1. Introduction
Staphylococcus aureus is a formidable pathogen capable of causing severe infections in both humans and animals. The increasing prevalence of multidrug-resistant strains, particularly methicillin-resistant
S. aureus (MRSA), in clinical settings poses a significant public health concern [
1,
2]. Consequently, gaining a deeper understanding of MRSA’s physiology is critical for developing alternative strategies to combat antibiotic-resistant infections.
Among the potential targets for novel antibacterial agents is the staphylococcal Gcp protein. Gcp and its homologs are essential for the viability of several bacterial species, including
S. aureus [
3,
4],
Streptococcus pneumoniae [
3],
Escherichia coli [
5],
Bacillus subtilis [
6,
7],
Francisella novicida [
8],
Pseudomonas aeruginosa [
9], and
Mycoplasma genitalium [
10]. These homologs possess diverse functions. For example, the Gcp of
Mannheimia haemolytica exhibits glycoprotease activity, specifically cleaving O-sialoglycosylated proteins [
11]. As members of the ASKHA (acetate and sugar kinases, HSP70, and actin) superfamily, Gcp homologs, such as
Pyrococcus abyssi Pa-Kae1, can bind iron and ATP and demonstrate DNA-binding and apurinic endonuclease activity [
12]. In yeast, the Gcp homolog Kae1 is a core component of the KEOPS/EKC complex, which plays a role in transcription and chromatin regulation [
13,
14].
Our previous studies have demonstrated that Gcp, also known as TsaD, is indispensable for
S. aureus viability under in vitro conditions and is involved in regulating bacterial autolysis [
4] and the N
6-threonyl carbamoyl adenosine (t
6A) modification of tRNA [
15]. Furthermore, our proteomic studies have revealed that Gcp modulates the biosynthesis of branched-chain amino acids (BCAAs), which are vital for bacterial growth and metabolism [
15]. In
B. subtilis, BCAA biosynthesis is orchestrated by the
ilvBHC-
leuABCD (
ilv-
leu) operon and related genes, such as
ilvA,
ilvD,
ybgE, and
ywaA [
16,
17,
18].
S. aureus harbors a homologous gene cluster,
ilvDBHC-
leuABCD-
ilvA, with
ilvE serving a role analogous to
ybgE and
ywaA in
B. subtilis [
15]. Gcp depletion was shown to enhance the production of key enzymes in this pathway, as revealed by a proteomic analysis, and to suppress
ilv-
leu operon transcription, based on qPCR, promoter–lux fusions, and gel-shift assays [
15]. Notably, Gcp depletion also upregulated CcpA, a known activator of
ilv-
leu expression, without affecting the CodY levels [
15].
BCAA metabolism plays a critical role in bacterial physiology, as these hydrophobic amino acids are essential for membrane protein structure and function [
19,
20]. Their intermediates, branched-chain α-keto acids, contribute to membrane biosynthesis via branched-chain fatty acids [
21,
22], and serve as precursors for cofactors such as pantothenate and coenzyme A [
23,
24]. To elucidate whether Gcp’s essentiality is due to its repression of the ILV biosynthesis pathway, we deleted the
ilv-
leu operon. Surprisingly, this did not alter Gcp’s essential role, suggesting that its function extends beyond ILV regulation [
15]. In
E. coli, Gcp homologs (TsaD) participate in the synthesis of t
6A, a conserved tRNA modification essential for translation fidelity [
25,
26]. Our research revealed that Gcp is similarly required for t
6A biosynthesis in
S. aureus [
15], and its interaction with YeaZ (TsaB) is crucial for bacterial viability [
27,
28]. Taken together, these findings indicate that Gcp/TsaD is multifunctional in bacteria.
In this study, we further investigated the role of Gcp/TsaD in S. aureus by assessing its effects on cell morphology using scanning and transmission electron microscopy. We also performed kinetic transcriptomic analyses via RNA sequencing to examine its global transcriptional impact. Together, these approaches provided a comprehensive view of the multifaceted role of Gcp/TsaD in staphylococcal physiology and highlight potential avenues for therapeutic intervention.
