–204. [Google Scholar] [CrossRef]
- Vehapoglu, A.; Turel, O.; Sahin, T.U.; Kutlu, N.O.; Iscan, A. Clinical Significance of Human Metapneumovirus in Refractory Status Epilepticus and Encephalitis: Case Report and Review of the Literature. Case Rep. Neurol. Med. 2015, 2015, 131780. [Google Scholar] [CrossRef]
- Shibuya, M.; Togashi, N.; Inui, T.; Okubo, Y.; Endo, W.; Miyabayashi, T.; Sato, R.; Takezawa, Y.; Kodama, K.; Ikeda, M.; et al. Multiple cerebral hemorrhages and white matter lesions developing after severe hMPV pneumonia in a patient with trisomy 13: A case report and review of the literature. Tohoku J. Exp. Med. 2022, 258, 49–54. [Google Scholar] [CrossRef]
- Dhariwal, K. Human Metapneumovirus Associated With Acute Hemorrhagic Oedema of Infancy: A Case Report. Cureus 2024, 16, e73296. [Google Scholar] [CrossRef]
- Chidambaram, A.C.; Bhowmick, R.; Parameswaran, N.; Gunasekaran, D. A rare case of metapneumovirus-induced rhabdomyolysis and multi-organ dysfunction in a 4-year-old child. Paediatr. Int. Child Health 2021, 41, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mao, N.-Y.; Zhang, C.; Yang, M.-J.; Wang, M.; Xu, W.-B.; Ma, X.-J. The development of a GeXP-based multiplex reverse transcription-PCR assay for simultaneous detection of sixteen human respiratory virus types/subtypes. BMC Infect. Dis. 2012, 12, 189. [Google Scholar] [CrossRef] [PubMed]
- Popowitch, E.B.; Kaplan, S.; Wu, Z.; Tang, Y.W.; Miller, M.B. Comparative Performance of the Luminex NxTAG Respiratory Pathogen Panel, GenMark eSensor Respiratory Viral Panel, and BioFire FilmArray Respiratory Panel. Microbiol. Spectr. 2022, 10, e0124822. [Google Scholar] [CrossRef]
- Sugimoto, S.; Kawase, M.; Suwa, R.; Kakizaki, M.; Kume, Y.; Chishiki, M.; Ono, T.; Okabe, H.; Norito, S.; Hosoya, M.; et al. Development of a duplex real-time RT-PCR assay for the detection and identification of two subgroups of human metapneumovirus in a single tube. J. Virol. Methods 2023, 322, 114812. [Google Scholar] [CrossRef]
- Song, Q.; Zhu, R.; Sun, Y.; Zhao, L.; Wang, F.; Deng, J.; Qian, Y. Identification of human metapneumovirus genotypes A and B from clinical specimens by reverse transcription loop-mediated isothermal amplification. J. Virol. Methods 2014, 196, 133–138. [Google Scholar] [CrossRef]
- Jiao, S.-L.; Xie, L.; Mao, Y.; Ni, H.-X.; Li, Y.-D. Establishment of a Reverse Transcriptase-Mediated Isothermal Amplification Fluorescence Assay for Detection of Human Metapneumovirus. 2023. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20231623480 (accessed on 30 April 2025).
- Available online: https://scholar.google.com/scholar_lookup?journal=Chin%20J%20Zoonos&title=Establishment%20of%20a%20reverse%20transcriptase-mediated%20isothermal%20amplication%20fluorescence%20assay%20for%20detection%20of%20Human%20metapneumovirus&author=SL%20Jiao&author=L%20Xie&author=Y%20Mao&author=%20NI%20HX,%20Li%20YD,&volume=39&publication_year=2023&pages=780-783& (accessed on 30 April 2025).
