Enhancement of Perylenequinonoid Compounds Production from Strain of Pseudoshiraia conidialis by UV-Induced Mutagenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Preparation of Spore Suspension and UV Mutagenesis
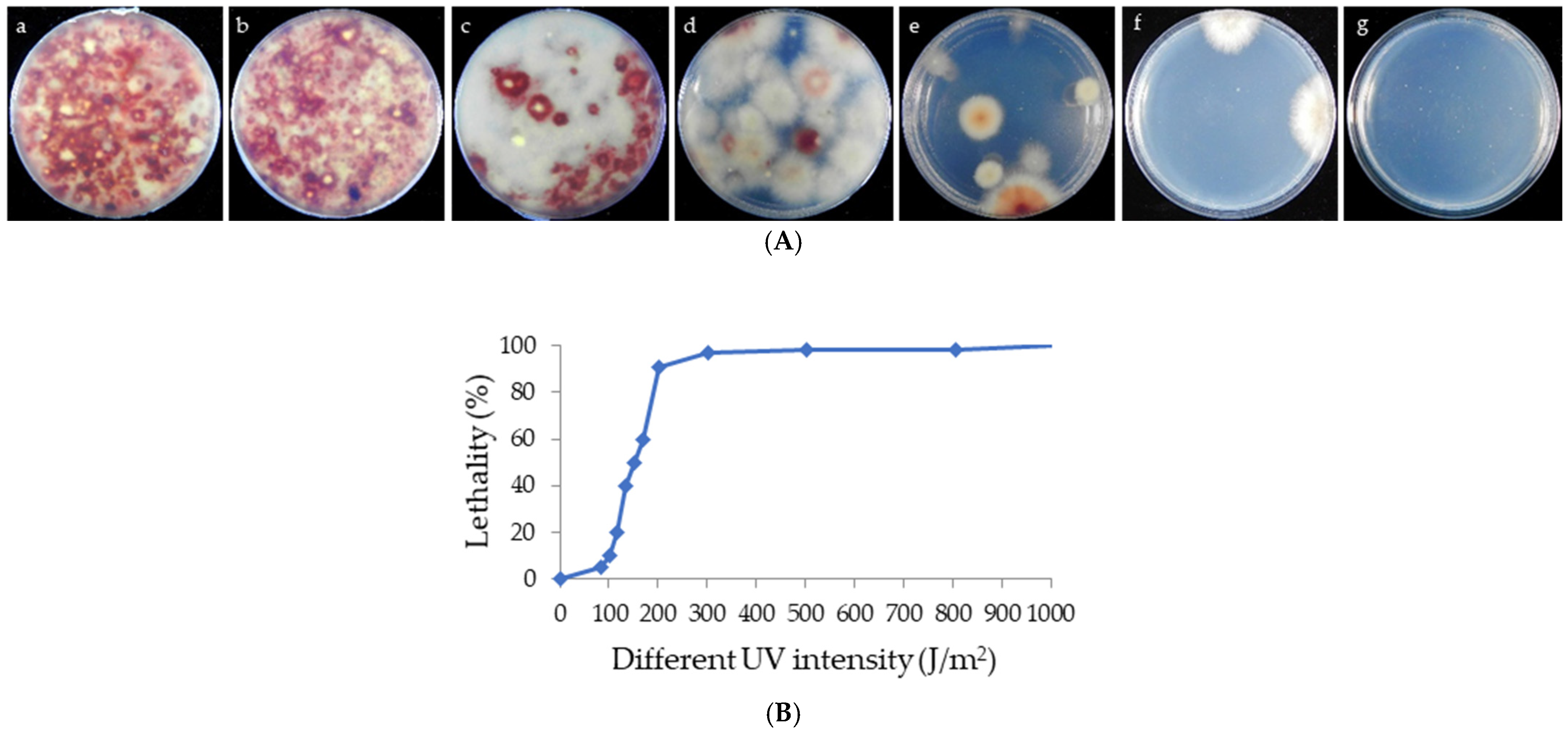
2.3. Lethality Assay
2.4. Preliminary Screening of High-Yield Mutants
2.5. Growth Rate Determination and Microscopic Observations of Mycelial Morphology
2.6. Analysis of Fermentation Time and Mycelial Biomass
2.7. Extraction of Intracellular Perylenequinonoid Compounds
2.8. HPLC Analysis Conditions
2.9. Analysis of Perylenequinonoid Compounds
2.10. Genetic Stability Test of Perylenequinonoid Compounds from Mutant Strains
3. Results
3.1. Selection of Mutant Strains and Lethality Analysis
3.2. Preliminary Screening of High-Yield Mutant Strains
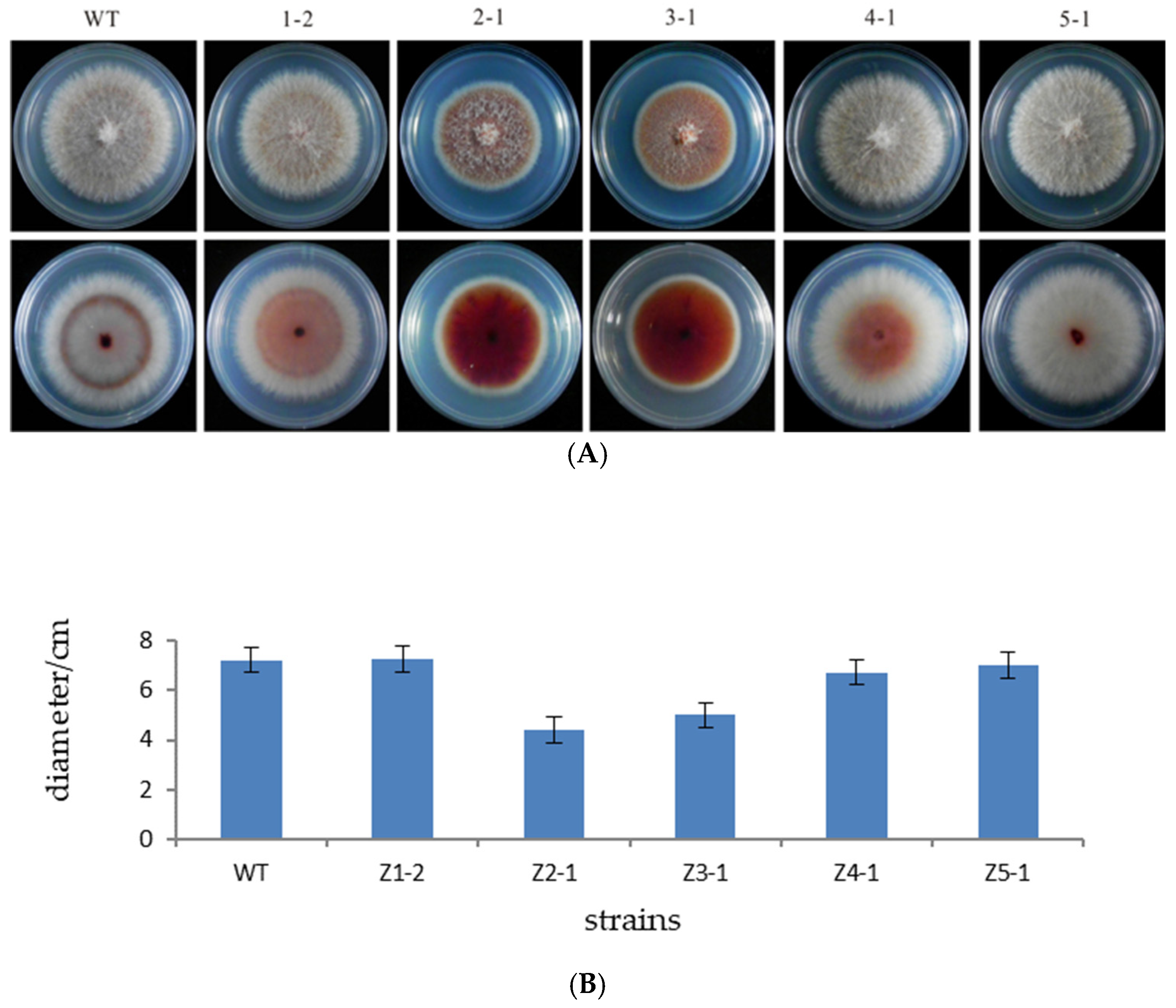
3.3. Determination of Growth Rate
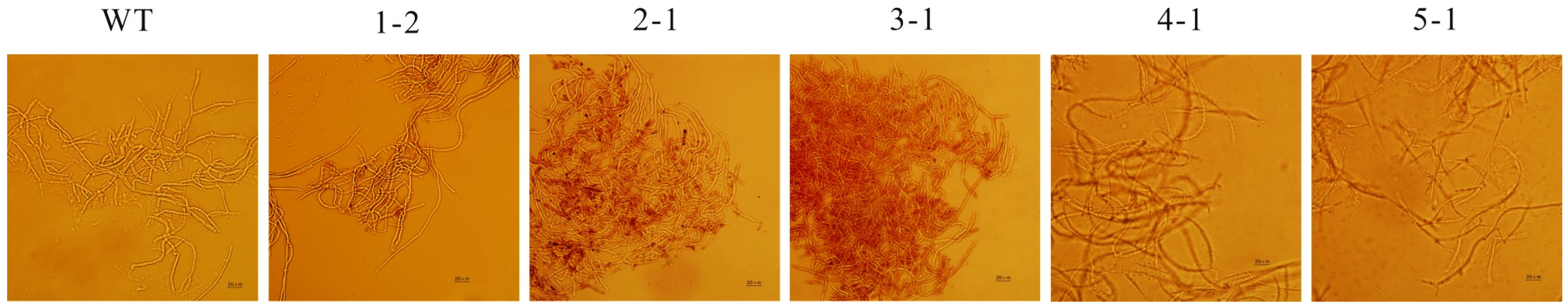
3.4. Microscopic Observations of Mycelium Morphology
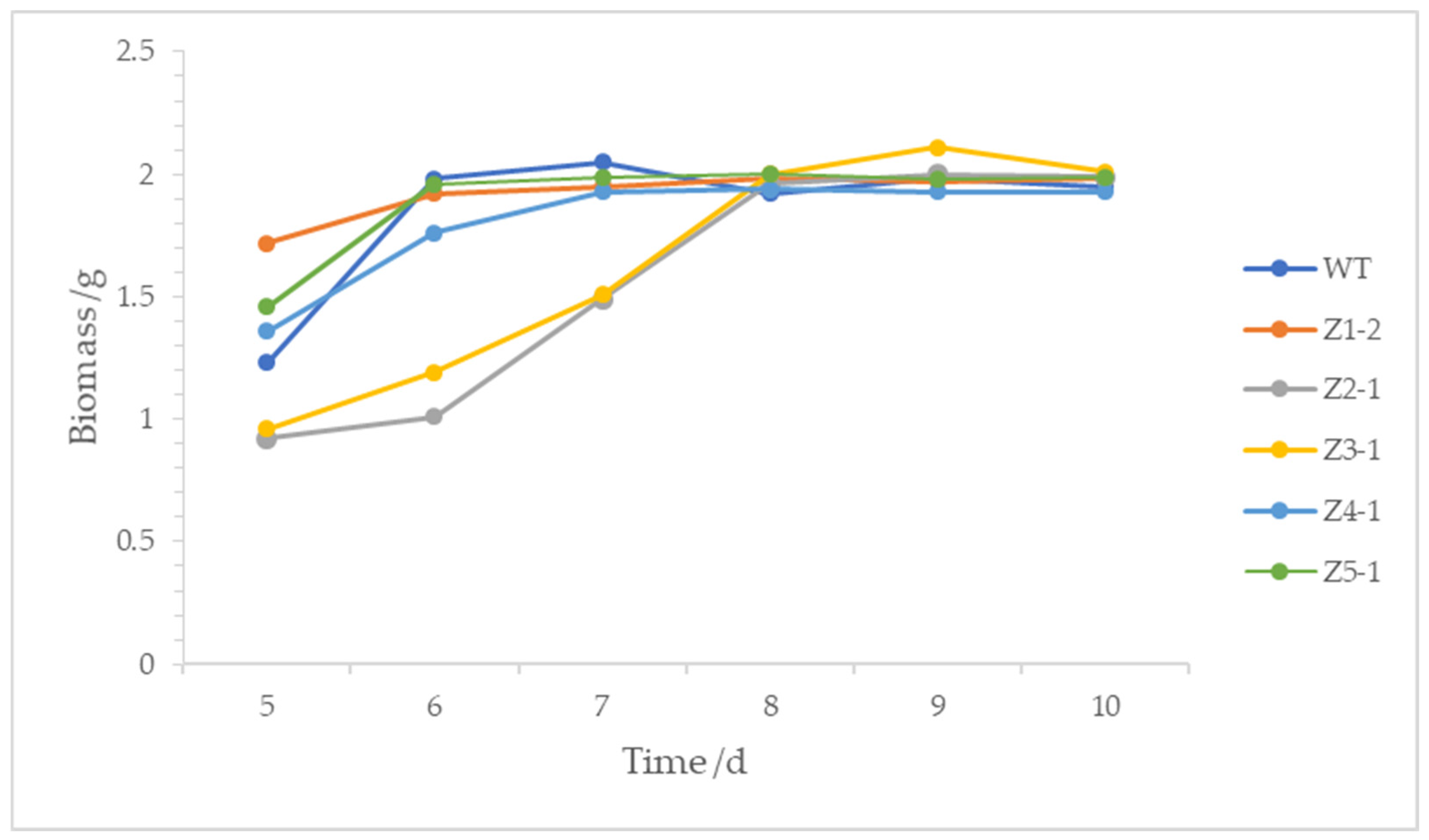
3.5. Analysis of Fermentation Time and Mycelia Biomass
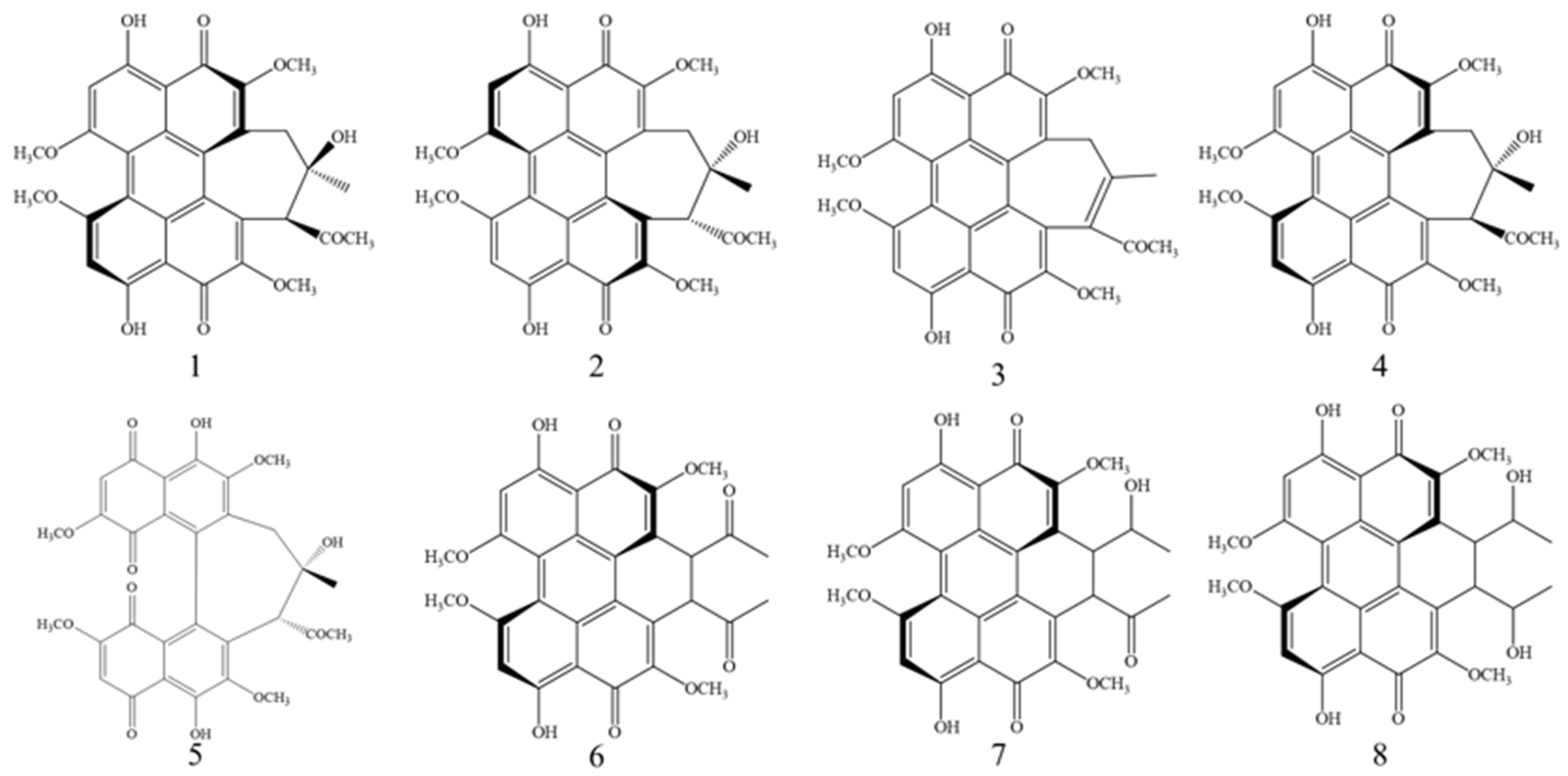
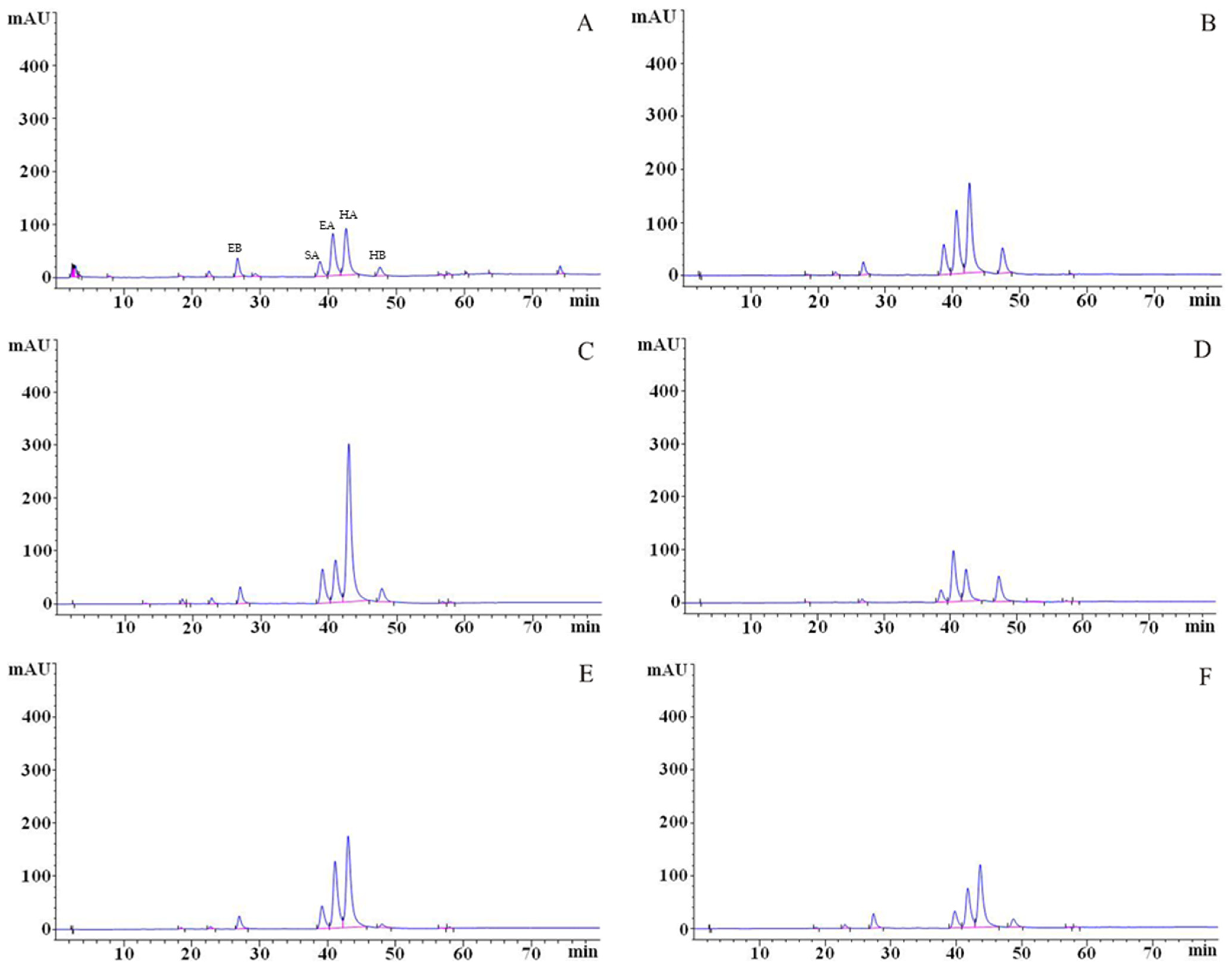
3.6. Identification and Analysis of Perylenequinonoid Compounds
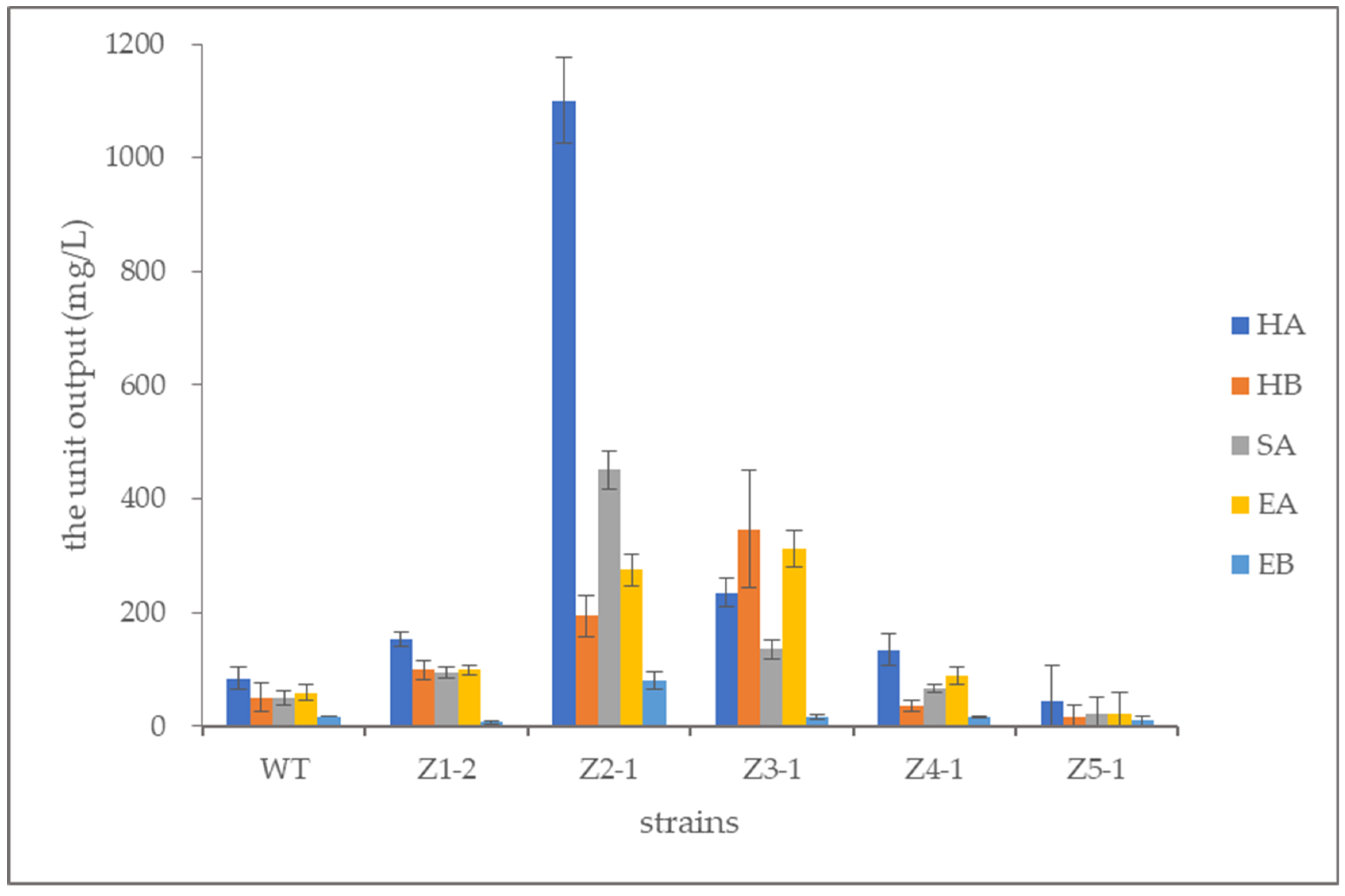
3.7. Quantitative Determination of Perylenequinonoid Compounds
3.8. Genetic Stability of Perylenequinone-Producing Mutant Strains Z2-1 and Z3-1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnone, A.; Camarda, L.; Nasini, G.; Merlini, L. Cheminform abstract: Secondary mold metabolites. Part 13. Fungal perylenequinones: Phleichrome, isophleichrome, and their endoperoxides. J. Chem. Soc. Perkin Trans. 1985, 16, 1387–1392. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, B.T.; Kim, J.A.; Chung, H.J.; Park, S.M.; Yang, M.S.; Hwang, K.J.; Kim, D.H. Cultural characteristics and extraction of the fungal pigment phleichrome from the phytopathogenic fungus Cladosporium phlei. Biotechnol. Bioprocess Eng. 2007, 12, 508–515. [Google Scholar] [CrossRef]
- Chen, W.X.; Cheng, Y.T.; Wan, X.Y.; Friedrichs, E.; Puff, H.; Breitmaier, E. Die Struktur des Hypocrellins und seines Photooxidationsproduktes Peroxyhypocrellin. Liebigs Ann. Chem. 1981, 1981, 1880–1885. [Google Scholar]
