A Collection and Analysis of Simplified Data for a Better Understanding of the Complex Process of Biofilm Inactivation by Ultraviolet and Visible Irradiation
Abstract
1. Introduction
- Does the irradiation of water reduce or prevent biofilm formation in water works and water distribution systems?
- Is it possible to prevent biofilm formation by irradiation in the long term (weeks or months) and what are the best parameters?
- Is biofilm prevention or reduction possible by all UV and VIS wavelengths?
- Which irradiation wavelength is the best?
- Are multi-species biofilms more irradiation resistant than single species biofilms?
- Are cells in biofilms more radiation resistant than planktonic cells?
- Which mathematical model describes the relation between irradiation and biofilm-reduction best? Is there a maximum reduction that cannot be increased even by higher irradiation doses?
- Is there an influence of the substrate below the biofilm?
- Are there differences in the biofilm sensitivity towards irradiation between microorganisms like bacteria or fungi or even between Gram+ and Gram- bacteria?
2. Data Collection and Analysis
2.1. Data Collection
2.2. Data Analysis
3. Results
3.1. Water Irradiation for Biofilm Prevention/Delay
| Reference | Irradiation Wavelength, Irradiance, Dose | Microorganisms | Biofilm Age, Thickness, Cells | Biofilm Substrate | Reduction |
|---|---|---|---|---|---|
| [23] | 254 nm, 0.003 mJ/cm2 | natural microbiome | 24 h–38 d, ≈107 cells/cm2 | steel, cement (in drinking water) | no significant biofilm reduction by water irradiation |
| [24] | 254 nm, 40 mJ/cm2 | natural microbiome | 4 w–6 m, ≈106–107 cells/cm2 | PVC, PE, steel, copper (in drinking water) | no significant biofilm reduction by water irradiation |
| [25] | 254 nm, 40 mJ/cm2 | natural microbiome | 20 w, ≈100 µg dry weight/cm2 | membrane (in water) | water irradiation reduced biofilm formation |
| [26] | 254 nm | natural microbiome | up to 30 d, ≈105–106 cells/cm2 | PVC, steel (in drinking water) | no significant biofilm reduction by water irradiation |
| [27] | 254 nm, up to 80 mJ/cm2 | natural microbiome | 19 d, ≈105 cells/cm2 | polyamide membrane (in waste water) | water irradiation increased biofilm formation |
| [28] | 254 nm, up to 259 mJ/cm2 | natural microbiome | 2 h, 30 d, ≈106 cells/cm2 | PVC (in drinking water) | no significant biofilm reduction by water irradiation (nutrient availability in UV-irradiated water higher; no effect on biofilm density in the long run) |
| [29] | 254 nm, up to 150 mJ/cm2 | P. aeruginosa | 24 h | PS | higher UVC doses led to stronger biofilm formation |
| [30] | 254 nm | mixture of P. aeruginosa, E. coli, Flavobacterium breve, Aeromonas hydrophila | up to 72 h, ≈105 cells/cm2 | PC | water irradiation reduced biofilm formation (difference ≤ 1 log/cm2 after 72 h) |
| [31] | 254 nm, 40 mJ/cm2 | natural microbiome | 4 w–6 m, ≈105–106 cells/cm2 | PC, iron | no biofilm reduction by UV alone |
| [32] | 254 nm, 40 mJ/cm2 | natural microbiome | 3 m, ≈5 × 104–7 × 106 cells/cm2 | steel, copper | depending on detection technique and parameters no biofilm reduction |
| [33] | 254 nm, 1900 mJ/cm2 every 30 min (pulsed) | natural microbiome | 32 d | hollow fiber membrane (surface water) | water irradiation prevented biofilm formation for 32 days |
| [34] | 254 nm, 49 mW/cm2; up to 29,000 mJ/cm2 | natural microbiome | 3 h | hollow fiber membrane (in waste water) | water irradiation reduced biofilm formation |
| [35] | 254 nm; broadband UVC (MP Hg) | natural microbiome | up to 200 d | unknown coupons (in drinking water) | water irradiation did not lead to a biofilm decrease; especially for broadband UVC there even seemed to be an increased biofilm formation |
| [36] | broadband UVC (MP Hg), 80 mJ/cm2 | natural microbiome | up to 4 m, 120–230 µm; 106–107 cells/cm2 | membranes (in brackish water) | water irradiation reduced biofilm formation |
| [37] | 220 nm, 260 nm, 280 nm, broadband UVC (MP Hg), up to 8.8 mJ/cm2 | isolates of natural microbiome | 24 h–38 d, ≈109 cells/cm2 | glass, PP in sea water | 280 nm water irradiation decreased biomass; other wavelength had no larger effect or even increased biofilm formation |
| [38] | 220 nm, 239 nm, 254 nm, 260 nm, 270 nm, 280 nm, broadband UVC (MP Hg), up to ≈15 mJ/cm2 | P. aeruginosa | up to 34 h | PS MTP | water irradiation reduced biofilm formation; 254 nm, 270 nm and broadband UVC were most effective (higher bacterial concentration led to stronger biofilm formation) |
| [39] | broadband UVC (MP Hg), ≈0.135 mW/cm2, up to 8 mJ/cm2; filtered UV > 295 nm, ≈0.045 mW/cm2, up to 40 mJ/cm2; | P. aeruginosa | up to 9 d | plastic MTP | water irradiation reduced biofilm formation (UV pretreatment of bacteria resulted in lower concentrations and reduced biofilm formation; in the long term: the UV treatment was unable to prevent biofilm formation) |
| [40] | broadband UVC (MP Hg) | P. aeruginosa | 24 h, 15–20 µm | glass, PVC, steel (in drinking water) | ≥99.9% biofilm volume reduction by water irradiation (decisive for biofilm formation: bacterial concentration, but not whether bacteria were previously irradiated) |
| [41] | broadband UVC (MP Hg), 137 mJ/cm2 | natural microbiome | ≈10 µm | membrane in brackish water | irradiation (alone) did not lead to biofilm reduction |
| [42] | 254 nm, 42 mJ/cm2 | natural microbiome | (20 m) | PE in drinking water | water irradiation reduced biofilm formation |
| [43] | 254 nm; broadband UVC (MP Hg), 40 mJ/cm2 | natural microbiome | up to 170 d | membrane in water | water irradiation reduced biofilm formation (membrane running time was increased by factor 6x) |
| [44] | 275 nm, up to ≈30 mW/cm2 (pulsed and continuous) | natural microbiome | up to 11 d | membrane in water | water irradiation reduced biofilm formation |
| [45] | 278 nm, 2 mJ/cm2 | natural microbiome | up to 15 d, 108–109 cells/cm2 | membrane (in tap water) | water irradiation reduced biofilm formation |
| [46] | 254 nm; 283 nm, 40 mJ/cm2 | natural microbiome | 5 d | PC in waste water | water irradiation reduced/retarded biofilm formation (no significant difference between irradiated and not irradiated water in the long run) |
| [47] | 280 nm; 40 mJ/cm2 | E. coli | 5 d | membrane in contaminated water | water irradiation reduced/delayed biofilm formation—higher UV doses led to more biofilm |
3.2. Surface Irradiation for Biofilm Prevention
| Reference | Irradiation Wavelength, Irradiance, Dose | Microorganisms | Biofilm Age, Thickness, Cells | Biofilm Substrate | Reduction |
|---|---|---|---|---|---|
| [55] | 222 nm, 0.236 mW/cm2; up to 354 mJ/cm2 | P. aeruginosa, S. aureus | 24 h, 48 h, ≈20 µm | steel | biofilm formation observed under continuous far-UVC irradiation, but formation much slower than biofilm formation in the dark |
| [50] | 254 nm, ≤0.0008 mW/cm2 | natural microbiome | 5 w–4 m | copper, silicone, epoxy | continuous irradiation prevented biofilm formation on most materials; 1 min irradiation per day reduced biofilm formation |
| [63] | 254 nm, 1.15 mW/cm2; up to 18.4 mJ/cm2 per vehicle run | natural microbiome | 1 m | steel, copper (in seawater) | successful after two weeks, but biofilm increase after 4 weeks (mobile UVC vehicle) |
| [64] | 254 nm | natural microbiome | 1–2 m | PVC (in seawater) | no biofilm after 2 months continuous UV irradiation; UV reduced existing biofilms |
| [56] | 254 nm, up to 1.47 mW/cm2 | natural microbiome | 2 d–7 d, ≈106 cells/cm2 after 7 d | glass | irradiation reduced biofilm formation (>99% less biofilm cells after 7 d); however, even 1.47 mW/cm2 did not completely stop biofilm formation for 7 d |
| [65] | 254 nm, up to 2 mW/cm2 | natural microbiome | 24 d, 106–107 cells/cm2 | quartz (in sea water) | antifouling impact starts for >10 µW/cm2; however, even 0.8 mW/cm2 did not prevent biofilm formation completely |
| [58] | 254 nm, up to 0.350 mW/cm2 | E. coli | 2 d | glass (in drinking water) | 95% less biofilm volume @ 50.5 µW/cm2 |
| [59] | 254 nm, up to ≈0.15 mW/cm2 | E. coli | 2 d, 12 d, up to 27 µm | flow cell | 0.06 mW/cm2 significantly reduced biofilm formation; however, biofilm formation even observed at 0.1 mW/cm2 and UVC is probably unable to stop biofilm formation in the long run (only 23 °C results) |
| [54] | 265 nm | P. aeruginosa, E. coli | agar plate | 4.3 mJ/cm2 to prevent biofilm (bacterial lawn) formation; (Irradiation via fibers) | |
| [66] | 265 nm, up to 21 mJ/cm2 | P. aeruginosa | 3 h | Teflon tubes | 100% @ 1 mJ/cm2 (Teflon); no bacteria observed for 3–4 d; higher doses necessary for other materials (high NaCl concentration (20%) for light guide approach) |
| [48] | 265 nm, 275 nm, 300 nm, 365 nm, up to 0.156 mW/cm2 (pulsed or continous) | mixture: P. aeruginosa, Ralstonia insidiosa, Burkholderia multivorans, Cupriavidus metallidurans, Methylobacterium fujisawaense | up to 6 d, ≈0.3 mm; 6.2 × 106 cells/cm2 | steel | 265/275 nm: significant biofilm prevention at about 10 µW/cm2 (continuous/pulsed) at least for 6 days; 300/365 nm: no biofilm prevention but biofilm increase (irradiation via optical fibers; no total biofilm prevention even above 10 µW/cm2) |