- Du, Y.; Liu, X.; Gao, H.; Liu, X.; Huang, M.; Chai, Q.; Xing, Z.; Zhang, T.; Ma, D. Rapid and one-tube detection of human metapneumovirus using the RT-RPA and CRISPR/Cas12a. J. Virol. Methods 2024, 329, 115001. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Huang, J.; Wang, T.; He, X.; Xu, G.; Li, Y. Visual detection of human metapneumovirus using CRISPR-Cas12a diagnostics. Virus Res. 2021, 305, 198568. [Google Scholar] [CrossRef]
- Xu, Y.; Lewandowski, K.; Jeffery, K.; Downs, L.O.; Foster, D.; Sanderson, N.D.; Kavanagh, J.; Vaughan, A.; Salvagno, C.; Vipond, R.; et al. Nanopore metagenomic sequencing to investigate nosocomial transmission of human metapneumovirus from a unique genetic group among haematology patients in the United Kingdom. J. Infect. 2020, 80, 571–577. [Google Scholar] [CrossRef]
- Rajagopala, S.V.; Bakhoum, N.G.; Pakala, S.B.; Shilts, M.H.; Rosas-Salazar, C.; Mai, A.; Boone, H.H.; McHenry, R.; Yooseph, S.; Halasa, N. Metatranscriptomics to characterize respiratory virome, microbiome, and host response directly from clinical samples. Cell Rep. Methods 2021, 1, 100091. [Google Scholar] [CrossRef]
- Jeong, S.; Park, M.-J.; Song, W.; Kim, H.-S. Advances in laboratory assays for detecting human metapneumovirus. Ann. Transl. Med. 2020, 8, 608. [Google Scholar] [CrossRef]
- Sakshi; Mavi, A.K.; Chowdhury, S.; Kumar, N.; Singh, P.; Kumar, D.; Preethi, L.; Kumar, U. Novel diagnostic methods for emerging respiratory viral infection. In Emerging Human Viral Diseases, Volume I: Respiratory and Haemorrhagic Fever; Springer: Berlin/Heidelberg, Germany, 2023; pp. 565–585. [Google Scholar]
- Brestovac, B.; Lawrence, C.; Speers, D.J.; Sammels, L.M.; Mulrennan, S. Respiratory viral infections in Western Australians with cystic fibrosis. Respir. Med. 2020, 161, 105854. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.F.; Hiatt, P.W.; Jewell, A.; Schoonover, S.L.; Cron, S.G.; Riggs, M.; Grace, S.; Oermann, C.M.; Piedra, P.A. Human metapneumovirus and respiratory syncytial virus infections in older children with cystic fibrosis. Pediatr. Pulmonol. 2007, 42, 66–74. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, C.; Banos-Lara, M.d.R. HMPV in Immunocompromised Patients: Frequency and Severity in Pediatric Oncology Patients. Pathogens 2020, 9, 51. [Google Scholar] [CrossRef]
- Kim, S.; Sung, H.; Im, H.J.; Hong, S.-J.; Kim, M.-N. Molecular epidemiological investigation of a nosocomial outbreak of human metapneumovirus infection in a pediatric hemato-oncology patient population. J. Clin. Microbiol. 2009, 47, 1221–1224. [Google Scholar] [CrossRef]
- Ali, M.; Baker, J.M.; Richardson, S.E.; Weitzman, S.; Allen, U.; Abla, O. Human metapneumovirus (hMPV) infection in children with cancer. J. Pediatr. Hematol. 2013, 35, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Hijano, D.R.; Maron, G.; Hayden, R.T. Respiratory Viral Infections in Patients with Cancer or Undergoing Hematopoietic Cell Transplant. Front. Microbiol. 2018, 9, 3097. [Google Scholar] [CrossRef]
- Dokos, C.; Masjosthusmann, K.; Rellensmann, G.; Werner, C.; Schuler-Lüttmann, S.; Müller, K.-M.; Schiborr, M.; Ehlert, K.; Groll, A.H. Fatal human metapneumovirus infection following allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2013, 15, E97–E101. [Google Scholar] [CrossRef]
- Cohen, C.; Moyes, J.; Tempia, S.; Groome, M.; Walaza, S.; Pretorius, M.; Naby, F.; Mekgoe, O.; Kahn, K.; von Gottberg, A.; et al. Epidemiology of Acute Lower Respiratory Tract Infection in HIV-Exposed Uninfected Infants. Pediatrics 2016, 137. [Google Scholar] [CrossRef] [PubMed]
