Abstract
Accurate label claims are essential for consumer trust in probiotic efficacy, yet limited datasets are available for poultry formulations marketed in the United Kingdom. We quantified and identified the viable bacteria in twelve commercial probiotics, seven for poultry and five for human use, using selective plate counts and MALDI-TOF MS. Observed colony forming units (CFU) were compared with declared values using one-sample t-tests, adopting a practical acceptance range of ±0.5 log CFU. Poultry products largely met or exceeded their labels (e.g., P5: 1.4 × 1010 CFU g−1 vs. 2 × 109 CFU g−1 declared), whereas human products delivered greater variability in both species composition and stated CFU count; one contained no detectable viable bacteria. All products deviated significantly from their label claims (p < 0.05); however, 11 of 12 met the ±0.5 log10 CFU benchmark—10 within the range and 1 above its “≥” value—leaving only one probiotic below the threshold. MALDI-TOF MS confirmed the presence of most labelled species, though Bifidobacterium bifidum was absent from one human product and Bacillus isolates were re-assigned to B. velezensis/B. amyloliquefaciens. These findings indicate robust quality assurance in UK poultry probiotics, but substantial under-delivery in the human probiotics, underscoring the need for harmonized viability standards and tighter post-market surveillance.
1. Introduction
According to the FAO/WHO, probiotics are defined as “live microorganisms which, when consumed in adequate amounts, confer health benefits on the host” [1]. The presence of viable microorganisms is therefore crucial [2]. Beneficial bacteria must remain alive in commercial products to deliver effects such as improving gut health, stimulating immunity, competing with pathogens and producing bioactive metabolites [2,3].
Label claims for viable counts and species, however, are often unreliable because most probiotic products are regulated as foods rather than medicines [4]. Numerous surveys of human probiotics have documented large discrepancies between declared and observed viable counts, with some products lacking the stated species altogether [2,5,6,7,8,9,10]. By contrast, data on poultry probiotics, particularly those marketed in the United Kingdom, remain scarce. The only UK study identified evaluated seven human products in 2016; fewer than half of those met their declared culture concentrations [11]. No recent, systematic assessment of poultry formulations has been published yet.
Enumeration of probiotic organisms is typically culture-dependent, employing selective agar media to determine colony-forming units (CFU) per dose [2,7,8,9,12,13]. Plate counting is the sole International Organization for Standardization (ISO)-validated standard for probiotic enumeration [14] and is endorsed by the European Commission for use in animal-feed research [15]. For species confirmation, matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF MS) offers rapid, reliable and cost-effective identification and is now routinely applied in diagnostic microbiology [16,17,18].
This study therefore evaluated the label accuracy of twelve UK-market probiotics—seven formulated for poultry and five for humans—using plate enumeration on selective agars and MALDI-TOF MS. We hypothesized that (i) observed viable counts would be significantly different than declared values and (ii) some labelled species would be absent. The results provide a much-needed baseline for functional studies and for post-market quality control of commercial probiotics before their dietary use.
2. Materials and Methods
2.1. Ethics Statement
Ethical approval was obtained from the Animal Welfare and Ethical Review Body at Newcastle University, United Kingdom (Project ID #948).
2.2. Probiotic Products Selection and Handling
Twelve commercial probiotic products (coded P1–P12; seven intended for poultry and five for human use) were obtained in the United Kingdom via commonly used online sources available to UK consumers for both human and poultry probiotics. Selection was by convenience sampling, stratified to cover both applications (human and poultry), multiple formulation types (capsule, tablet/chewable, powder) and differing taxa/technologies (non-spore-forming lactic acid bacteria and spore-forming Bacillus; single- and multi-strain).
For each product, one commercial batch was analysed. From that batch, technical replicates were prepared in parallel (n = 3 for multi-strain products; n = 4 for single-strain products). Lot numbers and expiry dates were recorded prior to analysis. All products were factory-sealed on arrival and tested before expiry. To ensure the accurate growth and identification of the target organisms, specific selective media and incubation conditions were used, as detailed in Table 1. Declared total counts and labelled species are listed in Table 2 (poultry probiotics) and Table 3 (human probiotics). Storage metadata (expiry date, label storage instruction, actual storage temperature and storage time prior to testing) are provided in Supplementary Table S1 and cross-referenced to product codes and their intended use (human or poultry) in Table 4.
Products were stored in their original packaging and under the manufacturer’s recommended storage conditions.
2.3. Optimisation of Selective Media and Incubation Conditions
Agar media were prepared according to manufacturer’s instructions. Molten agar (~55 °C) was poured into sterile 90 mm three-vent Petri dishes (Thermo Scientific, Newport, UK, P11309283) within a Class II microbiological safety cabinet (MSC 12, Jouan SA, Saint-Herblain, France). Selective supplements (e.g., vancomycin, lithium-mupirocin) were applied as detailed in Table 1, which also lists the target organisms and their incubation conditions.
