Clinical Features of Multidrug-Resistant Gram-Negative Bacteremia: A Comparative Study of Cancer and Non-Cancer Patients
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Data Collection
2.4. Statistical Analysis
2.5. Microbiological Identification and Susceptibility Testing
2.6. Ethical Considerations
3. Results
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bassetti, M.; Peghin, M.; Vena, A.; Giacobbe, D.R. Treatment of Infections Due to MDR Gram-Negative Bacteria. Front. Med. 2019, 6, 74. [Google Scholar] [CrossRef]
- Reynolds, D.; Burnham, J.P.; Guillamet, C.V.; McCabe, M.; Yuenger, V.; Betthauser, K.; Micek, S.T.; Kollef, M.H. The threat of multidrug-resistant/extensively drug-resistant Gram-negative respiratory infections: Another pandemic. Eur. Respir. Rev. 2022, 31, 220068. [Google Scholar] [CrossRef]
- Tohamy, S.T.; Aboshanab, K.M.; El-Mahallawy, H.A.; El-Ansary, M.R.; Afifi, S.S. Prevalence of multidrug-resistant Gram-negative pathogens isolated from febrile neutropenic cancer patients with bloodstream infections in Egypt and new synergistic antibiotic combinations. Infect. Drug Resist. 2018, 11, 783–790. [Google Scholar] [CrossRef]
- Paterson, D.L.; Bonomo, R.A. Extended-spectrum β-lactamases: A clinical update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef] [PubMed]
- Awada, B.; Abarca, J.; Mumtaz, S.; Al-Khirbash, A.; Al-Sayegh, H.; Milupi, M.; Garcia, A.E.; Al Harthy, M.; Al Qarshoubi, I.; Al Baimani, K.; et al. Predictors and outcomes of multi-drug–resistant gram-negative bacteremia in patients with cancer: A retrospective cohort study at a tertiary cancer center in Oman. IJID Reg. 2024, 12, 100399. [Google Scholar] [CrossRef] [PubMed]
- Gudiol, C.; Tubau, F.; Calatayud, L.; Garcia-Vidal, C.; Cisnal, M.; Sanchez-Ortega, I.; Duarte, R.; Calvo, M.; Carratala, J. Bacteraemia due to multidrug-resistant gram-negative bacilli in cancer patients: Risk factors, antibiotic therapy and outcomes. J. Antimicrob. Chemother. 2011, 66, 657–663. [Google Scholar] [CrossRef]
- Yuan, F.; Xiao, W.; Wang, X.; Fu, Y.; Wei, X. Clinical Characteristics and Prognosis of Bloodstream Infection with Carbapenem-Resistant Pseudomonas aeruginosa in Patients with Hematologic Malignancies. Infect. Drug Resist. 2023, 16, 4943–4952. [Google Scholar] [CrossRef]
- Perez, F.; Adachi, J.; Bonomo, R.A. Antibiotic-resistant gram-negative bacterial infections in patients with cancer. Clin. Infect. Dis. 2014, 59, S335–S339. [Google Scholar] [CrossRef]