| [57] | 267 nm, 1 mW/cm2; up to 60 mJ/cm2 | C. auris | 24 h | steel, PS, poly-cotton | 5–60 mJ/cm2 needed for a significant reduction in biofilm formation, depending on surface structure |
| [67] | 272 nm, up to 0.48 mW/cm2 (pulsed or continuous) | natural microbiome | up to 24 w | quartz (in sea water) | almost no biofilm after 69 d @ 0.48 mW/cm2 |
| [52] | 273 nm, <0.2 mW/cm2 | natural microbiome | up to 19 w | seachest with antifouling coating (in sea water) | UV-irradiation prevented/delayed biofilm formation |
| [49] | 275 nm, up to 0.25 mW/cm2 (pulsed or continuous) | P. aeruginosa | up to 3 d, ≈250 µm | steel | significant biofilm prevention at about 8 µW/cm2 (irradiation via optical fibers; no total biofilm prevention even above 8 µW/cm2) |
| [51] | 278 nm, 0.0174 mW/cm2 | natural microbiome | up to 47 d | plastic (in sea water) | biofilm prevented for 47 d |
| [53] | 278 nm | natural microbiome | up to 10 m | silicone (in sea water) | 9 cm disk quite biofilm-free after 4 weeks in water with an average irradiation of 0.005 mW/cm2 |
| [68] | 280 nm, up to 0.093 mW/cm2; up to 167 mJ/cm2 | natural microbiome | 9 m | quartz | UV reduced biofilm formation; even 0.0005 µW/cm2 seemed to have an impact |
| [69] | UVC LED, ≈0.1 mW/cm2 | natural microbiome | 20 d | glass/polymer | biofilm CFU 1.8 log lower compared to unirradiated control after 20 d |
| [70] | 281 nm, up to 0.108 mW/cm2; up to 18,700 mJ/cm2 | Navicula incerta | up to 5 d, ≈105 algae/cm2 | tiles | 1 log-reduction (biofilm cell) @ 42,000 mJ/cm2, 3 log-reduction @ 5 d and 5.77 µW (2500 mJ/cm2) |
| [71] | 285 nm | natural microbiome | 1 w–19 w | quartz (in sea water) | UV reduced biofilm formation |
| [72] | 285 nm, 0.025 mW/cm2 up to 180 J/cm2 | natural microbiome | 112 d | quartz (in sea water) | irradiation delayed biofilm formation |
| [60] | 385 nm, 420 nm, 2.5 mW/cm2; 216 J/cm2 | E. coli | up to 24 h | silicone (in urine mucine medium in MTP) | 2.5 mW/cm2 (216 J/cm2) reduced bacteria on silicone/medium and prevented biofilm formation |
| [73] | broadband blue (380–440 nm with peak @ 405 nm), 30.9 mW/cm2; 9.26 J/cm2 | S. mutans | 12–16 h | PS in medium in MTP | irradiation reduced biofilm formation (biofilm recovered for 2–6 h before analysis; tryptic soy broth might contain photosensitizer?) |
| [62] | 405 nm, up to 160 mW/cm2 up to 1728 J/cm2 | Proteus mirabilis | silicone (in artificial urine) | 1 log-reduction in biofilm formation @ 18–32 mW/cm2 (194–346 J/cm2); 1.7 log-reduction @ 30–50 mW/cm2 (324–540 J/cm2); total biofilm prevention @ 160 mW/cm2 (1,728 J/cm2) | |
| [74] | 405 nm, 26 mW/cm2; up to 748.8 J/cm2 | L. monocytogenes | 24 h | steel and acryl in salmon exudate | irradiation reduced biofilm formation by ≈1 log @ 26 mW/cm2 or 748.8 J/cm2 (irradiation impact slightly temperature dependent) |
| [75] | 410 nm, 455 nm, 100 mW/cm2; up to 450 J/cm2 | P. aeruginosa | 6 h | PS MTP | biofilm formation prevention: 410 nm: 6.6 log @ 450 J/cm2; 450 nm: 3.8 log @ 450 J/cm2; |
| [76] | 445 nm (laser), 970 nm (laser), different irradiances; up to 120 J/cm2 | P. aeruginosa | 24 h, 72 h | MTP, flow cell, wound | one time 445 nm irradiation inhibited growth up to 18 h, but had mostly no larger effect after 24 h besides a small biomass reduction; no effect by 970 nm irradiation; |
| [77] | 450 nm (pulsed), 2 mW/cm2; 7.6 J/cm2 three times per day over three days (68.4 J/cm2 total) | S. aureus, P. acnes | 3 d | PS MTP | no significant impact on forming biofilms for the first three days |
| [78] | 450 nm, 525 nm, 625 nm, up to 240 J/cm2 | C. albicans | 24 h | MTP | 450 nm irradiation led to an average reduction of up to 0.43 log @ 240 J/cm2; no effects for other wavelengths; |
| [79] | “blue”, up to 1300 lux | E. coli | 24 h | MTP | blue light reduced biofilm formation |
3.3. Biofilm Irradiation for Biofilm Reduction
| Reference | Irradiation Wavelength, Irradiance, Dose | Microorganisms | Biofilm Age, Thickness, Cells | Biofilm Substrate | Reduction |
|---|---|---|---|---|---|
| [98] | 222 nm, up to 0.6 mW/cm2; up to 179.3 mJ/cm2 | E. coli, S. epidermis | 5 h, ≈106 cells/cm2 | PS MTP | E. coli: 2.10 log @ 179.3 mJ/cm2, S. epidermis: 2.03 log @ 179.3 mJ/cm2 |
| [99] | 222 nm, 254 nm, up to 600 mJ/cm2 | F. nucleatum, P. gingivalis | 72 h, 25 µm, 38 µm | plastic MTP | reduction in biofilm thickness: 222 nm: F. nucleatum and P. gingivalis; 254 nm: F. nucleatum |
| [87] | 222 nm, 254 nm, 260 nm, 270 nm, 282 nm | P. aeruginosa | 1 d–5 d | PC, quartz | ≈1 log @ 55 mJ/cm2, 222 nm, 72 h ≈1 log @ 8.2 mJ/cm2, 270 nm, 72 h |
| [100] | 249–338 nm in 5 nm steps (UVC, UVB, UVA), up to 2110 mJ/cm2 | P. aeruginosa | 24 h, 48 h, ≈100 µm (48 h) | cellulose nitrate membrane filter | for 24 h biofilm @126–170 mJ/cm2: UVC: 0.36 log; UVB (296 nm): up to 2.4 log @ 296 nm; UVA: no significant reduction; 48 h biofilm much more resistant; |
| [101] | 254 nm | L. monocytogenes | 7 d | steel | cells in biofilm reduced |
| [102] | 254 nm, up to 1800 mJ/cm2 | L. monocytogenes | 24 h | steel, egg shell | steel: 0.26 log @ 300 mJ/cm2; 0.42 log @ 600 mJ/cm2; 1.12 log @ 1200 mJ/cm2; 1.47 log @ 1800 mJ/cm2; egg shell: 0.23 log @ 300 mJ/cm2; 0.40 log @ 600 mJ/cm2; 0.74 log @ 1200 mJ/cm2; 1.14 log @ 1800 mJ/cm2; |
| [103] | 254 nm, 1.3 mW/cm2; up to 390 mJ/cm2 | L. monocytogenes | 24 h, ≈106 cells/cm2 | lettuce, cabbage | cell reduction in biofilm on both surfaces: » 4.0 log @ 390 mJ/cm2 |
| [104] | 254 nm | L. monocytogenes | 6 d, 12 d, ≈106 cells/cm2 (12 d) | steel | ≥5 log cell reduction in biofilm |
| [105] | 254 nm, up to 60 mJ/cm2 | V. parahaemolyticus | 24 h, ≈107 cells/cm2 | shrimp, crab | shrimp: 1.37 log @ 5 mJ/cm2; 1.56 log @ 10 mJ/cm2; 1.84 log @ 30 mJ/cm2; 2.53 log @ 60 mJ/cm2; crab: 0.75 log @ 5 mJ/cm2; 0.94 log @ 10 mJ/cm2; 1.37 log @ 30 mJ/cm2; 1.94 log @ 60 mJ/cm2; |
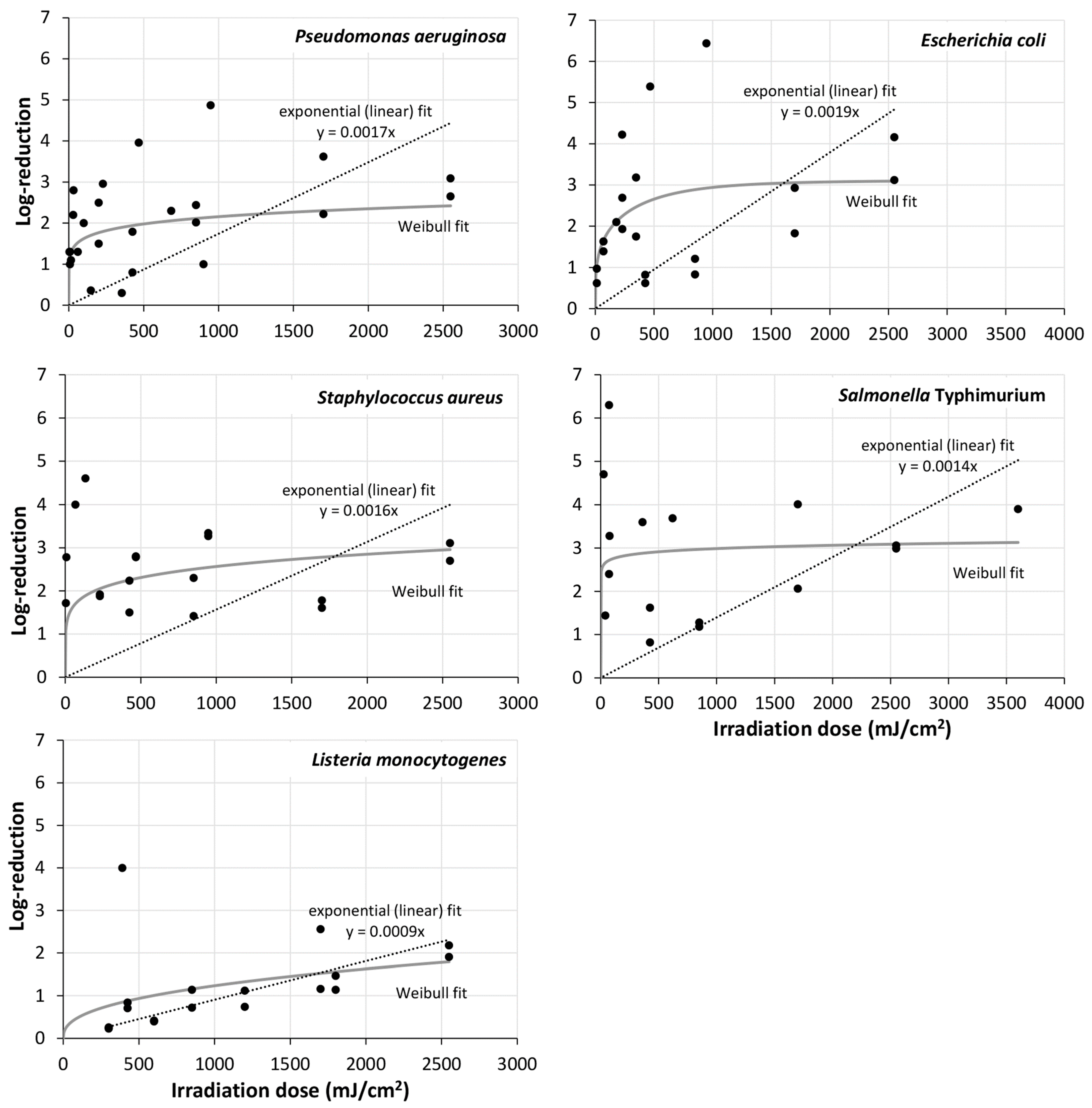
| [106] | 254 nm, 0.236 mW/cm2; up to 2549 mJ/cm2 | P. aeruginosa, S. aureus, E. coli, L. monocytogenes, S. Typhimurium | 24 h | biofilms from agar transferred to steel, PP | steel: P. aeruginosa: 0.80 log @ 425 mJ/cm2; 2.02 log @ 850 mJ/cm2; 2.22 log @ 1700 mJ/cm2; 2.65 log @ 2549 mJ/cm2; S. aureus: 2.24 log @ 425 mJ/cm2; 1.42 log @ 850 mJ/cm2; 1.61 log @ 1700 mJ/cm2; 2.70 log @ 2549 mJ/cm2; E. coli: 0.62 @ 425 mJ/cm2; 0.83 log @ 850 mJ/cm2; 1.83 log @ 1700 mJ/cm2 3.12 log @ 2549 mJ/cm2; L. monocytogenes: 0.84 @ 425 mJ/cm2; 1.14 log @ 850 mJ/cm2; 2.56 log @ 1700 mJ/cm2; 2.18 log @ 2549 mJ/cm2; S. Typhimurium: 0.82 log @ 425 mJ/cm2; 1.28 log @ 850 mJ/cm2; 2.06 log @ 1700 mJ/cm2; 3.06 log @ 2549 mJ/cm2; polypropylene: P. aeruginosa: 1.79 log @ 425 mJ/cm2; 2.44 log @ 850 mJ/cm2; 3.62 log @ 1700 mJ/cm2; 3.09 log @ 2549 mJ/cm2; S. aureus: 1.50 log @ 425 mJ/cm2; 2.30 log @ 850 mJ/cm2; 1.78 log @ 1700 mJ/cm2; 3.11 log @ 2549 mJ/cm2; E. coli: 0.82 @ 425 mJ/cm2; 1.21 log @ 850 mJ/cm2; 2.93 log @ 1700 mJ/cm2; 4.16 log @ 2549 mJ/cm2; L. monocytogenes: 0.71 @ 425 mJ/cm2; 0.72 log @ 850 mJ/cm2; 1.16 log @ 1700 mJ/cm2; 1.91 log @ 2549 mJ/cm2; S. Typhimurium: 1.62 log @ 425 mJ/cm2; 1.18 log @ 850 mJ/cm2; 4.01 log @ 1700 mJ/cm2; 2.99 log @ 2549 mJ/cm2; |
| [89] | 254 nm (irradiation from top or bottom for up to 60 min), up to 0.63 mW/cm2; up to 1400 mJ/cm2 | P. aeruginosa | 4 d | on quartz Petri dish | 0.3 log @ ≈354 mJ/cm2; 1 log @ ≈900 mJ/cm2; 100% @ 1300 mJ/cm2; (“inside out” irradiation more effective; planktonic cells more sensitive than cells in biofilm) |
| [82] | 254 nm, up to 40 mJ/cm2 | C. neoformans | up to 48 h | PS | 0.13 log @ 40 mJ/cm2; (planktonic cells more sensitive than cells in biofilm) |
| [91] | 254 nm | F. solani | up to 48 h | PS MTP | cells in biofilm are reduced (planktonic cells more sensitive than cells in biofilm) |
| [107] | 254 nm | P. aeruginosa, S. aureus, S. epidermis, A. baumannii, E. coli | 24 h | MTP | strong cell reduction in all biofilms, (no large change in biomass) |
| [94] | 254 nm | P. aeruginosa, E. coli, S. aureus MSSA, S. aureus MRSA, S. epidermis MRSE, C. albicans | 24 h | steel | P. aeruginosa: 2.96 log @ 228.6 mJ/cm2; 3.96 log @ 467.8 mJ/cm2; 4.87 log @ 946.7 mJ/cm2; E. coli: 4.22 log @ 228.6 mJ/cm2; 5.39 log @ 467.8 mJ/cm2; 6.44 log @ 946.7 mJ/cm2; S. aureus (MSSA): 1.88 log @ 228.6 mJ/cm2; 2.78 log @ 467.8 mJ/cm2; 3.34 log @ 946.7 mJ/cm2; S. aureus (MRSA): 1.92 log @ 228.6 mJ/cm2; 2.80 log @ 467.8 mJ/cm2; 3.27 log @ 946.7 mJ/cm2; S. epidermis: 1.21 log @ 228.6 mJ/cm2; 2.29 log @ 467.8 mJ/cm2; 3.88 log @ 946.7 mJ/cm2; C. albicans: 1.43 log @ 228.6 mJ/cm2; 3.38 log @ 467.8 mJ/cm2; 3.62 log @ 946.7 mJ/cm2; |
| [83] | 254 nm, 1.4 mW/cm2; up to 2600 mJ/cm2 | A. acidoterrestris, A. herbarius, A. cycloheptanicus, A. acidocaldarius | 72 h | steel, rubber | steel: 2.5 log @ 2600 mJ/cm2; rubber: 2.7 log @ 2600 mJ/cm2 (planktonic spores much more sensitive than cells in biofilm) |
| [56] | 254 nm, up to 1.47 mW/cm2 | natural microbiome | 2 d, ≈5 × 105 cells/cm2 | glass | 84%/0.8 log cell reduction in 2 d biofilm @ 2646 mJ/cm2; |
| [108] | 254 nm, up to 6,000,000 mJ/cm2 | natural microbiome | >100 d, ≈104 cells/cm2 | steel (in ground water) | ≈1.6 CFU log-reduction @ 6,000,000 mJ/cm2 |
