Bioconversion of a Dairy By-Product (Scotta) into Mannitol-Stabilized Violacein via Janthinobacterium lividum Fermentation
Abstract
1. Introduction
2. Materials and Methods
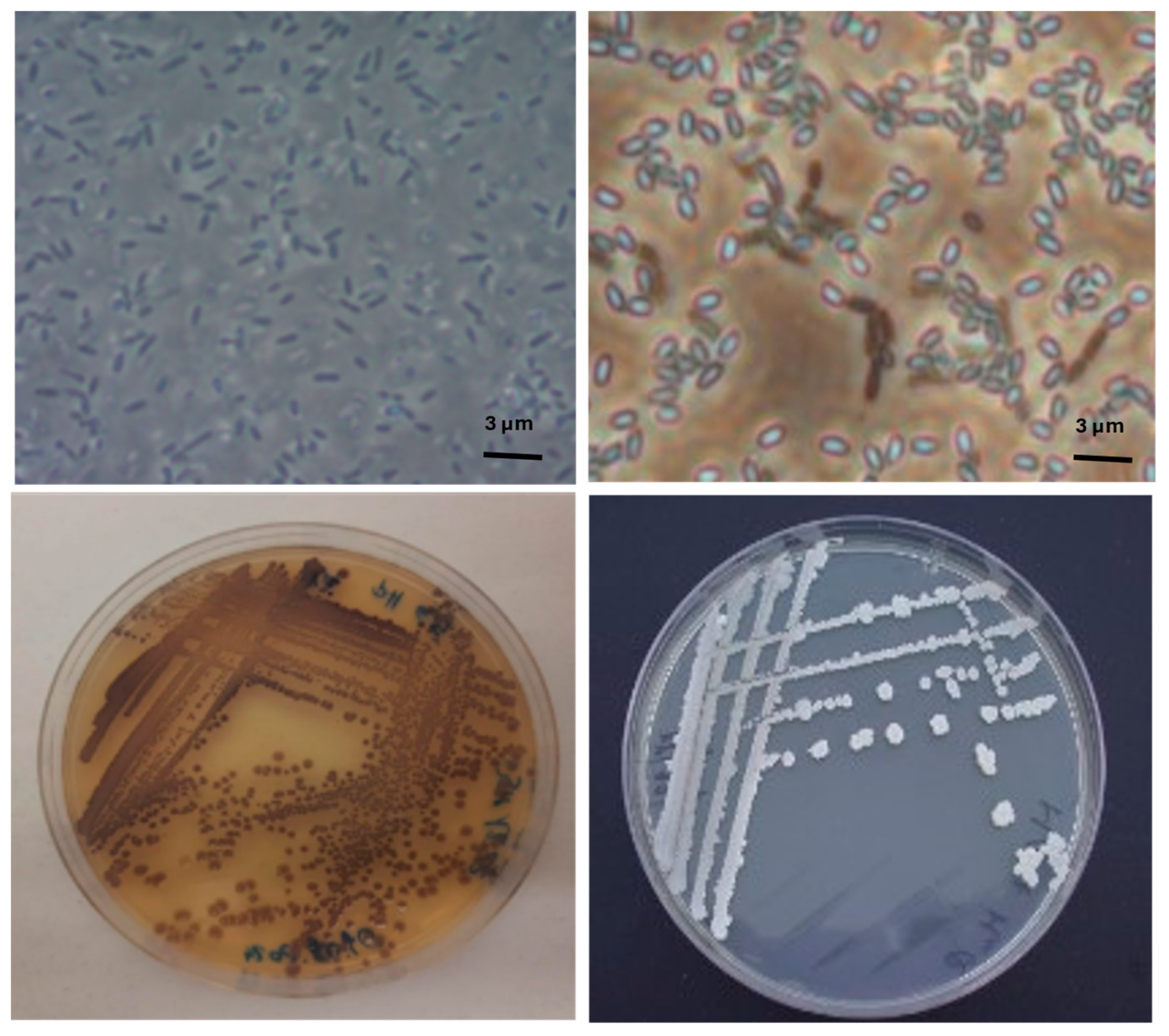
2.1. Bacterial Strains
2.2. Physical–Chemical Characterization of Dairy By-Products (Scotta)
2.3. Shaking-Flask Cultivation
2.3.1. Evaluation of the Effects of Different Temperatures on Violet Pigmentation, Using Different Scotta from Ricotta-Cheese Production
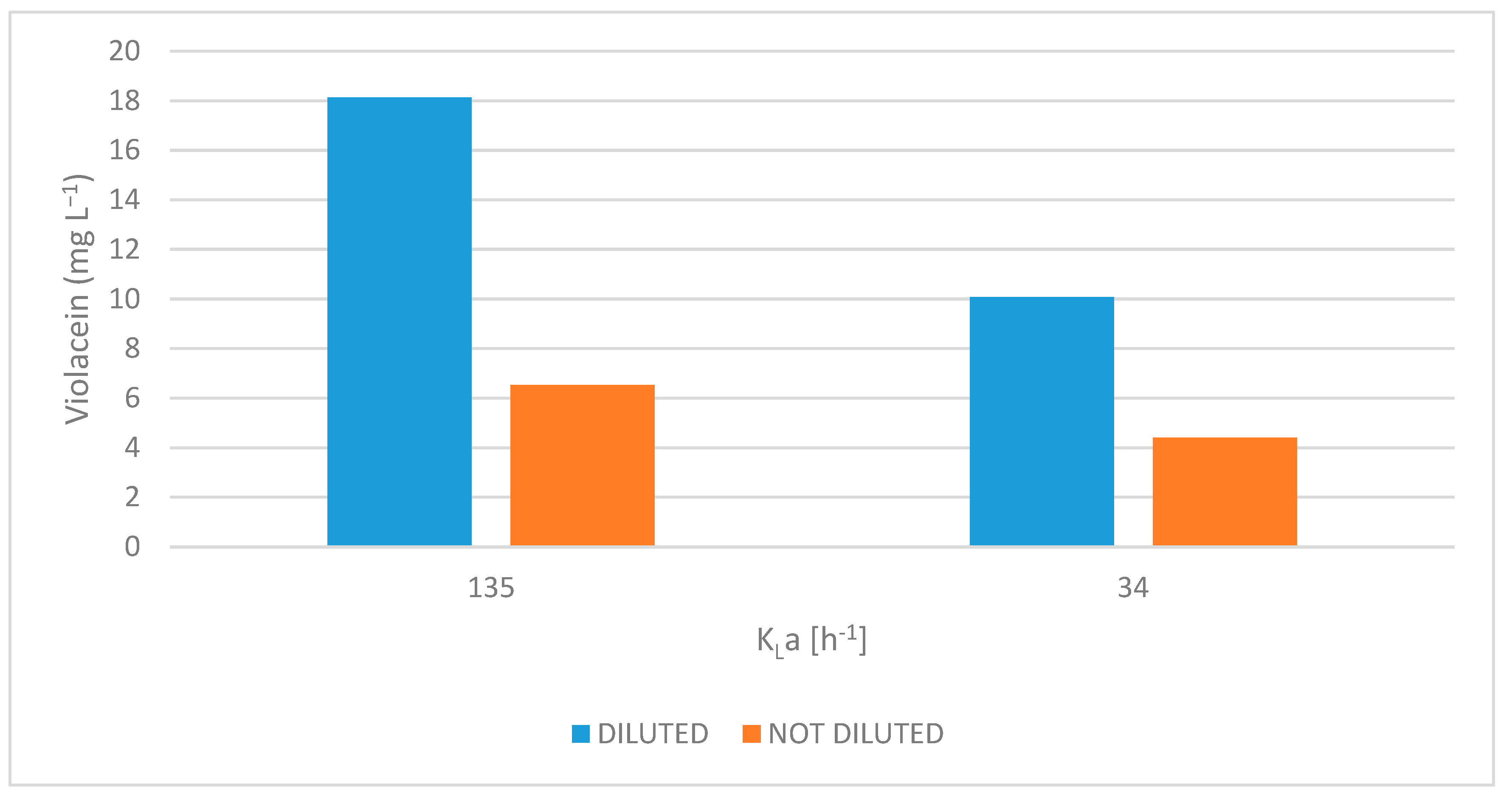