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, Antibiotics, and Growth Conditions
S. aureus WCUH29, a methicillin-resistant clinical isolate (MRSA), was used in this study [
15]. The control strain JW29011 (WCUH29 attB::pLH1) and the
gcp/
tsaD conditional expression mutant JW290111 (WCUH29 Δ
gcp attB::
Pspac-gcp, carrying plasmid pYH4-lacI) were cultured in Tryptic Soy Broth (TSB) at 37 °C with shaking at 225 rpm [
15]. The antibiotics were purchased from Sigma-Aldrich (St. Louis, MO, USA). Where applicable, the cultures were supplemented with 5 µg/mL erythromycin, 2.5 µg/mL tetracycline, and 100 µM isopropyl β-D-1-thiogalactopyranoside (IPTG, Sigma-Aldrich) to induce
gcp/
tsaD expression.
2.2. Scanning Electron Microscopy (SEM)
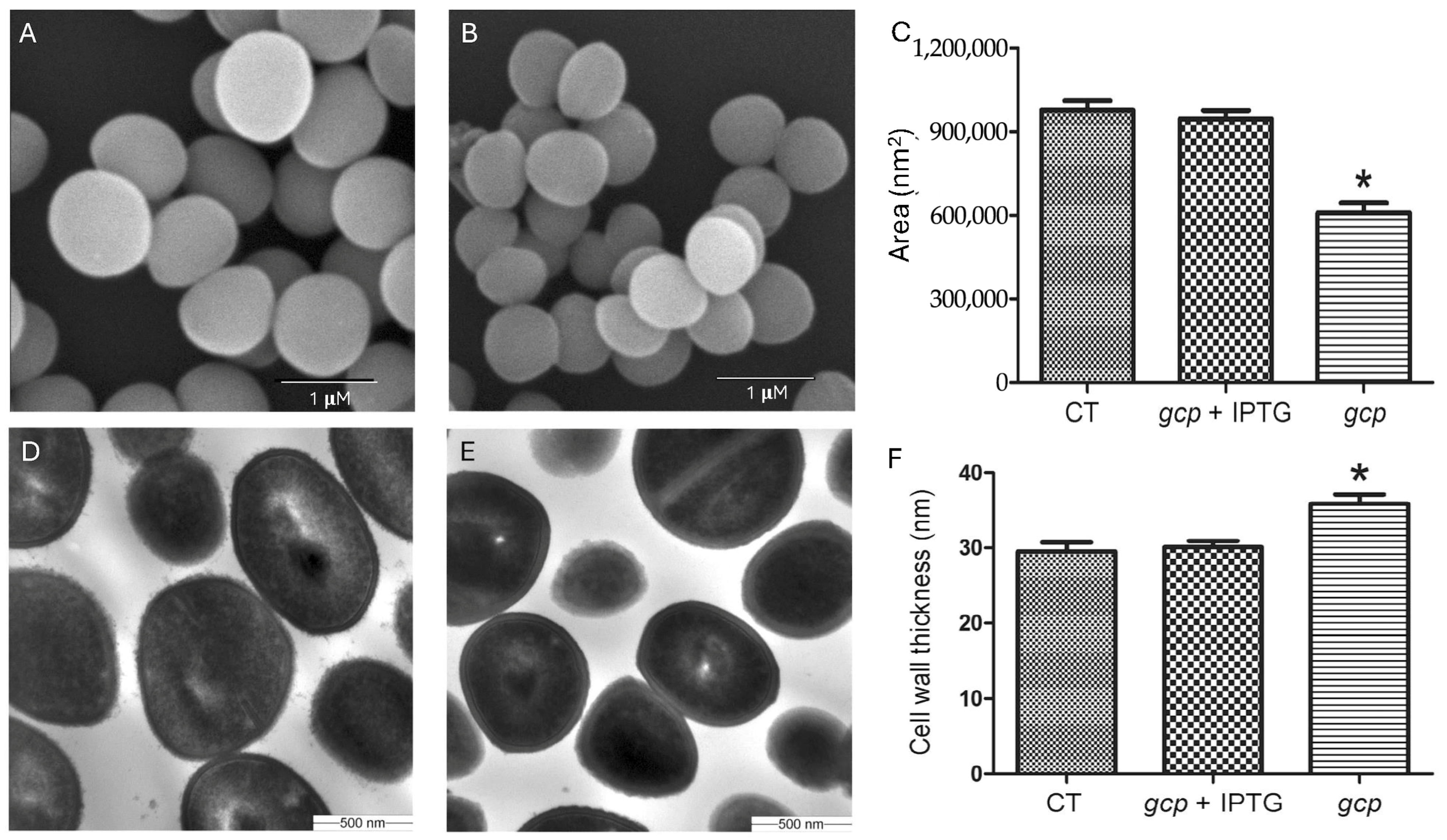
Overnight cultures of JW290111 were diluted 1:100 in fresh TSB with or without 100 µM IPTG and grown to the mid-log phase (OD600 = ~0.5). The cells were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (Electron Microscopy Sciences, EMS, Hatfield, PA, USA) overnight at 4 °C, washed, and post-fixed in 1% osmium tetroxide (EMS) in the same buffer. After washing, the samples were dehydrated through a graded ethanol series (25 to 100%), treated with hexamethyldisilazane (HMDS; EMS), air-dried on coverslips, and mounted on SEM stubs. The samples were sputter-coated with platinum (Ted Pella Inc., Redding, CA, USA) and visualized using a Hitachi S3500N scanning electron microscope (Hitachi, Tokyo, Japan). The images were acquired using Quartz PCI software Version 8 (Quartz Imaging Corp., Vancouver, BC, Canada), and the cell surface areas were quantified using iTEM software the 2013 version (Olympus SIS, Münster, Germany).
2.3. Transmission Electron Microscopy (TEM)