| [109] | 254 nm, 0.4 mW/cm2; up to 2160 mJ/cm2 | natural patient biofilm | mature | silicone urinary catheter | ≈0.96 log @ 12 mJ/cm2; ≈2 log @ 1400 mJ/cm2; (planktonic cells more sensitive than cells in biofilm) |
| [110] | 254 nm, 0.7 mW/cm2; up to 210 mJ/cm2 | C. albicans | 24 h | PMMA | 1.3 log @ 21 mJ/cm2; 1.9 log @ 84 mJ/cm2; 2.9 log @210 mJ/cm2; |
| [111] | 254 nm, 6.4 mW/cm2; 1920 mJ/cm2 | S. aureus, S. epidermis | 24 h | plastic | reduction below ≈5% (irradiation details unclear) |
| [112] | 254 nm, up to 620 mJ/cm2 | S. Typhimurium | 48 h, 3 × 106 cells/cm2 | steel | 1.44 log @ 39.5 mJ/cm2; 3.28 log @ 76.4 mJ/cm2; 3.69 log @ 620.4 mJ/cm2; |
| [113] | 254 nm, 1.2 mW/cm2; up to 360 mJ/cm2 | S. Typhimurium, cultivable indigenous microorganisms (CIM) | 72 h (steel) ≈107 cells/cm2, 24 h (lettuce) ≈3 × 104–7 × 106 cells/cm2 | steel, lettuce | steel: S. Typhimurium: 4.7 log @ 24 mJ/cm2; 6.3 log @ 72 mJ/cm2; S. Typhimurium mixed: 4.3 log @ 24 mJ/cm2; 6.0 log @ 72 mJ/cm2; lettuce: S. Typhimurium: 2.4 log @ 72 mJ/cm2; 3.6 log @ 360 mJ/cm2; S. Typhimurium mixed: 1.2 log @ 72 mJ/cm2; 1.8 log @ 360 mJ/cm2; (multi-species biofilms less sensitive) |
| [114] | 254 nm, 3.5 mW/cm2 | C. auris | 48 h | PS | 3.5 log @ 3864 mJ/cm2; 7.2 log @ 7728 mJ/cm2; 6.7 log @ 11,592 mJ/cm2; |
| [115] | UVC LED (254 nm?), irradiation up to 20 min | mixture: S. mutans, S. aureus, E. coli, C. albicans | 24 h | silicone | significant biofilm reduction for 20 min UVC |
| [116] | 254 nm, 3.1 mW/cm2, up to 11,160 mJ/cm2 | Navicula incerta | 60 min | glass | biofilm reduction |
| [117] | 254 nm, 0.625 mW/cm2, up to 200 mJ/cm2; 270 nm, 0.038 mW/cm2, up to 100 mJ/cm2; 405 nm, 75.5 mW/cm2, up to 225 J/cm2 | P. aeruginosa, natural microbiome | 3 d, P. aeruginosa: 1.8 × 108 CFU/cm2; mixed culture: 1.4 × 105 CFU/cm2 | PC, PTFE, PVC, quartz | P. aeruginosa biofilm on PC: 254 nm: 1.1 log @ 15 mJ/cm2 1.3 log @ 60 mJ/cm2 1.5 log @ 200 mJ/cm2 270 nm: 1.3 log @ 4.5 mJ/cm2 2.2 log @ 30 mJ/cm2 2.0 log @ 100 mJ/cm2 2.5 log @ 200 mJ/cm2 405 nm: 0.3 log @ 22 J/cm2 1.7 log @ 67 J/cm2 2.7 log @ 135 J/cm2 3.8 log @ 225 J/cm2 dual species biofilm on PC: 254 nm: 1.1 log @ 15 mJ/cm2 1.65 log @ 100 mJ/cm2 1.9 log @ 200 mJ/cm2 270 nm: 0.9 log @ 15 mJ/cm2 1.5 log @ 50 mJ/cm2 1.9 log @ 100 mJ/cm2 405 nm: 0.14 log @ 22 J/cm2 1.3 log @ 135 J/cm2 1.8 log @ 225 J/cm2 |
| [118] | 255 nm, 0.088 mW/cm2; up to 135 mJ/cm2 | S. aureus, A. baumannii | PVC | S. aureus: 1.72 log @ 3.7 mJ/cm2; 2.78 log @ 7.4 mJ/cm2; 4.0 log @ 66.7 mJ/cm2; 4.6 log @ 133 mJ/cm2; A. baumanni: 0.34 log @ 5.0 mJ/cm2; 0.92 log @ 17.4 mJ/cm2; 1.5 log @ 66.7 mJ/cm2; 1.5 log @ 133 mJ/cm2; dual species: 1.5 log @ 7.4 mJ/cm2; 2.1 log @ 17.4 mJ/cm2; 3.4 log @ 66.7 mJ/cm2; 3.7 log @ 133 mJ/cm2; | |
| [119] | 265 nm, up to 1570 mJ/cm2 | P. aeruginosa | 3 d | Teflon and silicone urinary catheter | ≈4 log @ 7.9 mJ/cm2 (high NaCl concentrations of up to 20% to achieve light guide effect ⇒ therefore values not included in analysis) |
| [120] | 265 nm | P. aeruginosa | 48 h | PC | ≈1.3 log @ 8 mJ/cm2 ≈2.8 log @ 32 mJ/cm2 |
| [121] | 265 nm, 1.93 mW/cm2; up to 231.6 mJ/cm2 | P. aeruginosa | 48 h | chamber well slides | irradiation led to dead biomass; no increase in dead biomass after about 13 mJ/cm2 |
| [122] | 266 nm (UVC), up to 1000 mJ/cm2; 296 nm (UVB), up to 2000 mJ/cm2; | P. aeruginosa | 24 h, 48 h, 72 h, ≈200 µm | cellulose nitrate membrane filter | UVC: ≈1 log @ 1000 mJ/cm2 (24 h) UVB: ≈1 log @ 63.8 mJ/cm2 (24 h) ≈4.1 log @ 200 mJ/cm2 (24 h); 48 h and 72 h biofilm more resistant |
| [93] | 268 nm (UVC) 275 nm (UVC) 312 nm (UVB) 370 nm (UVA) | E. coli | 24 h, ≈431 nm | PES membrane | 268 nm: 0.62 log @ 12 mJ/cm2; 1.39 log @ 69 mJ/cm2; 1.93 log @ 230 mJ/cm2; 1.75 log @ 347 mJ/cm2; 275 nm: 0.97 log @ 12 mJ/cm2; 1.63 log @ 69 mJ/cm2; 2.69 log @ 230 mJ/cm2; 3.18 log @ 347 mJ/cm2; 312 nm: 0.66 log @ 23 mJ/cm2; 0.95 log @ 69 mJ/cm2; 1.17 log @ 150 mJ/cm2; 1.25 log @ 230 mJ/cm2; 370 nm: 0.02 log @ 23 mJ/cm2; 0.38 log @ 69 mJ/cm2; 1.17 log @ 150 mJ/cm2; 1.25 log @ 230 mJ/cm2; |
| [123] | 275 nm (pulsed), 6 mW/cm2; 455 nm (pulsed), 291 mW/cm2 | S. Typhimurium, A. australiensis | up to 6 d, ≥107 cells/cm2 depending on biofilm and time | steel | S. Typhimurium: 275 nm: 3.9 log @ 3600 mJ/cm2; 455 nm: 2.8 log @ 349.2 J/cm2; A. australiensis: 275 nm: 2.8 log @ 3600 mJ/cm2; 455 nm: 5.6 log @ 87.3 J/cm2; dual species: 275 nm: 2.1 log @ 1800 mJ/cm2; 455 nm: 4.3 log @ 87.3 J/cm2; |
| [124] | 280 nm, 0.57 mW/cm2; up to 684 mJ/cm2 | P. aeruginosa, L. citreum | 24 h, 108–109 cells/cm2 | cellulose ester membranes | P. aeruginosa: 2.3 log @ 684 mJ/cm2; L. citreum: 2.2 log @ 684 mJ/cm2; |
| [72] | 285 nm, 0.025 µW/cm2; up to 180 mJ/cm2 (one time irradiation) | natural microbiome | 14 d | quartz | irradiation reduced further biofilm growth |
| [125] | 365 nm, 2.5 mW/cm2; up to 216 J/cm2 | P. aeruginosa | 0.5 h, 1 h, 24 h, ≥108 cells/cm2 | glass | UVA irradiation slightly promoted biofilm formation |
| [92] | 365 nm, 2 mW/cm2; up to 21.6 J/cm2 | P. aeruginosa | 24 h | glass | ≈1.5 log @ 21.6 J/cm2 |
| [126] | 365 nm pulsed and CW, 0.28 mW/cm2; 1008 mJ/cm2 | E. coli, C. albicans | E. coli: 48 h; C. albicans: 72 h; | MTP | E. coli: 3.4 log @ 1008 J/cm2; C. albicans: 3.1 log @ 1008 J/cm2; (100 Hz more effective than cw) |
| [60] | 385 nm, 420 nm, 2.5 mW/cm2; 216 J/cm2; | E. coli | up to 24 h | silicone (in urine mucine medium in MTP) | 24 h biofilms: in urine mucin medium: no reduction @ 216 J/cm2 for both wavelengths; in PBS: 2.2 log @ 216 J/cm2 of 385 nm; 1.3 log @ 216 J/cm2 of 405 nm; |
| [80] | 400 nm, 60 mW/cm2; up to 216 J/cm2 | P. aeruginosa, S. aureus, E. coli, A. baumannii, amongst others | 72 h | PP | @ 54/108/162/216 J/cm2: P. aeruginosa: 0.68/0.94/0.85/0.87; S. aureus: 0.32/0.44/0.58/0.63; E. coli: 1.13/1.15/1.23/1.28; A. baumannii: 0.31/0.7/0.83/1.0; (planktonic cells more sensitive than cells in biofilm) |
| [127] | 400 nm, 420 nm, 570 nm, 583 nm, 698 nm, up to 29.2 mW/cm2; up to 420.5 J/cm2 | P. fluorescens, S. epidermis | 24 h, P. fluorescens ≈108 cells/cm2; S. epidermis ≈107 cells/cm2 | PS | P. fluorescens @ 400 nm: 1 log @ ≈140 J/cm2, 29.1 mW/cm2; 6.8 log @ ≈420.5 J/cm2, 29.1 mW/cm2; less strong reduction at 420 nm, no reduction at other wavelengths; S. epidermis @ 400 nm: 1 log @ ≈130 J/cm2, 29.1 mW/cm2; 3.7 log @ ≈420.5 J/cm2, 29.1 mW/cm2; no reduction at other wavelengths |
| [128] | 405 nm (laser), 300 mW/cm2; up to 270 J/cm2 | S. aureus | 3 d | urethral stent in broth | 1.2 log @ 90 J/cm2; 2.2 log @ 180 J/cm2; 3.2 log @ 270 J/cm2; |
| [88] | 400 nm: up to 99.7 J/cm2; 470 nm: up to 306.3 J/cm2, 522 nm, 644 nm | P. fluorescens | 24 h, 107–108 cells/cm2 | PS (hydrated) | no significant changes in biofilm (planktonic cells (more) sensitive to violet light) |
| [129] | 402 nm, 440 nm, 35 mW/cm2; up to 252 J/cm2 | A. baumannii | 24 h | MTP | 402 nm: 1.9 @ 189 J/cm2; 4.8 log @ 252 J/cm2; 440 nm: 0.9 log @ 189 J/cm2; 1.7 log @ 252 J/cm2; |
| [130] | 403 nm laser, 141 mW/cm2; up to 21.16 J/cm2 | S. aureus | 8 h–48 h | MTP | 24 h biofilm: 0.86 log @ 21.2 J/cm2 48 h biofilm: 0.26 log @ 21.2 J/cm2 |
| [131] | 405 nm: 84 mW/cm2; 379–452 nm: 62 mW/cm2; | P. aeruginosa, S. aureus, E. coli, A. baumannii | 72 h | PP | average log-reduction @ 513 J/cm2 of 405 nm (“SWA”): P. aeruginosa 0.64; S. aureus 0.4; E. coli 0.97; A. baumannii 0.63; 395 nm exhibits similar antimicrobial impact; other wavelengths less antimicrobial; (not included in analysis because of seemingly inhomogeneous irradiation) |
| [132] | 405 nm, 80 mW/cm2; 144 J/cm2 | P. acnes | up to 7 d | PET membrane | 3.9 log @ 144 J/cm2 |
| [133] | 405 nm, 60 mW/cm2; 216 J/cm2 | S. aureus | 48 h | MTP | 0.62 log @ 108 J/cm2 1.28 log @ 216 J/cm2 |
| [134] | 405 nm, 1050 mW/cm2; | S. aureus | 48 h | titanium | 0.74 log @ 63 J/cm2; 1.55 log @ 315 J/cm2; |
| [135] | 405 nm, 150 mW/cm2; up to 3240 J/cm2; | S. aureus | 72 h | skin/titanium | 1.63 log @ 3240 J/cm2 |
| [90] | 405 nm, 60 mW/cm2, up to 216 J/cm2 | M. catarrhalis | 24 h | MTP | ≈3.6 @ 216 J/cm2 (planktonic cells somewhat more light sensitive) |
| [74] | 405 nm, 26 mW/cm2; up to 748.8 J/cm2 | L. monocytogenes | 24 h | steel and acryl in salmon exudate | @ 25 °C: steel: 1.5 log @ 748.8 J/cm2 acryl: 1.6 log @ 748.8 J/cm2 |
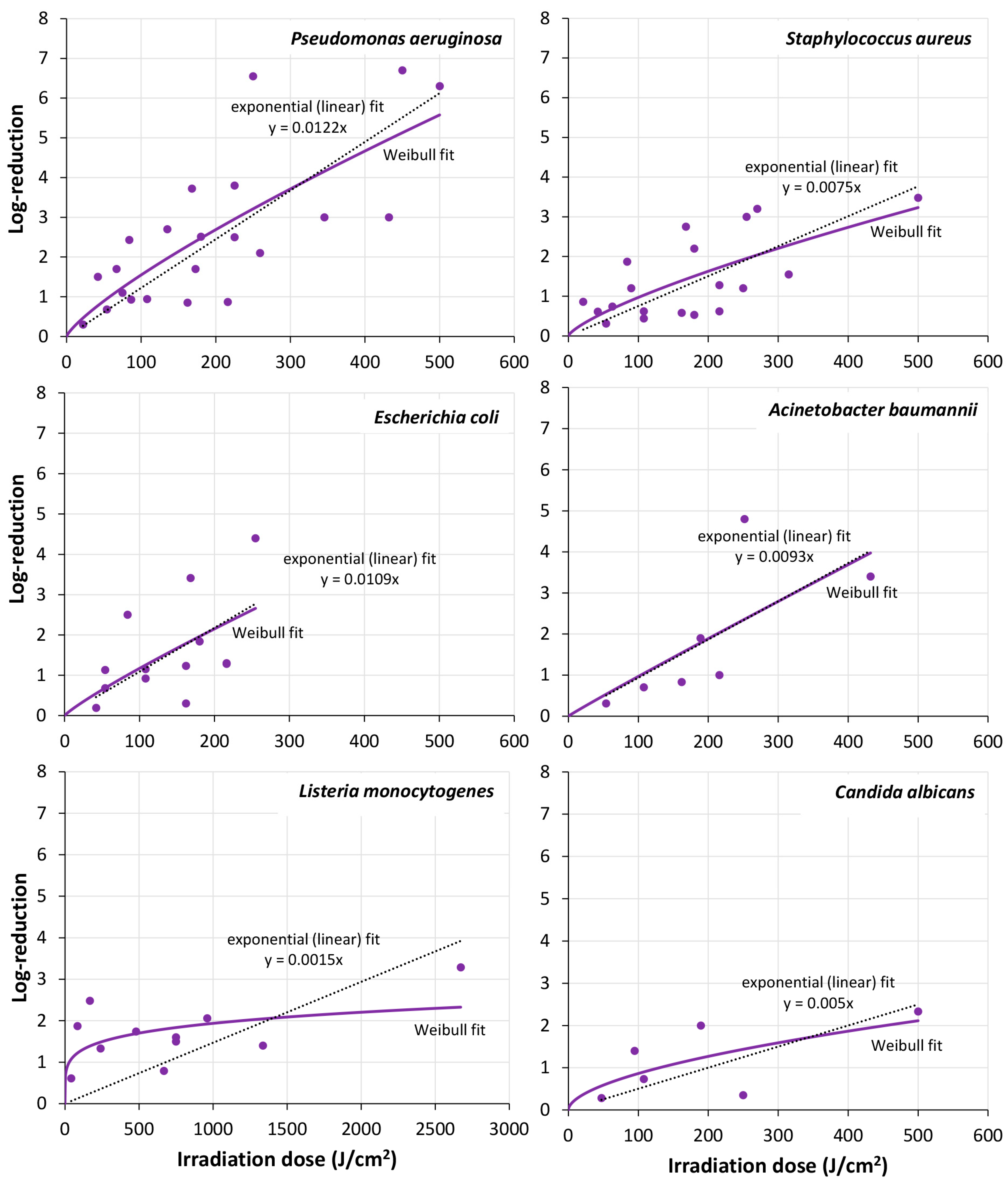
| [96] | 405 nm, 24 mW/cm2; up to 432 J/cm2 | P. aeruginosa | 24 h + 48 h | steel | @ 25 °C: 0.93 log @ 86.4 J/cm2; 1.7 log @ 172.8 J/cm2; 2.1 log @ 259.2 J/cm2; 3.0 log @ 345.6 J/cm2; (cells in biofilm less light resistant than planktonic cells) |
| [136] | 405 nm, 60 mW/cm2; up to 108 J/cm2 | C. albicans | 48 h | MTP | 0.73 log @ 108 J/cm2 planktonic cells more sensitive than cells in biofilm |
| [137] | 405 nm, up to 92.6 mW/cm2; up to 500 J/cm2 | P. aeruginosa, S. aureus, C. albicans | 24 h, 48 h, 107–108 cells/cm2 | MTP, PC | @ 24 h biofilm after 250/500 J/cm2: P. aeruginosa: 6.55/6.3 log S. aureus: 1.2/3.48 log C. albicans: 0.35/2.33 log; P. aeruginosa and S. aureus: P. aeruginosa: 3.94/3.4 log S. aureus: 1.42/2.37 log P. aeruginosa and C. albicans: P. aeruginosa: 5.67/6.34 log C. albicans: 2.46/3.11 @ 48 h MTP biofilm after 500 J/cm2: (biofilms grown on PC in CDC bioreactor slightly more resistant) |