2.3.2. Evaluation of Different Volumetric Mass Transfer Coefficients (kLa) and the Effect of Scotta Dilution on Violacein Production
2.3.3. Indirect Impact of Lactose Hydrolysis on Pigmentation
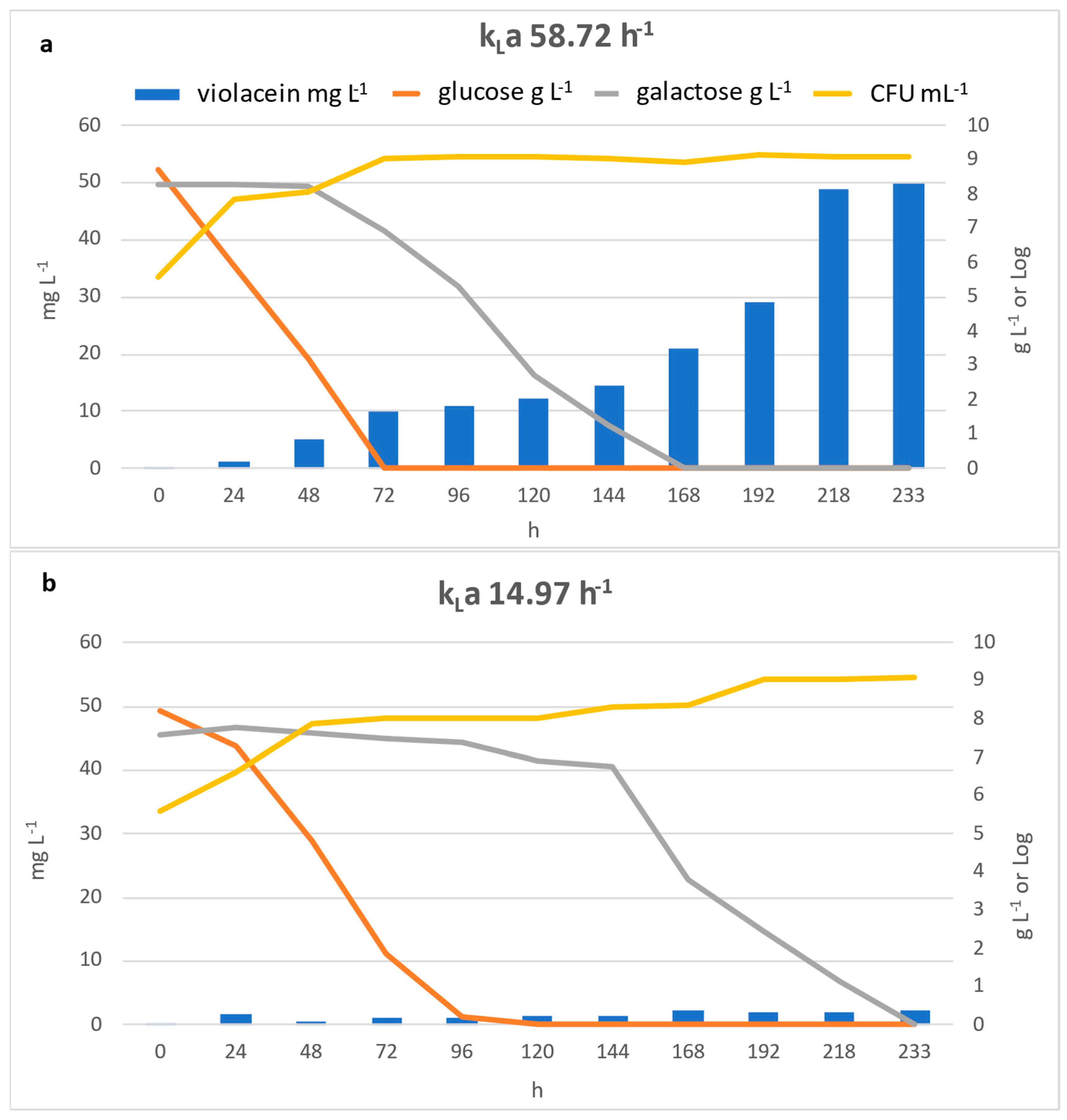
2.4. Violacein Production in a Bioreactor at Two Different kLa
- kLa = volumetric liquid-side mass transfer coefficient (h−1);
- Pg = total impeller power consumption (W);
- V = liquid volume in vessel (m3);
- νs = superficial gas velocity (m h−1).
- C, α, β are empirical constants, and depend on the geometrical parameters of the vessel; they are 0.026, 0.4 and 0.5, respectively.
