BCL-2 Multi-Strain Probiotics for Immunomodulation In Vitro and In Vivo Alleviation of Atopic Dermatitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Preparation
2.2. In Vitro Cell Culture and Cytokine Analysis
2.3. Animals and Experimental Design
2.4. Body and Spleen Weight and Clinical Signs Assessment
2.5. Flow Cytometry Analysis
2.6. Inflammatory Cytokine Gene Expression Analysis
2.7. Evaluation of Histopathological Lesions
2.8. Statistical Analysis
3. Results
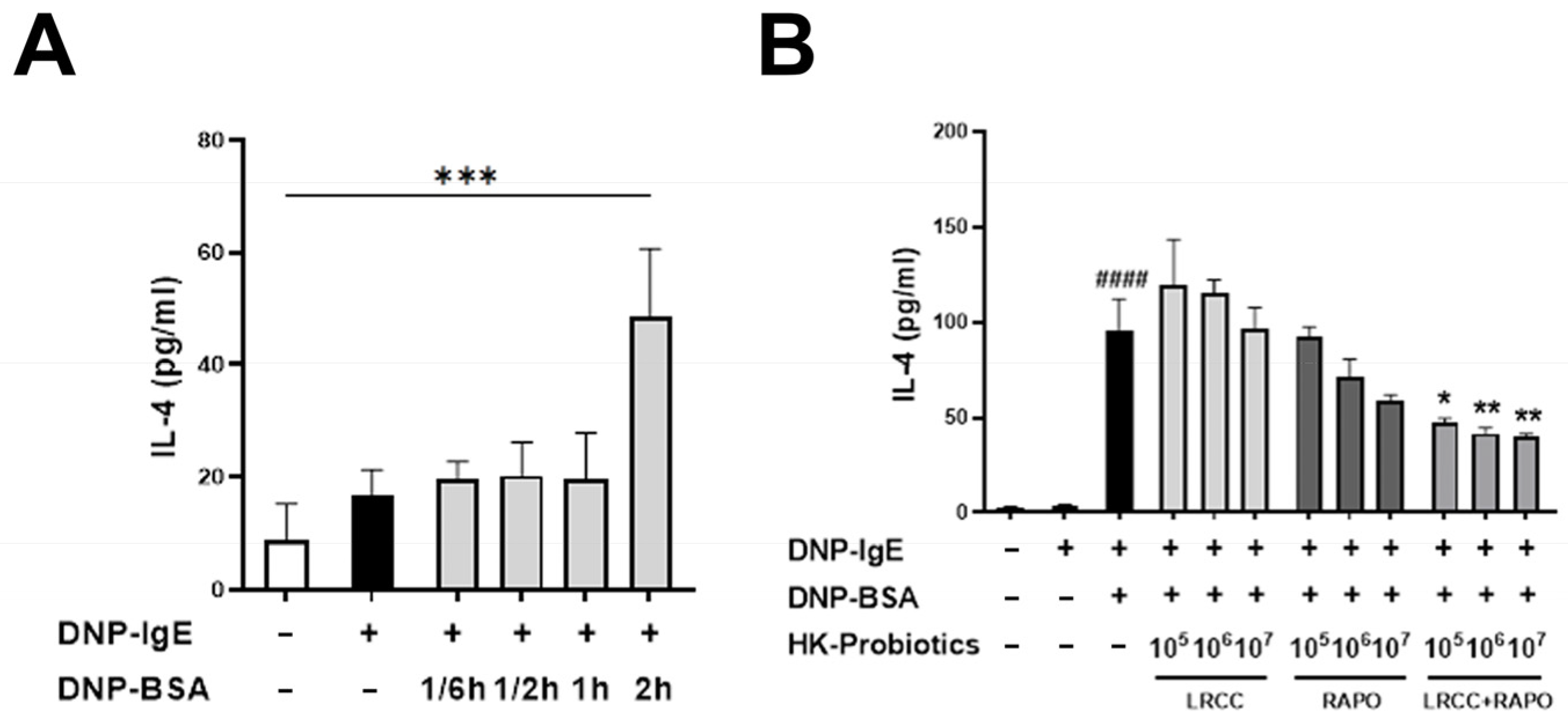
3.1. Synergistic Anti-Allergic Effects of LRCC and RAPO in RBL-2H3 Cells
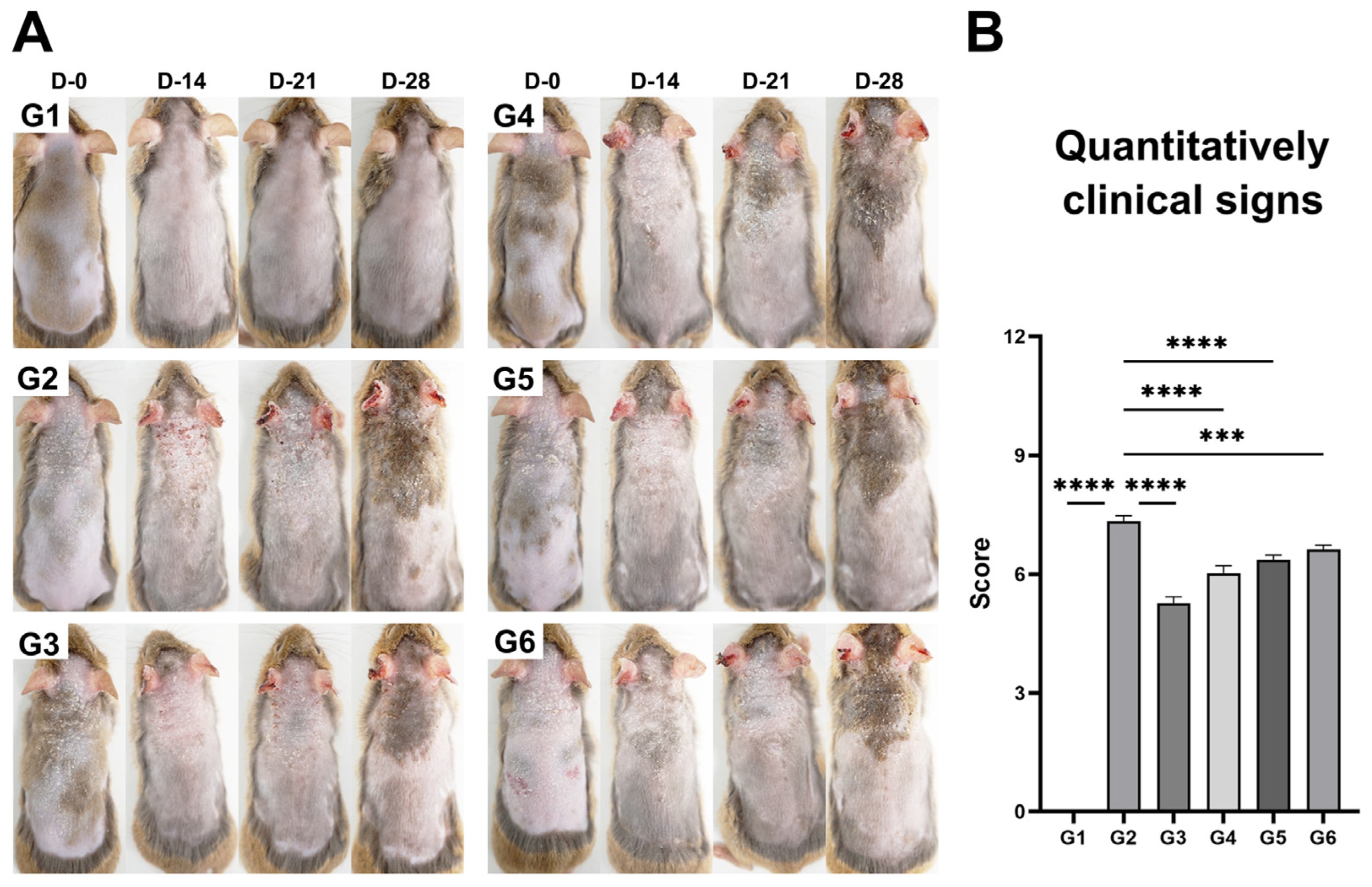
3.2. Evaluation of Body and Spleen Weight and Clinical Signs
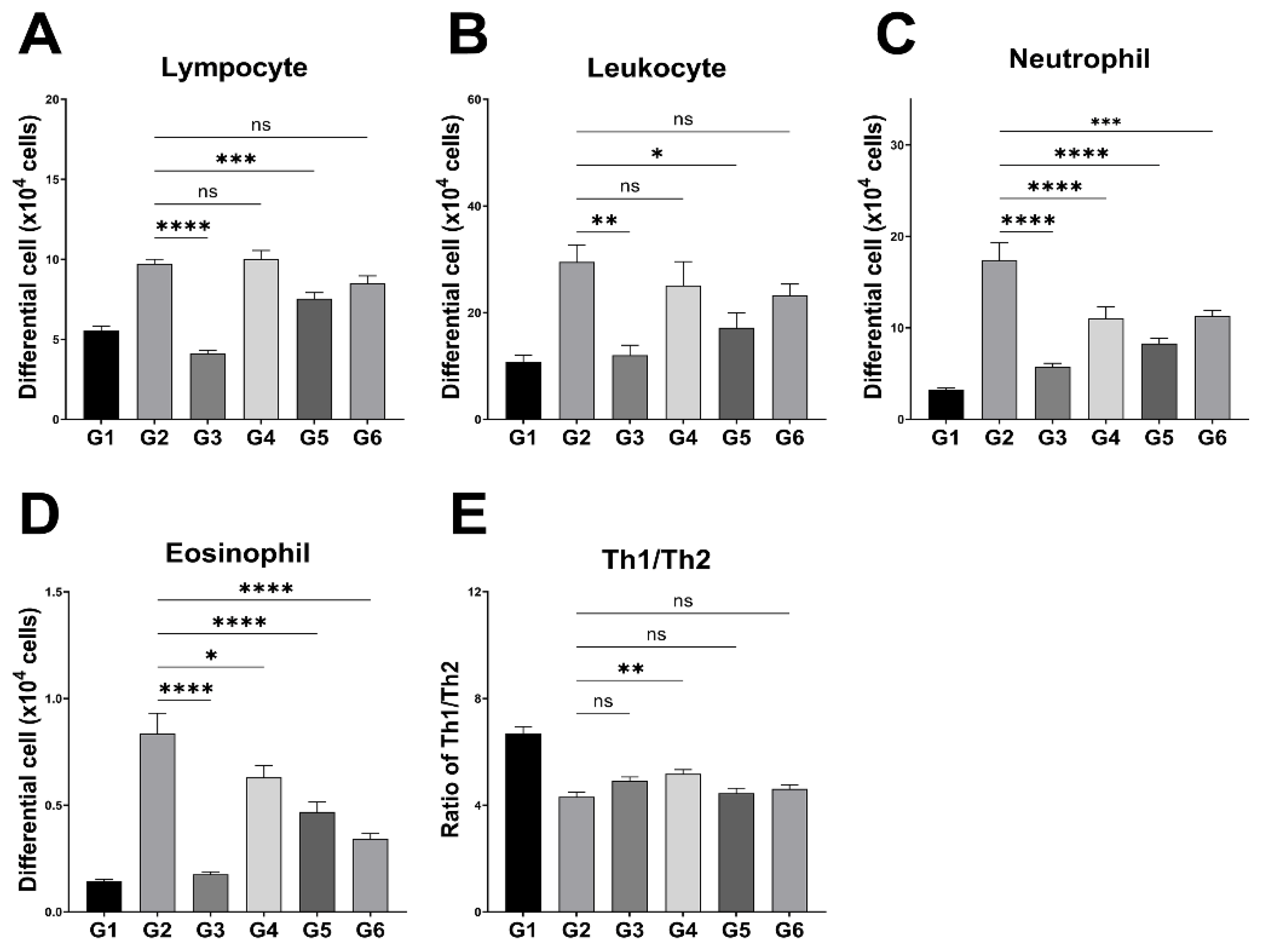
3.3. Peripheral Leukocyte Profiles and Splenic Th1/Th2 Ratio
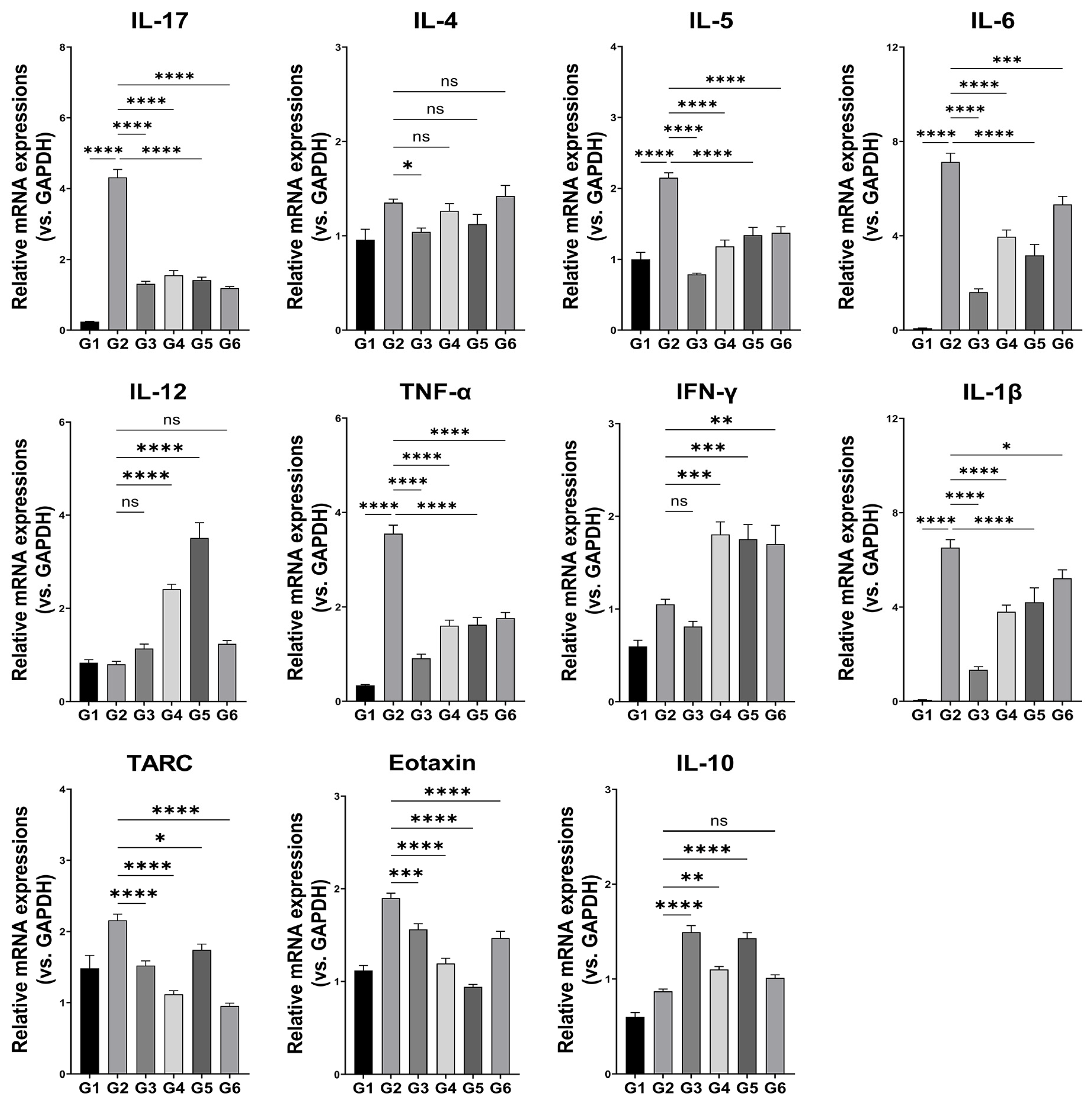
3.4. Inflammatory Cytokine mRNA Expression
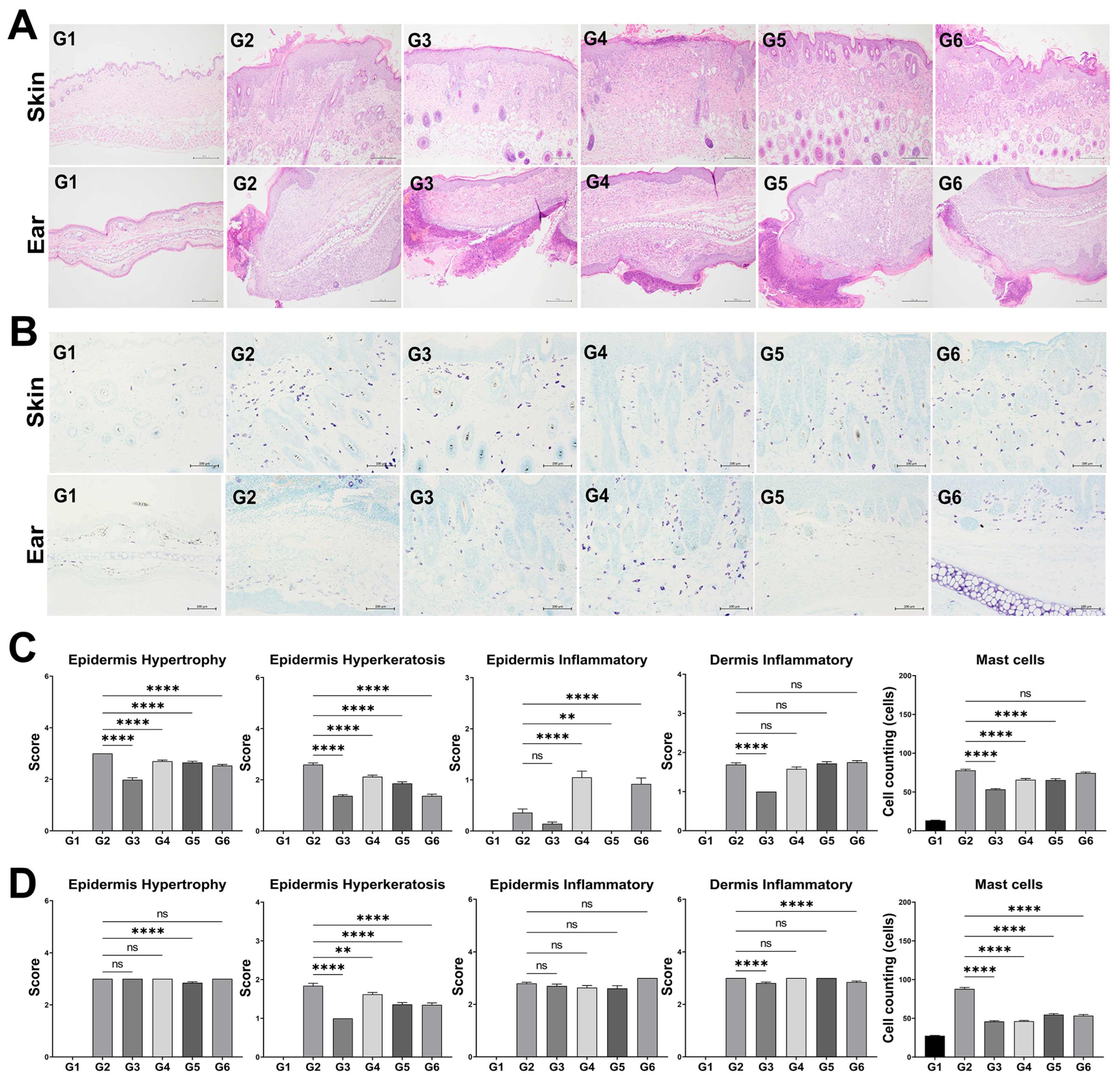
3.5. Histological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Atopic Dermatitis |
| ANOVA | Analysis of Variance |
| BL-RAPO | Bifidobacterium longum RAPO |
| DNP | Dinitrophenyl |
| DNP-BSA | Dinitrophenyl–Bovine Serum Albumin |
| EDTA | Ethylenediaminetetraacetic Acid |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EMEM | Eagle’s Minimum Essential Medium |
| FBS | Fetal Bovine Serum |
| LP-5264 | Lactiplantibacillus plantarum LRCC5264 |
| MRS | de Man, Rogosa, and Sharpe (agar/broth) |
| PBS | Phosphate-Buffered Saline |
| PMA | Phorbol 12-Myristate 13-Acetate |
| PRR | Pattern Recognition Receptor |
| SDS | Sodium Dodecyl Sulfate |
| SEM | Standard Error of the Mean |
| TLR | Toll-Like Receptor |
| TOS | Transgalactooligosaccharide |
