Abstract
Six aromatic plants (Lavandula pedunculata subsp. sampaioana, Lavandula stoechas subsp. luisieri, Mentha × piperita, Origanum vulgare subsp. virens, Thymus mastichina, and Thymus zygis subsp. sylvestris) were analyzed to evaluate their essential oil (EO) yield, chemical composition, antioxidant activity, and antifungal capacity against two mold species, green mold (Penicillium digitatum) and blue mold (Penicillium italicum). The antioxidant activity was evaluated using the ABTS and DPPH methods, and the antifungal activity was determined using the disk diffusion method. The results of the antioxidant activity tests showed that the essential oil of Th. zygis subsp. sylvestris has the highest value for the ABTS method (161.70 ± 0.15 mM TROLOX eq. and 864.20 ± 0.81 g TROLOX eq/g EO) and the L. stoechas subsp. luisieri essential oil in the DPPH method (33.91 ± 1.21 mM TROLOX eq. and 184.99 ± 6.58 g TROLOX eq/g EO). Furthermore, the essential oils with lower antioxidant activity were L. pedunculata subsp. sampaioana for the ABTS method (3.84 ± 0.26 mM TROLOX eq. and 20.79 ± 1.41 g TROLOX eq/g EO) and Th. mastichina for DPPH method (0.96 ± 0.03 mM TROLOX eq. and 5.31 ± 0.16 g TROLOX eq/g EO). Th. zygis subsp. sylvestris exhibited the strongest antifungal activity, with medium inhibition halo values of 60.50 ± 5.77 mm and 54.33 ± 2.93 mm for P. digitatum and P. italicum, respectively.
Keywords:
antifungal activity; antioxidant; essential oil; lavandula; mentha; origanum; penicillium; thymus 1. Introduction
Postharvest fruit and vegetable diseases caused by fungal infections (Aspergillus, Penicillium, Fusarium, Alternaria, …) due to wounds or insect bites produce an elevated loss of food during storage, distribution, and sale [1,2,3,4,5,6,7]. Citrus major sources of postharvest diseases are green mold (Penicillium digitatum (Pers.) Sacc.) and blue mold (Penicillium italicum Wehmer), which cause economic losses of 15–30% and affect 50–90% of production, particularly in developing countries [8,9,10,11,12,13]. Traditionally, several methods based on synthetic chemical fungicides have been developed to reduce post-harvest losses; however, the intensive use of these methods generates resistance, reducing their effectiveness [9,14,15]. Moreover, consumer trends demand products that are free of chemical residues and more environmentally friendly. Together with legislative restrictions on the use of phytosanitary products, this creates the need for new, more effective and environmentally friendly postharvest management. These alternatives include biocontrol strategies involving the use of antagonist yeast or bacteria, immersion in aqueous extracts of medicinal plants or citrus fruits, vaporization of essential oils from medicinal plants, wax coatings containing essential oils or plant extracts, new biopolymers and heat treatments, among others [6,11,16,17,18,19,20,21].
Essential oils and medicinal plant extracts are composed of mixtures of volatile organic compounds. These include alcohols, ethers, oxides, aldehydes, ketones, esters, amines, amides, phenols and heterocycles, as well as mainly terpenes [22,23]. The presence of these compounds varies depending on the plant species and, within the same species, the percentage of each chemical compound is influenced by the plant’s geographical origin, environmental conditions, and the storage process of the plant material and essential oil [24,25,26,27]. Therefore, it is crucial to understand the chemical composition of any essential oil, regardless of the plant species it comes from.
Work is currently underway to identify active ingredients of natural origin that are more environmentally friendly to produce and use. The aim is to develop effective treatments for antibiotic resistance in human and animal health and herbicides for agricultural crops [28,29,30,31]. This has prompted research into the chemical composition of essential oils, their antifungal, antibacterial, and antimicrobial properties, and their potential use in treating respiratory diseases, etc. [32,33,34,35,36].
Studies of the antifungal capacity of medicinal plants extracts or essential oils have reported the ability of fight various fungal infections caused by Aspergillus spp., Candida spp., Cryptococcus spp., Epidermophyton spp., Fusarium spp., Microsporum spp. Penicillium spp., and Trichophyton spp. [37,38,39,40,41]. Several studies have shown that essential oils from species such thyme, oregano, clove, cinnamon or citrus have a high inhibitory capacity against the in vitro growth of fungal colonies from Penicillium species such as P. digitatum and P. italicum) [42,43,44,45,46,47,48,49,50]. The antifungal properties of these essential oils have contributed to the development of new research aimed at preventing post-harvest infections caused by P. digitatum and P. italicum [51,52,53,54,55,56,57,58,59].
The use of essential oils from medicinal plants whose chemical composition includes antifungal compounds (e.g., thymol, carvacrol, terpinen-4-ol, etc. [59]) makes it possible to search for local medicinal species commonly used in traditional medicine that are rich in these kinds of compounds, creating a new local employment opportunity that are more environmentally friendly. For that purpose, the main objective of this research is to evaluate the inhibitory and antioxidant activities of different aromatic plants, native to the SW from the Iberian Peninsula, against two mold species (P. digitatum and P. italicum), which cause postharvest disease in citrus fruits (e.g., oranges).
2. Materials and Methods
2.1. Plant Material, Essential Oil Extraction, and Chemical Characterization of Essential Oils
Aerial parts of six aromatic plants (Lavandula pedunculata subsp. sampaioana (Rozeira) Franco, Lavandula stoechas subsp. luisieri (Rozeira) Rozeira, Mentha × piperita L., Origanum vulgare subsp. virens (Hoffmans. & Link) Bonnier & Layens, Thymus mastichina (L.) L., and Thymus zygis subsp. sylvestris (Hoffmanns. & Link) Cout.) were collected from the experimental crops at Institute of Agrarian Research “La Orden-Valdesequera” (CICYTEX) (near of Guadajira, Spain). Representative samples were collected during the flowering stage, which took place between May and June 2024.
Fresh stems, leaves, and flowers from each specie were cut into small pieces and submitted to hydro-distillation in Clevenger-type apparatus for 2 h. The essential oils (EOs) were stored in amber vial at 4 °C.