A short pilot study was run for every probiotic to assess growth and selectivity on the chosen selective agars and to establish their appropriate dilution range. For products P9 and P12 (containing Lactobacillus salivarius, Bifidobacterium animalis and Enterococcus faecium), standard Bifidobacterium Selective Medium (BSM) agar plus BSM supplement supported B. animalis but also permitted E. faecium growth; selectivity was restored by adding lithium-mupirocin (5 mL/100 mL), as previously reported [19]. Conversely, supplementing MRS (De Man–Rogosa–Sharpe) agar with vancomycin (20 mg L−1) inhibited E. faecium while allowing L. salivarius to grow [20].
For Bacillus spp. products, Tryptone Soya Agar (TSA) plates were pre-incubated at 37 °C for approximately 13 h, then inoculated and cultured at 33 °C instead of 37 °C. This two-step protocol prevented B. subtilis swarming and enabled accurate enumeration; swarming was not observed under the adjusted conditions.

Table 1.
Agar media and incubation conditions for the assessed probiotic bacteria.
Table 1.
Agar media and incubation conditions for the assessed probiotic bacteria.
| Agar Medium | Supplements | Target Organism(s) | Incubation Conditions |
|---|---|---|---|
| BSM Agar (Sigma, Gillingham, UK) | BSM Supplement (Sigma, UK) | Bifidobacterium spp. | Anaerobic, 37 °C, 72 h |
| ChromoSelect Agar Base (Sigma, Gillingham, UK) | E. faecium selective supplement (Sigma, UK) | Enterococcus faecium | Aerobic, 37 °C, 24 h |
| MRS Agar (Sigma, Gillingham, UK) | — | Lactobacillus spp. | Anaerobic, 37 °C, 72 h |
| Tryptone Soya Agar (TSA; Thermo Scientific, Basingstoke, UK) | — | Bacillus spp. | Aerobic, 33 °C, 17 h 1 |
| MRS Agar (Sigma, Gillingham, UK) | Vancomycin hydrochloride (20 mg L−1) 2 | Ligilactobacillus salivarius | Anaerobic, 37 °C, 72 h |
| BSM Agar (Sigma, Gillingham, UK) | BSM Supplement; lithium mupirocin (5 mL/100 mL) 3 | Bifidobacterium animalis | Anaerobic, 37 °C, 72 h |
1 TSA plates were pre-incubated at 37 °C for ~13 h before use to prevent B. subtilis swarming, then incubated at 33 °C. 2 Used to selectively grow Lactobacillus salivarius from probiotics 9 and 12. 3 Used to selectively grow Bifidobacterium animalis only from P9 and P12.

Table 2.
Details and viable counts of commercial poultry probiotic products.
Table 2.
Details and viable counts of commercial poultry probiotic products.
| Product Code | Species Declared on the Label | Declared Label Total (CFU/g) | MALDI-TOF MS ID 2 | Observed Viable Plate Counts (Mean ± SD) 3 |
|---|---|---|---|---|
| P3 | Bacillus subtilis | 2.5 × 107 | Bacillus spp. | 1.375 × 107 ± 0.206 |
| P4 | Bacillus subtilis | 3 × 108 | Bacillus spp. | 1.900 × 108 ± 0.182 |
| P5 | Bacillus subtilis | 2 × 109 | Bacillus spp. | 1.400 × 1010 ± 0.258 |
| P9 | Bifidobacterium animalis | Ratio 1 3/10 | Bifidobacterium animalis | 1.300 × 108 ± 0.258 |
| Lactobacillus salivarius | Ratio 1/10 | Ligilactobacillus salivarius | 3.200 × 107 ± 0.365 | |
| Enterococcus faecium | Ratio 6/10 | Enterococcus faecium | 1.055 × 109 ± 0.044 | |
| Total bacteria | 1 × 109 | Total bacteria | 1.212 × 109 ± 0.042 | |
| P10 | Enterococcus faecium | 2 × 1010 | Enterococcus faecium | 5.800 × 1010 ± 0.455 |
| P11 | Bacillus subtilis | 2 × 108 | Bacillus spp. | 5.950 × 108 ± 0.311 |
| P12 | Bifidobacterium animalis | Ratio 1 3/10 | Bifidobacterium animalis | 1.080 × 107 ± 0.148 |
| Lactobacillus salivarius | Ratio 1/10 | Ligilactobacillus salivarius | 1.375 × 106 ± 0.432 | |
| Enterococcus faecium | Ratio 6/10 | Enterococcus faecium | 5.300 × 108 ± 0.432 | |
| Total bacteria | 2 × 108 | Total bacteria | 5.418 × 108 ± 0.421 |
1 For products P9 and P12, the manufacturer declared the total CFU per gram of product but did not specify individual CFU counts for each bacterial species. Instead, the label listed each species as a ratio of the total bacterial population. 2 Identification of isolates by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. 3 Mean ± standard deviation per gram. Observed means are based on n = 3 replicates for multi-species products and n = 4 for single-species products.