- Wan, X.Y.; Chen, Y.T. Hypocrellin A-A new drug for photochemotherapy. Chin. Sci. Bull. 1981, 26, 1040–1042. [Google Scholar]
- Wan, X.Y.; Zhang, W.L.; Wang, Q.F. Isolation and identification of hypocrellin B from Hypocrella bambusae. J. Yunnan Univ. 1985, 4, 461–463. [Google Scholar]
- Diwu, Z.J.; Lown, J.W. Hypocrellins and their use in photosensitization. Photochem. Photobiol. 1990, 52, 609–616. [Google Scholar] [CrossRef]
- Du, W.; Liang, J.; Han, Y.; Yu, J.; Liang, Z. Nitric oxide mediates hypocrellin accumulation induced by fungal elicitor in submerged cultures of Shiraia bambusicola. Biotechnol. Lett. 2015, 37, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lao, X.F.; Wang, Q.W.; Lu, R.R.; Shen, C.; Zhang, F.; Liu, M.; Jia, L. The shiraiachromes: Novel fungal perylenequinone pigments from Shiraia bambusicola. J. Nat. Prod. 1989, 52, 948–951. [Google Scholar] [CrossRef]
- Kishi, T.; Tahara, S.; Taniguchi, N.; Tsuda, M.; Tanaka, C.; Takahashi, S. New perylenequinones from Shiraia bambusicola. Planta Med. 1991, 57, 376–379. [Google Scholar] [CrossRef]
- Fang, L.Z.; Qing, C.; Shao, H.J.; Yang, Y.D.; Dong, Z.J.; Wang, F.; Zhao, W.; Yang, W.Q.; Liu, J.K. Hypocrellin D, a cytotoxic fungal pigment from fruiting bodies of the ascomycete Shiraia bambusicola. J. Antibiot. 2006, 59, 351–354. [Google Scholar] [CrossRef]
- Su, Y.J.; Si, S.H.; Qiao, L.W.; Cai, Y.J.; Xu, Z.M.; Yang, Y.J. The effect of a hypocrellin A enriched diet on egg yolk quality and hypocrellin A distributions in the meat of laying hens. Eur. Food Res. Technol. 2011, 232, 935–940. [Google Scholar] [CrossRef]
- Khiralla, A.; Mohammed, A.O.; Yagi, S. Fungal perylenequinones. Mycol. Prog. 2022, 21, 38. [Google Scholar] [CrossRef]
- Bao, Z.; Xie, Y.; Xu, C.; Zhang, Z.; Zhu, D. Biotechnological production and potential applications of hypocrellins. Appl. Microbiol. Biotechnol. 2023, 107, 6421–6438. [Google Scholar] [CrossRef]
- Xie, W.; Wei, S.; Liu, J.; Ge, X.; Zhou, L.; Zhou, J.; Shen, J. Combination anticancer therapy activity studies for the complex of hypocrellin A and gallium ion. Dye. Pigment. 2014, 101, 43–50. [Google Scholar] [CrossRef]