| [138] | 405 nm, 141.5 mW/cm2; up to 504 J/cm2 | P. aeruginosa, S. aureus, E. coli, L. monocytogenes | 4–72 h, glass: 106–108 cells/cm2; acrylic: 104–105 cells/cm2 | glass, acryl | P. aeruginosa 24 h glass: 1.5 @ 42 J/cm2; 2.43 @ 84 J/cm2; 3.72 @ 168 J/cm2; L. monocytogenes 24 h glass: 0.61 @ 42 J/cm2; 1.87 @ 84 J/cm2; 2.48 @ 168 J/cm2; E. coli 24 h glass: 0.19 log @ 42 J/cm2; 2.5 log @ 84 J/cm2; 3.41 log 168 J/cm2; 4.4 log @ 254.7 J/cm2; S. aureus 24 h glass: 0.61 @ 42 J/cm2; 1.87 @ 84 J/cm2; 2.75 @ 168 J/cm2; 3.0 log @ 254.7 J/cm2; E. coli and S. aureus 24 h glass: 2.2 log @ 254.7 J/cm2; (mixed biofilm more resistant; biofilms became more resistant with maturity) |
| [139] | 405 nm, 60 mW/cm2; up to 162 J/cm2 | E. coli, K. pneumoniae, K. oxytoca | 72 h | PP | @ 162/54/108 J/cm2: E. coli: 0.30/0.68/0.92; K. pneumoniae: 0.21/0.46/0.91; K. oxytoca: 0.99/0.69/1.06; |
| [140] | 405 nm, 280 mW/cm2; up to 284.4 J/cm2 | C. albicans, C. glabrata, | 24 h | PMMA in artificial saliva | mono-species biofilms: C. albicans reduction: 0.28 log @ 47.4 J/cm2; 1.4 log @ 94.8 J/cm2; 2 log @ 189.6 J/cm2; C. glabrata reduction: 0.25 log @ 94.8 J/cm2; 2 log @ 189.6 J/cm2; no biofilm after 30 min (284 J/cm2) irradiation |
| [141] | 405 nm, 280 mW/cm2; up to 379.7 J/cm2 | S. mutans, C. albicans | 24 h | PMMA in artificial saliva | dual-species biofilm: 3.64 log @ 189.6 J/cm2 for C. albicans and 3.66 log @ 189.6 J/cm2 for S. mutans in dual species biofilm; faster reduction in C. albicans for higher doses; |
| [142] | 405 nm, 280 mW/cm2; up to 379.7 J/cm2 | S. mutans, C. albicans | 24 h | PMMA in artificial saliva | mono-species biofilms: S. mutans: 3.6 log @ 379.7 J/cm2; C. albicans: 3.55 log @ 379.7 J/cm2; cell reduction in dual-species biofilms: S. mutans: 3.4 log @ 379.7 J/cm2; C. albicans: 3.57 log @ 379.7 J/cm2; |
| [143] | 405 nm, 370.6 mW/cm2; up to 222 J/cm2 | B. bruxellensis | 30 d | steel, oak in wine or yeast medium | steel and yeast medium: 0.8 log @ 22 J/cm2; 2.6 log @ 44.5 J/cm2; 3.7 log @ 111 J/cm2; 3.8 log @ 222 J/cm2; wood and wine: 0.25 log @ 22 J/cm2; 0.5 log @ 44.5 J/cm2; 2.9 log @ 111 J/cm2; 4.7 log @ 222 J/cm2; |
| [97] | 405 nm, up to 100 mW/cm2; up to 360 J/cm2 | V. vulnificus | 48 h MTP; 6 h wound | MTP, wounds | 1 log @ ≈60 J/cm2; 3 log @ ≈162 J/cm2 (no large sensitivity differences between planktonic cells and cells in biofilms) |
| [144] | 405 nm 420 nm 460 nm | L. monocytogenes | 48 h, ≈6.5 µm | steel, PVC, silicone, PE, PS | steel: 405 nm: 0.79 log @ 668 J/cm2; 1.40 log @ 1336 J/cm2; 3.29 log @ 2672 J/cm2; 420 nm: 1.33 log @ 240 J/cm2; 1.74 log @ 480 J/cm2; 2.06 log @ 960 J/cm2; 460 nm: 1.27 log @ 200 J/cm2; 1.67 log @ 400 J/cm2; 1.72 log @ 800 J/cm2; significant biomass reduction for all wavelengths; |
| [75] | 410 nm, 455 nm, 100 mW/cm2; up to 450 J/cm2 | P. aeruginosa | 6 h | PS MTP | 410 nm: 1.1 log @ 75 J/cm2; 2.5 log @ 225 J/cm2; 6.7 log @ 450 J/cm2; 455 nm: 1.1 log @ 450 J/cm2 |
| [145] | 415 nm, up to 100 mW/cm2; up to 540 J/cm2 | P. aeruginosa, A. baumannii | 24 h, 72 h | MTP, wounds | MTP—P. aeruginosa: ≈3 log @ 432 J/cm2 for 24 and 72 h biofilm; MTP—A. baumannii: ≈3.6 and 3.2 log @ 432 J/cm2 for 24 and 72 h biofilm, respectively; wound—A. baumannii: ≈3 log @ 360–540 J/cm2 |
| [146] | 415 nm, 445 nm, 525 nm, 623 nm, up to 110 J/cm2 | P. aeruginosa, S. aureus | plastic | 415 nm: P. aeruginosa PAO1: ≥2 log @ 60 J/cm2 P. aeruginosa LESB65: ≥2 log @ 60 J/cm2 S. aureus CF-MRSA: ≥2 log @ 60 J/cm2 S. aureus USA300: ≈1 log@60 J/cm2, ≈1.5 log@110 J/cm2 445 nm: P. aeruginosa: ≈1 log @ 60 J/cm2 S. aureus: ≈1 log @ 60 J/cm2 525 nm: P. aeruginosa LESB65: ≈1 log @ 60 J/cm2; no reduction for other strains; 623 nm: no reduction | |
| [86] | 420 nm, 212 mW/cm2; up to 763 J/cm2 | P. fluorescens | 60 h | PS MTP in medium | ≈0.7 log @ 763 J/cm2 (planktonic bacteria more light sensitive than bacteria in biofilms) |
| [147] | 420 nm, 93 mW/cm2; 2 × 72 J/cm2 per day over 5 days (720 J/cm2 total) | S. mutans | 5 d | saliva-coated hydroxyapatite | 1 log @ 720 J/cm2 (in total); 42% biomass reduction; |
| [95] | 420 nm, 455 nm, 480 nm, 50 mW/cm2; up to 180 J/cm2 | P. aeruginosa, S. aureus, S. epidermis, E. coli | 24 h | MTP in medium | 420 nm @ 180 J/cm2 P. aeruginosa: 2.51; S. aureus: 0.53; S. epidermis: 1.63; E. coli: 1.84; 455 nm @ 180 J/cm2: P. aeruginosa: 0.83; S. aureus: 0.48; S. epidermis: 0.52; E. coli: 0.41; 480 nm @ 180 J/cm2: P. aeruginosa: 0.61; S. aureus: 0.69; S. epidermis: 0.63; E. coli: 0.85; (cells in biofilms more light sensitive than planktonic cells) |
| [76] | 445 nm (laser), 380–490 nm (LED), 970 nm (laser), different irradiances; up to 120 J/cm2 | P. aeruginosa | 0.5 h, 24 h | MTP, wound | 445 nm irradiation significantly reduced cells in 24 h biofilms in MTP with higher doses leading to a larger reduction; irradiated wound also exhibits reduced bacteria |
| [148] | 450 nm, 57 mW/cm2; 100 J/cm2 | P. aeruginosa | 48 h | MTP | no significant biofilm reduction |
| [77] | 450 nm (pulsed), 2 mW/cm2; 7.6 J/cm2 three times per day over three days (68.4 J/cm2 total) | S. aureus, P. acnes | 24 h | PS MTP | MRSA: 0.276 log @ 68.4 J/cm2 (total); P. acnes: 0.194 log @ 68.4 J/cm2 (total); (cells in biofilms more light sensitive than planktonic cells) |
| [78] | 450 nm, 525 nm, 625 nm, up to 240 J/cm2 | C. albicans | 24 h | MTP | 450 nm: of 0.41 log @ 240 J/cm2; no antimicrobial effects for other wavelengths; |
| [149] | 455 nm, 50 mW/cm2; 4 × 12 mJ/cm2 | natural patient biofilm | 3 d | MTP in medium | 0.28 log @ 48 J/cm2 (biofilm microbiome constitution changed after irradiation) |
| [150] | 455 nm, 75 mW/cm2; up to 45.2 J/cm2 | S. aureus, C. albicans | 14 d | bone | S. aureus: 3.2 log @ 45.2 J/cm2; C. albicans: 2.3 log @ 45.2 J/cm2 |
| [151] | 460 nm, red light, 60 mW/cm2; up to 240 J/cm2 | C. albicans | 24 h, 48 h, 72 h | 460 nm reduced cells in biofilm; no visible impact of red light | |
| [152] | 390–480 nm (peak at 460 nm), 1000 mW/cm2; 60 J/cm2; | E. faecalis | 3 w | teeth | 0.05 log @ 60 J/cm2 |
| [153] | blue light around 470 nm, 620 mW/cm2; up to 262 J/cm2 | S. mutans | 24 h, ≈85 µm | MTP in medium | biofilm regrowth increased after blue irradiation; however, bacterial viability decreased; blue light seemed to have a delayed antimicrobial impact |
| [81] | broadband blue (400–520 nm), 500 mW/cm2; up to 60 J/cm2 | A. actinomycetemcomitans, F. nucleatum, P. gingivalis | 7 d, up to 45 µm | MTP in medium | irradiation reduced mostly P. gingivalis cells in biofilm: 0.95 log @ 60 J/cm2; (planktonic cells much more light sensitive than cells in biofilm) |
| [154] | broadband blue (400–500 nm), 1140 mW/cm2; up to 68 J/cm2 | S. mutans | 24 h | MTP in medium | no effect on biofilm |
| [155] | 400–500 nm, 1217 mW/cm2; 146 J/cm2; | F. nucleatum, P. gingivalis, S. sanguinis, A. naeslundii | 48 h/72 h | hydroxyapatite in saliva | mono-species biofilms: P. gingivalis 0.2 log @146 J/cm2; no reduction for the other mono-species biofilms; (irradiation of the multi-species biofilm changed its bacterial composition) |
| [156] | broadband blue (400–500 nm), 1140 mW/cm2; up to 680 J/cm2; | S. mutans | 24 h | MTP in medium | blue light seemed to have a delayed antimicrobial impact |
| [157] | broadband blue (400–500 nm), 623 mW/cm2; 112 J/cm2; | S. mutans, S. sanguinis | 24 h, ≈200 µm | enamel (in PBS) | irradiation reduced viable cells in mono- and multi-species biofilm (biofilm recovered for 24–48 h before analysis) |
| [158] | pulsed (unknown spectrum) | P. aeruginosa | 8 h, 48 h biofilms | MTP, PC membrane | up to 100% reduction @ unknown irradiation parameters; (mature biofilms more resistant) |
| [159] | pulsed Xenon (220–520 nm) | P. aeruginosa, S. aureus, E. coli | up to 72 h | PVC | reductions in several logs achieved |
| [160] | pulsed Xenon (200–1100 nm), 1270 mJ/pulse at a distance that was not applied against biofilms | E. coli, L. monocytogenes | 24 h, 48 h | lettuce, PP | cell reduction in several logs in both bacteria; E. coli more sensitive than L. monocytogenes, mature biofilms more resistant; reduction slightly higher on polyethylene than on lettuce |
| [85] | pulsed Xenon (200–1000 nm) | A. niger, P. glaucum | 8 h, 48 h | MTP, PC membrane | irradiation reduced cells in biofilm independent of biofilm maturity (planktonic much more sensitive than cells in biofilm) |
| [84] | pulsed Xenon (200–1000 nm), up to 40.7 mJ/cm2 per pulse; up to 21,978 J/cm2 | S. aureus, B. cereus, B. thuringiensis, L. moncytogenes, P. acidilacti, L. brevis, E. faecium | 8 h and 48 h | MTP, PC membrane | irradiation reduced cells in biofilm; more mature biofilm more resistant (planktonic cell more sensitive than cells in biofilm) |
| [161] | pulsed Xenon (220–520 nm), 16.2 J/pulse | C. albican, C. parapsilosis | 48 h, 72 h | steel, PVC | 3–4 log @ 6.48 µJ/cm2 (irradiation dose correct?) |
4. Discussion
5. Conclusions
- The irradiation of water reduces or delays biofilm formation only in some situations or for some water conditions.