The bacterial cultures were processed as described for the SEM until the dehydration step, which was performed using an acetone gradient (25 to 100%). The samples were infiltrated with 2:1 acetone–Embed 812 resin (EMS) for 1 h, subsequently transferred to a 1:2 acetone–Embed 812 resin mixture for 1 h, and infiltrated with 100% resin. The resin-embedded samples were polymerized overnight at 58 °C in gelatin capsules. Ultrathin sections (60–70 nm) were prepared using a Leica UC6 ultramicrotome (Deerfield, IL, USA) and mounted on 200-mesh copper grids (EMS). The sections were stained with 5% uranyl acetate and Sato lead citrate, then examined with a JEOL 1200 EX II transmission electron microscope (Peabody, MA, USA) ggplot2. The images were captured using a Veleta 2K × 2K camera (Lakewood, CO, USA) and iTEM software, and measurements of cell area, perimeter, and peptidoglycan thickness were performed using the same software.
2.4. RNA Isolation and Purification
Overnight cultures were diluted 1:100 in fresh TSB, with or without 100 µM IPTG, and harvested at OD600 ≈ 0.2 (early), 0.5 (mid-log), and 1.0 (early stationary phase). The total RNA was isolated using either an SV Total RNA Isolation System (Promega, Madison, WI, USA) or a RiboPure™-Bacteria Kit (Thermo Fisher Scientific, Waltham, MA, USA). The genomic DNA was removed with two rounds of TURBO DNase treatment (Ambion, Austin, TX, USA), and the RNA concentration was measured at 260 nm.
2.5. RNA Sequencing (RNA-Seq) and Data Analysis
2.5.1. RNA Sequencing
The total RNA from three growth phases was purified from three biological replicates. The ribosomal RNA was depleted using a Ribo-off rRNA Depletion Kit (Bacteria), followed by cDNA synthesis and library preparation with a VAHTS™ Stranded mRNA-seq Library Prep Kit for Illumina
® (San Diego, CA, USA). The sequencing was performed on the Illumina platform [
29].
2.5.2. Differential Gene Expression Analysis
Differential expression was analyzed using DESeq [
30], with significance thresholds set at q-value ≤ 0.05 and |log
2 fold change| ≥ 1.