- Hirayama, J.; Ikebuchi, K.; Abe, H.; Kwon, K.W.; Ohnishi, Y.; Horiuchi, M.; Shinagawa, M.; Ikuta, K.; Kamo, N.; Sekiguchi, S. Photoinactivation of virus infectivity by hypocrellin A. Photochem. Photobiol. 1997, 66, 697–700. [Google Scholar] [CrossRef]
- Hudson, J.B.; Zhou, J.; Chen, J.; Harris, L.; Yip, L.; Towers, G.H.N. Hypocrellin, from Hypocrella bambuase, is phototoxic to human immunodeficiency virus. Photochem. Photobiol. 1994, 60, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, K.I.; Higaki, K. Nanoparticle-Based Photodynamic Therapy: Current Status and Future Application to Improve Outcomes of Cancer Treatment. Chem. Pharm. Bull. 2017, 65, 637–641. [Google Scholar] [CrossRef]
- Guo, L.Y.; Yan, S.Z.; Li, Q.; Xu, Q.; Lin, X.; Qi, S.S.; Yu, S.Q.; Chen, S.L. Poly(lactic-co-glycolic) acid nanoparticles improve oral bioavailability of hypocrellin A in rat. RSC Adv. 2017, 7, 42073–42082. [Google Scholar] [CrossRef]
- Lin, X.; Yan, S.Z.; Qi, S.S.; Xu, Q.; Han, S.S.; Guo, L.Y.; Zhao, N.; Chen, S.L.; Yu, S.Q. Transferrin-Modified Nanoparticles for Photodynamic Therapy Enhance the Antitumor Efficacy of Hypocrellin A. Front. Pharmacol. 2017, 8, 815–831. [Google Scholar] [CrossRef]
- Mulrooey, C.A.; O’Brien, E.M.; Morgan, B.J.; Kozlowski, M.C. Perylenequinones: Isolation, Synthesis, and Biological Activity. Eur. J. Org. Chem. 2012, 21, 3887–3904. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.E.; Cho, H.J.; Yi, E.; Kim, D.D.; Jheon, S. Hypocrellin B and paclitaxel-encapsulated hyaluronic acid-ceramide nanoparticles for targeted photodynamic therapy in lung cancer. J. Photochem. Photobiol. B 2016, 158, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Leung, A.W.; Wang, X.; Zhang, H.; Xu, C. Effect of photodynamic therapy with hypocrellin B on apoptosis, adhesion, and migration of cancer cells. Int. J. Radiat. Biol. 2014, 90, 575–579. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, L. Photogeneration of singlet oxygen (1O2) and free radicals (Sen·−, O·−2) by tetra-brominated hypocrellin B derivative. Free Radic. Res. 2001, 35, 767–777. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, C.; Li, S.; Jiao, Y.; Qi, T.; Wei, G.; Han, G. Effects of Photodynamic Therapy Using Yellow LED-light with Concomitant Hypocrellin B on Apoptotic Signaling in Keloid Fibroblasts. Int. J. Biol. Sci. 2017, 13, 319–326. [Google Scholar] [CrossRef]