The chemical analysis of the essential oils was carried out using a combination of two gas chromatography techniques (GC-FID + GC-MS), chemical compounds were identified by CG-MS and quantified by CG-FID. The analysis was performed on Agilent 8890 GC paired with the 5977B MSD (Mass Selective Detector). Polar column DB-WAX UI (60 m long, 0.25 mm diameter and 0.5 µm film thicknesses) was employed using Helium carrier gas at constant flow of 2 mL/min. Apolar column HP-5MS UI (60 m long, 0.25 mm diameter and 0.25 µm film thicknesses) was employed using Helium carrier gas at constant flow of 1 mL/min. The column temperature started at 50 °C and increased to 240 °C (polar column) and 285 °C (apolar column).
2.2. Antioxidant Activity
The antioxidant activity of each essential oil sample was determined by ABTS and DPPH assay method. The absorbance was measured using a spectrophotometer (Beckman Coulter DU® 730, Beckman Coulter, Inc., Brea, CA, USA).
The standard line from each assay was designed using Trolox (6-hidroxy-2,5,7,8-tetramethylchroman-carboxylic acid) (Sigma-Aldrich 238813, Sigma-Aldrich, Inc., St. Louis, MO, USA) between 1 mM and 2 mM concentration and measured the absorbance at 734 nm (ABTS) and 517 nm (DPPH).
All the essential oil samples were analyzed in triplicate. The sample volume used was 3 mL (2.95 mL from DPPH/ABTS + 50 μL from essential oil sample). The results, from both analyses (ABTS and DPPH) were presented as millimoles (mM) of Trolox equivalents and grams of Trolox equivalents per gram of essential oil, with the main objective of developing a data matrix comparable between each other.
2.2.1. ABTS [2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)] Assay
ABTS assay is based on the ability of molecules to scavenge the free radical of ABTS in comparison with Trolox [60]. Absolute ethanol was used to prepare the working solution of ABTS (Sigma-Aldrich A1888) at a concentration of 7 mM, which was then adjusted to obtain a final absorbance of 0.7 ± 0.02 (at 734 nm). To determine antioxidant activity, the essential oil samples remained in the dark at ambient temperature for 30 min and, thereafter, the absorbance was measured at 734 nm.
2.2.2. DPPH (2,2-Diphenyl-1-picrylhydrazyl) Assay
The DPPH protocol to measure antioxidant activity was based on the description in reference [61]. Methanol (100%) was used as the solvent to prepare a working solution 75 µmol/L of DPPH (Sigma-Aldrich D9132), which was then adjusted to a final absorbance of 0.7 ± 0.02 (at 517 nm). For antioxidant activity determination, the samples remained in the dark at an ambient temperature for 120 min, after which the absorbance was measured at 517 nm.
2.3. In Vitro Antifungal Activity Assay
2.3.1. Fungal Isolation
The fungal species used are P. digitatum and P. italicum, obtained from infected Citrus aurantium L. fruit. The isolation was realized in Petri dishes containing Sabouraud Dextrose agar (6%) and incubated for 7 days at 27 °C ± 1 °C, in complete darkness. The differential isolations were transferred to new Petri dishes containing Sabouraud Dextrose agar and re-sown each week until a pure fungal culture of each species was obtained. Finally, the standardization of the fungal colonies was achieved using a 0.85% saline solution suspension to obtain the 0.5 McFarland standard (1.5 × 108 CFU/mL) [62]. Morphological characterization (macro and microscopic) was performed using dichotomous keys as a reference [63,64,65].
2.3.2. Antifungal Activity–Disk Diffusion Method
The disk diffusion method was used to evaluate the antifungal activity of each of the essential oils [66,67]. The fungal suspension was sown in Petri dishes (90 mm diameter) containing 25 mL of Sabouraud Dextrose Agar. A sterile swab was used to spread the mold suspension (1.5 × 108 CFU/mL) evenly across the surface of the dish to ensure a homogeneous development of the mold. Essential oils were inoculated using a 10 mm diameter filter disk soaked with 25 μL of each essential oil sample and placed in the center of the Petri dish. A control group was included in the study to ensure that the mold growth was unaffected by the essential oil. The control group was processed in the same way as the study group, but with distilled water instead of essential oil.
The study included a control group and a study group with 3 repetitions of each for each species of essential oil. Thus, 12 Petri dishes were used for each essential oil species (3 control dishes + 3 study dishes for each one of the analyzed molds, P. digitatum and P. italicum). The Petri dishes were incubated for 5 days (96 h) in an incubator chamber at 27 °C ± 1 °C in complete darkness and in the normal position (not inverted) to avoid affecting the mold growth. Finally, the Petri dishes were checked, photographed, and measured every 24 h. Measurements were taken by evaluating the inhibitory halo of growth around the filter disk using a caliper.
2.4. Statistical Analysis
Descriptive and inferential statistical analysis were performed using R v 4.3.3 software [68] to determine the relationship between the inhibitory halo of growth results and the antioxidant activity obtained from the samples. The 48 h data from the inhibitory halo of growth were used to develop statistical analysis (to ensure a correct understanding of the data and avoid mixing up inhibition and natural absence of growth). This also ensures the correct effect of the essential oil and prevents it from evaporating during the process.
3. Results
3.1. Essential Oil Composition
Table 1 shows the essential oil yield obtained for each aromatic plant, expressed in grams of essential oil per kilogram of fresh plant and as a percentage (w/w). The highest yields were obtained in Th. mastichina, L. pedunculata subsp. sampaioana, and Th. zygis subsp. sylvestris with values of 2.43%, 1.28%, and 0.88%, respectively. The species with the lowest yields were M. × piperita, L. stoechas subsp. luisieri, and O. vulgare subsp. virens (0.62%, 0.42% and 0.41%, respectively).

Table 1.
Yield of the essential oil extraction by hydrodistillation.
The essential oils have a rich monoterpene-based chemical composition (Table 2). The majority of the detected compounds are: thymol (68.83% in Th. zygis subsp. sylvestris and 36.72% in O. vulgare subsp. virens), 1.8-cineole (66.06% and 17.71% in Th. mastichina and L. stoechas subsp. luisieri, respectively), camphor and fenchone (35.51% and 34.20%, respectively, in L. pedunculata subsp. sampaioana), gamma-terpinene (30.69% in O. vulgare subsp. virens), menthone and L-menthol (29.12% and 27.56%, respectively, in M. × piperita), and trans-alpha-necrodyl acetate (20.46% in L. stoechas subsp. luisieri).

Table 2.
Composition of the essential oils. (Note 1: value in %). (Note 2: OVV: O. vulgare subsp. virens, LPS: L. pedunculata subsp. sampaioana, LSL: L. stoechas subsp. luisieri, TM: Th. mastichina, TZS: Th. zygis subsp. sylvestris, MP: M. × piperita).