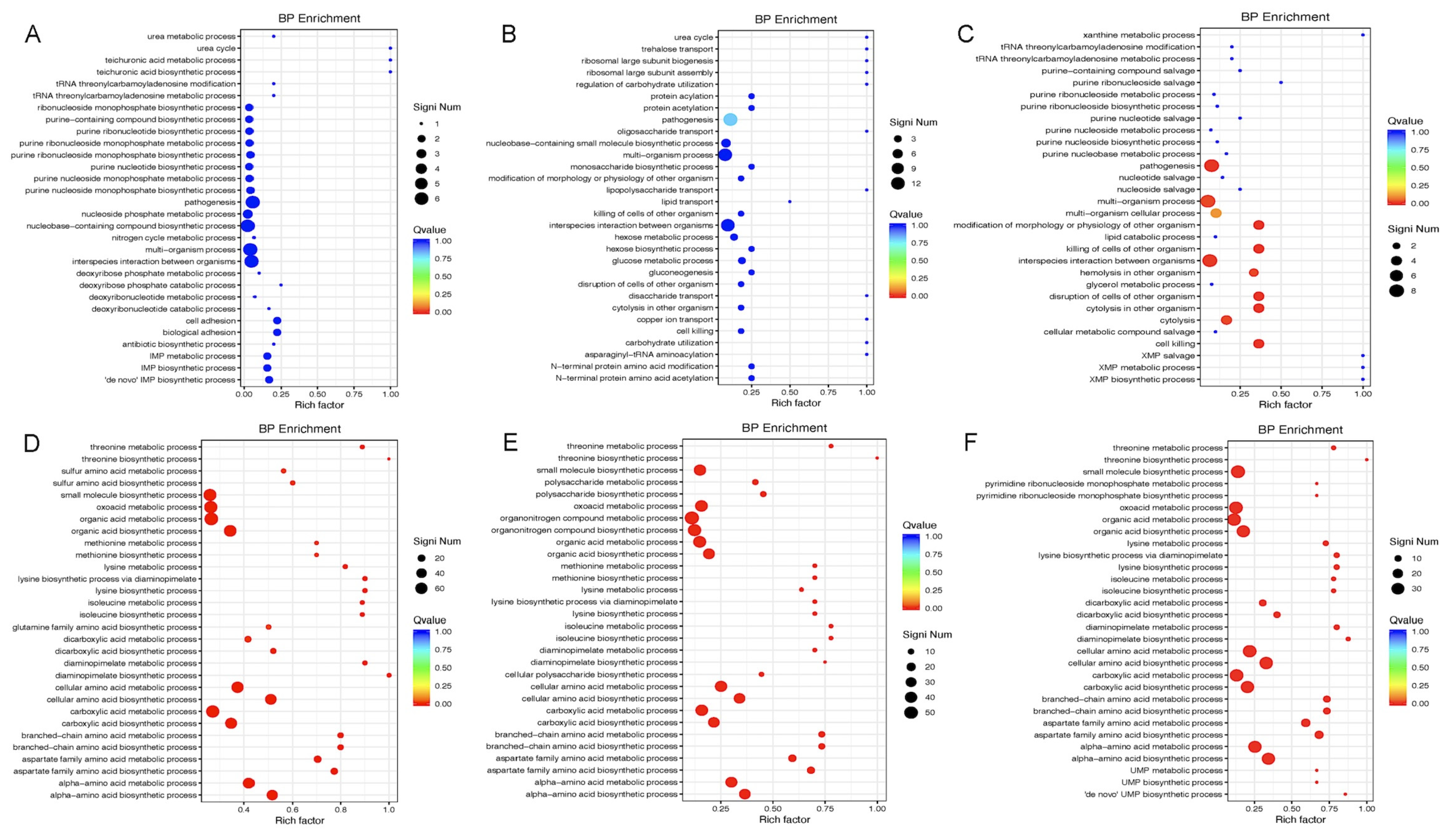
2.5.3. Functional Enrichment Analysis
The differentially expressed genes were subjected to a KEGG and Gene Ontology (GO) Biological Process (BP) enrichment analysis using the R language cluster Profiler. Fisher’s exact test was used to evaluate the statistical significance, and visualization was performed using ggplot2.