- Weiss, U.; Flon, H.; Burger, W.C. The photodynamic pigment of some species of Elsinoë and Sphaceloma. Arch. Biochem. Biophys. 1957, 69, 311–319. [Google Scholar] [CrossRef]
- Weiss, U.; Ziffer, H.; Batterham, T.J.; Blumer, M.; Hackeng, W.H.; Copier, H.; Salemink, C.A. Pigments of Elsinoë species. I. Pigment production by Elsinoë species; Isolation of pure elsinochromes A, B, C. Can. J. Microbiol. 1965, 11, 57–66. [Google Scholar] [CrossRef]
- Lousberg, R.J.J.C.; Paolillo, L.; Kon, H.; Weiss, U.; Salemink, C.A. Pigments of Elsinoe species. Part IV. Confirmatory evidence for the structure of elsinochrome A and its ethers from studies of nuclear magnetic resonance (solvent and overhauser effects) and electron spin resonance. J. Chem. Soc. C Org. 1970, 16, 2154–2159. [Google Scholar] [CrossRef]
- Ma, F.; Zhou, L.; Wang, W.; Feng, Y.Y.; Zhou, J.H.; Wang, X.H.; Shen, J. Spectroscopic studies on the interaction of Elsinochrome A with myoglobin. Spectrosc. Spect. Anal. 2011, 31, 1601–1605. [Google Scholar] [CrossRef]
- Li, T.; Deng, H.; Zhao, J.; Gu, Y. Elsinochrome A photosensitizers: Alternative drugs for photodynamic therapy. J. Innov. Opt. Health Sci. 2015, 8, 1530001–1530010. [Google Scholar] [CrossRef]
- Chooi, Y.H.; Zhang, G.; Hu, J.; Muria-Gonzalez, M.J.; Tran, P.N.; Pettitt, A.; Maier, A.G.; Barrow, R.A.; Solomon, P.S. Functional genomics-guided discovery of a light-activated phytotoxin in the wheat pathogen Parastagonospora nodorum via pathway activation. Environ. Microbiol. 2017, 19, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.Y.; Cheng, Y.L.; Cai, C.J.; Fan, L.; Gao, J.; Hou, C.L. Diversity and Antimicrobial Activity of Culturable Endophytic Fungi Isolated from Moso Bamboo Seeds. PLoS ONE 2014, 9, e95838. [Google Scholar] [CrossRef]
- Tong, X.; Wang, Q.T.; Shen, X.Y.; Hou, C.L.; Cannon, P.F. Phylogenetic Position of Shiraia-Like Endophytes on Bamboos and the Diverse Biosynthesis of Hypocrellin and Hypocrellin Derivatives. J. Fungi 2021, 7, 563. [Google Scholar] [CrossRef]