3.2. Antioxidant Activity
The obtained results (Table 3) show that the essential oils of the L. stoechas subsp. luisieri, O. vulgare subsp. Virens, and Th. zygis subsp. sylvestris species have higher antioxidant activity. These species have in common a high percentage of the chemical’s thymol, gamma-terpinene and trans-alpha-necrodyl acetate in their essential oils.

Table 3.
Antioxidant Activity Results (ABTS and DPPH methods). (Note 1: mean ± standard deviation) (Note 2: EO: Essential oil, OVV: O. vulgare subsp. virens, LPS: L. pedunculata subsp. sampaioana, LSL: L. stoechas subsp. luisieri, TM: Th. mastichina, TZS: Th. zygis subsp. sylvestris, MP: M. × piperita).
The essential oils tested showed a higher antioxidant capacity using the ABTS method than the DPPH method. In the ABTS method, the essential oils with the highest antioxidant capacity values are as follows: Th. zygis subsp. sylvestris (161.70 ± 0.15 mM TROLOX eq.), O. vulgare subsp. virens (76.45 ± 3.02 mM TROLOX eq.), and L. stoechas subsp. luisieri (24.06 ± 0.64 mM TROLOX eq.). By contrast, the results obtained using the DPPH method show a smaller difference between the essential oils with the highest antioxidant capacity belong to the same species of medicinal plants: L. stoechas subsp. luisieri (33.91 ± 1.21 mM TROLOX eq.), Th. zygis subsp. sylvestris (25.34 ± 1.08 mM TROLOX eq.), and O. vulgare subsp. virens (25.15 ± 1.69 mM TROLOX eq.).
In contrast, the species Th. mastichina, L. pedunculata subsp. sampaioana, and M. × piperita exhibited low antioxidant activity values using both methods, with higher values obtained using the ABTS than the DPPH method. The essential oil of Th. mastichina (0.96 ± 0.03 mM TROLOX eq.) exhibited the lowest antioxidant activity value in the DPPH method, followed by L. pedunculata subsp. sampaioana and M. × piperita (2.17 ± 0.16 mM TROLOX eq. and 3.83 ± 0.13 mM TROLOX eq., respectively). In the ABTS method, the essential oils of L. pedunculata subsp. sampaioana and M. × piperita have the lowest antioxidant capacity (3.84 ± 0.26 mM TROLOX eq. and 4.83 ± 0.09 mM TROLOX eq., respectively), whereas Th. mastichina. has a greater reducing capacity in this method than in the DPPH method (9.76 ± 0.41 mM TROLOX eq.).
3.3. In Vitro Antifungal Activity Assay
3.3.1. Fungal Isolation
The P. italicum species was observed to grow more quickly in in vitro conditions than P. digitatum (Figure 1).

Figure 1.
Fungal isolation: (a) P. digitatum; (b) P. italicum.
3.3.2. Antifungal Activity
The Petri dishes were photographed every 24 h, and it could be observed that the filter disk infused with the essential oils is able to inhibit the fungal species development (inhibition halo) and delay conidiospore development of both species. Figure 2 shows an example of the essential oil effects with different antifungal effects: Th. zygis subsp. sylvestris (high antifungal effects) and M. × piperita (low antifungal effects), both compared with the control group.

Figure 2.
Photographic progression of P. digitatum and P. italicum (time in hours). (Note: TZS: Th. zygis subsp. sylvestris, MP: M. × piperita).
Table 4 displays statistical parameters from the inhibition halo measurements obtained after 48 h of growth (the 48 h data were used to ensure a correct understanding of the data and avoid mixing up inhibition and natural absence of growth as well as to ensure a correct effect of the essential oil and avoid the evaporation of it during the process). Measurement results show a higher inhibitory capacity from essential oil over P. digitatum species than P. italicum.

Table 4.
Statistical parameters from inhibition halo measurement (Note 1: x̅ = mean, s = standard deviation, Me = median, Max = maximum value, Min = minimum value, SEM = standard error) (Note 2: OVV: O. vulgare subsp. virens, LPS: L. pedunculata subsp. sampaioana, LSL: L. stoechas subsp. luisieri, TM: Th. mastichina, TZS: Th. zygis subsp. sylvestris, MP: M. × piperita).
Finally, the data distribution in relation to each essential oil species is presented in a boxplot for each fungal species (Figure 3). It is possible to observe that the essential oil of Th. zygis subsp. sylvestris produced the largest inhibition halo (60.50 ± 5.77 mm and 54.33 ± 2.93 mm for P. digitatum and P. italicum, respectively), with a clear difference compared to the rest of the samples. The next species showing the highest antifungal activity were L. stoechas subsp. luisieri (30.67 ± 1.15 mm and 37.33 ± 2.52 mm for P. digitatum and P. italicum, respectively) and O. vulgare subsp. virens (31.33 ± 3.21 mm and 27.00 ± 6.38 mm for P. digitatum and P. italicum, respectively).
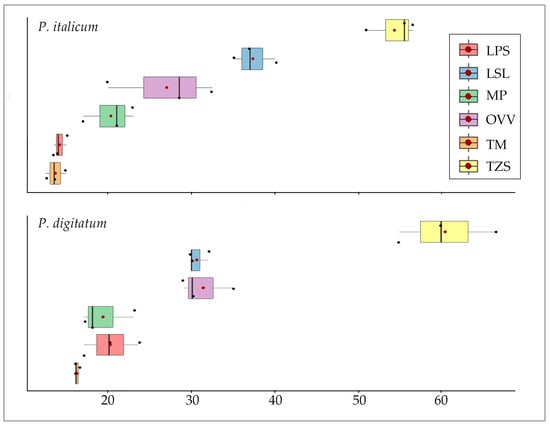
Figure 3.
Inhibition halo boxplot produced by the different essential oil samples. (Note 2: OVV: O. vulgare subsp. virens, LPS: L. pedunculata subsp. sampaioana, LSL: L. stoechas subsp. luisieri, TM: Th. mastichina, TZS: Th. zygis subsp. sylvestris, MP: M. × piperita).
The essential oil of Th. mastichina demonstrated the lowest level of antifungal activity, exhibiting an inhibition halo of 13.67 ± 1.26 mm over P. italicum y 16.17 ± 0.29 mm over P. digitatum.