- Huang, C.; Lin, L.; Kuo, S. Risk factors for mortality in Stenotrophomonas maltophilia bacteremia–a meta-analysis. Infect. Dis. 2024, 56, 335–347. [Google Scholar] [CrossRef]
- Lubwama, M.; Holte, S.E.; Zhang, Y.; Mubiru, K.R.; Katende, G.; Orem, J.; Kateete, D.P.; Bwanga, F.; Phipps, W. Etiology, Risk Factors, and Outcomes of Bacteremia in Patients with Hematologic Malignancies and Febrile Neutropenia in Uganda. Open Forum Infect. Dis. 2024, 11, ofae682. [Google Scholar] [CrossRef]
- Smit, J.; Søgaard, M.; Schønheyder, H.C.; Nielsen, H.; Frøslev, T.; Thomsen, R.W. Diabetes and risk of community-acquired Staphylococcus aureus bacteremia: A population-based case-control study. Eur. J. Endocrinol. 2016, 174, 631–639. [Google Scholar] [CrossRef]
- Choi, H.; Choi, M.H.; Kim, D.; Lee, K.H.; Jeong, S.H. Shifting trends in bloodstream infection-causing microorganisms and their clinical impact in patients with haematologic malignancies in South Korea: A propensity score-matched study. Int. J. Antimicrob. Agents 2024, 64, 107212. [Google Scholar] [CrossRef]
- Kanj, S.S.; Bassetti, M.; Kiratisin, P.; Rodrigues, C.; Villegas, M.V.; Yu, Y.; van Duin, D. Clinical data from studies involving novel antibiotics to treat multidrug-resistant Gram-negative bacterial infections. Int. J. Antimicrob. Agents 2022, 60, 106633. [Google Scholar] [CrossRef]
- Park, K.H.; Jung, Y.J.; Lee, H.J.; Kim, H.J.; Maeng, C.H.; Baek, S.K.; Han, J.J.; Jeon, W.; Kim, D.Y.; Lee, Y.-M.; et al. Impact of multidrug resistance on outcomes in hematologic cancer patients with bacterial bloodstream infections. Sci. Rep. 2024, 14, 15622. [Google Scholar] [CrossRef] [PubMed]
- Lopera, C.; Monzó, P.; Aiello, T.F.; Chumbita, M.; Peyrony, O.; Gallardo-Pizarro, A.; Pitart, C.; Cuervo, G.; Morata, L.; Bodro, M.; et al. Prevalence and impact of multidrug-resistant bacteria in solid cancer patients with bloodstream infection: A 25-year trend analysis. Microbiol. Spectr. 2024, 12, e0296123. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, M.; Shou, C.; Shi, F.; Song, X.; Hu, Q.; Wang, Y.; Chen, Y.; Tong, X. Pathogenic spectrum and drug resistance of bloodstream infection in patients with acute myeloid leukaemia: A single centre retrospective study. Front. Cell. Infect. Microbiol. 2024, 14, 1390053. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, R.M.; Bayoumi, A.; Abdellateif, M.S.; A Nooh, H.; Refaat, L.; Kandeel, E.Z.; Hassan, S.S. Bacterial co-infections in cancer patients with COVID-19: Predictors and antimicrobial resistance trends. J. Infect. Dev. Ctries. 2024, 18, 1185–1195. [Google Scholar] [CrossRef]