2.5. Extraction and Spray-Drying of Violet Pigment
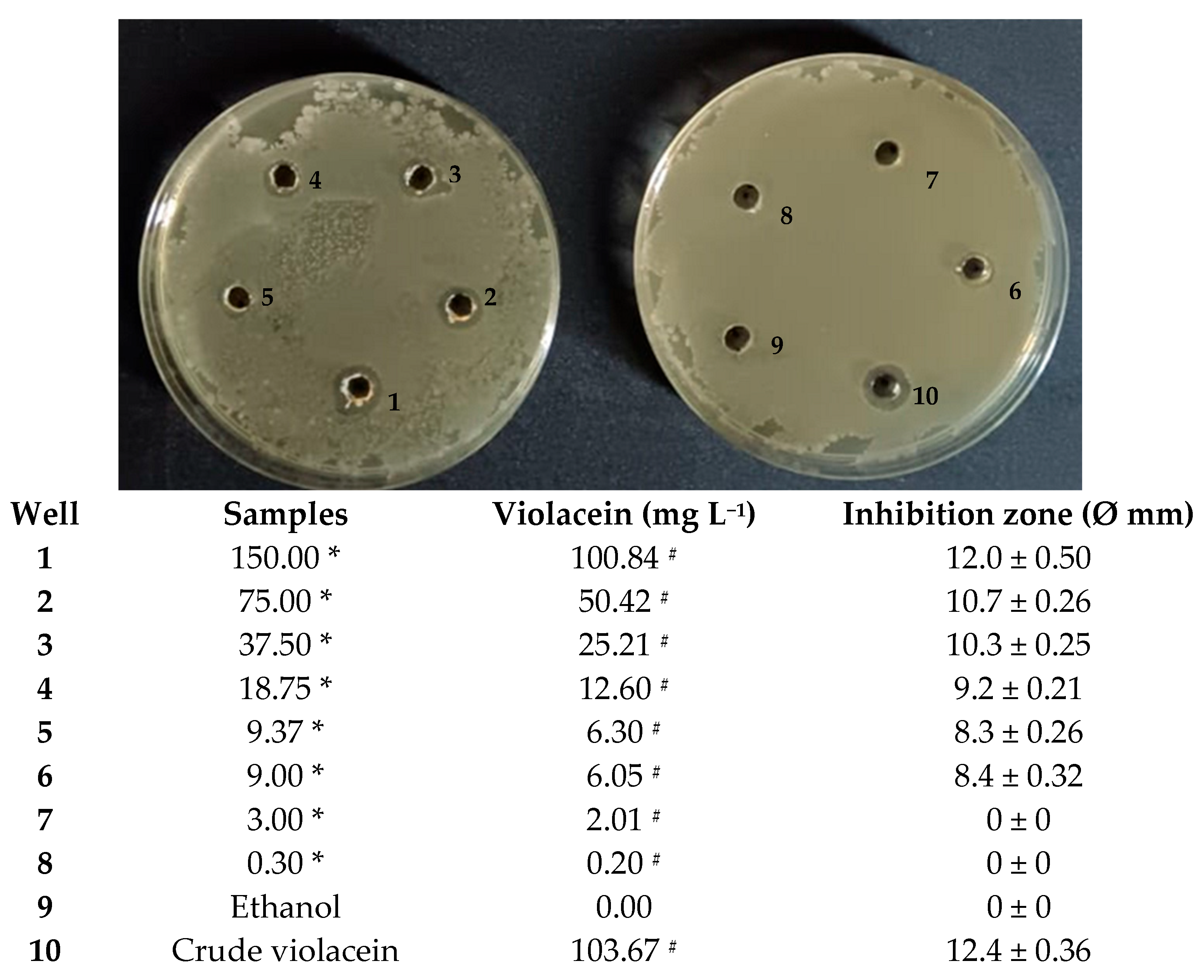
2.6. Antimicrobial Activity of Spray-Dried Pigment Against Bacillus Subtilis
2.7. Sugars HPLC Determination
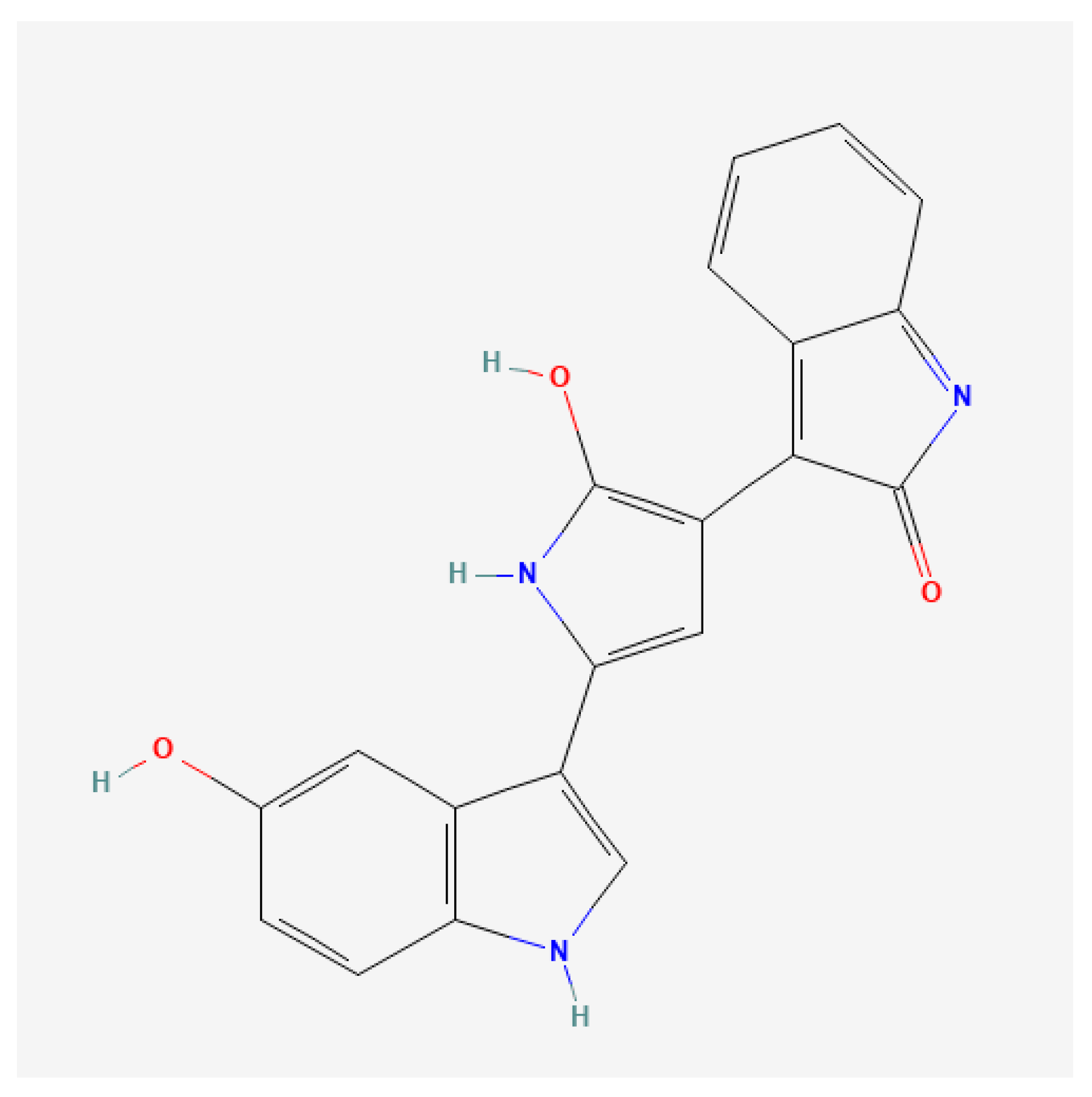
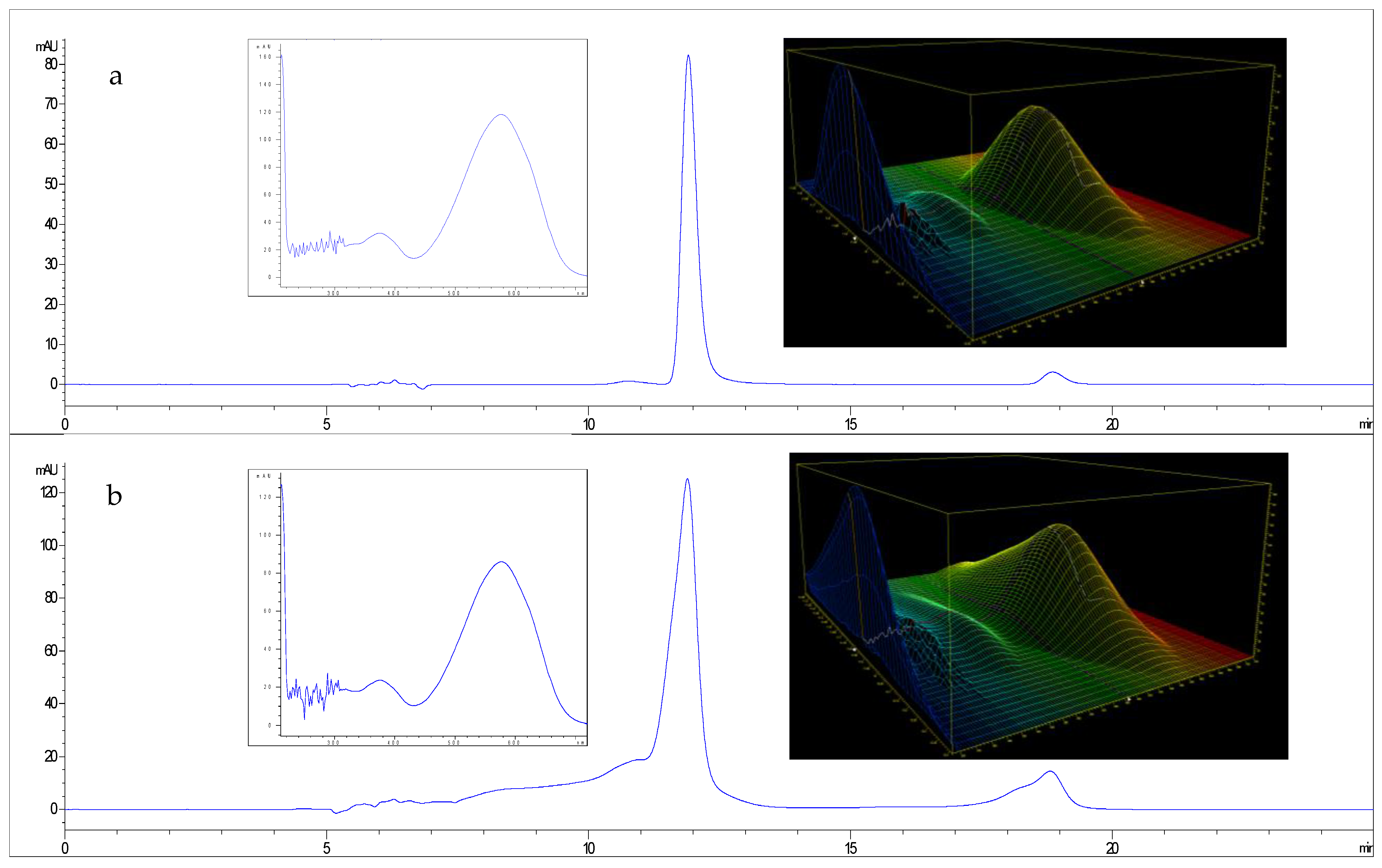
2.8. Pigment Determination
3. Results and Discussion
3.1. Physico-Chemical Characterization of Dairy By-Products (Scotta)
3.2. Shaking-Flask Cultivations
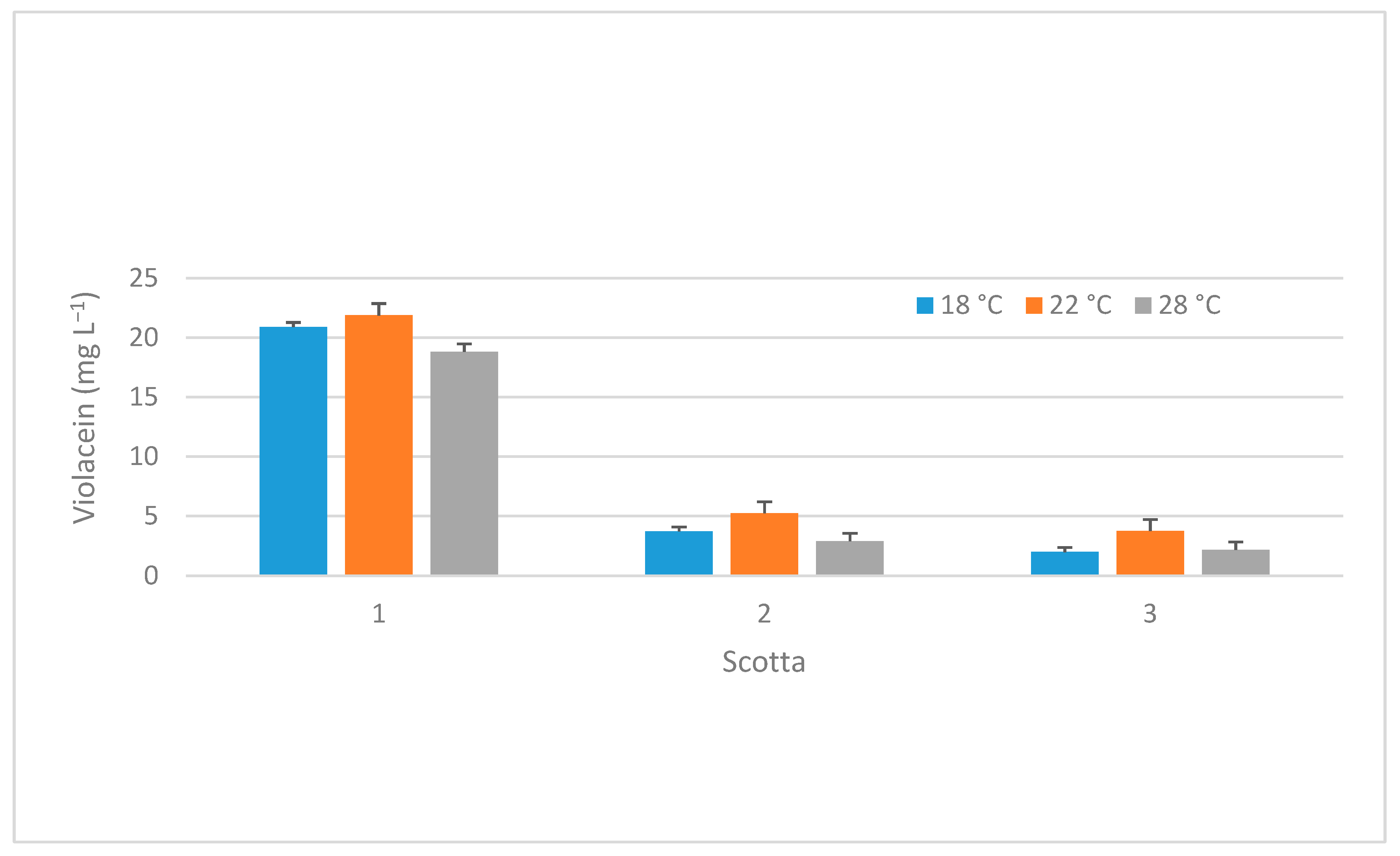
3.2.1. Evaluation of Differences in Temperature on Violet Pigmentation, Using Different Scotta from Ricotta-Cheese Production
3.2.2. Evaluation of Different Volumetric Mass Transfer Coefficients (kLa) and the Effect of Scotta Dilution on Violacein Production
3.2.3. Indirect Impact of Lactose Hydrolysis on Pigmentation
3.3. Violacein Production in a Bioreactor at Two Different kLa Values
3.4. Spray Drying of Violet Pigment and Its Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, S.Y.; Yoon, K.H.; Lee, J.I.; Mitchell, R.J. Violacein: Properties and Production of a Versatile Bacterial Pigment. BioMed Res. Int. 2015, 2015, 465056. [Google Scholar] [CrossRef]
- Ramsey, J.P.; Mercurio, A.; Holland, J.A.; Harris, R.N.; Minbiole, K.P.C. The Cutaneous Bacterium Janthinobacterium Lividum Inhibits the Growth of Trichophyton Rubrum in Vitro. Int. J. Dermatol. 2015, 54, 156–159. [Google Scholar] [CrossRef]
- Venegas, F.A.; Köllisch, G.; Mark, K.; Diederich, W.E.; Kaufmann, A.; Bauer, S.; Chavarría, M.; Araya, J.J.; García-Piñeres, A.J. The Bacterial Product Violacein Exerts an Immunostimulatory Effect Via TLR8. Sci. Rep. 2019, 9, 13661. [Google Scholar] [CrossRef] [PubMed]
- Füller, J.J.; Röpke, R.; Krausze, J.; Rennhack, K.E.; Daniel, N.P.; Blankenfeldt, W.; Schulz, S.; Jahn, D.; Moser, J. Biosynthesis of Violacein, Structure and Function of L-Tryptophan Oxidase VioA from Chromobacterium Violaceum. J. Biol. Chem. 2016, 291, 20068–20084. [Google Scholar] [CrossRef] [PubMed]
- Masuelli, L.; Pantanella, F.; La Regina, G.; Benvenuto, M.; Fantini, M.; Mattera, R.; Di Stefano, E.; Mattei, M.; Silvestri, R.; Schippa, S.; et al. Violacein, an Indole-Derived Purple-Colored Natural Pigment Produced by Janthinobacterium Lividum, Inhibits the Growth of Head and Neck Carcinoma Cell Lines Both in Vitro and in Vivo. Tumor Biol. 2016, 37, 3705–3717. [Google Scholar] [CrossRef]
- Park, H.A.; Park, S.A.; Yang, Y.H.; Choi, K.Y. Microbial Synthesis of Violacein Pigment and Its Potential Applications. Crit. Rev. Biotechnol. 2021, 41, 879–901. [Google Scholar] [CrossRef]
- Durán, N.; Nakazato, G.; Durán, M.; Berti, I.R.; Castro, G.R.; Stanisic, D.; Brocchi, M.; Fávaro, W.J.; Ferreira-Halder, C.V.; Justo, G.Z.; et al. Multi-Target Drug with Potential Applications: Violacein in the Spotlight. World J. Microbiol. Biotechnol. 2021, 37, 151. [Google Scholar] [CrossRef] [PubMed]
- Linhares, T.S.T.; de Lima, K.Y.G.; Marques, K.K.G.; Castro, I.O.; de Souza, R.R.M.; Borchardt, H.; Vasconcelos, U. Violacein—Part I: Theoretical Foundations and Foundations on Pigment. Themes Focus. Interdiscip. Sustain. Dev. Worldw. 2024, 2, 523–530. [Google Scholar] [CrossRef]
- Bagoghli, N.; Hosseini-Abari, A. Improvement of Violacein Production Using Abiotic Stresses and Microbial Adaptation. World J. Microbiol. Biotechnol. 2024, 40, 149. [Google Scholar] [CrossRef]
- Wang, H.; Wang, F.; Zhu, X.; Yan, Y.; Yu, X.; Jiang, P.; Xing, X.H. Biosynthesis and Characterization of Violacein, Deoxyviolacein and Oxyviolacein in Heterologous Host, and Their Antimicrobial Activities. Biochem. Eng. J. 2012, 67, 148–155. [Google Scholar] [CrossRef]
- Aranda, S.; Montes-Borrego, M.; Landa, B.B. Purple-Pigmented Violacein-Producing Duganella Spp. Inhabit the Rhizosphere of Wild and Cultivated Olives in Southern Spain. Microb. Ecol. 2011, 62, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Lim, S.; Yoon, K.; Lee, J.I.; Mitchell, R.J. Biotechnological Activities and Applications of Bacterial Pigments Violacein and Prodigiosin. J. Biol. Eng. 2021, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Lyakhovchenko, N.S.; Travkin, V.M.; Senchenkov, V.Y.; Solyanikova, I.P. Bacterial Violacein: Properties, Biosynthesis and Application Prospects. Appl. Biochem. Microbiol. 2022, 58, 692–700. [Google Scholar] [CrossRef]