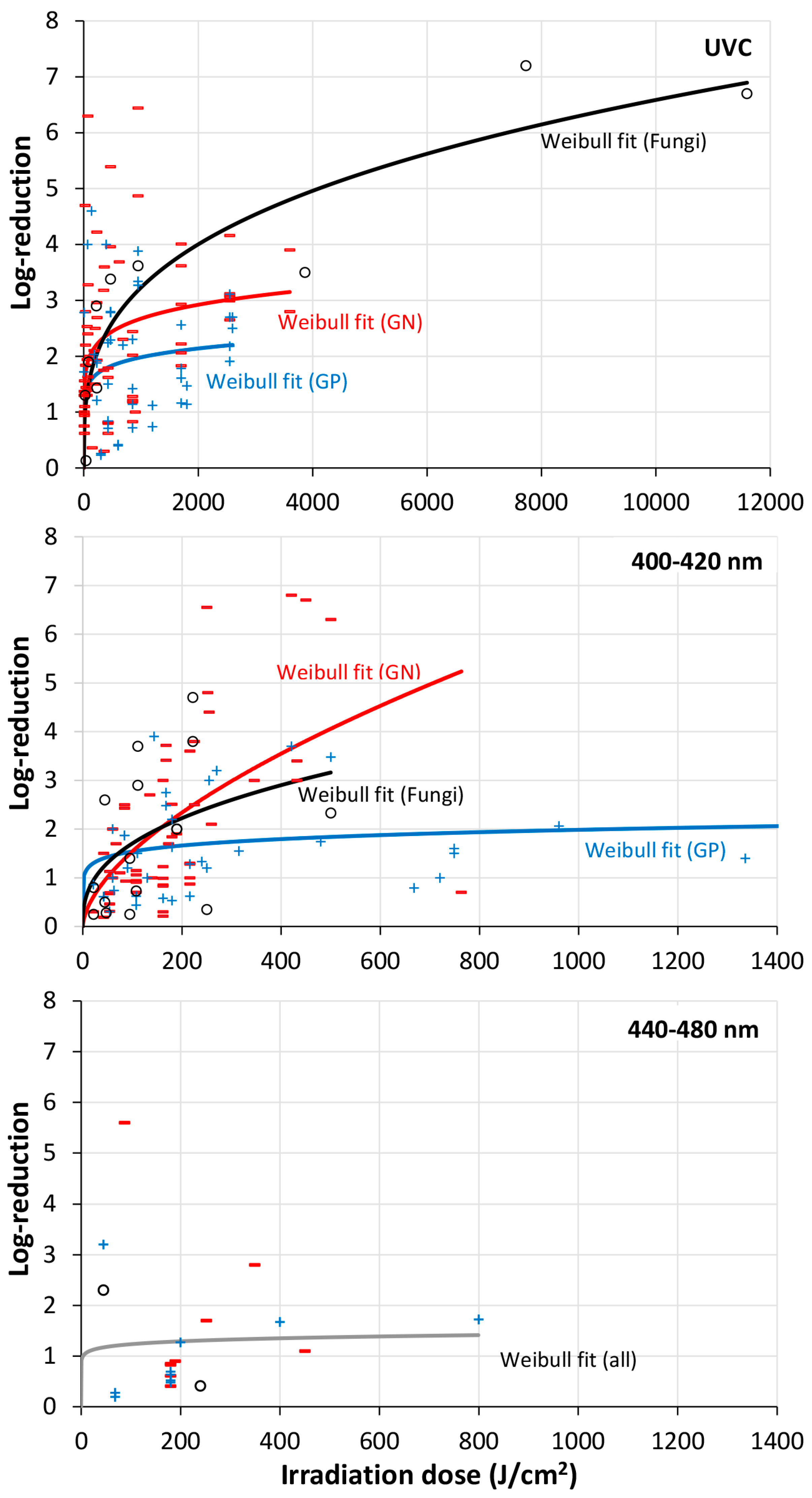
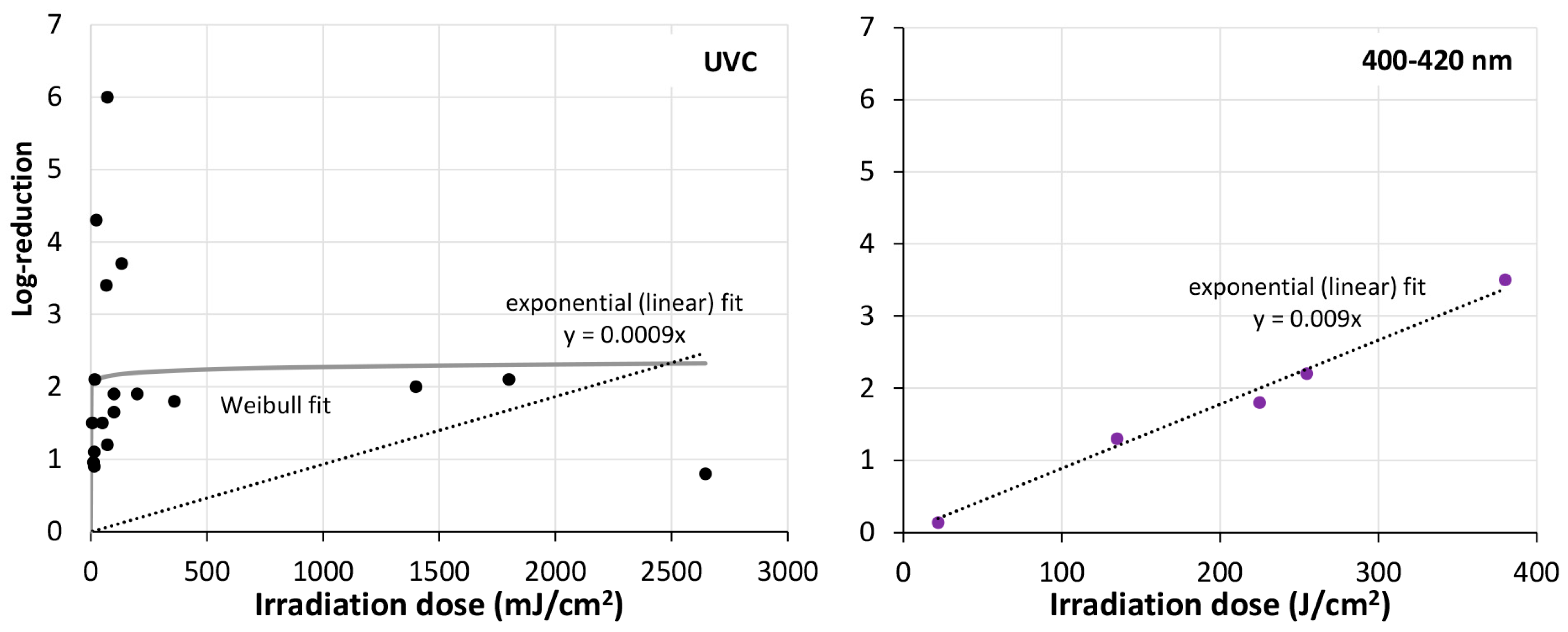
- Irradiation of surfaces reduces or delays biofilm formation. This is true for the spectral range 200–525 nm if the irradiation intensity is high enough.
- UVC seems to be much more efficient in biofilm reduction than visible blue/violet light, but it seems still unclear which wavelength is best for biofilm irradiation and reduction.
- Multi-species biofilms might be more irradiation resistant than mono-species biofilms, but the difference seems to be small.
- Compared to the scattering of the results, there are no large differences between the photosensitivities of Gram+ bacterial, Gram- bacterial, and fungal biofilms.
- Cells in biofilms are more radiation resistant than planktonic cells.
- The impact of the biofilm substrate seems to be rather low.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Funari, R.; Shen, A.Q. Detection and Characterization of Bacterial Biofilms and Biofilm-Based Sensors. ACS Sens. 2022, 7, 347–357. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Cámara, M.; Green, W.; MacPhee, C.E.; Rakowska, P.D.; Raval, R.; Richardson, M.C.; Slater-Jefferies, J.; Steventon, K.; Webb, J.S. Economic significance of biofilms: A multidisciplinary and cross-sectoral challenge. NPJ Biofilms Microbiomes 2022, 8, 42. [Google Scholar] [CrossRef]
- Li, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Biofilms formation in plant growth-promoting bacteria for alleviating agro-environmental stress. Sci. Total Environ. 2024, 907, 167774. [Google Scholar] [CrossRef]
- Flemming, H.-C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nat. Rev. Microbiol. 2023, 21, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Jagger, J. Introduction to Research in Ultraviolet Photobiology. Photochem. Photobiol. 1968, 7, 413. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Tomb, R.M.; White, T.A.; Coia, J.E.; Anderson, J.G.; MacGregor, S.J.; Maclean, M. Review of the Comparative Susceptibility of Microbial Species to Photoinactivation Using 380-480 nm Violet-Blue Light. Photochem. Photobiol. 2018, 94, 445–458. [Google Scholar] [CrossRef]
- Hessling, M.; Spellerberg, B.; Hoenes, K. Photoinactivation of bacteria by endogenous photosensitizers and exposure to visible light of different wavelengths—A review on existing data. FEMS Microbiol. Lett. 2016, 364, fnw270. [Google Scholar] [CrossRef] [PubMed]
- Gora, S.L.; Ma, B.; Lanzarini-Lopes, M.; Torkzadeh, H.; Zhao, Z.; Ley Matthews, C.; Westerhoff, P.; Linden, K.; Barbeau, B.; Simons, R.; et al. Control of biofilms with UV light: A critical review of methodologies, research gaps, and future directions. Environ. Sci. Water Res. Technol. 2024, 10, 3056–3073. [Google Scholar] [CrossRef]
- Grzelak, A.; Rychlik, B.; Bartosz, G. Light-dependent generation of reactive oxygen species in cell culture media. Free. Radic. Biol. Med. 2001, 30, 1418–1425. [Google Scholar] [CrossRef]
- Bigelow, W.D. The logarithmic nature of thermal death time curves. J. Infect. Dis. 1921, 29, 528–536. [Google Scholar] [CrossRef]
- Mafart, P.; Couvert, O.; Gaillard, S.; Leguerinel, I. On calculating sterility in thermal preservation methods: Application of the Weibull frequency distribution model. Int. J. Food Microbiol. 2002, 72, 107–113. [Google Scholar] [CrossRef]
- Geeraerd, A.H.; Valdramidis, V.P.; van Impe, J.F. GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int. J. Food Microbiol. 2005, 102, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Garre, A.; Fernández, P.S.; Lindqvist, R.; Egea, J.A. Bioinactivation: Software for modelling dynamic microbial inactivation. Food Res. Int. 2017, 93, 66–74. [Google Scholar] [CrossRef]
- Garre, A.; Clemente-Carazo, M.; Fernández, P.S.; Lindqvist, R.; Egea, J.A. Bioinactivation FE: A free web application for modelling isothermal and dynamic microbial inactivation. Food Res. Int. 2018, 112, 353–360. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.N.; Parenté, F.J.; Schmitt, C.J. A monte carlo study on the robustness of four MANOVA criterion tests. J. Stat. Comput. Simul. 1982, 15, 183–192. [Google Scholar] [CrossRef]
- Haase, R.F.; Ellis, M.V. Multivariate analysis of variance. J. Couns. Psychol. 1987, 34, 404–413. [Google Scholar] [CrossRef]
- Rencher, A.C. A Review of “Methods of Multivariate Analysis, Second Edition”. IIE Trans. 2005, 37, 1083–1085. [Google Scholar] [CrossRef]
- Ateş, C.; Kaymaz, Ö.; Kale, H.E.; Tekindal, M.A. Comparison of Test Statistics of Nonnormal and Unbalanced Samples for Multivariate Analysis of Variance in terms of Type-I Error Rates. Comput. Math. Methods Med. 2019, 2019, 2173638. [Google Scholar] [CrossRef]
- Statistics Kingdom. Statistics Online. Available online: https://www.statskingdom.com/index.html (accessed on 24 July 2025).
- Momba, M.N.B.; Cloete, T.E.; Venter, S.N.; Kfir, R. Evaluation of the impact of disinfection processes on the formation of biofilms in potable surface water distribution systems. Water Sci. Technol. 1998, 38, 283–289. [Google Scholar] [CrossRef]
- Schwartz, T.; Hoffmann, S.; Obst, U. Formation of natural biofilms during chlorine dioxide and u.v. disinfection in a public drinking water distribution system. J. Appl. Microbiol. 2003, 95, 591–601. [Google Scholar] [CrossRef]
- Marconnet, C.; Houari, A.; Seyer, D.; Djafer, M.; Coriton, G.; Heim, V.; Di Martino, P. Membrane biofouling control by UV irradiation. Desalination 2011, 276, 75–81. [Google Scholar] [CrossRef]
- Wenjun, S.; Wenjun, L. Impact of the Ultraviolet Disinfection Process on Biofilm Control in a Model Drinking Water Distribution System. Environ. Eng. Sci. 2009, 26, 809–816. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Chen, Z.; Li, X.; Wang, Y.-H.; Liu, B.; Chen, G.-Q.; Luo, L.-W.; Wang, H.-B.; Tong, X.; Bai, Y.; et al. Effect of ultraviolet disinfection on the fouling of reverse osmosis membranes for municipal wastewater reclamation. Water Res. 2021, 195, 116995. [Google Scholar] [CrossRef]
- Pozos, N.; Scow, K.; Wuertz, S.; Darby, J. UV disinfection in a model distribution system: Biofilm growth and microbial community. Water Res. 2004, 38, 3083–3091. [Google Scholar] [CrossRef]
- Saidi, N.; Kouki, S.; Mehri, I.; Ben Rejeb, A.; Belila, A.; Hassen, A.; Ouzari, H. Biofilm and siderophore effects on secondary waste water disinfection. Curr. Microbiol. 2011, 63, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Vankerckhoven, E.; Verbessem, B.; Crauwels, S.; Declerck, P.; Muylaert, K.; Willems, K.A.; Rediers, H. Exploring the potential synergistic effects of chemical disinfectants and UV on the inactivation of free-living bacteria and treatment of biofilms in a pilot-scale system. Water Sci. Technol. 2011, 64, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Rand, J.L.; Sharafimasooleh, M.; Walsh, M.E. Effect of water hardness and pipe material on enhanced disinfection with UV light and chlorine. J. Water Supply Res. Technol. 2013, 62, 426–432. [Google Scholar] [CrossRef]
- Jungfer, C.; Friedrich, F.; Varela Villarreal, J.; Brändle, K.; Gross, H.-J.; Obst, U.; Schwartz, T. Drinking water biofilms on copper and stainless steel exhibit specific molecular responses towards different disinfection regimes at waterworks. Biofouling 2013, 29, 891–907. [Google Scholar] [CrossRef]
- Yu, W.; Campos, L.C.; Graham, N. Application of pulsed UV-irradiation and pre-coagulation to control ultrafiltration membrane fouling in the treatment of micro-polluted surface water. Water Res. 2016, 107, 83–92. [Google Scholar] [CrossRef]
- Benito, A.; Garcia, G.; Gonzalez-Olmos, R. Fouling reduction by UV-based pretreatment in hollow fiber ultrafiltration membranes for urban wastewater reuse. J. Membr. Sci. 2017, 536, 141–147. [Google Scholar] [CrossRef]
- Metz, D.H.; Reynolds, K.; Meyer, M.; Dionysiou, D.D. The effect of UV/H2O2 treatment on biofilm formation potential. Water Res. 2011, 45, 497–508. [Google Scholar] [CrossRef]
- Harif, T.; Elifantz, H.; Margalit, E.; Herzberg, M.; Lichi, T.; Minz, D. The effect of UV pre-treatment on biofouling of BWRO membranes: A field study. Desalination Water Treat. 2011, 31, 151–163. [Google Scholar] [CrossRef]
- Kviatkovski, I.; Mamane, H.; Lakretz, A.; Sherman, I.; Beno-Moualem, D.; Minz, D. Resistance of a multiple-isolate marine culture to ultraviolet C irradiation: Inactivation vs biofilm formation. Lett. Appl. Microbiol. 2018, 67, 278–284. [Google Scholar] [CrossRef]
- Lakretz, A.; Ron, E.Z.; Mamane, H. Biofouling control in water by various UVC wavelengths and doses. Biofouling 2010, 26, 257–267. [Google Scholar] [CrossRef]
- Lakretz, A.; Ron, E.Z.; Mamane, H. Biofilm control in water by a UV-based advanced oxidation process. Biofouling 2011, 27, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Friedman, L.; Harif, T.; Herzberg, M.; Mamane, H. Mitigation of Biofilm Colonization on Various Surfaces in a Model Water Flow System by Use of UV Treatment. Water Air Soil Pollut 2016, 227, 43. [Google Scholar] [CrossRef]
- Lakretz, A.; Mamane, H.; Asa, E.; Harif, T.; Herzberg, M. Biofouling control by UV/H2O2 pretreatment for brackish water reverse osmosis process. Environ. Sci. Water Res. Technol. 2018, 4, 1331–1344. [Google Scholar] [CrossRef]
- Lund, V.; Ormerod, K. The influence of disinfection processes on biofilm formation in water distribution systems. Water Res. 1995, 29, 1013–1021. [Google Scholar] [CrossRef]
- Otaki, M.; Takizawa, S.; Ohgaki, S. Control and modeling of membrane fouling due to microorganism growth by UV pretreatment. Water Sci. Technol. 1998, 38, 405–412. [Google Scholar] [CrossRef]
- Sperle, P.; Khan, M.S.; Skibinski, B.; Wurzbacher, C.; Drewes, J.E. Optimizing UVC-disinfection using LEDs as an energy efficient pre-treatment for biofouling control in spiral-wound membrane systems. Desalination 2023, 557, 116589. [Google Scholar] [CrossRef]
- Sperle, P.; Wurzbacher, C.; Drewes, J.E.; Skibinski, B. Reducing the Impacts of Biofouling in RO Membrane Systems through In Situ Low Fluence Irradiation Employing UVC-LEDs. Membranes 2020, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Randall, T.; Shlomo, I.; Wells, E.; Real, B.; Ma, B.; Linden, Y.; Gamboa, J.; Friedler, E.; Linden, K.G. Evaluation of UVLED disinfection for biofouling control during distribution of wastewater effluent. Water Reuse 2024, 14, 80–94. [Google Scholar] [CrossRef]
- Karim, N.S.; Sarker, N.R.; Asker, D.; Hatton, B.; Bilton, A.M. Can UVC-LEDs mitigate biofouling in community-scale photovoltaic-powered reverse osmosis systems? Water Supply 2025, 25, 779–791. [Google Scholar] [CrossRef]
- Zhao, Z.; Rho, H.; Shapiro, N.; Ling, L.; Perreault, F.; Rittmann, B.; Westerhoff, P. Biofilm inhibition on surfaces by ultraviolet light side-emitted from optical fibres. Nat. Water 2023, 1, 649–657. [Google Scholar] [CrossRef]
- Zhao, Z.; Luo, Y.-H.; Wang, T.-H.; Sinha, S.; Ling, L.; Rittmann, B.; Alvarez, P.; Perreault, F.; Westerhoff, P. Phenotypic and Transcriptional Responses of Pseudomonas aeruginosa Biofilms to UV-C Irradiation via Side-Emitting Optical Fibers: Implications for Biofouling Control. Environ. Sci. Technol. 2023, 57, 15736–15746. [Google Scholar] [CrossRef]
- Hunsucker, K.Z.; Braga, C.; Gardner, H.; Jongerius, M.; Hietbrink, R.; Salters, B.; Swain, G. Using ultraviolet light for improved antifouling performance on ship hull coatings. Biofouling 2019, 35, 658–668. [Google Scholar] [CrossRef]
- Ryan, E.; Turkmen, S.; Benson, S. An Investigation into the application and practical use of (UV) ultraviolet light technology for marine antifouling. Ocean Eng. 2020, 216, 107690. [Google Scholar] [CrossRef]
- Piola, R.; Salters, B.; Grandison, C.; Ciacic, M.; Hietbrink, R. Assessing the Use of Low Voltage UV-Light Emitting Miniature LEDs for Marine Biofouling Control: Technical Report; Defence Science and Technology Group: Melbourne, Australia, 2016.