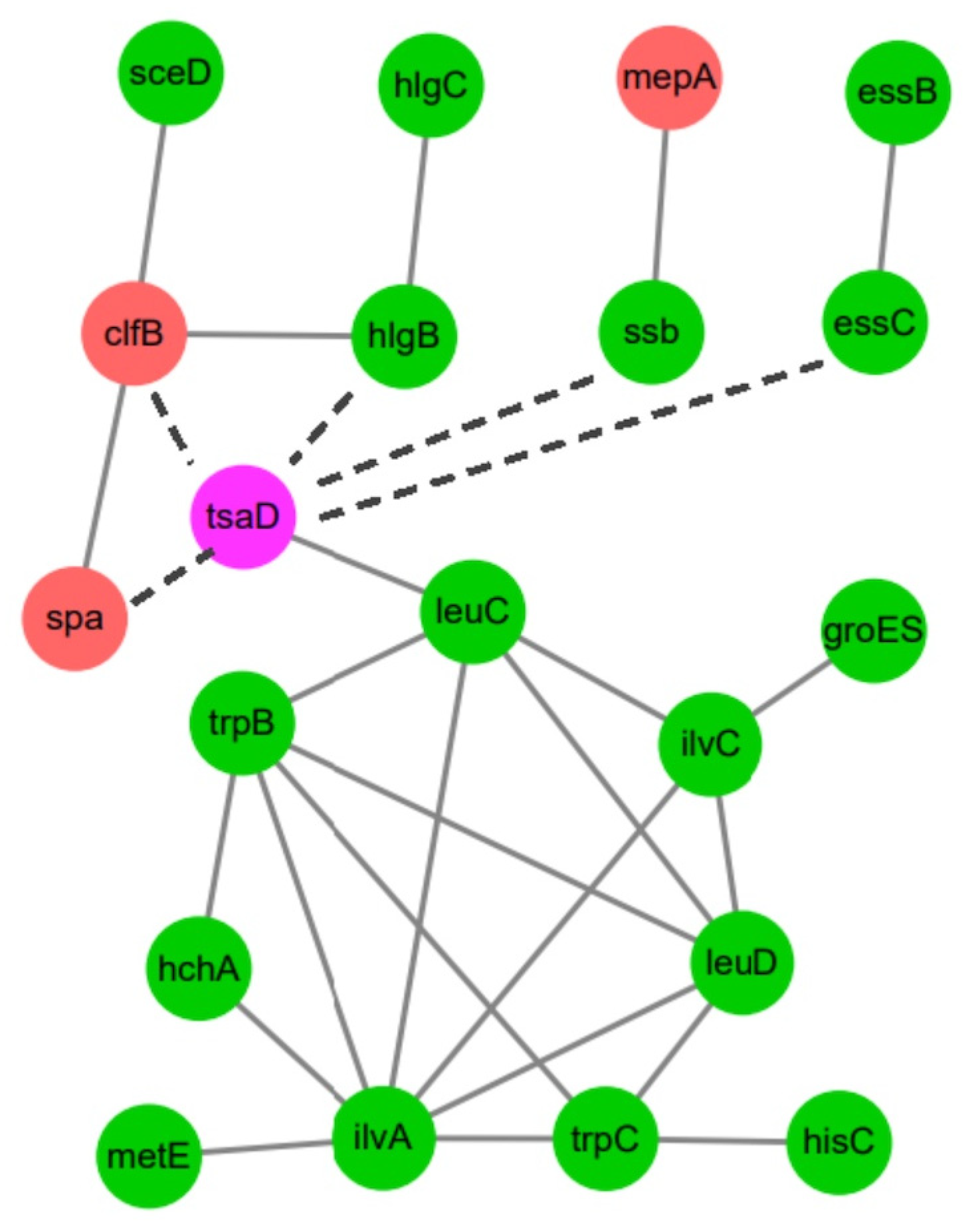
2.5.4. Protein–Protein Interaction (PPI) Network Analysis
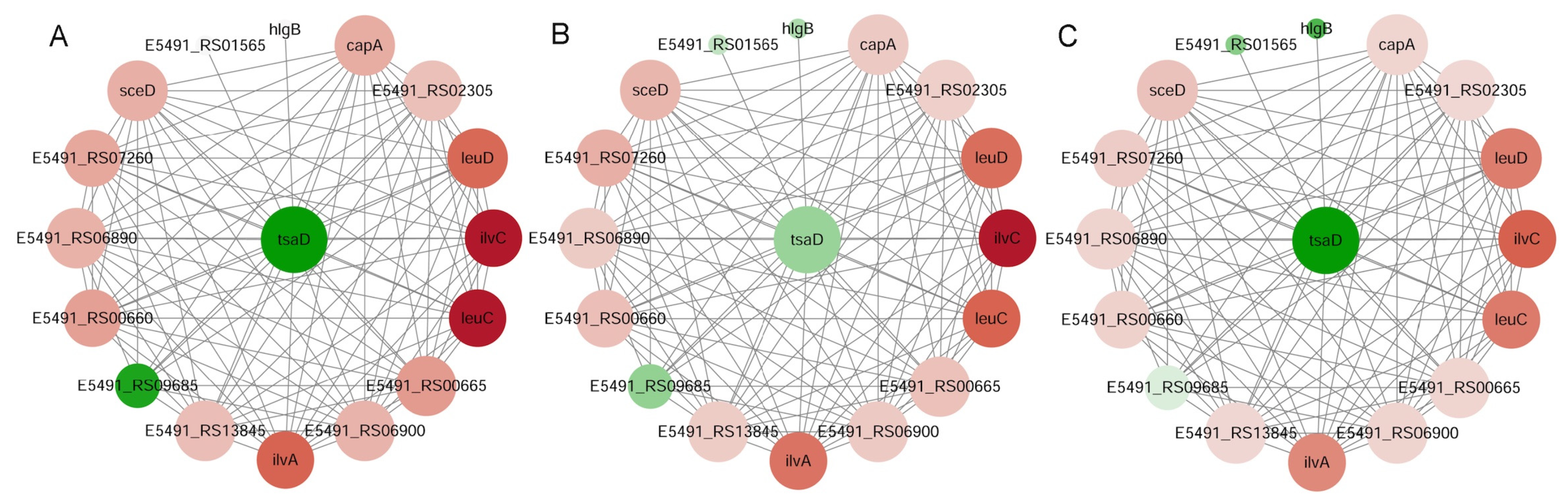
The PPI networks were constructed using the STRING 11.5 database, referencing S. aureus NCTC 8325. A confidence score threshold of ≥0.4 was applied. The network diagrams were generated using Cytoscape v3.6.1.
2.6. Semi-Quantitative Real-Time RT-PCR (qPCR)
To validate RNA-seq results, selected genes were analyzed by qPCR [
29]. Cultures were grown to mid-log phase (OD600 = ~0.5), and RNA was isolated as described. cDNA synthesis was carried out with SuperScript III (Invitrogen, Waltham, MA, USA) and random primers. Reactions were run in duplicate using VeriQuest SYBR Green Master Mix (Affymetrix, Santa Clara, CA, USA) on Stratagene Mx3000P system (Stratagene, La Jolla, CA, USA). Primers (
Table 1) were designed to yield 100–200 bp amplicons. Relative expression was calculated using ΔΔCt method, with 16S rRNA as internal control.
2.7. Minimum Inhibitory Concentration (MIC) Assays
S. aureus strains were grown overnight in TSB, diluted to ~10
5 CFU/mL in Mueller–Hinton broth (MHB), and exposed to serial dilutions of test compounds in 96-well microtiter plates as described in [
31]. MICs were defined as lowest concentration that inhibited visible growth after 18 h incubation at 37 °C.
4. Discussion
Our study provides compelling evidence that Gcp/TsaD is a key contributor to multiple functions of
S. aureus biology. Previous work from our group showed that Gcp/TsaD forms a complex with its operon partner YeaZ/TsaB, co-encoded in the
tsaBCDE operon in
S. aureus [
28]. We further demonstrated that YeaZ/TsaB directly binds to the
ilv-leu promoter to enhance its transcription [
40]. Although Gcp/TsaD does not bind to the promoter, its depletion leads to a significant increase in
ilv-leu transcription [
15], suggesting that the Gcp-YeaZ complex may have co-functional roles in regulating ILV biosynthesis. Taken together, these observations suggest the possibility that Gcp/TsaD participates in broader regulatory circuits beyond its canonical role in tRNA threonyl-carbamoylation. Through the combination of a morphological analysis, transcriptomic profiling, network-based bioinformatic analysis, and antibiotic susceptibility assays, we demonstrated that Gcp/TsaD is involved in a wide array of physiological processes, including cell morphology, cell wall homeostasis, transcriptional regulation, virulence expression, and antibiotic response.