- Aruldass, C.A.; Masalamany, S.R.L.; Venil, C.K.; Ahmad, W.A. Antibacterial Mode of Action of Violacein from Chromobacterium Violaceum UTM5 against Staphylococcus Aureus and Methicillin-Resistant Staphylococcus Aureus (MRSA). Environ. Sci. Pollut. Res. 2018, 25, 5164–5180. [Google Scholar] [CrossRef]
- Kanelli, M.; Mandic, M.; Kalakona, M.; Vasilakos, S.; Kekos, D.; Nikodinovic-Runic, J.; Topakas, E. Microbial Production of Violacein and Process Optimization for Dyeing Polyamide Fabrics with Acquired Antimicrobial Properties. Front. Microbiol. 2018, 9, 1495. [Google Scholar] [CrossRef]
- Baricz, A.; Teban, A.; Chiriac, C.M.; Szekeres, E.; Farkas, A.; Nica, M.; Dascălu, A.; Oprișan, C.; Lavin, P.; Coman, C. Investigating the Potential Use of an Antarctic Variant of Janthinobacterium Lividum for Tackling Antimicrobial Resistance in a One Health Approach. Sci. Rep. 2018, 8, 15272. [Google Scholar] [CrossRef]
- Sasidharan, A.; Sasidharan, N.K.; Amma, D.B.N.S.; Vasu, R.K.; Nataraja, A.V.; Bhaskaran, K. Antifungal Activity of Violacein Purified from a Novel Strain of Chromobacterium Sp. NIIST (MTCC 5522). J. Microbiol. 2015, 53, 694–701. [Google Scholar] [CrossRef]
- Di Salvo, E.; Lo Vecchio, G.; De Pasquale, R.; De Maria, L.; Tardugno, R.; Vadalà, R.; Cicero, N. Natural Pigments Production and Their Application in Food, Health and Other Industries. Nutrients 2023, 15, 1923. [Google Scholar] [CrossRef]
- Magarelli, R.A.; Trupo, M.; Ambrico, A.; Larocca, V.; Martino, M.; Palazzo, S.; Balducchi, R.; Joutsjoki, V.; Pihlanto, A.; Bevivino, A. Designing a Waste-Based Culture Medium for the Production of Plant Growth Promoting Microorganisms Based on Cladodes Juice from Opuntia Ficus-Indica Pruning. Fermentation 2022, 8, 225. [Google Scholar] [CrossRef]
- Ahmad, W.A.; Yusof, N.Z.; Nordin, N.; Zakaria, Z.A.; Rezali, M.F. Production and Characterization of Violacein by Locally Isolated Chromobacterium Violaceum Grown in Agricultural Wastes. Appl. Biochem. Biotechnol. 2012, 167, 1220–1234. [Google Scholar] [CrossRef] [PubMed]
- Gohil, N.; Bhattacharjee, G.; Gayke, M.; Narode, H.; Alzahrani, K.J.; Singh, V. Enhanced Production of Violacein by Chromobacterium Violaceum Using Agro-industrial Waste Soybean Meal. J. Appl. Microbiol. 2022, 132, 1121–1133. [Google Scholar] [CrossRef]
- Garcia-Saravia Ortiz-de-Montellano, C.; Samani, P.; van der Meer, Y. How Can the Circular Economy Support the Advancement of the Sustainable Development Goals (SDGs)? A Comprehensive Analysis. Sustain. Prod. Consum. 2023, 40, 352–362. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kajla, P.; Luthra, A.; Siwach, R. Whey: As a Low-Cost Substrate for the Production of Biosurfactants BT. In Whey Valorization: Innovations, Technological Advancements and Sustainable Exploitation; Poonia, A., Trajkovska Petkoska, A., Eds.; Springer Nature: Singapore, 2023; pp. 285–310. ISBN 978-981-99-5459-9. [Google Scholar]
- Ramos, G.L.D.P.A.; Guimarães, J.T.; Pimentel, T.C.; da Cruz, A.G.; de Souza, S.L.Q.; Vendramel, S.M.R. Whey: Generation, Recovery, and Use of a Relevant by-Product. In Valorization of Agri-Food Wastes and By-Products; Elsevier: Amsterdam, The Netherlands, 2021; pp. 391–414. [Google Scholar]
- Poonia, A.; Rao, V.; Mann, B. Whey Valorization. In Whey Valorization; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Zandona, E.; Blažić, M.; Režek Jambrak, A. Whey Utilisation: Sustainable Uses and Environmental Approach. Food Technol. Biotechnol. 2021, 59, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Warncke, M.; Keienburg, S.; Kulozik, U. Cold-Renneted Milk Powders for Cheese Production: Impact of Casein/Whey Protein Ratio and Heat on the Gelling Behavior of Reconstituted Rennet Gels and on the Survival Rate of Integrated Lactic Acid Bacteria. Foods 2021, 10, 1606. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J. An Integrated Approach for the Valorization of Cheese Whey. Foods 2021, 10, 564. [Google Scholar] [CrossRef]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy By-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Maragkoudakis, P.; Vendramin, V.; Bovo, B.; Treu, L.; Corich, V. Potential Use of Scotta, the by-Product of the Ricotta Cheese Manufacturing Process, for the Production of Fermented Drinks. J. Dairy Res. 2015, 83, 104–108. [Google Scholar] [CrossRef]