Table 3.
Details and viable counts of commercial human probiotic products.
Table 3.
Details and viable counts of commercial human probiotic products.
| Product Code | Form | Species Declared on the Label | Declared Label Total (CFU/form 1) | MALDI-TOF MS ID 3 | Observed Viable Plate Counts (Mean ± SD) 4 |
|---|---|---|---|---|---|
| P1 | Tablets | Lactobacillus acidophilus | 2 × 1011 | NA | No CFU detected 2 |
| P2 | Capsule | Lactobacillus acidophilus | ND 5 | Lactobacillus acidophilus | 3.483 × 109 ± 0.277 |
| Bifidobacterium animalis | ND | Bifidobacterium animalis | 6.533 × 108 ± 0.351 | ||
| Bifidobacterium bifidum | ND | ||||
| Total bacteria | 1 × 1010 | Total bacteria | 4.137 × 109 ± 0.297 | ||
| P6 | Capsules | Lactobacillus acidophilus | ND | Lactobacillus acidophilus | 3.300 × 109 ± 0.100 |
| Lactobacillus salivarius | ND | Lactobacillus salivarius | 2.067 × 106 ± 0.462 | ||
| Bifidobacterium animalis | ND | Bifidobacterium animalis | 2.100 × 107 ± 0.200 | ||
| Lactobacillus Bulgaricus | ND | ||||
| Total bacteria | 3 × 109 | Total bacteria | 3.320 × 109 ± 0.100 | ||
| P7 | Capsules | Lactobacillus acidophilus | ND | Lactobacillus acidophilus | 5.667 × 109 ± 0.306 |
| Bifidobacterium bifidum | ND | Bifidobacterium bifidum | 8.100 × 108 ± 0.436 | ||
| Total bacteria | 1 × 1010 | Total bacteria | 6.477 × 109 ± 0.337 | ||
| P8 | Chewable Tablets | Lactobacillus acidophilus | ND | Lactobacillus acidophilus | 5.533 × 108 ± 0.152 |
| Bifidobacterium animalis | ND | Bifidobacterium animalis | 2.800 × 107 ± 0.100 | ||
| Total bacteria | 1 × 109 | Total bacteria | 5.813 × 108 ± 0.156 |
1 Per form (capsule or tablet, as specified in the “Form” column, except for P1, where label showed counts per gram). 2 For product P1, viability testing was conducted using two sample types: (i) a single tablet, and (ii) a composite of seven tablets equivalent to 1 g, as the label declared viable count per gram. No colony growth was observed in either case on any agar medium. 3 Identification of isolates by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. 4 Mean ± standard deviation per form. Observed means are based on n = 3 replicates for multi-species products and n = 4 for single-species products. 5 ND = Not declared on label; the manufacturer provided a total count but did not specify counts for individual species.

Table 4.
Observed vs. declared viable counts (log10 CFU) in probiotic products (P1–P12).
Table 4.
Observed vs. declared viable counts (log10 CFU) in probiotic products (P1–P12).