The transmission and scanning electron microscopy revealed striking morphological defects in the Gcp/TsaD-depleted strain, including reduced cell size and increased cell wall thickness. These findings indicate that Gcp/TsaD plays a crucial role in coordinating proper cell division and peptidoglycan biosynthesis. Our findings are consistent with previous reports that have shown the effects of depleted YgjD (Gcp’s homolog in
E. coli) on bacterial cell morphology [
41,
42,
43]. The increased cell wall thickness is likely a compensatory response to cell envelope stress and structural instability, a phenomenon that has also been observed in mutants defective in wall teichoic acid synthesis or penicillin-binding proteins [
44,
45]. Importantly, cell wall modifications are a common bacterial strategy to counteract environmental stresses, including host immune pressures and exposure to antibiotics [
46]. The morphological phenotype in our Gcp/TsaD-depleted strain, therefore, likely reflects broader perturbations in the cell envelope integrity pathway. The changed cell wall structure might contribute to the increased resistance of autolysis and antibiotics-induced cell lysis in Gcp/TsaD-downregulated
S. aureus [
4].
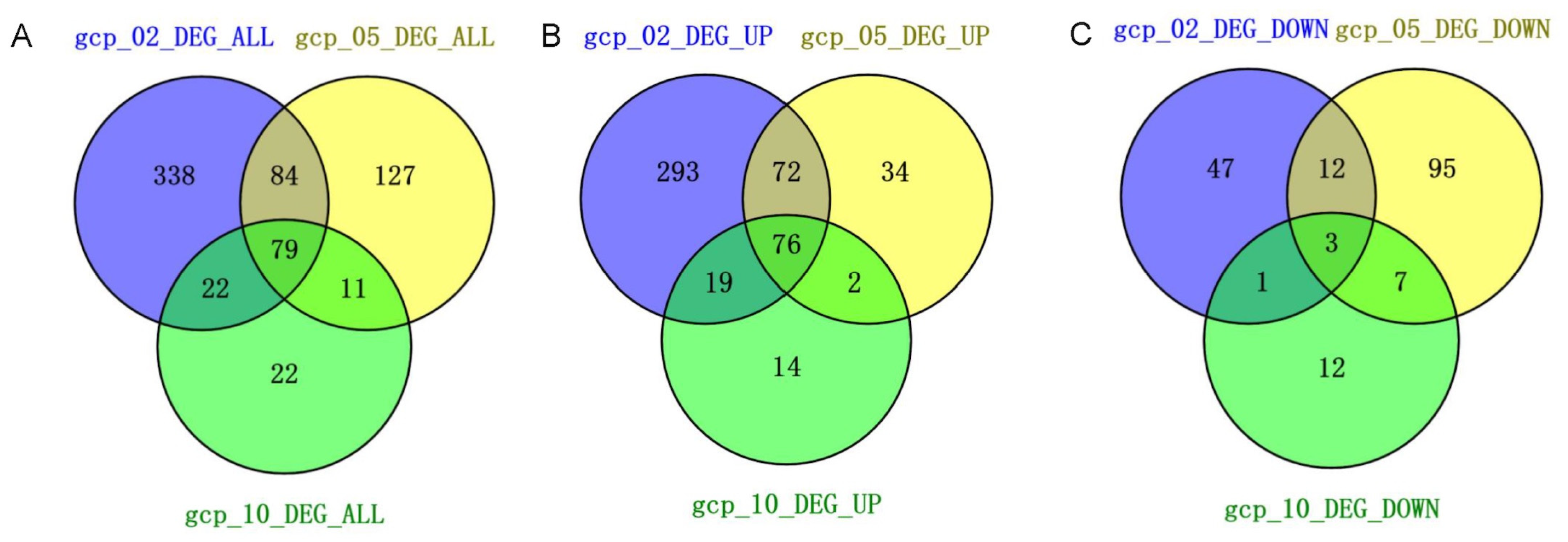
The RNA-seq analysis revealed widespread changes in gene expression upon Gcp/TsaD depletion. The most dramatic alterations occurred during the early log phase, suggesting that Gcp/TsaD is particularly critical during rapid growth when the biosynthetic and translational demands are high. Notably, 79 genes were consistently differentially expressed across all three growth phases, indicating a core transcriptional response likely tied to Gcp/TsaD function. Among these, the genes involved in translation, energy production, and amino acid biosynthesis were prominently affected, consistent with Gcp/TsaD’s essential role in tRNA modification, specifically in the formation of the universally conserved threonylcarbamoyl adenosine (t
6A) at position 37 of ANN-decoding tRNAs [
25,
33,
47]. The t
6A modification is essential for proper decoding during translation and for maintaining reading frame fidelity. In organisms ranging from bacteria to humans, loss of this modification results in translational stress, codon misreading, and ribosomal pausing [
48,
49,
50]. The observed downregulation of multiple tRNA genes, including several involved in decoding ANN codons, provides further evidence that Gcp/TsaD depletion induces translational stress in