- Pescuma, M.; de Valdez, G.F.; Mozzi, F. Whey-Derived Valuable Products Obtained by Microbial Fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 6183–6196. [Google Scholar] [CrossRef]
- Sommella, E.; Pepe, G.; Ventre, G.; Pagano, F.; Conte, G.M.; Ostacolo, C.; Manfra, M.; Tenore, G.C.; Russo, M.; Novellino, E.; et al. Detailed Peptide Profiling of “Scotta”: From a Dairy Waste to a Source of Potential Health-Promoting Compounds. Dairy Sci. Technol. 2016, 96, 763–771. [Google Scholar] [CrossRef]
- Hoshino, T. Violacein and Related Tryptophan Metabolites Produced by Chromobacterium Violaceum: Biosynthetic Mechanism and Pathway for Construction of Violacein Core. Appl. Microbiol. Biotechnol. 2011, 91, 1463–1475. [Google Scholar] [CrossRef]
- kLa Calculator. Available online: https://www.presens.de/support-services/kla-calculator (accessed on 17 July 2025).
- Riet, K.V. Review of Measuring Methods and Results in Nonviscous Gas-Liquid Mass Transfer in Stirred Vessels. Ind. Eng. Chem. Process Des. Dev. 1979, 18, 357–364. [Google Scholar] [CrossRef]
- Venil, C.K.; Arul, C.; Hazerin, M.; Halim, A.; Reza, A.; Akmar, Z.; Azlina, W. International Biodeterioration & Biodegradation Spray Drying of Violet Pigment from Chromobacterium Violaceum UTM 5 and Its Application in Food Model Systems. Int. Biodeterior. Biodegrad. 2015, 102, 324–329. [Google Scholar] [CrossRef]
- Sar, T.; Harirchi, S.; Ramezani, M.; Bulkan, G.; Akbas, M.Y.; Pandey, A.; Taherzadeh, M.J. Potential Utilization of Dairy Industries By-Products and Wastes through Microbial Processes: A Critical Review. Sci. Total Environ. 2022, 810, 152253. [Google Scholar] [CrossRef] [PubMed]
- Secchi, N.; Giunta, D.; Pretti, L.; Ruiz, M.; Tonina, G.; Mannazzu, I.; Catzeddu, P. Bioconversion of Ovine Scotta into Lactic Acid with Pure and Mixed Cultures of Lactic Acid Bacteria. J. Ind. Microbiol. Biotechnol. 2012, 39, 175–181. [Google Scholar] [CrossRef]
- Beers, V.A.; Pasta, C.; Caccamo, M.; Petriglieri, R.; Difalco, A.; Farina, G.; Belvedere, G.; Marino, G.; Marino, V.M.; Garavaldi, A.; et al. Sicilian Whey: Utilization of Ricotta Whey in the Production of Value-Added Artisanal Beers. Fermentation 2024, 10, 19. [Google Scholar]
- Mangione, G.; Caccamo, M.; Natalello, A. Graduate Student Literature Review: History, Technologies of Production, and Characteristics of Ricotta Cheese. J. Dairy Sci. 2023, 106, 3807–3826. [Google Scholar] [CrossRef]
- Balibar, C.J.; Walsh, C.T. In Vitro Biosynthesis of Violacein from L-Tryptophan by the Enzymes VioA—E from Chromobacterium V Iolaceum. Biochemistry 2006, 45, 15444–15457. [Google Scholar] [CrossRef]
- Somerville, G.A.; Proctor, R.A. Cultivation Conditions and the Diffusion of Oxygen into Culture Media: The Rationale for the Flask-to-Medium Ratio in Microbiology. BMC Microbiol. 2013, 13, 9. [Google Scholar] [CrossRef]
- Garcia-ochoa, F.; Gomez, E.; Santos, V.E.; Merchuk, J.C. Oxygen Uptake Rate in Microbial Processes: An Overview. Biochem. Eng. J. 2010, 49, 289–307. [Google Scholar] [CrossRef]
- Schulte, A.; Jordan, A.; Klöckner, W.; Schumacher, M. Effect of High-Speed Shaking on Oxygen Transfer in Shake Flasks. Biotechnol. J. 2025, 20, e70013. [Google Scholar] [CrossRef]
- De León, M.E.; Wilson, H.S.; Jospin, G.; Eisen, J.A. Genome Sequencing and Multifaceted Taxonomic Analysis of Novel Strains of Violacein-Producing Bacteria and Non-Producing Close Relatives. Microb. Genom. 2023, 9, 000971. [Google Scholar] [CrossRef]