References
- Biedermann, T.; Skabytska, Y.; Kaesler, S.; Volz, T. Regulation of T cell immunity in atopic dermatitis by microbes: The Yin and Yang of cutaneous inflammation. Front. Immunol. 2015, 6, 353. [Google Scholar] [CrossRef]
- Wasserer, S.; Jargosch, M.; Mayer, K.E.; Eigemann, J.; Raunegger, T.; Aydin, G.; Eyerich, S.; Biedermann, T.; Eyerich, K.; Lauffer, F. Characterization of high and low IFNG-expressing subgroups in atopic dermatitis. Int. J. Mol. Sci. 2024, 25, 6158. [Google Scholar] [CrossRef]
- Sugaya, M. The role of Th17-related cytokines in atopic dermatitis. Int. J. Mol. Sci. 2020, 21, 1314. [Google Scholar] [CrossRef]
- Facheris, P.; Jeffery, J.; Del Duca, E.; Guttman-Yassky, E. The translational revolution in atopic dermatitis: The paradigm shift from pathogenesis to treatment. Cell Mol. Immunol. 2023, 20, 448–474. [Google Scholar] [CrossRef]
- Wollenberg, A.; Thomsen, S.F.; Lacour, J.-P.; Jaumont, X.; Lazarewicz, S. Targeting immunoglobulin E in atopic dermatitis: A review of the existing evidence. World Allergy Organ J. 2021, 14, 100519. [Google Scholar] [CrossRef]
- Wang, H.H.; Li, Y.C.; Huang, Y.C. Efficacy of omalizumab in patients with atopic dermatitis: A systematic review and meta-analysis. J. Allergy Clin. Immunol. 2016, 138, 1719–1722.e1. [Google Scholar] [CrossRef]
- Yu, S.H.; Drucker, A.M.; Lebwohl, M.; Silverberg, J.I. A systematic review of the safety and efficacy of systemic corticosteroids in atopic dermatitis. J. Am. Acad. Dermatol. 2018, 78, 733–740.e11. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of probiotics on gut microbiota: An overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Kim, H.W.; Hong, R.; Choi, E.Y.; Yu, K.; Kim, N.; Hyeon, J.Y.; Cho, K.K.; Choi, I.S.; Yun, C.