| Product Code | Application | Declared Total Bacteria (log10 CFU/unit 1) | Observed Total Viable Plate Counts (log10 CFU ± SD) 2 | p-Value 3 | 95% Confidence Interval (CI) | Acceptable Log10 Range (±0.5) 4 | Relative Difference (%) 5 |
|---|---|---|---|---|---|---|---|
| P1 | Human | 11.30 | No CFU | 0.000 | (0.00, 0.00) | 10.80–11.80 | −100.00 |
| P2 | Human | 10 | 9.61 ± 0.031 | 0.002 | (9.53, 9.69) | 9.50–10.50 | −3.90 |
| P3 | Poultry | 7.39 | 7.13 ± 0.067 | 0.005 | (7.02, 7.24) | 6.89–7.89 | −3.52 |
| P4 | Poultry | 8.47 | 8.27 ± 0.042 | 0.003 | (8.21, 8.34) | 7.97–8.97 | −2.36 |
| P5 6 | Poultry | min. 9.30 | 10.14 ± 0.081 | 0.001 | (10.01, 10.26) | 8.80–9.80 | 9.03 |
| P6 | Human | 9.47 | 9.52 ± 0.007 | 0.023 | (9.48, 9.55) | 8.97–9.97 | 0.53 |
| P7 | Human | 10 | 9.81 ± 0.022 | 0.005 | (9.75, 9.86) | 9.50–10.50 | −1.90 |
| P8 | Human | 9 | 8.76 ± 0.011 | 0.001 | (8.73, 8.79) | 8.50–9.50 | −2.67 |
| P9 | Poultry | 9 | 9.08 ± 0.015 | 0.002 | (9.05, 9.10) | 8.50–9.50 | 0.89 |
| P10 | Poultry | 10.30 | 10.76 ± 0.033 | 0.001 | (10.70, 10.81) | 9.80–10.80 | 4.46 |
| P11 | Poultry | min. 8.30 | 8.77 ± 0.023 | 0.001 | (8.73, 8.81) | 7.80–8.80 | 5.66 |
| P12 | Poultry | 8.30 | 8.73 ± 0.034 | 0.001 | (8.67, 8.78) | 7.80–8.80 | 5.18 |
1 As declared on label (per capsule or tablet for human probiotics, except for P1, their label showed counts per gram, therefore both units—gram and tablets—were tested for growth. Per gram for all poultry probiotics). 2 Observed means are based on n = 3 replicates for multi-species products and n = 4 for single-species products. 3 One-sample t-test comparing each observed mean with the declared log10 value (α = 0.05). 4 Acceptance threshold defined as ±0.5 log10 CFU, following Italian Ministry of Health guidance. 5 Relative difference = ((observed − declared)/declared) × 100%. 6 Declared counts marked “min.” represent manufacturer minimums; positive deviations above these values are compliant.
2.4. Sample Preparation, Culturing and Enumeration
For each probiotic, three independent aliquots (multi-species products) or four independent aliquots (single-species products) were weighed and processed in parallel. One gram of each poultry product, or one human capsule/tablet (contents emptied if encapsulated), was suspended in 20 mL sterile phosphate-buffered saline (PBS, pH 7.4; Sigma, P4417) containing three 6 mm sterile metal beads and vortex-mixed for 2–3 min until fully dispersed. Ten-fold serial dilutions (up to 10−9) were prepared in the same PBS; 20 µL of each dilution was spread onto the appropriate selective-agar sector (Table 1) with sterile L-shaped spreaders (Thermo Scientific, Loughborough, UK, P12322048). Cultured dilutions of Bacillus subtilis products underwent an additional 80 °C for 10 min heat activation step before plating, using a heat block (TECHNE, model DB100/3). Plates were incubated under genus-specific conditions (17–72 h, temperature and atmosphere per Table 1). Anaerobic plates were placed in 2.5 L Oxoid AnaeroJar™ jars (Thermo Scientific, Basingstoke, UK) with AnaeroGen™ sachets and resazurin indicator strips to verify anaerobiosis. Colony counts were expressed as CFU per gram, capsule or tablet using the recorded colony number and dilution factor. Negative controls—blank agar plus PBS only, plated in duplicate—were included in every run to verify the sterility of media, diluent and equipment.
2.5. Species Identification
Bacterial identification followed a three-step workflow: (i) growth on the selective agars listed in Table 1; (ii) presumptive recognition by colony morphology (shape, colour, size); and (iii) confirmation by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF MS).
After enumeration, ≥4 colonies were picked from each selective agar on at least two replicate plates for each probiotic product. Fresh colonies were transported aseptically on the same day to Freeman Hospital, Newcastle upon Tyne, UK, and analysed on a Bruker Biotyper instrument under NHS laboratory protocols. For each isolate, a well-isolated colony was transferred to a 96-spot stainless-steel target plate (Bruker), overlaid with 1 µL α-cyano-4-hydroxycinnamic acid (HCCA) matrix solution (Bruker), and allowed to air-dry. Spectra were acquired within 10 min using the manufacturer’s default settings and matched against the Bruker reference library.
Identification scores were interpreted with Bruker cut-offs: >2.0 = species level; 1.70–1.99 = secure genus level; <1.70 = unreliable [21].
2.6. Statistical Analysis
Observed colony-forming-unit (CFU) counts (per g, capsule or tablet) were first summarised in their original scale as means ± standard deviation (SD). Descriptive statistics are presented in Table 3. Counts were then converted to log10 CFU per unit. Prior to hypothesis testing, the normality of each log10-transformed dataset was assessed by the Ryan–Joiner test, with no dataset showing a significant departure from normality (p > 0.05). One-sample t-tests on the log10-transformed means assessed whether each product differed from its declared log10 label value, using α = 0.05 for significance. For comparisons with label claims, t-based 95% confidence intervals (CIs) are reported in Table 4 alongside p-values.