- Schober, I.; Julia, K.; Carbasse, J.S.; Ebeling, C.; Schmidt, M.L.; Podsta, A.; Gupta, R.; Ilangovan, V.; Overmann, J.; Reimer, L.C. Bac Dive in 2025: The Core Database for Prokaryotic Strain Data. Nucleic Acids Res. 2025, 53, 748–756. [Google Scholar] [CrossRef]
- Ambrožič Avguštin, J.; Žgur Bertok, D.; Kostanjšek, R.; Avguštin, G. Isolation and Characterization of a Novel Violacein-like Pigment Producing Psychrotrophic Bacterial Species Janthinobacterium svalbardensis Sp. Nov. Antonie Van Leeuwenhoek 2013, 103, 763–769. [Google Scholar] [CrossRef]
- Chernogor, L.; Bakhvalova, K.; Belikova, A.; Belikov, S. Isolation and Properties of the Bacterial Strain. Microorganisms 2022, 10, 1071. [Google Scholar] [CrossRef]
- Pantanella, F.; Berlutti, F.; Passariello, C.; Sarli, S.; Morea, C.; Schippa, S. Violacein and Biofilm Production in Janthinobacterium lividum. J. Appl. Microbiol. 2007, 102, 992–999. [Google Scholar] [CrossRef]
- Fender, J.E.; Bender, C.M.; Stella, N.A.; Lahr, R.M.; Kalivoda, E.J.; Shanks, R.M.Q.; Haddix, P. Serratia Marcescens Quinoprotein Glucose Dehydrogenase Activity Mediates Medium Acidification and Inhibition of Prodigiosin Production by Glucose. Appl. Environ. Microbiol. 2012, 78, 6225–6235. [Google Scholar] [CrossRef] [PubMed]
- Palukurty, A.; Sangivalasa, V.; Guru Mahesh Darsi, S.R.S.D. Effect of Aeration on Growth and Production of Violacein by Chromobacterium Violaceum Using a Bubble Column Reactor. In Proceedings of the International Journal of Management, Technology and Engineering, Visakhapatnam, India, 12 September 2019. [Google Scholar]
- Bru, R.; Titgemeyer, F. Carbon Catabolite Repression in Bacteria: Choice of the Carbon Source and Autoregulatory Limitation of Sugar Utilization. FEMS Microbiol. Lett. 2002, 209, 141–148. [Google Scholar] [PubMed]
- Deutscher, J. The Mechanisms of Carbon Catabolite Repression in Bacteria Josef Deutscher. Curr. Opin. Microbiol. 2008, 11, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chu, X.; Du, B.; Huang, C.; Xie, C.; Zhang, Z.; Jiang, L. Functional Characterization of a Novel Violacein Biosynthesis Operon. Appl. Microbiol. Biotechnol. 2022, 106, 2903–2916. [Google Scholar] [CrossRef]
- Tong, Y.; Zhou, J.; Zhang, L.; Xu, P. Engineering Oleaginous Yeast Yarrowia Lipolytica for Violacein Production: Extraction, Quantitative Measurement and Culture Optimization. bioRxiv 2019, 1, 687012. [Google Scholar]
- Fang, M.; Zhang, C.; Yang, S.; Cui, J.; Jiang, P.; Lou, K.; Wachi, M.; Xing, X. High Crude Violacein Production from Glucose by Escherichia Coli Engineered with Interactive Control of Tryptophan Pathway and Violacein Biosynthetic Pathway. Microb. Cell Factories 2015, 14, 8. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, D.; Xiong, B.; Zhang, C.; Bi, C. Engineering Corynebacterium Glutamicum for Violacein Hyper Production. Microb. Cell Fact. 2016, 15, 148. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kazakov, A.E.; Gushgari-doyle, S.; Yu, X.; Trotter, V.; Stuart, R.K.; Chakraborty, R. Comparative Genomics Reveals Insights into Induction of Violacein Biosynthesis and Adaptive Evolution in Janthinobacterium. Microbiol. Spectr. 2021, 9, e01414-21. [Google Scholar] [CrossRef]
- Durán, N.; Justo, G.Z.; Durán, M.; Brocchi, M.; Cordi, L.; Tasic, L.; Castro, G.R.; Nakazato, G. Advances in Chromobacterium Violaceum and Properties of Violacein-Its Main Secondary Metabolite: A Review. Biotechnol. Adv. 2016, 34, 1030–1045. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.L.; Trachtmann, N.; Becker, J.; Lohanatha, A.F.; Blotenberg, J.; Bolten, C.J.; Korneli, C.; De Souza, A.O.; Porto, L.M.; Sprenger, G.A.; et al. Systems Metabolic Engineering of Escherichia Coli for Production of the Antitumor Drugs Violacein and Deoxyviolacein. Metab. Eng. 2013, 20, 29–41. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, P.; Lu, Y.; Ruan, Z.; Jiang, R.; Xing, X.; Lou, K.; Wei, D. Optimization of Culture Conditions for Violacein Production by a New Strain of Duganella sp. B2. Biochem. Eng. J. 2009, 44, 119–124. [Google Scholar] [CrossRef]