-H. A probiotic mixture regulates T cell balance and reduces atopic dermatitis symptoms in mice. Front. Microbiol. 2018, 9, 2414. [Google Scholar] [CrossRef]
- Eslami, M.; Bahar, A.; Keikha, M.; Karbalaei, M.; Kobyliak, N.M.; Yousefi, B. Probiotics function and modulation of the immune system in allergic diseases. Allergol. Immunopathol. 2020, 48, 771–788. [Google Scholar] [CrossRef]
- An, S.B.; Yang, B.-G.; Jang, G.; Kim, D.-Y.; Kim, J.; Oh, S.-M.; Oh, N.; Lee, S.; Moon, J.-Y.; Kim, J.-A.; et al. Combined IgE neutralization and Bifidobacterium longum supplementation reduces the allergic response in models of food allergy. Nat. Commun. 2022, 13, 5669. [Google Scholar] [CrossRef]
- Jeong, Y.; Jhun, J.; Lee, S.-Y.; Na, H.S.; Choi, J.; Cho, K.-H.; Lee, S.Y.; Lee, A.R.; Park, S.-J.; You, H.J.; et al. Therapeutic potential of a novel Bifidobacterium identified through microbiome profiling of RA patients with different RF levels. Front. Immunol. 2021, 12, 736196. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, B.K.; Park, H.J.; Park, Y.H.; Kim, B.O.; Pyo, S. Atopic dermatitis-mitigating effects of new Lactobacillus strain, Lactobacillus sakei probio 65 isolated from Kimchi. J. Appl. Microbiol. 2013, 115, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Kim, J.-E.; Yoon, Y.-S.; Kim, T.H.; Seo, J.-G.; Chung, M.-J.; Yum, D.-Y. Improvement of atopic dermatitis-like skin lesions by IL-4 inhibition of P14 protein isolated from Lactobacillus casei in NC/Nga mice. Appl. Microbiol. Biotechnol. 2015, 99, 7089–7099. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.H.; Lim, J.; Shin, D.H.; Maeng, J.; Lee, K. Dimerized translationally controlled tumor protein-binding peptide ameliorates atopic dermatitis in NC/Nga mice. Int. J. Mol. Sci. 2017, 18, 256. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, M.J.; Bak, D.H.; Lee, B.C.; Ko, E.J.; Ahn, G.R.; Ahn, S.W.; Kim, M.J.; Na, J.; Kim, B.J. Topical administration of EGF suppresses immune response and protects skin barrier in DNCB-induced atopic dermatitis in NC/Nga mice. Sci. Rep. 2018, 8, 11895. [Google Scholar] [CrossRef]
- Islamzada, E.; Matthews, K.; Lamoureux, E.S.; Duffy, S.P.; Scott, M.D.; Ma, H. Red blood cells with reduced deformability are selectively cleared from circulation in a mouse model. Blood Adv. 2025, 9, 2988–2996. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Ju, D.B.; Kye, Y.-C.; Ju, Y.-J.; Kim, C.G.; Lee, I.K.; Park, S.-M.; Choi, I.S.; Cho, K.K.; Lee, S.H.; et al. Galectin-9 induced by dietary probiotic mixture regulates immune balance to reduce atopic dermatitis symptoms in mice. Front. Immunol. 2019, 10, 3063. [Google Scholar] [CrossRef]
- Falda, A.; Doretto, P. Automated analysis for differentiating leukocytes in body fluids using the software “biological liquid application” on ADVIA2120/2120i hematology analyzer. J. Clin. Lab. Anal. 2018, 32, e22578. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.; Kim, W. Cream cheese-derived Lactococcus chungangensis CAU 28 modulates the gut microbiota and alleviates atopic dermatitis in BALB/c mice. Sci. Rep. 2019, 9, 446, Erratum in Sci. Rep. 2019, 9, 7001. [Google Scholar] [CrossRef]