- Flórez Riaño, A.F.; Rojas Castro, O.J.; Ospina, S.; Ramírez-Sánchez, I.C. Association between inappropriate empirical antimicrobial therapy and mortality in gram-negative bloodstream infections in patients with febrile neutropenia and hematological malignancy. J. Infect. Chemother. 2024, 31, 102538. [Google Scholar] [CrossRef]
- Terrance Walker, G.; Rockweiler, T.J.; Kersey, R.K.; Frye, K.L.; Mitchner, S.R.; Toal, D.R.; Quan, J. Rapid Molecular Screening for Gram-Negative Antimicrobial-Resistance Genes with Commercially Available Methods. Clin. Microbiol. Newsl. 2015, 37, 169–173. [Google Scholar] [CrossRef]
- Lin, Y.C.; Yang, K.Y.; Peng, C.K.; Chan, M.-C.; Sheu, C.-C.; Feng, J.-Y.; Wang, S.-H.; Huang, W.-H.; Chen, C.-M.; Chen, D.-H.; et al. Clinical outcomes of carbapenem-resistant gram-negative bacterial bloodstream infection in patients with end-stage renal disease in intensive care units: A multicenter retrospective observational study. Infection 2024, 53, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mu, M.; Zhu, J.; Yang, J.; Tao, Y.; Chen, Y.; Hu, Q.; Zhou, H.; Zhao, A.; Niu, T. Adult acute leukemia patients with gram-negative bacteria bloodstream infection: Risk factors and outcomes of antibiotic-resistant bacteria. Ann. Hematol. 2024, 103, 4021–4031. [Google Scholar] [CrossRef] [PubMed]
- Gill, E.L.; Gill, C.M.; McEvoy, C. Validation of a Stenotrophomonas maltophilia bloodstream infection prediction score in the hematologic malignancy population. Ann. Hematol. 2024, 103, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
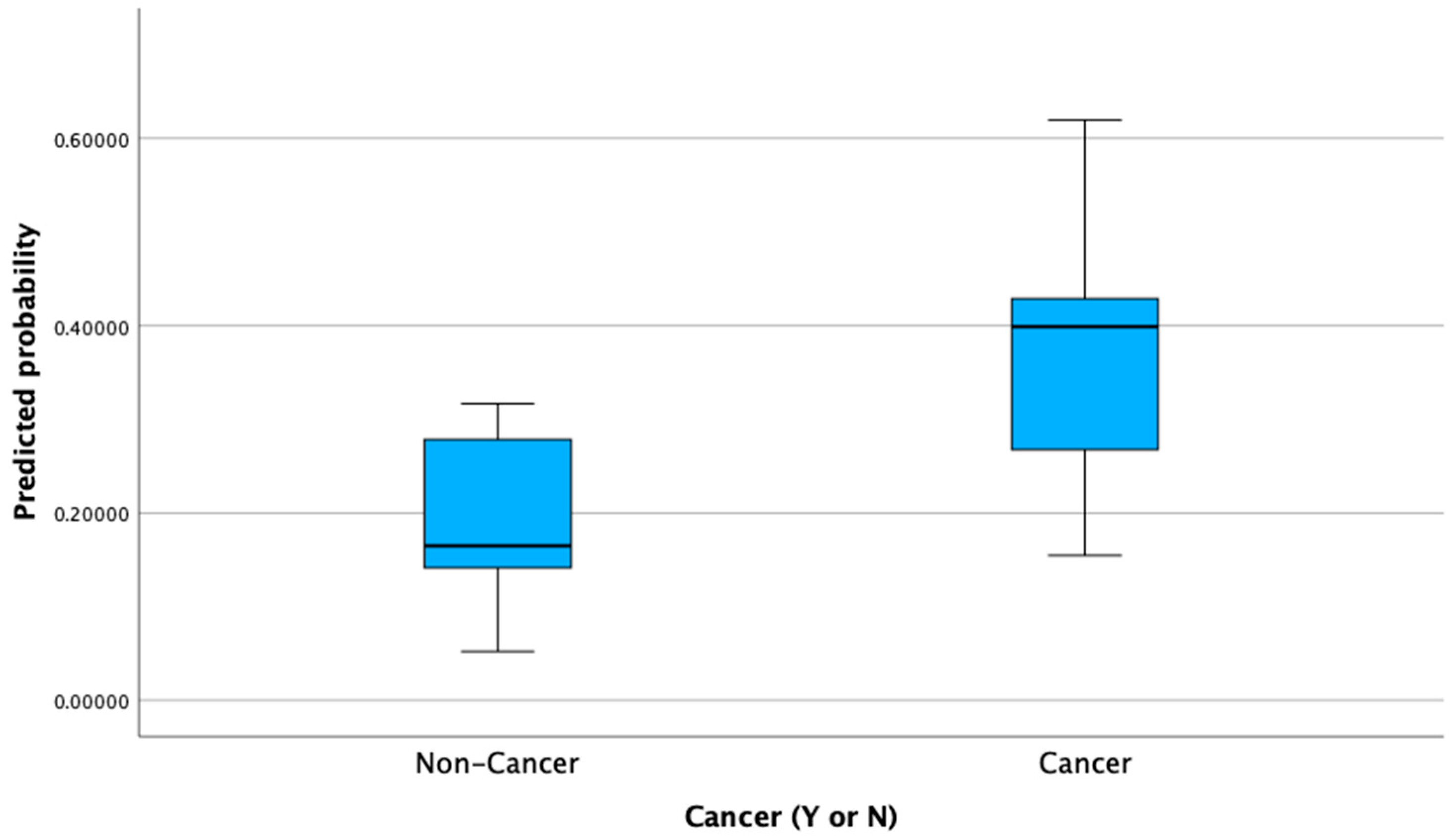
| Variable | Cancer (n = 31) | Non-Cancer (n = 81) | p-Value |
|---|---|---|---|
| Age | 67.82 ± 11.43 | 55.20 ± 16.70 | <0.001 |
| Race | |||
| Black | 15 (48.4%) | 59 (72.8%) | 0.031 |
| White | 14 (45.2%) | 21 (25.9%) | 0.031 |
| Hispanic | 2 (6.5%) | 1 (1.2%) | 0.031 |
| Sex | |||
| Male | 23 (74.2%) | 47 (58.0%) | 0.114 |
| Female | 8 (25.8%) | 34 (42.0%) | 0.114 |
| Comorbidities | |||
| Diabetes Mellitus | 7 (22.6%) | 39 (48.1%) | 0.014 |
| Hypertension | 20 (64.5%) | 52 (64.2%) | 0.975 |
| Stroke History | 0 (0.0%) | 18 (22.2%) | 0.004 |
| End-Stage Renal Disease | 0 (0.0%) | 11 (13.6%) | 0.031 |
| HIV Positive | 0 (0.0%) | 2 (2.5%) | 0.377 |
| COPD | 4 (12.9%) | 10 (12.3%) | 0.936 |
| CAD/Heart Failure | 8 (25.8%) | 17 (21.0%) | 0.584 |