In line with the Italian Ministry of Health guidance on probiotics and prebiotics [8,22], a label claim was deemed acceptable if the observed log10 count remained within ±0.5 log10 CFU, a margin intended to cover viable count loss over shelf life. Although the UK has no formal tolerance for probiotic viability, the Italian Ministry of Health guideline is the only European document that specifies a quantitative limit (±0.5 log10 CFU) for shelf-life losses. We therefore adopted this well-recognized benchmark as a pragmatic reference for products sold on the UK market. All analyses were performed in Minitab software v21.4.
3. Results
Poultry probiotics generally met or exceeded label claims (Table 2). For example, P5 (Bacillus subtilis) delivered 1.40 × 1010 CFU g−1 versus the declared 2 × 109 CFU g−1, and P11 likewise exceeded its minimum declaration. In contrast, four of five human products under-delivered the declared CFU count (Table 3). Most notably, P1 yielded no colony growth on any agar plate, despite testing composite samples equivalent to 1 g (seven tablets), which were labelled as containing 2 × 1011 CFU g−1. The best-performing human product, P6, slightly exceeded its declaration (3.32 × 109 ± 0.11 CFU capsule−1 vs. 3 × 109 CFU capsule−1).
For inferential comparisons with label claims, the results are summarized in Table 4, which reports t-based 95% confidence intervals (CIs) alongside p-values. Relative differences calculated as ((observed − declared)/declared) × 100%—ranged from −3.52% to +9.03% in poultry products and from −100% to +0.53% in human products (Table 4). One-sample t-tests on log-transformed counts confirmed significant deviations (p < 0.05) for every product; however, all but P1 met the adopted ±0.5 log10 benchmark—P5 exceeded the upper margin but was acceptable because its label specifies a minimum viable count (Table 4). A graphical summary of declared versus observed total counts is shown in Figure 1.
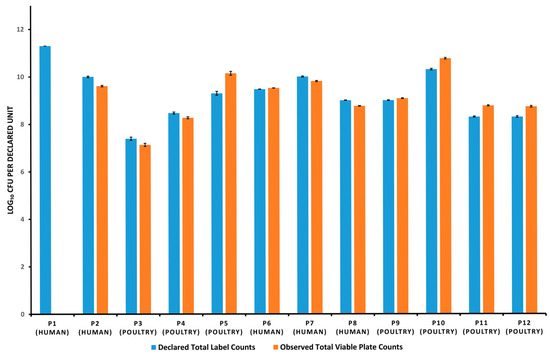
Figure 1.
Visual representation of declared versus observed total viable bacterial counts (log10 CFU per declared label unit) in Human and Poultry Probiotics (P1–P12). “Declared unit” refers to CFU (colony forming unit) per gram for poultry probiotics, or CFU per capsule/tablet for human products. For P1, both a single tablet and a composite of seven tablets (1 g) were tested, with no growth detected.
MALDI-TOF MS confirmed the labelled species in 10 of 12 products (Table 3 and Table 4). Bifidobacterium bifidum was not detected in P2, and Bacillus isolates from P3, P4, P5 and P11 were assigned to the B. velezensis/B. amyloliquefaciens group—both members of the B. subtilis group. No isolates outside the label-declared species were detected by MALDI-TOF MS, indicating that no contamination was present in any of the studied probiotics.
Products P9 and P12 employ ratio-based labelling (3:1:6 for B. animalis, L. salivarius and E. faecium, respectively). Observed counts closely matched these declared ratios, indicating accurate representation of relative composition.
4. Discussion
This study evaluated the viability and label accuracy of seven poultry and five human probiotic products by viable plate count, with bacterial identification confirmed by selective agars and MALDI-TOF MS. The primary goal was to verify whether manufacturers’ claims on viable counts and species composition were met or not. Determining effective dose and health impact is product and indication specific and requires targeted in vivo evaluation; these endpoints were not addressed in the present study, and accordingly we do not claim or refute efficacy for any product.
Significant discrepancies were observed between label claims and measured counts, particularly among the human products, in agreement with previous surveys of commercial probiotics [5,6,7,8,10]. For instance, product P1, labelled to contain 2 × 1011 CFU g−1 of Lactobacillus acidophilus, yielded no detectable viable bacteria (Table 3). By contrast, P6 and P7 displayed counts close to their declared values, suggesting that manufacturing practices and strain robustness strongly influence final viability of bacterial presence [23,24].