- Lu, Z.M.; Zhang, R.T.; Huang, X.B.; Cao, X.T.; Shen, X.Y.; Fan, L.; Hou, C.L. Optimisation of hypocrellin production in Shiraia-like fungi via genetic modification involving a transcription factor gene and a putative monooxygenase gene. Mycology 2023, 15, 272–281. [Google Scholar] [CrossRef]
- Cai, Y.; Liang, X.; Liao, X.; Ding, Y.; Sun, J.; Li, X. High-yield hypocrellin A production in solid-state fermentation by Shiraia sp. SUPER-H168. Appl. Biochem. Biotechnol. 2010, 160, 2275–2286. [Google Scholar] [CrossRef]
- Shen, X.Y.; Hu, Y.J.; Song, L.; Hou, C.L. Improvement of hypocrellin production by a new fungal source and optimization of cultivation conditions. Biotechnol. Biotechnol. Equip. 2016, 30, 819–826. [Google Scholar] [CrossRef]
- Liu, X.Y.; Shen, X.Y.; Fan, L.; Gao, J.; Hou, C.L. High-efficiency biosynthesis of hypocrellin A in Shiraia sp. using gamma-ray mutagenesis. Appl. Microbiol. Biotechnol. 2016, 100, 4875–4883. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Pan, W.; Zhao, Y.; Lei, X.; Chen, K.; Wang, J. Screening of higher hypocrellin A with strains of Shiraia bambusicola by genome-shuffling. Chin. J. Bioprocess Eng. 2012, 10, 25–29. [Google Scholar] [CrossRef]
- Liu, B.; Bao, J.; Zhang, Z.; Yan, R.; Wang, Y.; Yang, H.; Zhu, D. Enhanced production of perylenequinones in the endophytic fungus Shiraia sp. Slf14 by calcium/calmodulin signal transduction. Appl. Microbiol. Biotechnol. 2018, 102, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Liang, X.; Liu, J.; Zheng, X.; Fan, T.P.; Cai, Y. Advances and perspectives on perylenequinone biosynthesis. Front. Microbiol. 2022, 13, 1070110. [Google Scholar] [CrossRef]
- Shen, X.Y.; Cao, X.T.; Huang, X.B.; Zhuo, L.; Yang, H.M.; Fan, L.; Hou, C.L. Mitochondrial genome and transcription of Shiraia-like species reveal evolutionary aspects in protein-coding genes. IMA Fungus 2025, 16, e138572. [Google Scholar] [CrossRef]
- Cai, Y.; Liao, X.; Liang, X.; Ding, Y.; Sun, J.; Zhang, D. Induction of hypocrellin production by Triton X-100 under submerged fermentation with Shiraia sp. SUPER-H168. New Biotechnol. 2011, 28, 588–592. [Google Scholar] [CrossRef]