| History of COVID-19 (past 6 months) | 1 (3.2%) | 13 (16.0%) | 0.066 |
| Variable | Cancer (n = 31) | Non-Cancer (n = 81) | p-Value |
|---|---|---|---|
| Infection Characteristics | |||
| Bone Infection | 1 (3.2%) | 7 (8.6%) | 0.319 |
| CLABSI | 2 (6.5%) | 8 (9.9%) | 0.570 |
| Unknown Source | 9 (29.0%) | 13 (59.1%) | 0.122 |
| Heart Infection | 1 (3.2%) | 1 (1.2%) | 0.477 |
| Intra-abdominal Infection | 2 (6.5%) | 1 (1.2%) | 0.126 |
| Lung Infection | 3 (9.7%) | 10 (12.3%) | 0.693 |
| Genitourinary Infection | 13 (41.9%) | 42 (51.9%) | 0.348 |
| Skin Infection | 4 (4.9%) | 1 (3.2%) | 0.685 |
| Enterobacteriaceae spp. Isolates | 26 (83.9%) | 67 (82.7%) | 0.884 |
| Non-lactose fermenters | 5 (16.1%) | 14 (17.3%) | 0.884 |
| Quinolone Resistance | 23 (74.2%) | 51 (63.0%) | 0.261 |
| Clinical Symptoms | |||
| Fever | 18 (58.1%) | 52 (64.2%) | 0.549 |
| Chills/Rigors | 16 (51.6%) | 47 (58.0%) | 0.541 |
| Clinical Outcomes | |||
| ICU Admission | 8 (25.8%) | 40 (49.4%) | 0.024 |
| 30-Day Mortality | 12 (39.0%) | 13 (16.4%) | 0.017 |
| WBC < 12,000 | 17 (54.8%) | 28 (34.6%) | 0.050 |
| WBC > 12,000 | 14 (45.2%) | 53 (65.4%) | 0.050 |
| Platelets < 150,000 | 15 (48.4%) | 25 (31.6%) | 0.101 |
| Platelets > 150,000 | 16 (51.6%) | 54 (68.4%) | 0.101 |
| Procalcitonin < 0.5 | 5 (20.8%) | 7 (10.1%) | 0.178 |
| Procalcitonin > 0.5 | 19 (79.2%) | 62 (89.9%) | 0.178 |
| Lactic Acid < 2 | 9 (29.0%) | 25 (30.9%) | 0.850 |
| Lactic Acid > 2 | 22 (71.0%) | 56 (69.1%) | 0.850 |
| Recent Hospital Admission (Last 3 Months) | 21 (67.7%) | 33 (40.7%) | 0.011 |
| ID Consultation | 7 (22.6%) | 34 (42.0%) | 0.057 |
| Hospice Admission | 7 (22.6%) | 3 (3.7%) | 0.002 |
| Treatment and Antibiotic Use | |||
| Empiric Antibiotic Therapy | 16 (51.6%) | 52 (64.2%) | 0.222 |
| Appropriate Antibiotic Coverage Provided | 21 (67.7%) | 69 (85.2%) | 0.038 |
| Targeted Therapy Given | 27 (87.1%) | 77 (95.1%) | 0.143 |
| Days to Targeted Therapy Initiation | |||
| 1 Day | 9 (33.3%) | 34 (44.2%) | 0.169 |
| 2–3 Days | 14 (51.9%) | 21 (27.3%) | 0.169 |
| 4–6 Days | 3 (11.1%) | 18 (23.4%) | 0.169 |
| 7–9 Days | 0 (0.0%) | 2 (2.6%) | 0.169 |
| 10+ Days | 1 (3.7%) | 2 (2.6%) | 0.169 |
| (A) Patients with Cancer (n = 31) | ||
| Variable | Odds Ratio (OR) | 95% Confidence Interval |
| Diabetes Mellitus | 2.39 | 1.13–5.07 |
| ICU Admission | 2.16 | 1.06–4.40 |
| Recent Hospital Admission (Last 3 Months) | 3.06 | 1.28–7.32 |
| Hospice Admission | 7.58 | 1.82–31.62 |
| (B) Patients without Cancer (n = 81) | ||
| Variable | Odds Ratio (OR) | 95% Confidence Interval |