- Durán, N.; Justo, G.Z.; Ferreira, C.V.; Melo, S.; Cordi, L.; Martins, D. Violacein: Properties and Biological Activities. Biotechnol. Appl. Biochem. 2007, 133, 127–133. [Google Scholar] [CrossRef]
- Mendes, A.S.; De Carvalho, J.E.; Duarte, M.C.T.; Dur, N.; Roy, E. Factorial Design and Response Surface Optimization of Crude Violacein for Chromobacterium Violaceum Production. Biotechnol. Lett. 2001, 23, 1963–1969. [Google Scholar] [CrossRef]
- Scaramussa, S.A.D.L.; Kie, C.; Juliano, I.; Bicas, L. Studies of Violacein and Related Compounds; Springer: Dordrecht, The Netherlands, 2025; Volume 3, ISBN 0123456789. [Google Scholar]
- Aruldass, C.A.; Venil, C.K.; Ahmad, W.A. RSC Advances Violet Pigment Production from Liquid Pineapple. RSC Adv. 2015, 5, 51524–51536. [Google Scholar] [CrossRef]
- Cassarini, M.; Crônier, D.; Besaury, L.; Rémond, C. Protein-Rich Agro-Industrial Co-Products Are Key Substrates for Growth of Chromobacterium Vaccinii and Its Violacein Bioproduction. Waste Biomass Valorization 2022, 13, 4459–4468. [Google Scholar] [CrossRef]
- Carletto, R.A. Studio della Produzione di Poliidrossialcanoati da Siero di Latte; VCH: New York, NY, USA, 2014. [Google Scholar]
- Parent, G.; Le Bihan, G.; Häusler, O.; Lefevre, P. Study of the Long-Term Stability of the Compactibility of Spray-Dried Mannitol Powders Containing α and β Crystalline Polymorphs. In Proceedings of the 3rd European Conference on Pharmaceutics, Bologna, Italy, 25–26 March 2019; pp. 2–3. [Google Scholar] [CrossRef]
- Thakral, S.; Sonje, J.; Munjal, B.; Bhatnagar, B.; Suryanarayanan, R. Mannitol as an Excipient for Lyophilized Injectable Formulations. J. Pharm. Sci. 2023, 112, 19–35. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and Challenges of the Spray-Drying Technology for the Production of Pure Drug Particles and Drug-Loaded Polymeric Carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Homayoonfal, M.; Malekjani, N.; Baeghbali, V.; Ansarifar, E.; Hedayati, S.; Jafari, S.M. Optimization of Spray Drying Process Parameters for the Food Bioactive Ingredients. Crit. Rev. Food Sci. Nutr. 2024, 64, 5631–5671. [Google Scholar] [CrossRef]
- Cheng, K.C.; Hsiao, H.C.; Hou, Y.C.; Hsieh, C.W.; Hsu, H.Y.; Chen, H.Y.; Lin, S.P. Improvement in Violacein Production by Utilizing Formic Acid to Induce Quorum Sensing in Chromobacterium Violaceum. Antioxidants 2022, 11, 849. [Google Scholar] [CrossRef]
- Lou, X.; Zhou, Q.; Jiang, Q.; Lin, L.; Zhu, W.; Mei, X.; Xiong, J.; Gao, Y. Inhibitory Effect and Mechanism of Violacein on Planktonic Growth, Spore Germination, Biofilm Formation and Toxin Production of Bacillus Cereus and Its Application in Grass Carp Preservation. Int. J. Food Microbiol. 2025, 426, 110917. [Google Scholar] [CrossRef]
- Dodou, H.V.; de Morais Batista, A.H.; Sales, G.W.P.; de Medeiros, S.C.; Rodrigues, M.L.; Nogueira, P.C.N.; Silveira, E.R.; Nogueira, N.A.P. Violacein Antimicrobial Activity on Staphylococcus Epidermidis and Synergistic Effect on Commercially Available Antibiotics. J. Appl. Microbiol. 2017, 123, 853–860. [Google Scholar] [CrossRef]
- Abedin, S.M.M.; Tarafdar, M.R.; Saha, A.; Atiqua; Rahim, S.; Karim, M.M.; Khan, S.N. Isolation and Characterization of Chromobacterium Violaceum and Antibacterial Activities of Its Metabolite Violacein. Dhaka Univ. J. Biol. Sci. 2024, 33, 109–119. [Google Scholar] [CrossRef]
- Lyakhovchenko, N.S.; Efimova, V.A.; Seliverstov, E.S.; Anis’kov, A.A.; Solyanikova, I.P. Bacteriostatic Activity of Janthinobacterium Lividum and Purified Violacein Fraction against Clavibacter Michiganensis. Processes 2024, 12, 1116. [Google Scholar] [CrossRef]
- Chauhan, A.; Mathkor, D.M.; Joshi, H.; Chauhan, R.; Sharma, U.; Sharma, V.; Kumar, M.; Saini, R.V.; Saini, A.K.; Tuli, H.S.; et al. Mechanistic Insight of Pharmacological Aspects of Violacein: Recent Trends and Advancements. J. Biochem. Mol. Toxicol. 2025, 39, e70114. [Google Scholar] [CrossRef]
- Cauz, A.C.G.; Carretero, G.P.B.; Saraiva, G.K.V.; Park, P.; Mortara, L.; Cuccovia, I.M.; Brocchi, M.; Gueiros-Filho, F.J. Violacein Targets the Cytoplasmic Membrane of Bacteria. ACS Infect. Dis. 2019, 5, 539–549. [Google Scholar] [CrossRef]