- López-Timoner, R.; Santos-Juanes, L.; Amat, A.M.; Arfelli, F.; Cespi, D.; Passarini, F.; Polo, M.I.; Zuriaga, E.; Arques, A. Life cycle assessment of UVC-based advanced oxidation processes as quaternary treatments: Clostridium spp. inactivation and comparison with CECs removal. Sci. Total Environ. 2025, 972, 179029. [Google Scholar] [CrossRef]
- Menoni, M.; Alcoba, P.; Zuluaga, M.J.; Peluffo, R.D. Generation of cellular reactive oxygen and nitrogen species by exposure to ultraviolet radiation. Biophys. Rev. 2025, 17, 547–560. [Google Scholar] [CrossRef]
- Deng, H.; Chen, J.; Gao, R.; Liao, X.; Cai, Y. Adaptive Responses to Oxidative Stress in the Filamentous Fungal Shiraia bambusicola. Molecules 2016, 21, 1118. [Google Scholar] [CrossRef]
- Wang, W.J.; Li, X.P.; Shen, W.H.; Huang, Q.Y.; Cong, R.P.; Zheng, L.P.; Wang, J.W. Nitric oxide mediates red light-induced perylenequinone production in Shiraia mycelium culture. Bioresour. Bioprocess. 2024, 11, 2. [Google Scholar] [CrossRef]
- Wu, X.; Meng, X.; Xiao, Y.; Yang, H.; Zhang, Z.; Zhu, D. Energy Metabolism Enhance Perylenequinone Biosynthesis in Shiraia sp. Slf14 through Promoting Mitochondrial ROS Accumulation. Int. J. Mol. Sci. 2024, 25, 10113. [Google Scholar] [CrossRef] [PubMed]
- Beseli, A.; Noar, R.; Daub, M.E. Characterization of Cercospora nicotianae Hypothetical Proteins in Cercosporin Resistance. PLoS ONE 2015, 10, e0140676. [Google Scholar] [CrossRef]
- Tong, Z.W.; Mao, L.; Liang, H.; Zhang, Z.; Wang, Y.; Yan, R.; Zhu, D. Simultaneous Determination of Six Perylenequinones in Shiraiaia sp. Slf14 by HPLC. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 536–540. [Google Scholar] [CrossRef]
- Liu, X.Y.; Fan, L.; Gao, J.; Shen, X.Y.; Hou, C.L. Global identification of alternative splicing in Shiraia bambusicola and analysis of its regulation in hypocrellin biosynthesis. Appl. Microbiol. Biotechnol. 2020, 104, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Brandsberg, J.W.; French, M.E. In vitro susceptibility of isolates of Aspergillus fumigatus and Sporothrix schenckii to amphotericin B. Antimicrob. Agents Chemother. 