- Lenahan, J.L.; Englund, J.A.; Katz, J.; Kuypers, J.; Wald, A.; Magaret, A.; Tielsch, J.M.; Khatry, S.K.; LeClerq, S.C.; Shrestha, L.; et al. Human Metapneumovirus and Other Respiratory Viral Infections during Pregnancy and Birth, Nepal. Emerg. Infect. Dis. 2017, 23, 1341–1349. [Google Scholar] [CrossRef]
- Fuchs, A.; McLaren, R.J.; Saunders, P.; Karakash, S.; Minkoff, H. Human Metapneumovirus Infection and Acute Respiratory Distress Syndrome During Pregnancy. Obstet. Gynecol. 2017, 130, 630–632. [Google Scholar] [CrossRef]
- Hause, A.M.; Avadhanula, V.; Maccato, M.L.; Pinell, P.M.; Bond, N.; Santarcangelo, P.; Ferlic-Stark, L.; Munoz, F.M.; Piedra, P.A. A Cross-sectional Surveillance Study of the Frequency and Etiology of Acute Respiratory Illness Among Pregnant Women. J. Infect. Dis. 2018, 218, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Murphy, V.E.; Powell, H.; Wark, P.A.B.; Gibson, P.G. A prospective study of respiratory viral infection in pregnant women with and without asthma. Chest 2013, 144, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Emont, J.; Chung, K.; Rouse, D. A Report of Two Cases of Human Metapneumovirus Infection in Pregnancy Involving Superimposed Bacterial Pneumonia and Severe Respiratory Illness. J. Clin. Gynecol. Obstet. 2019, 8, 107–110. [Google Scholar] [CrossRef]
- Shivarame Gowda, S.K.; Abdalla, F.E.; Narasimhaiah, R. A Case of Human Meta-pneumovirus Infection in Pregnancy Involving Superimposed Bacterial Pneumonia and Respiratory Distress. Acta Sci. Med. Sci. 2023, 7, 135–138. [Google Scholar] [CrossRef]
- Dauby, N.; Flamand, V. From maternal breath to infant’s cells: Impact of maternal respiratory infections on infants ‘immune responses. Front. Pediatr. 2022, 10, 1046100. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Dhaliwal, A. Infections in Pregnancy With COVID-19 and Other Respiratory RNA Virus Diseases Are Rarely, If Ever, Transmitted to the Fetus: Experiences With Coronaviruses, Parainfluenza, Metapneumovirus Respiratory Syncytial Virus, and Influenza. Arch. Pathol. Lab. Med. 2020, 144, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Ramocha, L.M.; van den Hoogen, B.G.; Baillie, V.; van Nieuwkoop, S.; Cutland, C.L.; Jones, S.; Moultrie, A.; Izu, A.; Verwey, C.; Madhi, S.A.; et al. Fetal Transfer of Human Metapneumovirus-Neutralizing Antibodies Is Reduced From Mothers Living With HIV-1. J. Pediatr. Infect. Dis. Soc. 2022, 11, 341–344. [Google Scholar] [CrossRef]
- Funes, S.C.; Ríos, M.; Fernández-Fierro, A.; Rivera-Pérez, D.; Soto, J.A.; Valbuena, J.R.; Altamirano-Lagos, M.J.; Gómez-Santander, F.; Jara, E.L.; Zoroquiain, P.; et al. Female offspring gestated in hypothyroxinemia and infected with human Metapneumovirus (hMPV) suffer a more severe infection and have a higher number of activated CD8(+) T lymphocytes. Front. Immunol. 2022, 13, 966917. [Google Scholar] [CrossRef]
- Verma, M.; Panchal, N.S.; Yadav, P.K. Exploring Chemical Space to Identify Partial Binders Against hMPV Nucleocapsid Protein. J. Cell. Biochem. 2025, 126, e30618. [Google Scholar] [CrossRef]
- Martínez-Espinoza, I.; Babawale, P.I.; Miletello, H.; Cheemarla, N.R.; Guerrero-Plata, A. Interferon Epsilon-Mediated Antiviral Activity Against Human Metapneumovirus and Respiratory Syncytial Virus. Vaccines 2024, 12, 1198. [Google Scholar] [CrossRef]