4. Discussion
The obtained data shows the essential oils of Th. zygis subsp. sylvestris, O. vulgare subsp. virens and L. stoechas subsp. luisieri have high antioxidant activity for both ABTS and DPPH and elevated antifungal activity. Differences are observed in the results for each essential oil when using the ABTS and DPPH methods. This is due to the different reduction mechanisms used by each method: DPPH is based on proton elimination, while ABTS is based on proton interchange [69]. Conversely, the essential oils of Th. mastichina, M. × piperita and L. pedunculata subsp. sampaioana exhibited low antioxidant and antifungal activity against the two evaluated fungal species, P. italicum and P. digitatum.
In terms of their chemical composition, the essential oils of Th. zygis subsp. sylvestris and Origanum vulgare subsp. virens are rich in thymol (monoterpene phenol), while L. stoechas subsp. luisieri contains trans-alpha-necrodyl acetate (monoterpene ester). L. pedunculata subsp. sampaioana contains mainly fenchone and camphene (monoterpene ketones). Finally, Th. mastichina and M. × piperita contain mainly 1,8-cineole (a monoterpene ether), menthone (monoterpenic ketone), and menthol (monoterpene alcohol), respectively. Correlating the main component of each essential oil with its antioxidant and antifungal properties shows that phenolic monoterpenes and esters are more inhibitory than alcohol, ketone and ether monoterpenes.
High antifungal and antioxidant activity from Th. zygis has been widely recognized in several studies [70,71,72,73,74,75]. Th. zygis essential oil can have different chemotypes (thymol, carvacrol, carvacrol/thymol, linalool, geranyl acetate/geraniol, …) [72,74,75,76,77]. However, only the carvacrol, thymol, and carvacrol/thymol chemotypes, have demonstrated elevated antifungal and antioxidant capacity [60,62,63,64,65,66]. The presence of thymol and carvacrol compounds in essential oil from other species of Thymus L. genus is well known [47,78,79,80]. Furthermore, research into the antifungal activity indicates that they have a higher inhibitory capacity for fungal growth than the pure compounds thymol or carvacrol [71,78]. Regarding P. digitatum and P. italicum molds, Th. zygis subsp. sylvestris essential oil has an elevated inhibitory capacity against “in vitro” growth, as observed in other Penicillium species [47,50,70].
On the other hand, the other thyme species included in this study, Th. mastichina, has an essential oil rich in 1,8-cineole [73,81,82,83], with a poor antioxidant and antifungal capacity against the Penicillium species studied. However, other studies have shown it to have good antifungal properties against other fungal species, such as Sclerotinia spp., Fusarium spp., Alternaria spp. or Candida spp. [73,84,85,86]. This makes it possible to use it to fight fungal infections in crops or on the skin.
O. vulgare subsp. virens essential oil has a thymol/gamma-terpinene chemotype, which is unusual for this species [87,88]. This coincides with what was observed in research involving the carvacrol chemotype of O. vulgare subsp. vulgare, which exhibits high antioxidant and antifungal activity against P. digitatum and P. italicum [50,88,89,90,91].
The two Lavandula L. subspecies studied exhibit different antifungal capacities, with L. stoechas subsp. luisieri demonstrating greater activity than L. pedunculata subsp. sampaioana [92], and notably the inhibitory effect on P. digitatum growth is higher than on P. italicum. The antioxidant activity of L. stoechas subsp. luisieri essential oil is very high, mainly due to the presence of necrodiol derivatives [93]. On the other hand, L. pedunculata subsp. sampaioana has an essential oil rich in fenchone, camphor and 1,8-cineole, which are compounds with low antioxidant capacity [73,82].
M. × piperita essential oil exhibits the lowest of all the essential oils studied in the present research. However, other studies indicate good inhibitory capacity against several species of the Penicillium genus, including P. digitatum [94,95,96,97]. This divergence in results could be due to variation in the essential oil’s chemical composition, including different percentages of menthol, menthone, limonene, alpha-pinene, and betha-pinene, among others.
5. Conclusions
The Th. zygis subsp. sylvestris, O. vulgare subsp. Virens, and L. stoechas subsp. luisieri essential oils have a high antioxidant capacity and can effectively inhibit the “in vitro” growth of the molds (P. digitatum and P. italicum) that mainly cause postharvest damages in Citrus genus fruits. Furthermore, all the essential oils studied exhibited a higher inhibition response against green mold (P. digitatum) than blue mold (P. italicum).
Essential oils with a chemical composition rich in phenolic monoterpenes and ethers have greater antioxidant and antifungal capacity against P. digitatum and P. italicum molds, as they inhibit spore germination for a longer period of time.
Author Contributions
Conceptualization, M.d.C.G.-C. and F.M.-G.; methodology, M.d.C.G.-C. and F.M.-G.; software, M.d.C.G.-C. and D.G.-A.; formal analysis, M.d.C.G.-C., D.G.-A. and F.M.-G.; investigation, M.d.C.G.-C., F.M.-G., C.D.B.-T. and D.G.-A.; resources, F.M.V.P.; writing—original draft preparation, M.d.C.G.-C. and F.M.-G.; writing—review and editing, D.G.-A., C.D.B.-T. and F.M.V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study forms part of the AGROALNEXT program and was supported by MCIN with funding from European Union NextGenerationEU (PRTR-C17.I1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to acknowledge Alonso Martín Jabato and Julian Mor-cillo Solis for their help and maintenance of the assay crops used in this research. Additionally, we are grateful for the help of Pedro Del Viejo Esteban and Alicia Gil de los Santos during the development of the laboratory studies.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hodges, R.J.; Buzby, J.C.; Bennett, B. Postharvest losses and waste in developed and less developed countries: Opportunities to improve resource use. J. Agric. Sci. 2011, 149, 37–45. [Google Scholar] [CrossRef]