| Recent Hospital Admission (Last 3 Months) | 1.35 | 1.06–1.73 |
| Predictor | Adjusted Odds Ratio (aOR) | 95% CI | p-Value |
|---|---|---|---|
| Cancer (Yes vs. No) | 3.82 | 1.23–11.86 | 0.020 |
| Sex (Male vs. Female) | 1.06 | 0.40–2.81 | 0.913 |
| Diabetes Mellitus (Yes vs. No) | 0.96 | 0.35–2.59 | 0.930 |
| ICU Admission (Yes vs. No) | 2.35 | 0.88–6.29 | 0.090 |
| Recent Hospitalization (Yes vs. No) | 0.92 | 0.35–2.45 | 0.867 |
| Ethnicity (Non-White vs. White) | 0.48 | 0.16–1.45 | 0.194 |
| Age ≥ 40 y (vs. ≤39 y) * | 1.74 | 0.34–8.87 | 0.504 |
| Organism (Resistance Type) | Cancer (n = 31) | Non-Cancer (n = 81) | Total (N = 112) |
|---|---|---|---|
| Escherichia coli (ESBL) | 19 (61.3%) | 41 (50.6%) | 60 (53.6%) |
| Klebsiella pneumoniae (ESBL) | 3 (9.7%) | 16 (19.8%) | 19 (17.0%) |
| Klebsiella pneumoniae (CRO) | 1 (3.2%) | 2 (2.5%) | 3 (2.7%) |
| Klebsiella oxytoca (ESBL) | 1 (3.2%) | 2 (2.5%) | 3 (2.7%) |
| Klebsiella oxytoca (CRO) | 0 | 1 (1.2%) | 1 (0.9%) |
| Proteus mirabilis (ESBL) | 1 (3.2%) | 9 (11.1%) | 10 (8.9%) |
| Pseudomonas aeruginosa (CRO) | 3 (9.7%) | 7 (8.6%) | 10 (8.9%) |
| Acinetobacter baumannii/haemolyticus (CRO) | 3 (9.7%) | 8 (9.9%) | 11 (9.8%) |
| Serratia marcescens (CRO) | 0 | 1 (1.2%) | 1 (0.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dicharry, D.; Smith, D.G.; Khan, M.H.; Self, M.; Parikh, C.; Malek, A.E. Clinical Features of Multidrug-Resistant Gram-Negative Bacteremia: A Comparative Study of Cancer and Non-Cancer Patients. Microorganisms 2025, 13, 2110. https://doi.org/10.3390/microorganisms13092110
Dicharry D, Smith DG, Khan MH, Self M, Parikh C, Malek AE. Clinical Features of Multidrug-Resistant Gram-Negative Bacteremia: A Comparative Study of Cancer and Non-Cancer Patients. Microorganisms. 2025; 13(9):2110. https://doi.org/10.3390/microorganisms13092110
Chicago/Turabian StyleDicharry, Destyn, Deborah G. Smith, Muhammad H. Khan, Michelle Self, Cameron Parikh, and Alexandre E. Malek. 2025. "Clinical Features of Multidrug-Resistant Gram-Negative Bacteremia: A Comparative Study of Cancer and Non-Cancer Patients" Microorganisms 13, no. 9: 2110. https://doi.org/10.3390/microorganisms13092110
APA StyleDicharry, D., Smith, D. G., Khan, M. H., Self, M., Parikh, C., & Malek, A. E. (2025). Clinical Features of Multidrug-Resistant Gram-Negative Bacteremia: A Comparative Study of Cancer and Non-Cancer Patients. Microorganisms, 13(9), 2110. https://doi.org/10.3390/microorganisms13092110