S. aureus, thereby triggering global transcriptional reprogramming.
In response to this stress, we observed a compensatory upregulation of genes involved in cell envelope biosynthesis and modification. This included an increased expression of the
dltABCD operon, which mediates D-alanylation of teichoic acids and contributes to resistance to cationic antimicrobial peptides [
51], and upregulation of the
cap operon, responsible for capsule synthesis. Capsules are known virulence factors that also play protective roles against host immune defenses [
52,
53]. These findings support the hypothesis that Gcp/TsaD depletion disrupts envelope homeostasis, resulting in the activation of envelope stress response pathways that aim to preserve cellular integrity under hostile conditions.
Interestingly, we observed marked downregulation of several key virulence genes, including
spa (encoding protein A),
lukH (Panton–Valentine leukocidin component),
scpA (staphopain A), and
hlgA/
B/
C (gamma-hemolysin subunits). These genes are under the control of major virulence regulators, such as the SaeRS two-component system and the Agr quorum-sensing network [
37,
54]. Our data indicate that Gcp/TsaD depletion leads to downregulation of
saeS, suggesting that Gcp/TsaD may exert indirect control over virulence gene expression through modulation of regulatory circuits. Similar repression of virulence factors has been documented in
S. aureus under oxidative stress or in response to translational arrest [
55,
56]. Thus, Gcp/TsaD appears to play a dual role in both maintaining basal metabolic functions and enabling the expression of pathogenic traits under favorable conditions.
The perturbation of metabolic genes upon Gcp/TsaD downregulation is also noteworthy. The pathways involving sulfur metabolism, purine biosynthesis, and branched-chain amino acid synthesis were broadly repressed, which is consistent with our previous findings [
15]. These pathways are essential for nucleotide production, protein synthesis, and energy generation, especially under nutrient-limited conditions [
57,
58,
59]. The network analysis further identified
leuC,
ilvA, and
trpB as co-regulated hubs associated with Gcp/TsaD. These genes are key enzymes in leucine, isoleucine, and tryptophan biosynthesis, respectively, and have previously been linked to the virulence and persistence of
S. aureus [
60].
Importantly, our functional assays revealed that Gcp/TsaD depletion significantly increased susceptibility to fosfomycin, an antibiotic that targets the enzyme MurA, which catalyzes the first committed step in peptidoglycan biosynthesis [
61,
62]. This finding suggests that Gcp/TsaD may play a previously unappreciated role in maintaining cell envelope integrity or in the regulation of peptidoglycan precursor synthesis. The heightened susceptibility to fosfomycin supports a model in which Gcp/TsaD is functionally intertwined with cell wall biosynthetic pathways, potentially through direct or indirect regulation of enzymatic systems involved in precursor synthesis or membrane homeostasis. The precise mechanisms underlying these effects need to be further investigated. The hypersensitivity to fosfomycin likely reflects a compromised cell wall synthesis machinery and altered expression of cell wall biosynthetic genes. Given the increasing interest in adjuvant therapies that sensitize
S. aureus to β-lactams and other cell wall-targeting antibiotics, our findings raise the possibility that targeting Gcp/TsaD or its downstream pathways could be a viable therapeutic strategy [
63,
64].
In conclusion, this study demonstrates that Gcp/TsaD plays a critical role in modulating global gene expression, morphogenesis, virulence, and antibiotic susceptibility in S. aureus. Its essential nature and broad regulatory influence highlight Gcp/TsaD as a promising target for novel antimicrobial development. Future studies will focus on elucidating the molecular mechanisms of Gcp/TsaD-dependent regulation and assessing its potential for therapeutic exploitation against drug-resistant S. aureus infections.