- Choi, S.Y.; Im, H.; Mitchell, R.J. Violacein and Bacterial Predation: Promising Alternatives for Priority Multidrug Resistant Human Pathogens. Future Microbiol. 2017, 12, 835–838. [Google Scholar] [CrossRef]
- Maimouni Filali, S. Study of Solubility of Mannitol in Different Organic Solvents. Bachelor’s Theses, University of Barcelona, Barcelona, Spain, 2023. Available online: https://hdl.handle.net/2445/201726 (accessed on 2 September 2025).
- Berti, I.R.; Rodenak-Kladniew, B.; Perez, A.A.; Santiago, L.; Duran, N.; Castro, G.R. Development of Biocarrier for Violacein Controlled Release in the Treatment of Cancer. React. Funct. Polym. 2019, 136, 122–130. [Google Scholar] [CrossRef]
- Martins, D.; Costa, F.T.M.; Brocchi, M.; Durán, N. Evaluation of the Antibacterial Activity of Poly-(d,l-Lactide-Co-Glycolide) Nanoparticles Containing Violacein. J. Nanoparticle Res. 2011, 13, 355–363. [Google Scholar] [CrossRef]
- Party, P.; Piszman, Z.I.; Ambrus, R. Development of Mannitol-Based Microparticles for Dry Powder Inhalers: Enhancing Pulmonary Delivery of NSAIDs. Pharmaceuticals 2025, 18, 923. [Google Scholar] [CrossRef] [PubMed]
- Banat, H.; Csóka, I.; Kun-Szabó, F.; Fodor, G.H.; Somogyi, P.; Peták, F.; Party, P.; Sztojkov-Ivanov, A.; Ducza, E.; Berkecz, R.; et al. Mannitol-Leucine Synergy in Nanocrystal Agglomerates for Enhanced Systemic Delivery of Inhaled Ketoprofen: Pharmacokinetics and Safety in Ovalbumin-Sensitized Rats. Int. J. Pharm. 2025, 676, 125610. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.; Karlén, A. Discovery and Preclinical Development of New Antibiotics. Ups. J. Med. Sci. 2014, 119, 162–169. [Google Scholar] [CrossRef] [PubMed]

| Scotta | Lactose | pH | Density | EC |
|---|---|---|---|---|
| g L−1 | g mL−1 | mS cm−1 | ||
| S1 | 9.99 | 7.07 | 1.007 | 5.15 |
| S2 | 42.19 | 6.77 | 1.015 | 7.21 |
| S3 | 26.81 | 3.9 | 1.021 | 19.79 |
| S4 | 7.22 | 6.78 | 1.027 | 5.89 |
| Time | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 120 h | |||||||
| Scotta | Lactose (g L−1) | Glucose (g L−1) | Galactose (g L−1) | Lactose (g L−1) | Glucose (g L−1) | Galactose (g L−1) | Violacein (mg L−1) | |
| Not diluted | 3 | 26.81 ± 0.41 | ND | ND | 26.72 ± 0.32 | ND | ND | 6.53 ± 0.37 |
| 3+L | 26.81 ± 0.41 | ND | ND | ND | 6.49 ± 0.29 | 13.39 ± 0.41 | ND | |
| 4 | 7.22 ± 0.34 | 21.86 ± 0.46 | 20.36 ± 0.42 | 6.64 ± 0.11 | 9.97 ± 0.03 | 18.97 ± 0.27 | ND | |
| Diluted | 3 | 10.19 ± 8.66 | ND | ND | 10.33 ± 0.41 | ND | ND | 18.13 ± 0.63 |
| 3+L | 10.19 ± 0.34 | ND | ND | ND | ND | 5.27 ± 0.38 | 19.87 ± 0.13 | |
| 4 | 2.74 ± 0.12 | 8.3 ± 0.30 | 7.74 ± 0.41 | 2.65 ± 0.03 | 1.62 ± 0.21 | 6.95 ± 0.06 | 20.21 ± 0.44 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trupo, M.; Magarelli, R.A.; Palazzo, S.; Larocca, V.; Martino, M.; Spagnoletta, A.; Ambrico, A. Bioconversion of a Dairy By-Product (Scotta) into Mannitol-Stabilized Violacein via Janthinobacterium lividum Fermentation. Microorganisms 2025, 13, 2125. https://doi.org/10.3390/microorganisms13092125
Trupo M, Magarelli RA, Palazzo S, Larocca V, Martino M, Spagnoletta A, Ambrico A. Bioconversion of a Dairy By-Product (Scotta) into Mannitol-Stabilized Violacein via Janthinobacterium lividum Fermentation. Microorganisms. 2025; 13(9):2125. https://doi.org/10.3390/microorganisms13092125
Chicago/Turabian StyleTrupo, Mario, Rosaria Alessandra Magarelli, Salvatore Palazzo, Vincenzo Larocca, Maria Martino, Anna Spagnoletta, and Alfredo Ambrico. 2025. "Bioconversion of a Dairy By-Product (Scotta) into Mannitol-Stabilized Violacein via Janthinobacterium lividum Fermentation" Microorganisms 13, no. 9: 2125. https://doi.org/10.3390/microorganisms13092125
APA StyleTrupo, M., Magarelli, R. A., Palazzo, S., Larocca, V., Martino, M., Spagnoletta, A., & Ambrico, A. (2025). Bioconversion of a Dairy By-Product (Scotta) into Mannitol-Stabilized Violacein via Janthinobacterium lividum Fermentation. Microorganisms, 13(9), 2125. https://doi.org/10.3390/microorganisms13092125