In contrast to the human products, poultry products generally showed higher accuracy in terms of the declared bacterial types and numbers. This observation likely reflects their formulation with resilient strains, principally Bacillus spp. spores, known to tolerate heat, desiccation and gastric acidity [25]. Even the three poultry products lacking Bacillus (P9, P10, P12) performed well; all were produced by the same manufacturer and consisted of microencapsulated powders, a technology shown to enhance survival during processing and storage of similar probiotics [23,26]. Among the component species, Enterococcus faecium consistently exhibited the greatest viability, out-competing Lactobacillus salivarius and Bifidobacterium animalis on selective agar. Growth of this robust strain on BSM plates had to be suppressed with lithium-mupirocin, echoing earlier reports [19].
These findings contradict the initial hypothesis, drawn from the literature for human probiotics, that most products would fall short of their label claims. While little contemporary information exists for animal probiotics, earlier North American surveys reported poor compliance for equine [2] and companion animal products [13]. The higher conformity observed here for poultry products may reflect stricter EU regulations on feed additives [27], under which UK poultry probiotics are authorised, versus the weaker dietary-supplement framework governing many human products.
Beyond viability counts, species identification was generally concordant with labels, although Bifidobacterium bifidum (P2) and Lactobacillus bulgaricus (P6) were not recovered as CFU on agar plates. Absence on selective agar does not prove absence in the product: a low starting abundance or competitive overgrowth by faster-growing strains could mask the recovery of slow-growing strains [28,29]. Nevertheless, for consumers and for regulators, the practical issue is whether the claimed viable dose of each named species is delivered at the use-by date.
The practical implications of this study are significant. For consumers and clinicians, our findings highlight the importance of brand reputation and transparency, suggesting that products providing third-party quality verification may be more reliable. For veterinarians and poultry producers, the data provide confidence in the quality of currently regulated products but also emphasize the need for continued vigilance. For regulatory bodies, this research underscores the potential public health gap between the tightly controlled animal feed sector and the more loosely regulated human supplement market, supporting calls for harmonized standards and more robust post-market surveillance.
However, the findings should be interpreted with some limitations in mind. Because products were chosen by convenience sampling to span common applications and formulations, the panel is not probabilistic; therefore, results should be interpreted as a market snapshot rather than an exhaustive, brand-representative survey. Furthermore, a key limitation is the single-batch sampling per product, which precludes assessment of lot-to-lot variability; future surveillance should ideally include multiple batches to assess manufacturing consistency.
Looking ahead, several avenues for future research are apparent. To build on this market snapshot, longitudinal studies analysing multiple batches of the same product over time are essential to differentiate between sporadic quality control failures and systemic manufacturing issues. Additionally, the integration of molecular methods, such as quantitative PCR (qPCR), alongside plate counting would provide a more complete picture by enabling the quantification of viable but non-cultivable (VBNC) cells. Finally, stability trials that assess viability under simulated consumer storage conditions would offer valuable insights into the real-world performance of these products throughout their shelf life.
Based on these findings, the data highlight the importance of selecting hardy strains and appropriate formulation technologies. For poultry, spore-forming Bacillus and microencapsulated blends offer clear viability advantages under farm-level temperature and humidity fluctuations. Human products appear more vulnerable to viability loss, likely due to the use of non-spore-forming lactic acid bacteria that are less resistant to heat, desiccation and oxygen exposure during manufacturing and storage, and may require similar technological upgrades, such as inclusion of spore forming Bacillus strains and advanced microencapsulation techniques, to achieve the reliability seen in the animal sector.
Ultimately, these findings reinforce the necessity of rigorous quality control and standardized testing procedures across probiotic production. Ensuring accurate and transparent labelling of viable counts and species composition is crucial, not only to maintain regulatory compliance but also to uphold consumer trust and maximize the therapeutic potential of probiotic products.
5. Conclusions
The present study underlines marked variability in the viability of commercial probiotics sold in the UK, with human formulations displaying the greatest divergence from label claims. Several human products delivered markedly fewer viable bacteria than those declared on the labels, whereas poultry products generally conformed to their labels. This likely reflected the use of spore-forming or microencapsulated strains that better withstand the processing and storage of these products. These findings support calls for stronger regulatory oversight and lot-specific quality control testing to ensure that marketed probiotic products provide the promised health benefits. Future work should focus on refining enumeration methods and transferring proven veterinary technologies, such as microencapsulation and robust Bacillus strains, into human probiotics. By aligning manufacturing practices with rigorous viability standards, both the human and animal sectors can deliver more reliable, evidence-based probiotic interventions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13081933/s1, Table S1: Storage metadata for probiotic products (P1–P12), including expiry date, label storage instruction, actual storage temperature and storage time prior to testing.