- Sojati, J.; Parks, O.B.; Zhang, Y.; Walters, S.; Lan, J.; Eddens, T.; Lou, D.; Fan, L.; Chen, K.; Oury, T.D.; et al. IFN-λ drives distinct lung immune landscape changes and antiviral responses in human metapneumovirus infection. mBio 2024, 15, e0055024. [Google Scholar] [CrossRef]
- Bergeron, H.C.; Crabtree, J.; Nagy, T.; Martin, D.E.; Tripp, R.A. Probenecid Inhibits Human Metapneumovirus (HMPV) Replication In Vitro and in BALB/c Mice. Viruses 2024, 16, 1087. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Abeywickrema, P.; Bonneux, B.; Behera, I.; Anson, B.; Jacoby, E.; Fung, A.; Adhikary, S.; Bhaumik, A.; Carbajo, R.J.; et al. Structural and mechanistic insights into the inhibition of respiratory syncytial virus polymerase by a non-nucleoside inhibitor. Commun. Biol. 2023, 6, 1074. [Google Scholar] [CrossRef] [PubMed]
- Luck, M.I.; Subillaga, E.J.; Borenstein, R.; Sabo, Y. Ginkgolic acid inhibits orthopneumo- and metapneumo- virus infectivity. Sci. Rep. 2024, 14, 8230. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, Y.M.; Zhao, J.; Bai, Y.M.; Yan, C.Q.; Du, G.H.; Zheng, L.S.; Liu, A.L. Fumarprotocetraric acid and geraniin were identified as novel inhibitors of human respiratory syncytial virus infection in vitro. Front. Cell. Infect. Microbiol. 2024, 14, 1484245. [Google Scholar] [CrossRef]
- Bhuvaneshwari, V.; Amsaveni, R. Exploring quercetin based nano formulation in combating human Metapneumovirus infections. Int. Immunopharmacol. 2025, 153, 114510. [Google Scholar] [CrossRef]
- Hamelin, M.E.; Couture, C.; Sackett, M.; Kiener, P.; Suzich, J.; Ulbrandt, N.; Boivin, G. The prophylactic administration of a monoclonal antibody against human metapneumovirus attenuates viral disease and airways hyperresponsiveness in mice. Antivir. Ther. 2008, 13, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Abu-Shmais, A.A.; Guo, L.; Khalil, A.M.; Miller, R.J.; Janke, A.K.; Vukovich, M.J.; Bass, L.E.; Suresh, Y.P.; Rush, S.A.; Wolters, R.M.; et al. A potently neutralizing and protective human antibody targeting antigenic site V on RSV and hMPV fusion glycoprotein. Biorxiv Prepr. Serv. Biol. 2024. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC11565947/ (accessed on 2 April 2025). [CrossRef]
- Guo, L.; Li, L.; Liu, L.; Zhang, T.; Sun, M. Neutralising antibodies against human metapneumovirus. Lancet. Microbe 2023, 4, e732–e744. [Google Scholar] [CrossRef]
- Karron, R.A.; San Mateo, J.; Wanionek, K.; Collins, P.L.; Buchholz, U.J. Evaluation of a Live Attenuated Human Metapneumovirus Vaccine in Adults and Children. J. Pediatr. Infect. Dis. Soc. 2018, 7, 86–89. [Google Scholar] [CrossRef]
- Shapiro, C.; Sánchez-Crespo, N.; Ciarlet, M.; Hourguettes, N.; Wen, J.; Rida, W.; Price, J.; Engram, A.E.; Adams, E.M.; Kanesa-Thasan, N. A Randomized Phase 1 Clinical Trial of a Respiratory Syncytial Virus and Human Metapneumovirus Combination Protein-Based Virus-like Particle Vaccine in Adults 60–75 Years of Age. Open Forum Infect. Dis. 2025, 12, ofaf160. [Google Scholar] [CrossRef]
- Ogonczyk-Makowska, D.; Brun, P.; Vacher, C.; Chupin, C.; Droillard, C.; Carbonneau, J.; Laurent, E.; Dulière, V.; Traversier, A.; Terrier, O.; et al. Mucosal bivalent live attenuated vaccine protects against human metapneumovirus and respiratory syncytial virus in mice. NPJ Vaccines 2024, 9, 111. [Google Scholar] [CrossRef]