- Yin, Y.; Mitson-Salazar, A.; Prussin, C. Detection of intracellular cytokines by flow cytometry. Curr. Protoc. Immunol. 2015, 110, 6.24.1–6.24.18. [Google Scholar] [CrossRef]
- Alam, M.J.; Xie, L.; Yap, Y.-A.; Robert, R. A mouse model of MC903-induced atopic dermatitis. Curr. Protoc. 2023, 3, e695. [Google Scholar] [CrossRef]
- Jung, Y.O.; Jeong, H.; Cho, Y.; Lee, E.O.; Jang, H.W.; Kim, J.; Nam, K.T.; Lim, K.-M. Lysates of a probiotic, Lactobacillus rhamnosus, can improve skin barrier function in a reconstructed human epidermis model. Int. J. Mol. Sci. 2019, 20, 4289. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Kohno, K.; Inoue, S.; Koya-Miyata, S.; Okamoto, I.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Oral administration of royal jelly inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. Int. Immunopharmacol. 2003, 3, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Motulsky, H. Intuitive Biostatistics: A Nonmathematical Guide to Statistical Thinking, 4th ed.; Oxford University Press: New York, NY, USA, 2018. [Google Scholar]
- Sichetti, M.; De Marco, S.; Pagiotti, R.; Traina, G.; Pietrella, D. Anti-inflammatory effect of multistrain probiotic formulation (L. rhamnosus, B. lactis, and B. longum). Nutrition 2018, 53, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, N.; Irvine, A.D. Filaggrin and beyond: New insights into the skin barrier in atopic dermatitis and allergic diseases, from genetics to therapeutic perspectives. Ann. Allergy Asthma Immunol. 2024, 132, 187–195. [Google Scholar] [CrossRef]