1972, 2, 402–404. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Liu, J.Z.; Huang, J.S.; Mao, Z.W. Genome shuffling of Propionibacterium shermanii for improving vitamin B12 production and comparative proteome analysis. J. Biotechnol. 2010, 148, 139–143. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Zhao, X.; Ling, J. Genome shuffling improved the nucleosides production in Cordyceps kyushuensis. J. Biotechnol. 2017, 260, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fang, R.; Feng, R.; Li, Q.; Su, M.; Hou, C.; Zhuang, K.; Dai, Y.; Lei, N.; Jiang, Y.; et al. Cage-modified hypocrellin against multidrug-resistant Candida spp. with unprecedented activity in light-triggered combinational photodynamic therapy. Drug Resist. Updates 2022, 65, 100887. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Khan, S.I.; Jacob, M.R.; Tekwani, B.L.; Li, Z.; Pasco, D.S.; Walker, L.A.; Khan, I.A. Antimicrobial and antileishmanial activities of hypocrellins A and B. Antimicrob. Agents Chemother. 2004, 48, 4450–4452. [Google Scholar] [CrossRef] [PubMed]

| Time (min) | 0.1% Phosphoric Acid Water (%, v/v) | Methanol (%, v/v) |
|---|---|---|
| 0–10 | 40 | 60 |
| 10–15 | 40–30 | 60–70 |
| 15–25 | 30 | 70 |
| 25–45 | 30–25 | 70–75 |
| 45–60 | 25–0 | 75–100 |
| 60–80 | 0 | 100 |
| UV Intensity (J/m2) | Strain Names |
|---|---|
| 100 | Z1-1, Z1-2, Z1-3, Z1-4, Z1-5 |
| 120 | Z2-1, Z2-2, Z2-3, Z2-4, Z2-5 |
| 150 | Z3-1, Z3-2, Z3-3, Z3-4, Z3-5 |
| 300 | Z4-1, Z4-2, Z4-3, Z4-4, Z4-5 |
| 800 | Z5-1, Z5-2, Z5-3, Z5-4, Z5-5 |
| Strains | HA | HB | SA | EA | EB | Total Content |
|---|---|---|---|---|---|---|
| WT | 6.16 ± 1.38 | 3.67 ± 1.83 | 3.64 ± 0.82 | 4.32 ± 0.44 | 1.29 ± 0.06 | 19.09 ± 4.54 |
| Z1-2 | 11.78 ± 1.01 | 7.64 ± 1.24 | 7.26 ± 0.70 | 7.66 ± 0.63 | 0.57 ± 0.25 | 34.91 ± 3.81 |
| Z2-1 | 110.81 ± 7.69 | 19.53 ± 3.65 | 45.42 ± 3.43 | 27.71 ± 2.77 | 8.09 ± 1.56 | 211.57 ± 19.10 |
| Z3-1 | 23.35 ± 2.47 | 34.44 ± 10.25 | 13.41 ± 1.56 | 31.06 ± 3.10 | 1.60 ± 0.31 | 103.87 ± 17.69 |
| Z4-1 | 10.49 ± 2.10 | 2.70 ± 0.78 | 5.17 ± 0.58 | 6.89 ± 1.23 | 1.26 ± 0.13 | 26.50 ± 4.81 |
| Z5-1 | 3.23 ± 4.79 | 1.30 ± 1.49 | 1.63 ± 2.30 | 1.72 ± 2.79 | 0.71 ± 0.64 | 8.59 ± 12.01 |