Author Contributions
Conceptualization, M.W.T. and A.S.C.; methodology, M.W.T.; validation, M.W.T., E.C.L.M. and D.J.C.F.; formal analysis, M.W.T.; investigation, M.W.T., E.C.L.M. and D.J.C.F.; resources, A.S.C.; data curation, M.W.T.; writing—original draft preparation, M.W.T.; writing—review and editing, M.W.T. and A.S.C.; visualization, M.W.T.; supervision, A.S.C.; project administration, M.W.T.; funding acquisition, A.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Higher Committee for Education Development in Iraq (HCED) as part of the PhD sponsorship for Mostafa Waleed Taha at Newcastle University.
Institutional Review Board Statement
Ethical approval was obtained from the Animal Welfare and Ethical Review Body at Newcastle University (Project ID #948).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to institutional and ethical restrictions.
Acknowledgments
The authors would like to thank the technical staff at Newcastle University and Freeman Hospital (Newcastle upon Tyne) for their assistance with sample processing and logistical support. Special thanks are extended to Fiona Cuskin and Ieva Lelenaite for their invaluable training and methodological advice. We also thank Andrew Walker, Senior Research Technician at Newcastle University, for his technical support during the laboratory work. We are grateful to John D. Perry for performing the MALDI-TOF MS analyses and for his constructive suggestions on the manuscript. The authors are additionally grateful for the administrative support received throughout the study and appreciate the staff who provided access to laboratory facilities and equipment, which contributed significantly to the completion of this research.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hotel, A.; Cordoba, A. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention 2001, 5, 1–10. [Google Scholar]
- Berreta, A.; Kopper, J.J.; Alexander, T.L.; Kogan, C.J.; Burbick, C.R. Effect of an In Vitro Proximal Gastrointestinal Tract on Viability of Commercially Available Equine Probiotics. J. Equine Vet. Sci. 2021, 104, 103671. [Google Scholar] [CrossRef]
- Ronka, E.; Malinen, E.; Saarela, M.; Rinta-Koski, M.; Aarnikunnas, J.; Palva, A. Probiotic and milk technological properties of Lactobacillus brevis. Int. J. Food Microbiol. 2003, 83, 63–74. [Google Scholar] [CrossRef]
- Pendharkar, S.; Skafte-Holm, A.; Simsek, G.; Haahr, T. Lactobacilli and their probiotic effects in the vagina of reproductive age women. Microorganisms 2023, 11, 636. [Google Scholar] [CrossRef]
- Zawistowska-Rojek, A.; Zareba, T.; Mrowka, A.; Tyski, S. Assessment of the Microbiological Status of Probiotic Products. Pol. J. Microbiol. 2016, 65, 97–104. [Google Scholar] [CrossRef]
- Drago, L.; Rodighiero, V.; Celeste, T.; Rovetto, L.; de Vecchi, E. Microbiological evaluation of commercial probiotic products available in the USA in 2009. J. Chemother. 2010, 22, 373–377. [Google Scholar] [CrossRef]
- Korona-Glowniak, I.; Siwiec, R.; Luszczewska-Sierakowska, I.; Maciejewski, R.; Wrobel, R.; Malm, A. Microbiological evaluation of 10 commercial probiotic products available in Poland. Curr. Issues Pharm. Med. Sci. 2019, 32, 121–124. [Google Scholar] [CrossRef]
- Kesavelu, D., Sr.; Rohit, A.; Karunasagar, I.; Karunasagar, I. Composition and Laboratory Correlation of Commercial Probiotics in India. Cureus 2020, 12, e11334. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.; de Vecchi, E.; Rodighiero, V.; Drago, L. Microbiological and genetic identification of some probiotics proposed for medical use in 2011. J. Chemother. 2013, 25, 156–161. [Google Scholar] [CrossRef]
- Marinova, V.Y.; Rasheva, I.K.; Kizheva, Y.K.; Dermenzhieva, Y.D.; Hristova, P.K. Microbiological quality of probiotic dietary supplements. Biotechnol. Biotechnol. Equip. 2019, 33, 834–841. [Google Scholar] [CrossRef]
- Fredua-Agyeman, M.; Parab, S.; Gaisford, S. Evaluation of commercial probiotic products. Br. J. Pharm. 2016, 1, 84–89. [Google Scholar] [CrossRef]
- Metras, B.N.; Holle, M.J.; Parker, V.J.; Miller, M.J.; Swanson, K.S. Commercial kefir products assessed for label accuracy of microbial composition and density. JDS Commun. 2021, 2, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S.; Martin, H. Assessment of commercial probiotic bacterial contents and label accuracy. Can. Vet. J. 2011, 52, 43. [Google Scholar]
- ISO 20128: 2006 (IDF 192: 2006); Milk Products–Enumeration of Presumptive Lactobacillus acidophilus on a Selective Medium–Colony-Count Technique at 37 Degrees C. ISO-International Organization for Standardization: Geneva, Switzerland, 2006.