- Kwon, M.S.; Lim, S.K.; Jang, J.Y.; Lee, J.; Park, H.K.; Kim, N.; Yun, M.; Shin, M.-Y.; Jo, H.E.; Oh, Y.J.; et al. Lactobacillus sakei WIKIM30 ameliorates atopic dermatitis-like skin lesions by inducing regulatory T cells and altering gut microbiota structure in mice. Front. Immunol. 2018, 9, 1905. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chen, Y.T.; Li, K.Y.; Huang, H.W.; Lin, Y.C.; Chen, M.J. A heat-killed probiotic mixture regulates immune T cells balance and IgE production in house dust mite extraction-induced atopic dermatitis mice. Microorganisms 2022, 10, 1881. [Google Scholar] [CrossRef]
- He, H.; Del Duca, E.; Diaz, A.; Kim, H.J.; Gay-Mimbrera, J.; Zhang, N.; Wu, J.; Beaziz, J.; Estrada, Y.; Krueger, J.G.; et al. Mild atopic dermatitis lacks systemic inflammation and shows reduced nonlesional skin abnormalities. J. Allergy Clin. Immunol. 2021, 147, 1369–1380. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Maurelli, M.; Peris, K.; Girolomoni, G. Targeting IL-4 for the treatment of atopic dermatitis. Immunotargets Ther. 2020, 9, 151–156. [Google Scholar] [CrossRef]
- Kakinuma, T.; Nakamura, K.; Wakugawa, M.; Mitsui, H.; Tada, Y.; Saeki, H.; Torii, H.; Asahina, A.; Onai, N.; Matsushima, K.; et al. Thymus and activation-regulated chemokine in atopic dermatitis: Serum thymus and activation-regulated chemokine level is closely related with disease activity. J. Allergy Clin. Immunol. 2001, 107, 535–541. [Google Scholar] [CrossRef]
- Yamamura, Y.; Nakashima, C.; Otsuka, A. Interplay of cytokines in the pathophysiology of atopic dermatitis: Insights from murine models and humans. Front. Med. 2024, 11, 1342176. [Google Scholar] [CrossRef]
- Romagnani, P.; Annunziato, F.; Piccinni, M.P.; Maggi, E.; Romagnani, S. Th1/Th2 cells, their associated molecules, and role in pathophysiology. Eur. Cytokine Netw. 2000, 11, 510–511. [Google Scholar] [PubMed]