- Trinité, B.; Durr, E.; Pons-Grífols, A.; O’Donnell, G.; Aguilar-Gurrieri, C.; Rodriguez, S.; Urrea, V.; Tarrés, F.; Mane, J.; Ortiz, R.; et al. VLPs generated by the fusion of RSV-F or hMPV-F glycoprotein to HIV-Gag show improved immunogenicity and neutralizing response in mice. Vaccine 2024, 42, 3474–3485. [Google Scholar] [CrossRef] [PubMed]
- Swart, M.; Allen, J.; Reed, B.; Izquierdo Gil, A.; Verspuij, J.; Schmit-Tillemans, S.; Chakkumkal, A.; Findeis, M.; Hafner, A.V.; Harjivan, C.; et al. Plant Cell Culture-Derived Saponin Adjuvant Enhances Immune Response Against a Stabilized Human Metapneumovirus Pre-Fusion Vaccine Candidate. Vaccines 2024, 12, 1435. [Google Scholar] [CrossRef]
- August, A.; Shaw, C.A.; Lee, H.; Knightly, C.; Kalidindia, S.; Chu, L.; Essink, B.J.; Seger, W.; Zaks, T.; Smolenov, I.; et al. Safety and Immunogenicity of an mRNA-Based Human Metapneumovirus and Parainfluenza Virus Type 3 Combined Vaccine in Healthy Adults. Open Forum Infect. Dis. 2022, 9, ofac206. [Google Scholar] [CrossRef] [PubMed]
- Pantaleo, G.; Correia, B.; Fenwick, C.; Joo, V.S.; Perez, L. Antibodies to combat viral infections: Development strategies and progress. Nat. reviews. Drug Discov. 2022, 21, 676–696. [Google Scholar] [CrossRef] [PubMed]
- Mazur, N.I.; Terstappen, J.; Baral, R.; Bardají, A.; Beutels, P.; Buchholz, U.J.; Cohen, C.; Crowe, J.E., Jr.; Cutland, C.L.; Eckert, L.; et al. Respiratory syncytial virus prevention within reach: The vaccine and monoclonal antibody landscape. Lancet. Infect. Dis. 2023, 23, e2–e21. [Google Scholar] [CrossRef]
- Stepanova, E.; Matyushenko, V.; Rudenko, L.; Isakova-Sivak, I. Prospects of and Barriers to the Development of Epitope-Based Vaccines against Human Metapneumovirus. Pathogens 2020, 9, 481. [Google Scholar] [CrossRef]
- Spires, S.S.; Talbot, H.K.; Pope, C.A.; Talbot, T.R. Paramyxovirus Outbreak in a Long-Term Care Facility: The Challenges of Implementing Infection Control Practices in a Congregate Setting. Infect. Control Hosp. Epidemiol. 2017, 38, 399–404. [Google Scholar] [CrossRef]
- Sood, G.; Perl, T.M. Outbreaks in Health Care Settings. Infect. Dis. Clin. North Am. 2016, 30, 661–687. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, G.A.; Lee, S.H.; Park, Y.H. A systematic review on the causes of the transmission and control measures of outbreaks in long-term care facilities: Back to basics of infection control. PLoS ONE 2020, 15, e0229911. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.T.; Jackson, O.; Cohen, B.; Hutcheon, G.; Saiman, L.; Larson, E.; Neu, N. Impact of Infection Prevention and Control Initiatives on Acute Respiratory Infections in a Pediatric Long-Term Care Facility. Infect. Control. Hosp. Epidemiol. 2016, 37, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.P.; Shane, A.L. Infections Associated with Group Childcare. Princ. Pract. Pediatr. Infect. Dis. 2018, 25–32.e23. [Google Scholar] [CrossRef]
- Brecht, I.; Esther van, K.; Placide, M.; Kostas, D.; Ivalda, M.; Niel, H.; Eveline, C.; Marianne, A.B.v.d.S. Embedding risk monitoring in infectious disease surveillance for timely and effective outbreak prevention and control. BMJ Glob. Health 2025, 10, e016870. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. ECDC Strategic Framework for the Integration of Molecular and Genomic Typing into European Surveillance and Multi-Country Outbreak Investigations—2021–2027. 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/ecdc-strategic-framework-integration-molecular-and-genomic-typing-european (accessed on 22 June 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).