| Strains | HA | HB | SA | EA | EB | Total Content |
|---|---|---|---|---|---|---|
| WT | 84.15 ± 18.89 | 50.15 ± 25.06 | 49.79 ± 11.20 | 59.08 ± 14.34 | 17.67 ± 0.79 | 260.84 ± 70.28 |
| Z1-2 | 153.18 ± 13.10 | 99.29 ± 16.12 | 94.34 ± 9.04 | 99.61 ± 8.14 | 7.39 ± 3.20 | 453.81 ± 49.59 |
| Z2-1 | 1100.70 ± 76.40 | 194.02 ± 36.21 | 451.20 ± 34.04 | 275.28 ± 27.50 | 80.40 ± 15.49 | 2101.60 ± 189.64 |
| Z3-1 | 235.08 ± 24.83 | 346.68 ± 103.25 | 135.04 ± 15.72 | 312.68 ± 31.23 | 16.12 ± 3.11 | 1045.60 ± 178.14 |
| Z4-1 | 134.91 ± 27.02 | 34.75 ± 10.06 | 66.46 ± 7.43 | 88.59 ± 15.82 | 16.23 ± 1.62 | 340.94 ± 61.95 |
| Z5-1 | 42.91 ± 63.56 | 17.20 ± 19.79 | 21.63 ± 30.50 | 22.87 ± 37.09 | 9.38 ± 8.51 | 113.99 ±159.43 |
| Generation Numbers | Mutant Strain Z2-1 (mg/L) | Rate of Change (%) | Mutant Strain Z3-1 (mg/L) | Rate of Change (%) |
|---|---|---|---|---|
| 1 | 2101.6 | 0 | 1045.6 | 0 |
| 2 | 2198.7 | 4.62 | 1094.3 | 4.66 |
| 3 | 2237.5 | 6.47 | 1147.8 | 9.77 |
| 4 | 2045 | −2.69 | 978.2 | −6.45 |
| 5 | 2219.2 | 5.60 | 1121.8 | 7.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, X.; Shen, X.-Y.; Huang, M.-R.; Hou, C.-L. Enhancement of Perylenequinonoid Compounds Production from Strain of Pseudoshiraia conidialis by UV-Induced Mutagenesis. Microorganisms 2025, 13, 1999. https://doi.org/10.3390/microorganisms13091999
Tong X, Shen X-Y, Huang M-R, Hou C-L. Enhancement of Perylenequinonoid Compounds Production from Strain of Pseudoshiraia conidialis by UV-Induced Mutagenesis. Microorganisms. 2025; 13(9):1999. https://doi.org/10.3390/microorganisms13091999
Chicago/Turabian StyleTong, Xin, Xiao-Ye Shen, Man-Rong Huang, and Cheng-Lin Hou. 2025. "Enhancement of Perylenequinonoid Compounds Production from Strain of Pseudoshiraia conidialis by UV-Induced Mutagenesis" Microorganisms 13, no. 9: 1999. https://doi.org/10.3390/microorganisms13091999
APA StyleTong, X., Shen, X.-Y., Huang, M.-R., & Hou, C.-L. (2025). Enhancement of Perylenequinonoid Compounds Production from Strain of Pseudoshiraia conidialis by UV-Induced Mutagenesis. Microorganisms, 13(9), 1999. https://doi.org/10.3390/microorganisms13091999