- Leuschner, R.G.; Bew, J.; Simpson, P.; Ross, P.R.; Stanton, C. A collaborative study of a method for the enumeration of probiotic bifidobacteria in animal feed. Int. J. Food Microbiol. 2003, 83, 161–170. [Google Scholar] [CrossRef]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Million, M.; Henry, M.; Raoult, D. Rapid and accurate bacterial identification in probiotics and yoghurts by MALDI-TOF mass spectrometry. J. Food Sci. 2011, 76, M568–M572. [Google Scholar] [CrossRef]
- Loy, D.J.; Clawson, M.L. From Genomics to MALDI-TOF MS: Diagnostic Identification and Typing of Bacteria in Veterinary Clinical Laboratories. In Microbiological Identification Using MALDI-TOF and Tandem Mass Spectrometry: Industrial and Environmental Applications; Shah, H.N., Gharbia, S.E., Shah, A.J., Tranfield, E.Y., Thompson, K.C., Eds.; John Wiley & Sons: Chichester, UK, 2023; pp. 283–302. [Google Scholar] [CrossRef]
- Simpson, P.; Fitzgerald, G.; Stanton, C.; Ross, R. The evaluation of a mupirocin-based selective medium for the enumeration of bifidobacteria from probiotic animal feed. J. Microbiol. Methods 2004, 57, 9–16. [Google Scholar] [CrossRef]
- Hartemink, R.; Domenech, V.; Rombouts, F. LAMVAB—A new selective medium for the isolation of lactobacilli from faeces. J. Microbiol. Methods 1997, 29, 77–84. [Google Scholar] [CrossRef]
- Vianna, E.d.F.; Pentagna, L.S.d.S.; Menezes, N.I.M.; de Freitas, F.A.D.; Leite, C.d.C.F.; Albano, R.M.; Leão, R.S.; Marques, E.A. Decreasing the cut-off score value of MALDI-ToF MS increase the identities of burkholderia cepacia complex species. Curr. Microbiol. 2021, 78, 2259–2263. [Google Scholar] [CrossRef]
- della Salute, M. Direzione Generale per l’Igiene e la Sicurezza degli Alimenti e la Nutrizione—Ufficio 4. Linee guida su Probiotici e Prebiotici; Ministero della Salute: Rome, Italy, March 2018. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_1016_allegato.pdf (accessed on 1 July 2025).
- Sarkar, S. Approaches for enhancing the viability of probiotics: A review. Br. Food J. 2010, 112, 329–349. [Google Scholar] [CrossRef]
- Iaconelli, C.; Lemetais, G.; Kechaou, N.; Chain, F.; Bermúdez-Humarán, L.G.; Langella, P.; Gervais, P.; Beney, L. Drying process strongly affects probiotics viability and functionalities. J. Biotechnol. 2015, 214, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Popov, I.V.; Algburi, A.; Prazdnova, E.V.; Mazanko, M.S.; Elisashvili, V.; Bren, A.B.; Chistyakov, V.A.; Tkacheva, E.V.; Trukhachev, V.I.; Donnik, I.M.; et al. A Review of the Effects and Production of Spore-Forming Probiotics for Poultry. Animals 2021, 11, 1941. [Google Scholar] [CrossRef]
- Shori, A.B. Microencapsulation Improved Probiotics Survival During Gastric Transit. HAYATI J. Biosci. 2017, 24, 1–5. [Google Scholar] [CrossRef]
- Leuschner, R.G.K.; Kneifel, W.; Vernoux, J.-P.; Stanton, C.; Aldamiz, P. Methods for the Official Control of Probiotics (Microorganisms) Used as Feed Additives; Final Report, Volume III, Project SMT4-CT98-2235; European Commission, Community Research: Luxembourg, 2002; Available online: https://op.europa.eu/en/publication-detail/-/publication/bed1feec-4f66-4f3e-8c95-2710b525fd1a (accessed on 1 July 2025).
- Süle, J.; Kõrösi, T.; Hucker, A.; Varga, L. Evaluation of culture media for selective enumeration of bifidobacteria and lactic acid bacteria. Braz. J. Microbiol. 2014, 45, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Al-Hadeedy, I.Y.; Mohammed, A.K.; Al-Tikriti, S.S. Genetic Polymorphism of Estrogen Receptor Alpha Gene (ESRα) and Its Effect on Production and Biochemical Traits of White Quails. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2023; p. 072094. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).