- Snyder, A.B.; Worobo, R.W. Fungal Spoilage in Food Processing. J. Food Prot. 2018, 81, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Rizwana, H.; Bokahri, N.A.; Alsahli, S.A.; Al Showiman, A.S.; Alzahrani, R.M.; Aldehaish, H.A. Postharvest disease management of Alternaria spots on tomato fruit by Annona muricata fruit extracts. Saudi J. Biol. Sci. 2021, 28, 2236–2244. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, Q.; Shaheen, M.R.; Ali, S.; Ahmad, A.; Raheel, M.; Bajwa, R.T. Chapter 1—Postharvest management of fruits and vegetables. In Applications of Biosurfactant in Agriculture; Inamuddin, C.O.A., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 1–16. [Google Scholar] [CrossRef]
- Zakaria, L. Fusarium Species Associated with Diseases of Major Tropical Fruit Crops. Horticulturae 2023, 9, 322. [Google Scholar] [CrossRef]
- Pouris, J.; Kolyva, F.; Bratakou, S.; Vogiatzi, C.A.; Chaniotis, D.; Beloukas, A. The Role of Fungi in Food Production and Processing. Appl. Sci. 2024, 14, 5046. [Google Scholar] [CrossRef]
- Dijksterhuis, J.; Houbraken, J. Fungal Spoilage of Crops and Food. In Agricultural and Industrial Applications. The Mycota; Grüttner, S., Kollath-Leiß, K., Kempken, F., Eds.; Springer: Cham, Switzerland, 2025; Volume 16, pp. 31–66. [Google Scholar] [CrossRef]
- Palou, L.; Valencia-Chamorro, S.A.; Pérez-Gago, M.B. Antifungal edible coatings for fresh citrus fruit: A review. Coatings 2015, 5, 962–986. [Google Scholar] [CrossRef]
- Costa, J.H.; Wassano, C.I.; Angolini, C.F.F.; Scherlach, K.; Hertweek, C.; Fill, T.P. Antifungal potential of secondary metabolites involved in the interaction between citrus pathogens. Sci. Rep. 2019, 9, 18647. [Google Scholar] [CrossRef]
- Papoutsis, K.; Mathioudakis, M.M.; Hasperué, J.H.; Ziogas, V. Non-chemical treatments for preventing the postharvest fungal rotting of citrus caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Trends Food Sci. Technol. 2019, 86, 479–491. [Google Scholar] [CrossRef]
- Cheng, Y.; Lin, Y.; Cao, H.; Li, Z. Citrus Postharvest Green Mold: Recent Advances in Fungal Pathogenicity and Fruit Resistance. Microorganisms 2020, 8, 449. [Google Scholar] [CrossRef]
- Hanif, Z.; Ashari, H. Post-harvest losses of citrus fruits and perceptions of farmers in marketing decisions. E3S Web Conf. 2021, 306, 02059. [Google Scholar] [CrossRef]
- Kadhim, Z.R.; Ali, S.H.; AL-Rubaye, S.A. The economic impacts of the post-harvest losses of tangerines and Seville oranges crops in Iraq (Baghdad Governorate: As a case study). Bulg. J. Agric. Sci. 2025, 31, 237–244. [Google Scholar]
- Hall, D.J. Comparative activity of selected food preservatives as citrus postharvest fungicides. Proc. Fla. State Hortic. Soc. 1988, 101, 184–187. [Google Scholar]
- Holmes, G.J.; Eckert, J.W. Sensitivity of Penicillium digitatum and P. italicum to postharvest citrus fungicides in California. Phytopathology 1999, 89, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Talibi, I.; Boubaker, H.; Boudyach, E.H.; Ait Ben Aoumar, A. Alternative methods for the control of postharvest citrus diseases. J. Appl. Microbiol. 2014, 117, 1–17. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Chen, H.; Zeng, Z.; Li, Z.-L.; Jiang, H. Synthesis of Oxylipin Mimics and Their Antifungal Activity against the Citrus Postharvest Pathogens. Molecules 2016, 21, 254. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shen, Y.; Chen, C.; Wan, C. Inhibition of Key Citrus Postharvest Fungal Strains by Plant Extracts In Vitro and In Vivo: A Review. Plants 2019, 8, 26. [Google Scholar] [CrossRef]
- Kanashiro, A.M.; Akiyama, D.Y.; Kupper, K.C.; Fill, T.P. Penicillium italicum: An Underexplored Postharvest Pathogen. Front. Microbiol. 2020, 11, 606852. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sui, Y.; Li, J.; Tian, X.; Wang, Q. Biological control of postharvest fungal decays in citrus: A review. Crit. Rev. Food Sci. Nutr. 2020, 62, 861–870. [Google Scholar] [CrossRef]
- Strano, M.C.; Altieri, G.; Allegra, M.; Di Renzo, G.C.; Paterna, G.; Matera, A.; Genovese, F. Postharvest Technologies of Fresh Citrus Fruit: Advances and Recent Developments for the Loss Reduction during Handling and Storage. Horticulturae 2022, 8, 612. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Teles, S.; Pereira, J.A.; Santos, C.H.B.; Menezes, R.V.; Malheiro, R.; Lucchese, A.M.; Silva, F. Effect of geographical origin on the essential oil content and composition of fresh and dried Mentha × villosa Hudson leaves. Ind. Crop. Prod. 2013, 46, 1–7. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Sharifi-Rad, R.; Ayatollahi, S.A.; Iriti, M. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Heydari, Z.; Jafari, L.; Yavari, A. Diversity in Essential Oil Compounds in Relation to Different Geographic Origins and Plant Organs of Salvia sharifii. J. Med. Plants By Prod. 2023, 12, 83–92. [Google Scholar] [CrossRef]
- Tomou, E.-M.; Fraskou, P.; Dimakopoulou, K.; Dariotis, E.; Krigas, N.; Skaltsa, H. Chemometric Analysis Evidencing the Variability in the Composition of Essential Oils in 10 Salvia Species from Different Taxonomic Sections or Phylogenetic Clades. Molecules 2024, 29, 1547. [Google Scholar] [CrossRef]