- Salters, B.; Piola, R. UVC Light for Antifouling. Mar. Technol. Soc. J. 2017, 51, 59–70. [Google Scholar] [CrossRef]
- Lanzarini-Lopes, M.; Zhao, Z.; Perreault, F.; Garcia-Segura, S.; Westerhoff, P. Germicidal glowsticks: Side-emitting optical fibers inhibit Pseudomonas aeruginosa and Escherichia coli on surfaces. Water Res. 2020, 184, 116191. [Google Scholar] [CrossRef]
- Chen, H.; Moraru, C.I. Exposure to 222 nm far UV-C effectively inactivates planktonic foodborne pathogens and inhibits biofilm formation. Innov. Food Sci. Emerg. Technol. 2023, 87, 103411. [Google Scholar] [CrossRef]
- Patil, J.S.; Kimoto, H.; Kimoto, T.; Saino, T. Ultraviolet radiation (UV-C): A potential tool for the control of biofouling on marine optical instruments. Biofouling 2007, 23, 215–230. [Google Scholar] [CrossRef]
- Mariita, R.M.; Davis, J.H.; Lottridge, M.M.; Randive, R.V. Shining light on multi-drug resistant Candida auris: Ultraviolet-C disinfection, wavelength sensitivity, and prevention of biofilm formation of an emerging yeast pathogen. Microbiologyopen 2022, 11, e1261. [Google Scholar] [CrossRef]
- Torkzadeh, H.; Zodrow, K.R.; Bridges, W.C.; Cates, E.L. Quantification and modeling of the response of surface biofilm growth to continuous low intensity UVC irradiation. Water Res. 2021, 193, 116895. [Google Scholar] [CrossRef]
- Torkzadeh, H.; Cates, E.L. Biofilm growth under continuous UVC irradiation: Quantitative effects of growth conditions and growth time on intensity response parameters. Water Res. 2021, 206, 117747. [Google Scholar] [CrossRef] [PubMed]
- Vollmerhausen, T.L.; Conneely, A.; Bennett, C.; Wagner, V.E.; Victor, J.C.; O’Byrne, C.P. Visible and UVA light as a potential means of preventing Escherichia coli biofilm formation in urine and on materials used in urethral catheters. J. Photochem. Photobiol. B 2017, 170, 295–303. [Google Scholar] [CrossRef]
- European Union. Directive 2006/25/EC of the European Parliament and of the Council on the minimum health and safety requirements regarding the exposure of workers to risks arising from physical agents (artificial optical radiation). Off. J. Eur. Union 2006, 114, 38–59. [Google Scholar]
- Butement, J.T.; Noel, D.J.; Bryant, C.A.; Wilks, S.A.; Eason, R.W. A light-guiding urinary catheter for the inhibition of Proteus mirabilis biofilm formation. Front. Microbiol. 2022, 13, 995200. [Google Scholar] [CrossRef] [PubMed]
- Braga, C.; Hunsucker, K.; Erdogan, C.; Gardner, H.; Swain, G. The Use of a UVC Lamp Incorporated with an ROV to Prevent Biofouling: A Proof-of-Concept Study. Mar. Technol. Soc. J. 2020, 54, 76–83. [Google Scholar] [CrossRef]
- Braga, C.; Hunsucker, K.; Gardner, H.; Swain, G. A novel design to investigate the impacts of UV exposure on marine biofouling. Appl. Ocean. Res. 2020, 101, 102226. [Google Scholar] [CrossRef]
- Disalvo, L.H.; Cobet, A.B. Control of an estuarine microfouling sequence on optical surfaces using low-intensity ultraviolet irradiation. Appl. Microbiol. 1974, 27, 172–178. [Google Scholar] [CrossRef]
- Bak, J.; Ladefoged, S.D.; Begovic, T.; Winding, A. UVC fluencies for preventative treatment of Pseudomonas aeruginosa contaminated polymer tubes. Biofouling 2010, 26, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Hoeher, P.A.; Zenk, O.; Cisewski, B.; Boos, K.; Groeger, J. UVC-Based Biofouling Suppression for Long-Term Deployment of Underwater Cameras. IEEE J. Ocean. Eng. 2023, 48, 1389–1405. [Google Scholar] [CrossRef]
- Bueley, C.; Olender, D.; Bocking, B. In-Situ Trial of Uv-C as an Antifoulant to Reduce Biofouling Induced Measurement Error. J. Ocean. Technol. 2014, 2014, 49–67. [Google Scholar]
- Alidokht, L.; Fitzpatrick, K.; Butler, C.; Hunsucker, K.Z.; Braga, C.; Maza, W.A.; Fears, K.P.; Arekhi, M.; Lanzarini-Lopes, M. UV emitting glass: A promising strategy for biofilm inhibition on transparent surfaces. Biofilm 2024, 7, 100186. [Google Scholar] [CrossRef]
- Whitworth, P.; Aldred, N.; Reynolds, K.J.; Plummer, J.; Duke, P.W.; Clare, A.S. Importance of Duration, Duty-Cycling and Thresholds for the Implementation of Ultraviolet C in Marine Biofouling Control. Front. Mar. Sci. 2022, 8. [Google Scholar] [CrossRef]
- MacKenzie, A.F.; Maltby, E.A.; Harper, N.; Bueley, C.; Olender, D.; Wyeth, R.C. Periodic ultraviolet-C illumination for marine sensor antifouling. Biofouling 2019, 35, 483–493. [Google Scholar] [CrossRef]
- Purvis, K.; Curnew, K.H.; Trevors, A.L.; Hunter, A.T.; Wilson, E.R.; Wyeth, R.C. Single Ultraviolet-C light treatment of early stage marine biofouling delays subsequent community development. Biofouling 2022, 38, 536–546. [Google Scholar] [CrossRef]
- Gomez, G.F.; Huang, R.; MacPherson, M.; Ferreira Zandona, A.G.; Gregory, R.L. Photo Inactivation of Streptococcus mutans Biofilm by Violet-Blue light. Curr. Microbiol. 2016, 73, 426–433. [Google Scholar] [CrossRef]
- Li, X.; Kim, M.-J.; Bang, W.-S.; Yuk, H.-G. Anti-biofilm effect of 405-nm LEDs against Listeria monocytogenes in simulated ready-to-eat fresh salmon storage conditions. Food Control 2018, 84, 513–521. [Google Scholar] [CrossRef]
- Martegani, E.; Bolognese, F.; Trivellin, N.; Orlandi, V.T. Effect of blue light at 410 and 455 nm on Pseudomonas aeruginosa biofilm. J. Photochem. Photobiol. B 2020, 204, 111790. [Google Scholar] [CrossRef]
- Rupel, K.; Zupin, L.; Ottaviani, G.; Bertani, I.; Martinelli, V.; Porrelli, D.; Vodret, S.; Vuerich, R.; Passos da Silva, D.; Bussani, R.; et al. Blue laser light inhibits biofilm formation in vitro and in vivo by inducing oxidative stress. NPJ Biofilms Microbiomes 2019, 5, 29. [Google Scholar] [CrossRef]
- Bumah, V.V.; Masson-Meyers, D.S.; Enwemeka, C.S. Pulsed 450 nm blue light suppresses MRSA and Propionibacterium acnes in planktonic cultures and bacterial biofilms. J. Photochem. Photobiol. B 2020, 202, 111702. [Google Scholar] [CrossRef] [PubMed]
- Bapat, P.; Singh, G.; Nobile, C.J. Visible Lights Combined with Photosensitizing Compounds Are Effective against Candida albicans Biofilms. Microorganisms 2021, 9, 500. [Google Scholar] [CrossRef]
- Sun, W.; Shi, S.; Chen, J.; Zhao, W.; Chen, T.; Li, G.; Zhang, K.; Yu, B.; Liu, D.; Chen, Y.; et al. Blue Light Signaling Regulates Escherichia coli W1688 Biofilm Formation and l-Threonine Production. Microbiol. Spectr. 2022, 10, e0246022. [Google Scholar] [CrossRef]
- Halstead, F.D.; Thwaite, J.E.; Burt, R.; Laws, T.R.; Raguse, M.; Moeller, R.; Webber, M.A.; Oppenheim, B.A. Antibacterial Activity of Blue Light against Nosocomial Wound Pathogens Growing Planktonically and as Mature Biofilms. Appl. Environ. Microbiol. 2016, 82, 4006–4016. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-H.; Lee, J.-K.; Um, H.-S.; Chang, B.-S.; Lee, S.-Y.; Lee, M.-K. Phototoxic effect of blue light on the planktonic and biofilm state of anaerobic periodontal pathogens. J. Periodontal. Implant Sci. 2013, 43, 72–78. [Google Scholar] [CrossRef]
- Martinez, L.R.; Casadevall, A. Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl. Environ. Microbiol. 2007, 73, 4592–4601. [Google Scholar] [CrossRef]
- Prado, D.B.d.; Szczerepa, M.M.D.A.; Capeloto, O.A.; Astrath, N.G.C.; Santos, N.C.A.D.; Previdelli, I.T.S.; Nakamura, C.V.; Mikcha, J.M.G.; de Abreu Filho, B.A. Effect of ultraviolet (UV-C) radiation on spores and biofilms of Alicyclobacillus spp. in industrialized orange juice. Int. J. Food Microbiol. 2019, 305, 108238. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, S.; Soteyome, T.; Bai, C.; Liu, J.; Wang, Z.; Kjellerup, B.V.; Xu, Z. Intense pulsed light for inactivation of foodborne gram-positive bacteria in planktonic cultures and bacterial biofilms. LWT-Food Sci. Technol. 2021, 152, 112374. [Google Scholar] [CrossRef]
- Li, X.; Gu, N.; Ye, Y.; Lan, H.; Peng, F.; Peng, G. Intense pulsed light for inactivating planktonic and biofilm molds in food. Front. Microbiol. 2022, 13, 1104875. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-G.; Jiang, L.; Lin, S.; Jin, W.-G.; Gu, Q.; Chen, Y.-W.; Zhang, K.; Ettelaie, R. Ultra-efficient antimicrobial photodynamic inactivation system based on blue light and octyl gallate for ablation of planktonic bacteria and biofilms of Pseudomonas fluorescens. Food Chem. 2022, 374, 131585. [Google Scholar] [CrossRef]
- Ma, B.; Seyedi, S.; Wells, E.; McCarthy, D.; Crosbie, N.; Linden, K.G. Inactivation of biofilm-bound bacterial cells using irradiation across UVC wavelengths. Water Res. 2022, 217, 118379. [Google Scholar] [CrossRef]
- Angarano, V.; Akkermans, S.; Smet, C.; Chieffi, A.; van Impe, J.F.M. The potential of violet, blue, green and red light for the inactivation of P. fluorescens as planktonic cells, individual cells on a surface and biofilms. Food Bioprod. Process. 2020, 124, 184–195. [Google Scholar] [CrossRef]
- Jones, C.C.; Valdeig, S.; Sova, R.M.; Weiss, C.R. Inside-out Ultraviolet-C Sterilization of Pseudomonas aeruginosa Biofilm In Vitro. Photochem. Photobiol. 2016, 92, 835–841. [Google Scholar] [CrossRef]
- Liu, X.; Chang, Q.; Ferrer-Espada, R.; Leanse, L.G.; Goh, X.S.; Wang, X.; Gelfand, J.A.; Dai, T. Photoinactivation of Moraxella catarrhalis Using 405-nm Blue Light: Implications for the Treatment of Otitis Media. Photochem. Photobiol. 2020, 96, 611–617. [Google Scholar] [CrossRef]
- Córdova-Alcántara, I.M.; Venegas-Cortés, D.L.; Martínez-Rivera, M.Á.; Pérez, N.O.; Rodriguez-Tovar, A.V. Biofilm characterization of Fusarium solani keratitis isolate: Increased resistance to antifungals and UV light. J. Microbiol. 2019, 57, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Pezzoni, M.; Pizarro, R.A.; Costa, C.S. Protective role of extracellular catalase (KatA) against UVA radiation in Pseudomonas aeruginosa biofilms. J. Photochem. Photobiol. B 2014, 131, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Chen, W.; He, J.; Luo, X.; Mei, Y.; Zhang, B. Effective management of pre-existing biofilms using UV-LED through inactivation, disintegration and peeling. J. Hazard. Mater. 2024, 486, 136925. [Google Scholar] [CrossRef]
- Palma, F.; Díaz-Navarro, M.; Visedo, A.; Sanz-Ruíz, P.; Brandi, G.; Schiavano, G.F.; Guembe, M. Assessment of the anti-biofilm effect of UV-C irradiation (254 nm) against healthcare associated infections related microorganisms. Front. Microbiol. 2025, 16, 1570334. [Google Scholar] [CrossRef]
- Plattfaut, I.; Demir, E.; Fuchs, P.C.; Schiefer, J.L.; Stürmer, E.K.; Brüning, A.K.E.; Opländer, C. Characterization of Blue Light Treatment for Infected Wounds: Antibacterial Efficacy of 420, 455, and 480 nm Light-Emitting Diode Arrays Against Common Skin Pathogens Versus Blue Light-Induced Skin Cell Toxicity. Photobiomodulation Photomed. Laser Surg. 2021, 39, 339–348. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, S.; Xie, Y.; Wang, M.; Cai, T.; Li, J.; Guo, D.; Zhao, L.; Xu, Y.; Liang, S.; et al. Inactivation of Pseudomonas aeruginosa Biofilms by 405-Nanometer-Light-Emitting Diode Illumination. Appl. Environ. Microbiol. 2020, 86, e00092-20. [Google Scholar] [CrossRef]
- Dos Anjos, C.; Leanse, L.G.; Liu, X.; Miranda, H.V.; Anderson, R.R.; Dai, T. Antimicrobial Blue Light for Prevention and Treatment of Highly Invasive Vibrio vulnificus Burn Infection in Mice. Front. Microbiol. 2022, 13, 932466. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.; Oliveira, I.M.; Correia, L.; Gomes, I.B.; Sousa, C.A.; Braga, D.F.O.; Simões, M. Far-UV-C irradiation promotes synergistic bactericidal action against adhered cells of Escherichia coli and Staphylococcus epidermidis. Sci. Total Environ. 2024, 917, 170352. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, J.; Fujii, T.; Fukuda, S.; Yoneda, S.; Tamura, Y.; Shimizu, Y.; Yanai, A.; Kobayashi, Y.; Harada, K.; Kawasaki, K.; et al. Far-ultraviolet irradiation at 222 nm destroys and sterilizes the biofilms formed by periodontitis pathogens. J. Microbiol. Immunol. Infect. 2024, 57, 533–545. [Google Scholar] [CrossRef]