- Eom, J.E.; Shin, D.U.; Kim, G.D.; Yoon, J.H.; Shin, H.S.; Lee, S.Y. Pediococcus pentosaceus KF159 alleviates house dust mite-induced atopic dermatitis by promoting IL-10 production and regulatory T cell induction. Food Funct. 2024, 15, 6975–6987. [Google Scholar] [CrossRef] [PubMed]
- McAleer, M.A.; Irvine, A.D. The multifunctional role of filaggrin in allergic skin disease. J. Allergy Clin. Immunol. 2013, 131, 280–291. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Criteria Description | Score |
|---|---|---|
| Hypertrophy | No hypertrophy | 0 |
| Mild hypertrophy (slight thickening of the epidermis) | 1 | |
| Moderate hypertrophy (increased epidermal thickness) | 2 | |
| Severe hypertrophy (marked thickening of the epidermis) | 3 | |
| Hyperkeratosis | No hyperkeratosis | 0 |
| Mild hyperkeratosis (minimal keratin layer) | 1 | |
| Moderate hyperkeratosis (evident keratin layer) | 2 | |
| Severe hyperkeratosis (thickened, compact keratin layer) | 3 | |
| Inflammation | No inflammatory cell infiltration | 0 |
| Mild infiltration (few inflammatory cells in the dermis) | 1 | |
| Moderate infiltration (scattered inflammatory cells) | 2 | |
| Severe infiltration (dense aggregation of inflammatory cells) | 3 |
| Animal Groups | Body Weight/Experimental Duration (g/Days) | Organ Weight/Day28 | |||||
|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | Spleen (g) | % (vs. BW) | |
| G1 | 24.0 ± 0.4 | 24.8 ± 0.4 | 25.3 ± 0.4 | 25.9 ± 0.2 | 26.3 ± 0.3 | 0.1 ± 0.0 | 0.3 ± 0.0 |
| G2 | 23.7 ± 0.3 | 24.0 ± 0.5 | 24.2 ± 0.4 | 24.5 ± 0.4 | 24.9 ± 0.5 | 0.2 ± 0.0 *** | 0.7 ± 0.1 *** |