- Iseppi, R.; Mariani, M.; Condò, C.; Sabia, C.; Messi, P. Essential Oils: A Natural Weapon against Antibiotic-Resistant Bacteria Responsible for Nosocomial Infections. Antibiotics 2021, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Janotto, L.; de Melo Nazareth, T.; Meca, G.; Bittecourt Luciano, F.; Gonçalves Evangelista, A. Exploring the efficacy of antibiotic-essential oil combinations: Implications for combating antimicrobial resistance. Bioresour. Technol. Rep. 2023, 24, 101679. [Google Scholar] [CrossRef]
- Öner, E.K.; Yeşil, M. Effects of altitudes on secondary metabolite contents of Origanum majorana L. Sci. Rep. 2023, 13, 10765. [Google Scholar] [CrossRef]
- Miloudi, S.; Abbad, I.; Soulaimani, B.; Ferradous, A.; Abbad, A.; El Mouden, H. Optimization of herbicidal activity of essential oil mixtures from Satureja alpina, Thymus satureioides and Myrtus communis on seed germination and post-emergence growth of Amaranthus retroflexus L. Crop Prot. 2024, 180, 106642. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer Properties of Essential Oils and Other Natural Products. Evid. Based Complement. Altern. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef]
- Abd Rashed, A.; Rathi, D.-N.G.; Ahmad Nasir, N.A.H.; Abd Rahman, A.Z. Antifungal Properties of Essential Oils and Their Compounds for Application in Skin Fungal Infections: Conventional and Nonconventional Approaches. Molecules 2021, 26, 1093. [Google Scholar] [CrossRef]
- Mohamed Abdoul-Latif, F.; Ainane, A.; Houmed Aboubaker, I.; Mohamed, J.; Ainane, T. Exploring the Potent Anticancer Activity of Essential Oils and Their Bioactive Compounds: Mechanisms and Prospects for Future Cancer Therapy. Pharmaceuticals 2023, 16, 1086. [Google Scholar] [CrossRef]
- Ivanova, S.; Gvozdeva, Y.; Staynova, R.; Grekova-Kafalova, D.; Nalbantova, V.; Benbassat, N.; Koleva, N.; Ivanov, K. Essential oils—A review of the natural evolution of applications and some future perspectives. Pharmacia 2025, 72, 1–12. [Google Scholar] [CrossRef]
- Tejeswini, M.G.; Sowmya, H.V.; Swarnalatha, S.P.; Negi, P.S. Antifungal activity of essential oils and their combinations in vitro and in vivo conditions. Arch. Phytopathol. Plant Prot. 2013, 47, 564–570. [Google Scholar] [CrossRef]
- Felšöciová, S.; Vukovic, N.; Jeżowski, P.; Kačániová, M. Antifungal activity of selected volatile essential oils against Penicillium sp. Open Life Sci. 2020, 15, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.S.; Rosalen, P.L.; Benso, B.; de Cássia Orlandi Sardi, J.; Denny, C.; de Sousa, S.A.; Guerra, F.Q.S.; de Oliveira Lima, E.; Almeida Freires, I.; Dias de Castro, R. The Use of Essential Oils and Their Isolated Compounds for the Treatment of Oral Candidiasis: A Literature Review. Evid. Based Complement. Altern. Med. 2021, 2021, 1059274. [Google Scholar] [CrossRef]
- Hou, T.; Sana, S.S.; Li, H.; Xing, Y.; Nanda, A.; Netala, V.R.; Zhang, Z. Essential oils and its antibacterial, antifungal and anti-oxidant activity applications: A review. Food Biosci. 2022, 47, 101716. [Google Scholar] [CrossRef]
- Tran, H.M.; Le, D.H.; Nguyen, V.-A.T.; Vu, T.X.; Thanh, N.T.K.; Giang, D.H.; Dat, N.T.; Pham, H.T.; Muller, M.; Nguyen, H.Q.; et al. Penicillium digitatum as a Model Fungus for Detecting Antifungal Activity of Botanicals: An Evaluation on Vietnamese Medicinal Plant Extracts. J. Fungi 2022, 8, 956. [Google Scholar] [CrossRef]
- Daferera, D.J.; Ziogas, B.N.; Polissiou, M.G. GC-MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum. J. Agric. Food Chem. 2000, 48, 2576–2581. [Google Scholar] [CrossRef]
- Plaza, P.; Torres, R.; Usall, J.; Lamarca, N.; Vinas, I. Evaluation of the potential of commercial postharvest application of essential oils to control citrus decay. J. Hortic. Sci. Biotechnol. 2004, 79, 935–940. [Google Scholar] [CrossRef]
- Yahyazadeh, M.; Omidbaigi, R.; Zare, R.; Taheri, H. Effect of some essential oils on mycelial growth of Penicillium digitatum Sacc. World J. Microbiol. Biotechnol. 2008, 24, 1445–1450. [Google Scholar] [CrossRef]
- Jing, L.; Lei, Z.; Li, L.; Xie, R.; Xi, W.; Guan, Y.; Sumner, L.W.; Zhou, Z. Antifungal Activity of Citrus Essential Oils. J. Agric. Food Chem. 2014, 62, 3011–3033. [Google Scholar] [CrossRef]
- Tao, N.; Jia, L.; Zhou, H. Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem. 2014, 153, 265–271. [Google Scholar] [CrossRef]
- Boubaker, H.; Karim, H.; El Hamdaoui, A.; Msanda, F.; Leach, D.; Bombarda, I.; Vanloot, P.; Abbad, A.; Boudyach, E.H.; Ait Ben Aoumar, A. Chemical characterization and antifungal activities of four Thymus species essential oils against postharvest fungal pathogens of citrus. Ind. Crops Prod. 2016, 86, 95–101. [Google Scholar] [CrossRef]
- Moussa, H.; El Omari, B.; Chefchaou, H.; Tanghort, M.; Mzabi, A.; Chami, N.; Remmal, A. Action of thymol, carvacrol and eugenol on Penicillium and Geotrichum isolates resistant to commercial fungicides and causing postharvest citrus decay. Can. J. Plant Pathol. 2020, 43, 26–34. [Google Scholar] [CrossRef]
- Et-tazy, L.; Lamiri, A.; Satia, L.; Essahli, M.; Bencheqroun, S.K. In Vitro Antioxidant and Antifungal Activities of Four Essential Oils and Their Major Compounds against Post-Harvest Fungi Associated with Chickpea in Storage. Plants 2023, 12, 3587. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.A.; Bicas, J.L. Antifungal activity of essential oils of tea tree, oregano, thyme, and cinnamon, and their components. Braz. J. Food Technol. 2024, 27, e2023071. [Google Scholar] [CrossRef]