- Argyraki, A.; Markvart, M.; Stavnsbjerg, C.; Kragh, K.N.; Ou, Y.; Bjørndal, L.; Bjarnsholt, T.; Petersen, P.M. UV light assisted antibiotics for eradication of in vitro biofilms. Sci. Rep. 2018, 8, 16360. [Google Scholar] [CrossRef] [PubMed]
- Bernbom, N.; Vogel, B.F.; Gram, L. Listeria monocytogenes survival of UV-C radiation is enhanced by presence of sodium chloride, organic food material and by bacterial biofilm formation. Int. J. Food Microbiol. 2011, 147, 69–73. [Google Scholar] [CrossRef]
- Kim, M.; Park, S.Y.; Ha, S.-D. Synergistic effect of a combination of ultraviolet–C irradiation and sodium hypochlorite to reduce Listeria monocytogenes biofilms on stainless steel and eggshell surfaces. Food Control 2016, 70, 103–109. [Google Scholar] [CrossRef]
- Srey, S.; Park, S.Y.; Jahid, I.K.; Ha, S.-D. Reduction effect of the selected chemical and physical treatments to reduce L. monocytogenes biofilms formed on lettuce and cabbage. Food Res. Int. 2014, 62, 484–491. [Google Scholar] [CrossRef]
- Tajik, H.; Naghili, H.; Ghasemmahdi, H.; Moradi, M.; Badali, A. Effects of Zataria multiflora boiss essential oil, ultraviolet radiation and their combination on Listeria monocytogenes biofilm in a simulated industrial model. Int. J. Food Sci. Technol. 2015, 50, 2113–2119. [Google Scholar] [CrossRef]
- Roy, P.K.; Mizan, M.F.R.; Hossain, M.I.; Han, N.; Nahar, S.; Ashrafudoulla, M.; Toushik, S.H.; Shim, W.-B.; Kim, Y.-M.; Ha, S.-D. Elimination of Vibrio parahaemolyticus biofilms on crab and shrimp surfaces using ultraviolet C irradiation coupled with sodium hypochlorite and slightly acidic electrolyzed water. Food Control 2021, 128, 108179. [Google Scholar] [CrossRef]
- Bae, Y.-M.; Lee, S.-Y. Inhibitory effects of UV treatment and a combination of UV and dry heat against pathogens on stainless steel and polypropylene surfaces. J. Food Sci. 2012, 77, M61–M64. [Google Scholar] [CrossRef] [PubMed]
- Tingpej, P.; Tiengtip, R.; Kondo, S. Decontamination Efficacy of Ultraviolet Radiation against Biofilms of Common Nosocomial Bacteria. J. Med. Assoc. Thai. 2015, 98, 582–588. [Google Scholar] [PubMed]
- Murray, K.E.; Manitou-Alvarez, E.I.; Inniss, E.C.; Healy, F.G.; Bodour, A.A. Assessment of oxidative and UV-C treatments for inactivating bacterial biofilms from groundwater wells. Front. Environ. Sci. Eng. 2015, 9, 39–49. [Google Scholar] [CrossRef]
- Bak, J.; Ladefoged, S.D.; Tvede, M.; Begovic, T.; Gregersen, A. Dose requirements for UVC disinfection of catheter biofilms. Biofouling 2009, 25, 289–296. [Google Scholar] [CrossRef]
- Binns, R.; Li, W.; Wu, C.D.; Campbell, S.; Knoernschild, K.; Yang, B. Effect of Ultraviolet Radiation on Candida albicans Biofilm on Poly(methylmethacrylate) Resin. J. Prosthodont. 2020, 29, 686–692. [Google Scholar] [CrossRef]
- El-Azizi, M.; Khardori, N. Efficacy of ultraviolet C light at sublethal dose in combination with antistaphylococcal antibiotics to disinfect catheter biofilms of methicillin-susceptible and methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis in vitro. Infect. Drug Resist. 2016, 9, 181–189. [Google Scholar] [CrossRef]
- Silva-Espinoza, B.A.; Palomares-Navarro, J.J.; Tapia-Rodriguez, M.R.; Cruz-Valenzuela, M.R.; González-Aguilar, G.A.; Silva-Campa, E.; Pedroza-Montero, M.; Almeida-Lopes, M.; Miranda, R.; Ayala-Zavala, J.F. Combination of ultraviolet light-C and clove essential oil to inactivate Salmonella Typhimurium biofilms on stainless steel. J. Food Saf. 2020, 40, e12788. [Google Scholar] [CrossRef]
- Jahid, I.K.; Han, N.R.; Srey, S.; Ha, S.-D. Competitive interactions inside mixed-culture biofilms of Salmonella Typhimurium and cultivable indigenous microorganisms on lettuce enhance microbial resistance of their sessile cells to ultraviolet C (UV-C) irradiation. Food Res. Int. 2014, 55, 445–454. [Google Scholar] [CrossRef]
- Epelle, E.I.; Amaeze, N.; Mackay, W.G.; Yaseen, M. Efficacy of gaseous ozone and UVC radiation against Candida auris biofilms on polystyrene surfaces. J. Environ. Chem. Eng. 2024, 12, 113862. [Google Scholar] [CrossRef]
- Malateaux, G.; Salazar-Gamarra, R.E.; de Souza Silva, J.; Pecorari, V.G.A.; Suffredini, I.B.; Netto, F.P.; Neves, C.R.; Rodrigues de Souza, I.; de Mello Mesquita, A.M.; Dib, L.L. Ultraviolet C as a method of disinfecting medical silicone used in facial prostheses: An in vitro study—Part 2. J. Prosthet. Dent. 2024, 132, 844.e1–844.e6. [Google Scholar] [CrossRef]
- Richard, K.N.; Palmer, A.; Swain, G.; Hunsucker, K.Z. Assessing the impact of UV-C exposure on pre-existing cultured marine diatom biofilms. Biofilm 2025, 9, 100285. [Google Scholar] [CrossRef] [PubMed]
- Pousty, D.; Ma, B.; Mathews, C.; Halanur, M.; Mamane, H.; Linden, K.G. Biofilm inactivation using LED systems emitting germicidal UV and antimicrobial blue light. Water Res. 2024, 267, 122449. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.H.; Mayer, B.K.; Marshall, C.W.; Hristova, K.R. UV-LED inactivation of S. aureus and A. baumannii dual-species biofilm: Insights into the role of interspecies interactions. J. Environ. Chem. Eng. 2025, 13, 115689. [Google Scholar] [CrossRef]
- Bak, J.; Ladefoged, S.D.; Tvede, M.; Begovic, T.; Gregersen, A. Disinfection of Pseudomonas aeruginosa biofilm contaminated tube lumens with ultraviolet C light emitting diodes. Biofouling 2010, 26, 31–38. [Google Scholar] [CrossRef]
- Gora, S.L.; Rauch, K.D.; Ontiveros, C.C.; Stoddart, A.K.; Gagnon, G.A. Inactivation of biofilm-bound Pseudomonas aeruginosa bacteria using UVC light emitting diodes (UVC LEDs). Water Res. 2019, 151, 193–202. [Google Scholar] [CrossRef]
- Marasini, S.; Dean, S.J.; Swift, S.; Hussan, J.R.; Craig, J.P. In vitro anti-biofilm efficacy of therapeutic low dose 265 nm UVC. J. Photochem. Photobiol. B 2025, 263, 113091. [Google Scholar] [CrossRef] [PubMed]
- Argyraki, A.; Markvart, M.; Bjørndal, L.; Bjarnsholt, T.; Petersen, P.M. Inactivation of Pseudomonas aeruginosa biofilm after ultraviolet light-emitting diode treatment: A comparative study between ultraviolet C and ultraviolet B. J. Biomed. Opt. 2017, 22, 65004. [Google Scholar] [CrossRef]
- Prasad, A.; Roopesh, M.S. Bacterial biofilm reduction by 275 and 455 nm light pulses emitted from light emitting diodes. J. Food Saf. 2023, 43. [Google Scholar] [CrossRef]
- Labadie, M.; Marchal, F.; Merbahi, N.; Girbal-Neuhauser, E.; Fontagné-Faucher, C.; Marcato-Romain, C.-E. Cell density and extracellular matrix composition mitigate bacterial biofilm sensitivity to UV-C LED irradiation. Appl. Microbiol. Biotechnol. 2024, 108, 286. [Google Scholar] [CrossRef] [PubMed]
- Pezzoni, M.; Pizarro, R.A.; Costa, C.S. Exposure to low doses of UVA increases biofilm formation in Pseudomonas aeruginosa. Biofouling 2018, 34, 673–684. [Google Scholar] [CrossRef]
- Li, J.; Hirota, K.; Yumoto, H.; Matsuo, T.; Miyake, Y.; Ichikawa, T. Enhanced germicidal effects of pulsed UV-LED irradiation on biofilms. J. Appl. Microbiol. 2010, 109, 2183–2190. [Google Scholar] [CrossRef] [PubMed]
- Angarano, V.; Smet, C.; Akkermans, S.; Watt, C.; Chieffi, A.; van Impe, J.F.M. Visible Light as an Antimicrobial Strategy for Inactivation of Pseudomonas fluorescens and Staphylococcus epidermidis Biofilms. Antibiotics 2020, 9, 171. [Google Scholar] [CrossRef]
- Maknuna, L.; van Tran, N.; Lee, B.-I.; Kang, H.W. Inhibitory effect of 405 nm laser light on bacterial biofilm in urethral stent. Sci. Rep. 2023, 13, 3908. [Google Scholar] [CrossRef]
- Buchovec, I.; Vyčaitė, E.; Badokas, K.; Sužiedelienė, E.; Bagdonas, S. Application of Antimicrobial Photodynamic Therapy for Inactivation of Acinetobacter baumannii Biofilms. Int. J. Mol. Sci. 2022, 24, 722. [Google Scholar] [CrossRef] [PubMed]
- Astuti, S.D.; Hafidiana; Rulaningtyas, R.; Abdurachman; Putra, A.P.; Samian; Arifianto, D. The efficacy of photodynamic inactivation with laser diode on Staphylococcus aureus biofilm with various ages of biofilm. Infect. Dis. Rep. 2020, 12, 8736. [Google Scholar] [CrossRef]
- Halstead, F.D.; Hadis, M.A.; Marley, N.; Brock, K.; Milward, M.R.; Cooper, P.R.; Oppenheim, B.; Palin, W.M. Violet-Blue Light Arrays at 405 Nanometers Exert Enhanced Antimicrobial Activity for Photodisinfection of Monomicrobial Nosocomial Biofilms. Appl. Environ. Microbiol. 2019, 85, e01346-19. [Google Scholar] [CrossRef]
- Schafer, M.E.; McNeely, T. Combining Visible Light and Non-Focused Ultrasound Significantly Reduces Propionibacterium acnes Biofilm While Having Limited Effect on Host Cells. Microorganisms 2021, 9, 929. [Google Scholar] [CrossRef]
- Leanse, L.G.; Zeng, X.; Dai, T. Potentiated antimicrobial blue light killing of methicillin resistant Staphylococcus aureus by pyocyanin. J. Photochem. Photobiol. B 2021, 215, 112109. [Google Scholar] [CrossRef]
- Giannelli, M.; Landini, G.; Materassi, F.; Chellini, F.; Antonelli, A.; Tani, A.; Nosi, D.; Zecchi-Orlandini, S.; Rossolini, G.M.; Bani, D. Effects of photodynamic laser and violet-blue led irradiation on Staphylococcus aureus biofilm and Escherichia coli lipopolysaccharide attached to moderately rough titanium surface: In vitro study. Lasers Med. Sci. 2017, 32, 857–864. [Google Scholar] [CrossRef]
- Ong, J.; Godfrey, R.; Nazarian, A.; Tam, J.; Drake, L.; Isaacson, B.; Pasquina, P.; Williams, D. Antimicrobial blue light as a biofilm management therapy at the skin-implant interface in an ex vivo percutaneous osseointegrated implant model. J. Orthop. Res. 2023, 41, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Leanse, L.G.; Goh, X.S.; Dai, T. Quinine Improves the Fungicidal Effects of Antimicrobial Blue Light: Implications for the Treatment of Cutaneous Candidiasis. Lasers Surg. Med. 2020, 52, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Espada, R.; Liu, X.; Goh, X.S.; Dai, T. Antimicrobial Blue Light Inactivation of Polymicrobial Biofilms. Front. Microbiol. 2019, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, K.; Maclean, M.; Timoshkin, I.V.; Endarko, E.; MacGregor, S.J.; Anderson, J.G. Photoinactivation of bacteria attached to glass and acrylic surfaces by 405 nm light: Potential application for biofilm decontamination. Photochem. Photobiol. 2013, 89, 927–935. [Google Scholar] [CrossRef]
- Halstead, F.D.; Ahmed, Z.; Bishop, J.R.B.; Oppenheim, B.A. The potential of visible blue light (405 nm) as a novel decontamination strategy for carbapenemase-producing enterobacteriaceae (CPE). Antimicrob. Resist. Infect. Control 2019, 8, 14. [Google Scholar] [CrossRef]
- Tsutsumi-Arai, C.; Arai, Y.; Terada-Ito, C.; Takebe, Y.; Ide, S.; Umeki, H.; Tatehara, S.; Tokuyama-Toda, R.; Wakabayashi, N.; Satomura, K. Effectiveness of 405-nm blue LED light for degradation of Candida biofilms formed on PMMA denture base resin. Lasers Med. Sci. 2019, 34, 1457–1464. [Google Scholar] [CrossRef]
- Tsutsumi-Arai, C.; Arai, Y.; Terada-Ito, C.; Imamura, T.; Tatehara, S.; Ide, S.; Wakabayashi, N.; Satomura, K. Microbicidal effect of 405-nm blue LED light on Candida albicans and Streptococcus mutans dual-species biofilms on denture base resin. Lasers Med. Sci. 2022, 37, 857–866. [Google Scholar] [CrossRef]
- Tsutsumi-Arai, C.; Arai, Y.; Terada-Ito, C.; Imamura, T.; Tatehara, S.; Ide, S.; Shirakawa, J.; Wakabayashi, N.; Satomura, K. Inhibitory effect of 405-nm blue LED light on the growth of Candida albicans and Streptococcus mutans dual-species biofilms on denture base resin. Lasers Med. Sci. 2022, 37, 2311–2319. [Google Scholar] [CrossRef]