| G3 | 24.6 ± 0.7 | 23.4 ± 0.7 | 23.1 ± 0.5 ** | 23.0 ± 0.5 *** | 23.0 ± 0.5 ***,# | 0.1 ± 0.0 # | 0.2 ± 0.0 # |
| G4 | 22.7 ± 0.5 | 23.1 ± 0.4 | 23.6 ± 0.3 * | 23.9 ± 0.4 ** | 24.3 ± 0.5 ** | 0.1 ± 0.0 | 0.5 ± 0.0 |
| G5 | 24.8 ± 0.6 | 25.0 ± 0.7 | 25.0 ± 0.6 | 25.1 ± 0.6 | 25.3 ± 0.5 | 0.1 ± 0.0 | 0.5 ± 0.0 |
| G6 | 23.8 ± 0.4 | 24.3 ± 0.3 | 24.7 ± 0.2 | 24.5 ± 0.3 | 25.4 ± 0.3 | 0.1 ± 0.0 | 0.5 ± 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, M.; Sim, S.; Lim, A.; Moon, J.S.; Jeon, J.; Heo, K.; Kwak, W.; Park, M.S.; Kwak, J.; Park, E.; et al. BCL-2 Multi-Strain Probiotics for Immunomodulation In Vitro and In Vivo Alleviation of Atopic Dermatitis. Microorganisms 2025, 13, 1950. https://doi.org/10.3390/microorganisms13081950
Sung M, Sim S, Lim A, Moon JS, Jeon J, Heo K, Kwak W, Park MS, Kwak J, Park E, et al. BCL-2 Multi-Strain Probiotics for Immunomodulation In Vitro and In Vivo Alleviation of Atopic Dermatitis. Microorganisms. 2025; 13(8):1950. https://doi.org/10.3390/microorganisms13081950
Chicago/Turabian StyleSung, MinKyung, Seongrok Sim, Ahyoung Lim, Jin Seok Moon, JongIk Jeon, Keon Heo, Woongkwon Kwak, Myeong Soo Park, Jungki Kwak, EunYoung Park, and et al. 2025. "BCL-2 Multi-Strain Probiotics for Immunomodulation In Vitro and In Vivo Alleviation of Atopic Dermatitis" Microorganisms 13, no. 8: 1950. https://doi.org/10.3390/microorganisms13081950
APA StyleSung, M., Sim, S., Lim, A., Moon, J. S., Jeon, J., Heo, K., Kwak, W., Park, M. S., Kwak, J., Park, E., & Yoon, S. (2025). BCL-2 Multi-Strain Probiotics for Immunomodulation In Vitro and In Vivo Alleviation of Atopic Dermatitis. Microorganisms, 13(8), 1950. https://doi.org/10.3390/microorganisms13081950