- Arras, G.; Usai, M. Fungitoxic Activity of 12 Essential Oils against Four Postharvest Citrus Pathogens: Chemical Analysis of Thymus capitatus Oil and its Effect in Subatmospheric Pressure Conditions. J. Food Prot. 2001, 64, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Wills, R.B.H.; Bowyer, M.C.; Golding, J.B.; Kirkman, T.; Pristijono, P. Efficacy of Orange Essential Oil and Citral after Exposure to UV-C Irradiation to Inhibit Penicillium digitatum in Navel Oranges. Horticulturae 2020, 6, 102. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Palou, L.; Taberner, V.; Fernández-Catalán, A.; Argente-Sanchis, M.; Pitta, E.; Pérez-Gago, M.B. Natural Pectin-Based Edible Composite Coatings with Antifungal Properties to Control Green Mold and Reduce Losses of ‘Valencia’ Oranges. Foods 2022, 11, 1083. [Google Scholar] [CrossRef]
- Wardana, A.A.; Kingwascharapong, P.; Wigati, L.P.; Tanaka, F.; Tanaka, F. The antifungal effect against Penicillium italicum and characterization of fruit coating from chitosan/ZnO nanoparticle/Indonesian sandalwood essential oil composites. Food Packag. Shelf Life 2022, 32, 100849. [Google Scholar] [CrossRef]
- Olmedo, G.M.; Zhang, J.; Zhao, W.; Mattia, M.; Rosskopf, E.N.; Ritenour, M.; Plotto, A.; Bai, J. Application of Thymol Vapors to Control Postharvest Decay Caused by Penicillium digitatum and Lasiodiplodia theobromae in Grapefruit. Foods 2023, 12, 3637. [Google Scholar] [CrossRef] [PubMed]
- Gharzouli, M.; Aouf, A.; Mahmoud, E.; Ali, H.; Alsulami, T.; Badr, A.N.; Ban, Z.; Farouk, A. Antifungal effect of Algerian essential oil nanoemulsions to control Penicillium digitatum and Penicillium expansum in Thomson Navel oranges (Citrus sinensis L. Osbeck). Front. Plant Sci. 2024, 15, 1491491. [Google Scholar] [CrossRef]
- Maunpuii, C.V.L.; Maisnam, R.; Antuhu, Y.L.; Kumari, A.; López-Menchero, J.R.; González-Coloma, A.; Andrés, M.F.; Kaushik, N. Evaluating the efficiency of essential oils as fumigants in controlling Penicillium digitatum in citrus fruits. BIO Web Conf. 2024, 110, 02009. [Google Scholar] [CrossRef]
- Maswanganye, L.T.C.; Pillai, S.K.; Sivakumar, D. Chitosan Coating Loaded with Spearmint Essential Oil Nanoemulsion for Antifungal Protection in Soft Citrus (Citrus reticulata) Fruits. Coatings 2025, 15, 105. [Google Scholar] [CrossRef]
- Sánchez-Torres, P. Emerging alternatives to control fungal contamination. Curr. Opin. Food Sci. 2025, 61, 101255. [Google Scholar] [CrossRef]
- Pérez-Alonso, C.O.; Martínez-Romero, D.; Zapata, P.J.; Serrano, M.; Valero, D.; Castillo, S. The effects of essential oils carvacrol and thymol on growth of Penicillium digitatum and P. italicum involved in lemon decay. Int. J. Food Microbiol. 2012, 158, 101–106. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Morris, S.C.; Nicholls, P.J. An evaluation of optical density to estimate fungal spore concentrations in water suspensions. Phytopathology 1978, 68, 1240–1242. [Google Scholar] [CrossRef]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 4th ed.; APS Press: Saint Paul, MN, USA, 1998; 218p. [Google Scholar]
- Pitt, J.I. A Laboratory Guide to Common Penicillium Species, 2nd ed.; CSIRO Division of Food Processing: North Ryde, New South Wales, Australia, 1988; 187p. [Google Scholar]
- Romero, C.S. Hongos Fitopatógenos; Universidad Autónoma Chapingo: Chapingo, Estado de México, Mexico, 1988; 347p. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Hossain, T.J. Methods for screening and evaluation of antimicrobial activity: A review of protocols, advantages, and limitations. Eur. J. Microbiol. Immunol. 2024, 14, 97–115. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- El Hajli, F.; Chakir, S.; Annemer, S.; Assouguem, A.; Elaissaoui, F.; Ullah, R.; Ali, E.A.; Choudhary, R.; Hammani, K.; Lahlali, R.; et al. Tetraclinis articulata (Vahl) Mast., Mentha pulegium L. and Thymus zygis L. essential oils: Chemical composition, antioxidant and antifungal properties against postharvest fungal diseases of apple, and in vitro, in vivo, and in silico investigation. Open Chem. 2025, 23, 20250131. [Google Scholar] [CrossRef]
- Radi, F.Z.; Bouhrim, M.; Mechchate, H.; Al-zahrani, M.; Qurtam, A.A.; Aleissa, A.M.; Drioiche, A.; Handaq, N.; Zair, T. Phytochemical Analysis, Antimicrobial and Antioxidant Properties of Thymus zygis L. and Thymus willdenowii Boiss. Essential Oils. Plants 2022, 11, 15. [Google Scholar] [CrossRef]
- Gonçalves, M.J.; Cruz, M.T.; Cavaleiro, C.; Lopes, M.C.; Salgueiro, L. Chemical, antifungal and cytotoxic evaluation of the essential oil of Thymus zygis subsp. sylvestris. Ind. Crops Prod. 2010, 32, 70–75. [Google Scholar] [CrossRef]
- Pina-Vaz, C.; Gonçalves, A.; Pinto, E.; Costa-de-Oliveira, S.; Tavares, C.; Salgueiro, L.; Cavaleiro, C.; Gonçalves, M.J.; Martinez-de-Oliveira, J. Antifungal activity of Thymus oils and their major compounds. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 73–78. [Google Scholar] [CrossRef]
- Gourich, A.A.; Bencheikh, N.; Bouhrim, M.; Regragui, M.; Rhafouri, R.; Drioiche, A.; Asbabou, A.; Remok, F.; Mouradi, A.; Addi, M.; et al. Comparative Analysis of the Chemical Composition and Antimicrobial Activity of Four Moroccan North Middle Atlas Medicinal Plants’ Essential Oils: Rosmarinus officinalis L., Mentha pulegium L., Salvia officinalis L. and Thymus zygis subsp. gracilis (Boiss.) R. Morales. Chemistry 2022, 4, 1775–1788. [Google Scholar] [CrossRef]
- Sáez, F. Essential oil variability of Thymus zygis growing wild in southeastern Spain. Phytochemistry 1995, 40, 819–825. [Google Scholar] [CrossRef]