- Grangeteau, C.; Lebleux, M.; David, V.; Rousseaux, S.; Alexandre, H.; Beney, L.; Dupont, S. Ultra-high irradiance (UHI) blue light treatment: A promising method for inactivation of the wine spoilage yeast Brettanomyces bruxellensis. LWT-Food Sci. Technol. 2024, 117038. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Dev Kumar, G.; Hur, M.; Diez-Gonzalez, F. Inactivation of dried cells and biofilms of Listeria monocytogenes by exposure to blue light at different wavelengths and the influence of surface materials. Appl. Environ. Microbiol. 2023, 89, e0114723. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, X.; Chen, J.; Amin, R.; Lu, M.; Bhayana, B.; Zhao, J.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; et al. Antimicrobial Blue Light Inactivation of Gram-Negative Pathogens in Biofilms: In Vitro and In Vivo Studies. J. Infect. Dis. 2016, 213, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Treghini, C.; Insero, G.; Dell’Accio, A.; Micieli, M.; Riccobono, E.; Valzano, F.; Fusi, F.; Rossolini, G.M.; Pallecchi, L.; Romano, G. In vitro photoinactivation of Pseudomonas aeruginosa and Staphylococcus aureus biofilm by a novel multi-dose LED-based illumination method. Photodiagnosis Photodyn. Ther. 2024, 46, 104153. [Google Scholar] [CrossRef]
- de Sousa, D.L.; Lima, R.A.; Zanin, I.C.; Klein, M.I.; Janal, M.N.; Duarte, S. Effect of Twice-Daily Blue Light Treatment on Matrix-Rich Biofilm Development. PLoS ONE 2015, 10, e0131941. [Google Scholar] [CrossRef]
- Alves, F.; Nakada, P.J.T.; Marques, M.J.d.A.M.; Rea, L.d.C.; Cortez, A.A.; Pellegrini, V.d.O.A.; Polikarpov, I.; Kurachi, C. Complete photodynamic inactivation of Pseudomonas aeruginosa biofilm with use of potassium iodide and its comparison with enzymatic pretreatment. J. Photochem. Photobiol. B 2024, 257, 112974. [Google Scholar] [CrossRef]
- Fontana, C.R.; Song, X.; Polymeri, A.; Goodson, J.M.; Wang, X.; Soukos, N.S. The effect of blue light on periodontal biofilm growth in vitro. Lasers Med. Sci. 2015, 30, 2077–2086. [Google Scholar] [CrossRef]
- Rosa, L.P.; da Silva, F.C.; Viana, M.S.; Meira, G.A. In vitro effectiveness of 455-nm blue LED to reduce the load of Staphylococcus aureus and Candida albicans biofilms in compact bone tissue. Lasers Med. Sci. 2016, 31, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, Z.; Peng, Y.; Guo, Y.; Yao, M.; Dong, J. Application of 460 nm visible light for the elimination of Candida albicans in vitro and in vivo. Mol. Med. Rep. 2018, 18, 2017–2026. [Google Scholar] [CrossRef]
- Moradi, M.; Fazlyab, M.; Pourhajibagher, M.; Chiniforush, N. Antimicrobial action of photodynamic therapy on Enterococcus faecalis biofilm using curing light, curcumin and riboflavin. Aust. Endod. J. 2022, 48, 274–282. [Google Scholar] [CrossRef]
- Cohen-Berneron, J.; Steinberg, D.; Featherstone, J.D.B.; Feuerstein, O. Sustained effects of blue light on Streptococcus mutans in regrown biofilm. Lasers Med. Sci. 2016, 31, 445–452. [Google Scholar] [CrossRef]
- Steinberg, D.; Moreinos, D.; Featherstone, J.; Shemesh, M.; Feuerstein, O. Genetic and physiological effects of noncoherent visible light combined with hydrogen peroxide on Streptococcus mutans in biofilm. Antimicrob. Agents Chemother. 2008, 52, 2626–2631. [Google Scholar] [CrossRef]
- Shany-Kdoshim, S.; Polak, D.; Houri-Haddad, Y.; Feuerstein, O. Killing mechanism of bacteria within multi-species biofilm by blue light. J. Oral Microbiol. 2019, 11, 1628577. [Google Scholar] [CrossRef]
- Chebath-Taub, D.; Steinberg, D.; Featherstone, J.D.B.; Feuerstein, O. Influence of blue light on Streptococcus mutans re-organization in biofilm. J. Photochem. Photobiol. B 2012, 116, 75–78. [Google Scholar] [CrossRef]
- Vaknin, M.; Steinberg, D.; Featherstone, J.D.; Feuerstein, O. Exposure of Streptococcus mutans and Streptococcus sanguinis to blue light in an oral biofilm model. Lasers Med. Sci. 2020, 35, 709–718. [Google Scholar] [CrossRef]
- Liang, J.; Huang, T.Y.; Li, X.; Gao, Y. Germicidal effect of intense pulsed light on Pseudomonas aeruginosa in food processing. Front. Microbiol. 2023, 14, 1247364. [Google Scholar] [CrossRef]
- Garvey, M.; Rabbitt, D.; Stocca, A.; Rowan, N. Pulsed ultraviolet light inactivation of Pseudomonas aeruginosa and Staphylococcus aureus biofilms. Water Environ. J. 2015, 29, 36–42. [Google Scholar] [CrossRef]
- Montgomery, N.L.; Banerjee, P. Inactivation of Escherichia coli O157:H7 and Listeria monocytogenes in biofilms by pulsed ultraviolet light. BMC Res. Notes 2015, 8, 235. [Google Scholar] [CrossRef]
- Garvey, M.; Andrade Fernandes, J.P.; Rowan, N. Pulsed light for the inactivation of fungal biofilms of clinically important pathogenic Candida species. Yeast 2015, 32, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Chick, H. An Investigation of the Laws of Disinfection. J. Hyg. 1908, 8, 92–158. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.E. A Note on the Variation of the Rate of Disinfection with Change in the Concentration of the Disinfectant. J. Hyg. 1908, 8, 536–542. [Google Scholar] [CrossRef] [PubMed]
| Microorganism | Abbreviation | Taxonomic Class | Comments |
|---|---|---|---|
| Bacteria | |||
| Actinomyces naeslundii | A. naeslundii | Actinomycetes | GP, anaerobe or microaerophilic |
| Aeromonas australiensis | A. australiensis | Gammaproteobacteria | GN, anaerobe |
| Acinetobacter baumannii | A. baumannii | Gammaproteobacteria | GN, |
| Aeromonas hydrophilia | A. hydrophilia | Gammaproteobacteria | GN, facultative anaerobe |
| Aggregatibacter actinomycetemcomitans | A. actinomycetem-comitans | Gammaproteobacteria | GN, facultative anaerobe |
| Alicyclobacillus acidocaldarius | A. acidocaldarius | Bacilli | GP, strict aerobic, ESF |
| Alicyclobacillus acidoterrestris | A. acidoterrestris | Bacilli | GP, strict aerobic, ESF |
| Alicyclobacillus cycloheptanicus | A. cycloheptanicus | Bacilli | GP, strict aerobic, ESF |
| Alicyclobacillus herbarius | A. herbarius | Bacilli | GP, strict aerobic, ESF |
| Bacillus cereus | B. cereus | Bacilli | GP, aerobic or facultative anaerobe, ESF |
| Bacillus thuringiensis | B. thuringiensis | Bacilli | GP, aerobic, ESF |
| Burkholderia multivorans | B. multivorans | Betaproteobacteria | GN, aerobic, |
| Cupriavidus metallidurans | C. metallidurans | Betaproteobacteria | GN, aerobic |
| Enterococcus faecalis | E. faecalis | Bacilli | GP, facultative anaerobe |
| Escherichia coli | E. coli | Gammaproteobacteria | GN, facultative anaerobe |
| Flavobacterium breve | F. breve | Flavobacteriia | GN, strict aerobic |
| Fusobacterium nucleatum | F. nucleatum | Fusobacteriia | GN, anaerobe |
| Klebsiella oxytoca | K. oxytoca | Gammaproteobacteria | GN, facultative anaerobe |
| Klebsiella pneumoniae | K. pneumoniae | Gammaproteobacteria | GN, facultative anaerobe |
| Lactobacillus brevis | L. brevis | Bacilli | GP, facultative anaerobe |
| Leuconostoc citreum | L. citreum | Bacilli | GP, facultative anaerobe |
| Listeria monocytogenes | L. monocytogenes | Bacilli | GP, facultative anaerobe |
| Methylobacterium fujisawaense | M.fujisawaense | Alphaproteobacteria | GN, facultative anaerobe |
| Moraxella catarrhalis | M. catarrhalis | Gammaproteobacteria | GN, aerobic |
| Pediococcus acidilacti | P. acidilacti | Bacilli | GP, facultative anaerobe |
| Porphyromonas gingivalis | P. gingivalis | Bacterioidia | GN, anaerobe |
| Propionibacterium acnes 1 | P. acnes | Actinomycetes | GP, aerotolerant, |
| Proteus mirabilis | P. mirabilis | Gammaproteobacteria | GN, facultative anaerobe |
| Pseudomonas aeruginosa | P. aeruginosa | Gammaproteobacteria | GN, facultative anaerobe |
| Pseudomonas fluorescens | P. fluorescens | Gammaproteobacteria | GN, facultative anaerobe |
| Ralstonia insidiosa | R. insidiosa | Betaproteobacteria | GN, aerobic |
| Salmonella Typhimurium | S. Typhimurium | Gammaproteobacteria | GN, facultative anaerobe |
| Staphylococcus aureus | S. aureus | Bacilli | GP, facultative anaerobe |
| Staphylococcus epidermis | S. epidermis | Bacilli | GP, facultative anaerobe |
| Streptococcus mutans | S. mutans | Bacilli | GP, facultative anaerobe |
| Streptococcus sanguinis | S. sanguinis | Bacilli | GP, facultative anaerobe |
| Vibrio parahaemolyticus | V. parahaemo-lyticus | Gammaproteobacteria | GN, facultative anaerobe |
| Vibrio vulnificus | V. vulnificus | Gammaproteobacteria | GN, facultative anaerobe |
| Fungi | |||
| Aspergillus niger | A. niger | Eurotiomycetes | Aerobic |
| Brettanomyces bruxellensis | B. bruxellensis | Pichiomycetes | Facultative anaerobe |
| Candida albicans | C. albicans | Pichiomycetes | Facultative anaerobe |
| Candida auris 2 | C. auris | Pichiomycetes | Facultative anaerobe |
| Candida glabrata 3 | C. glabrata | Saccharomycetales | Facultative anaerobe |
| Candida parapsilosis | C. parapsilosis | Pichiomycetes | Anaerobe |
| Cryptococcus neoformans | C. neoformans | Tremellomycetes | Obligate aerobic |
| Fusarium solani | F. solani | Sordariomycetes | Facultative anaerobe |
| Penicillium glaucum | P. glaucum | Eurotiomycetes | Facultative anaerobe |
| Algae | |||
| Navicula incerta | N. incerta | Bacillariophyceae | |
| RMSE for: | “Log-Lin” Bigelow 1920 [13] | “Log-Lin + Tail” Geeraerd 2005 [15] | “Weibull” Mafart 2002 [14] |
|---|---|---|---|
| Pseudomonas aeruginosa | 1.647 | 1.09 | 1.02 |
| Escherichia coli | 2.121 | 1.544 | 1.533 |
| Staphylococcus aureus | 2.078 | 0.895 | 0.94 |
| Salmonella Typhimurium | 2.592 | 1.59 | 1.57 |
| Listeria monocytogenes | 0.9854 | 1.00 | 0.9433 |
| Gram+ bacteria | 1.651 | 1.104 | 1.115 |
| Gram- bacteria | 1.986 | 1.727 | 1.261 |
| fungi | 2.053 | 1.414 | 0.911 |
| multi-species biofilm | 2.479 | 1.319 | 1.41 |
| RMSE for: | “Log-Lin” Bigelow 1920 [13] | “Log-Lin + Tail” Geeraerd 2005 [15] | “Weibull” Mafart 2002 [14] |
|---|---|---|---|
| P. aeruginosa | 1.294 | 1.326 | 1.293 |
| E. coli | 1.033 | 1.27 | 1.076 |
| A. baumannii | 1.266 | 1.82 | 1.267 |
| S. aureus | 0.8005 | 0.8185 | 0.8003 |
| L. monocytogenes | 1.093 | 0.84 | 0.6989 |
| C. albicans | 0.7538 | 0.97 | 0.7496 |
| Gram+ bacteria | 1.578 | 0.907 | 0.9316 |
| Gram- bacteria | 1.521 | 1.308 | 1.398 |
| fungi | 1.546 | 1.341 | 1.335 |
| multi-species biofilm | 0.1343 | 0.155 | 0.1404 |
| Gram+ Bacteria | Gram- Bacteria | Fungi | Multi-Species | |
|---|---|---|---|---|
| Gram+ bacteria | ns PT: ns, HL: ns, RM: ns | ssd PT: ns, HL: ssd, RM: ssd | ssd PT: ns, HL: ssd, RM: ssd | |
| Gram- bacteria | ns PT: ns, HL: ns, RM: ns | ns PT: ns, HL: ns, RM: ns | ns PT: ns, HL: ns, RM: ns | |
| fungi | ssd PT: ns, HL: ssd, RM: ssd | ns PT: ns, HL: ns, RM: ns | ns PT: ns, HL: ns, RM: ns | |
| multi-species | ssd PT: ns, HL: ssd, RM: ssd | ns PT: ns, HL: ns, RM: ns | ns PT: ns, HL: ns, RM: ns |
| Gram+ Bacteria | Gram- Bacteria | Fungi | Multi-Species | |
|---|---|---|---|---|
| Gram+ bacteria | ssd PT: ns, HL: ssd, RM: ssd | ns PT: ns, HL: ns, RM: ns | ns PT: ns, HL: ns, RM: ns | |
| Gram- bacteria | ssd PT: ns, HL: ssd, RM: ssd | ssd PT: ssd, HL: ssd, RM: ssd | ns PT: ns, HL: ns, RM: ns | |
| fungi | ns PT: ns, HL: ns, RM: ns | ssd PT: ssd, HL: ssd, RM: ssd | ns PT: ns, HL: ns, RM: ns | |
| multi-species | ns PT: ns, HL: ns, RM: ns | ns PT: ns, HL: ns, RM: ns | ns PT: ns, HL: ns, RM: ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hessling, M.; Meulebroeck, W.; Alsanius, B. A Collection and Analysis of Simplified Data for a Better Understanding of the Complex Process of Biofilm Inactivation by Ultraviolet and Visible Irradiation. Microorganisms 2025, 13, 2048. https://doi.org/10.3390/microorganisms13092048
Hessling M, Meulebroeck W, Alsanius B. A Collection and Analysis of Simplified Data for a Better Understanding of the Complex Process of Biofilm Inactivation by Ultraviolet and Visible Irradiation. Microorganisms. 2025; 13(9):2048. https://doi.org/10.3390/microorganisms13092048
Chicago/Turabian StyleHessling, Martin, Wendy Meulebroeck, and Beatrix Alsanius. 2025. "A Collection and Analysis of Simplified Data for a Better Understanding of the Complex Process of Biofilm Inactivation by Ultraviolet and Visible Irradiation" Microorganisms 13, no. 9: 2048. https://doi.org/10.3390/microorganisms13092048
APA StyleHessling, M., Meulebroeck, W., & Alsanius, B. (2025). A Collection and Analysis of Simplified Data for a Better Understanding of the Complex Process of Biofilm Inactivation by Ultraviolet and Visible Irradiation. Microorganisms, 13(9), 2048. https://doi.org/10.3390/microorganisms13092048