- Rodrigues, V.; Cabral, C.; Évora, L.; Ferreira, I.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Chemical composition, anti-inflammatory activity and cytotoxicity of Thymus zygis L. subsp. sylvestris (Hoffmanns. & Link) Cout. essential oil and its main compounds. Arab. J. Chem. 2015, 12, 3236–3243. [Google Scholar] [CrossRef]
- Pérez-Sánchez, R.; Ubera, J.L.; Lafont, F.; Gálvez, C. Composition and Variability of the Essential Oil in Thymus zygis from Southern Spain. J. Essent. Oil Res. 2008, 20, 192–200. [Google Scholar] [CrossRef]
- Šegvić, M.; Kosalec, I.; Mastelić, J.; Piecková, E.; Pepeljnak, S. Antifungal activity of thyme (Thymus vulgaris L.) essential oil and thymol against moulds from damp dwellings. Lett. Appl. Microbiol. 2007, 44, 36–42. [Google Scholar] [CrossRef]
- Golparvar, A.R.; Hadipanah, A. A Review of the Chemical Composition of Essential Oils of Thymus Species in Iran. Res. Crop Ecophysiol. 2023, 18, 25–51. [Google Scholar] [CrossRef]
- Etri, K.; Pluhár, Z. Exploring Chemical Variability in the Essential Oils of the Thymus Genus. Plants 2024, 13, 1375. [Google Scholar] [CrossRef]
- Delgado, T.; Marinero, P.; Asensio-S.-Manzanera, M.C.; Asensio, C.; Herrero, B.; Pereira, J.A.; Ramalhosa, E. Antioxidant activity of twenty wild Spanish Thymus mastichina L. populations and its relation with their chemical composition. LWT Food Sci. Technol. 2014, 57, 412–418. [Google Scholar] [CrossRef]
- Macedo, S.; Piçarra, A.; Guerreiro, M.; Salvador, C.; Candeias, F.; Caldeira, A.T.; Martins, M.R. Toxicological and pharmacological properties of essential oils of Calamintha nepeta, Origanum virens and Thymus mastichina of Alentejo (Portugal). Food Chem. Toxicol. 2019, 133, 110747. [Google Scholar] [CrossRef]
- Mateus, D.; Costa, F.; de Jesus, V.; Malaquias, L. Biocides Based on Essential Oils for Sustainable Conservation and Restoration of Mural Paintings in Built Cultural Heritage. Sustainability 2024, 16, 11223. [Google Scholar] [CrossRef]
- Fraternale, D.; Giamperi, L.; Ricci, D. Chemical Composition and Antifungal Activity of Essential Oil Obtained from In Vitro Plants of Thymus mastichina L. J. Essent. Oil Res. 2003, 15, 278–281. [Google Scholar] [CrossRef]
- Rodrigues, M.; Lopes, A.C.; Vaz, F.; Filipe, M.; Alves, G.; Ribeiro, M.P.; Coutinho, P.; Araujo, A.R.T.S. Thymus mastichina: Composition and Biological Properties with a Focus on Antimicrobial Activity. Pharmaceuticals 2020, 13, 479. [Google Scholar] [CrossRef]
- Diánez, F.; Santos, M.; Parra, C.; Navarro, M.J.; Blanco, R.; Gea, F.J. Screening of antifungal activity of 12 essential oils against eight pathogenic fungi of vegetables and mushroom. Lett. Appl. Microbiol. 2018, 67, 400–410. [Google Scholar] [CrossRef]
- Machado, A.M.; Lopes, V.; Barata, A.M.; Póvoa, O.; Farinha, N.; Figueiredo, A.C. Essential Oils from Origanum vulgare subsp. virens (Hoffmanns. & Link) Ietsw. Grown in Portugal: Chemical Diversity and Relevance of Chemical Descriptors. Plants 2023, 12, 621. [Google Scholar] [CrossRef]
- Soltani, S.; Shakeri, A.; Iranshahi, M.; Boozari, M. A Review of the Phytochemistry and Antimicrobial Properties of Origanum vulgare L. and Subspecies. Iran J. Pharm. Res. 2001, 20, 268–285. [Google Scholar] [CrossRef]
- Kocić-Tanackov, S.D.; Dimić, G.R.; Tanackov, I.J.; Pejin, D.J.; Mojović, L.V.; Pejin, J.D. Antifungal activity of Oregano (Origanum vulgare L.) extract on the growth of Fusarium and Penicillium species isolated from food. Hemjska Ind. 2012, 66, 33–41. [Google Scholar] [CrossRef]
- Zulu, L.; Gao, H.; Zhu, Y.; Wu, H.; Xie, Y.; Liu, X.; Yao, H.; Rao, Q. Antifungal effects of seven plant essential oils against Penicillium digitatum. Chem. Biol. Technol. Agric. 2023, 10, 82. [Google Scholar] [CrossRef]
- Vitoratos, A.; Bilalis, D.; Karkanis, A.; Efthimiadou, A. Antifungal Activity of Plant Essential Oils Against Botrytis cinerea, Penicillium italicum and Penicillium digitatum. Not. Bot. Horti. Agrobot. 2013, 41, 86–92. [Google Scholar] [CrossRef]
- Domingues, J.; Goulão, M.; Delgado, F.; Gonçalves, J.C.; Gonçalves, J.; Pintado, C.S. Essential Oils of Two Portuguese Endemic Species of Lavandula as a Source of Antifungal and Antibacterial Agents. Processes 2023, 11, 1165. [Google Scholar] [CrossRef]
- Pombal, S.; Rodrigues, C.F.; Araújo, J.P.; Rocha, P.M.; Rodilla, J.M.; Diez, D.; Granja, Á.P.; Gomes, A.C.; Silva, L.A. Antibacterial and antioxidant activity of Portuguese Lavandula luisieri (Rozeira) Rivas-Martinez and its relation with their chemical composition. SpringerPlus 2016, 5, 1711. [Google Scholar] [CrossRef][Green Version]
- Tyagi, A.K.; Malik, A. Antimicrobial potential and chemical composition of Mentha piperita oil in liquid and vapour phase against food spoiling microorganisms. Food Control 2011, 22, 1707–1714. [Google Scholar] [CrossRef]
- Reddy, D.N.; Al-Rajab, A.J.; Sharma, M.; Moses, M.M.; Reddy, G.R.; Albratty, M. Chemical constituents, in vitro antibacterial and antifungal activity of Mentha × Piperita L. (peppermint) essential oils. J. King Saud Univ. Sci. 2019, 31, 528–533. [Google Scholar] [CrossRef]
- Zamanian, Z.; Bonyadian, M.; Moshtaghi, H.; Ebrahimi, A. Antifungal effects of essential oils of Zataria multiflora, Mentha pulegium, and Mentha piperita. J. Food Qual. Hazards Control 2021, 8, 41–44. [Google Scholar] [CrossRef]
- Kgang, I.E.; Mathabe, P.M.K.; Klein, A.; Kalombo, L.; Belay, Z.A.; Caleb, O.J. Effects of lemon (Citrus Limon L.), lemongrass (Cymbopogon citratus) and peppermint (Mentha piperita L.) essential oils against of Botrytis cinerea and Penicillium expansum. JSFA Rep. 2022, 2, 405–414. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).