Physico-Chemical and Antimicrobial Evaluation of Ozonated Olive Oil Produced with a Medical-Grade Generator for Veterinary Purposes
Abstract
1. Introduction
2. Materials and Methods
2.1. Ozonation Method of Olive Oil
2.2. Physico-Chemical Analysis
2.2.1. Peroxide Value
2.2.2. Acid Value
2.2.3. Iodine Value
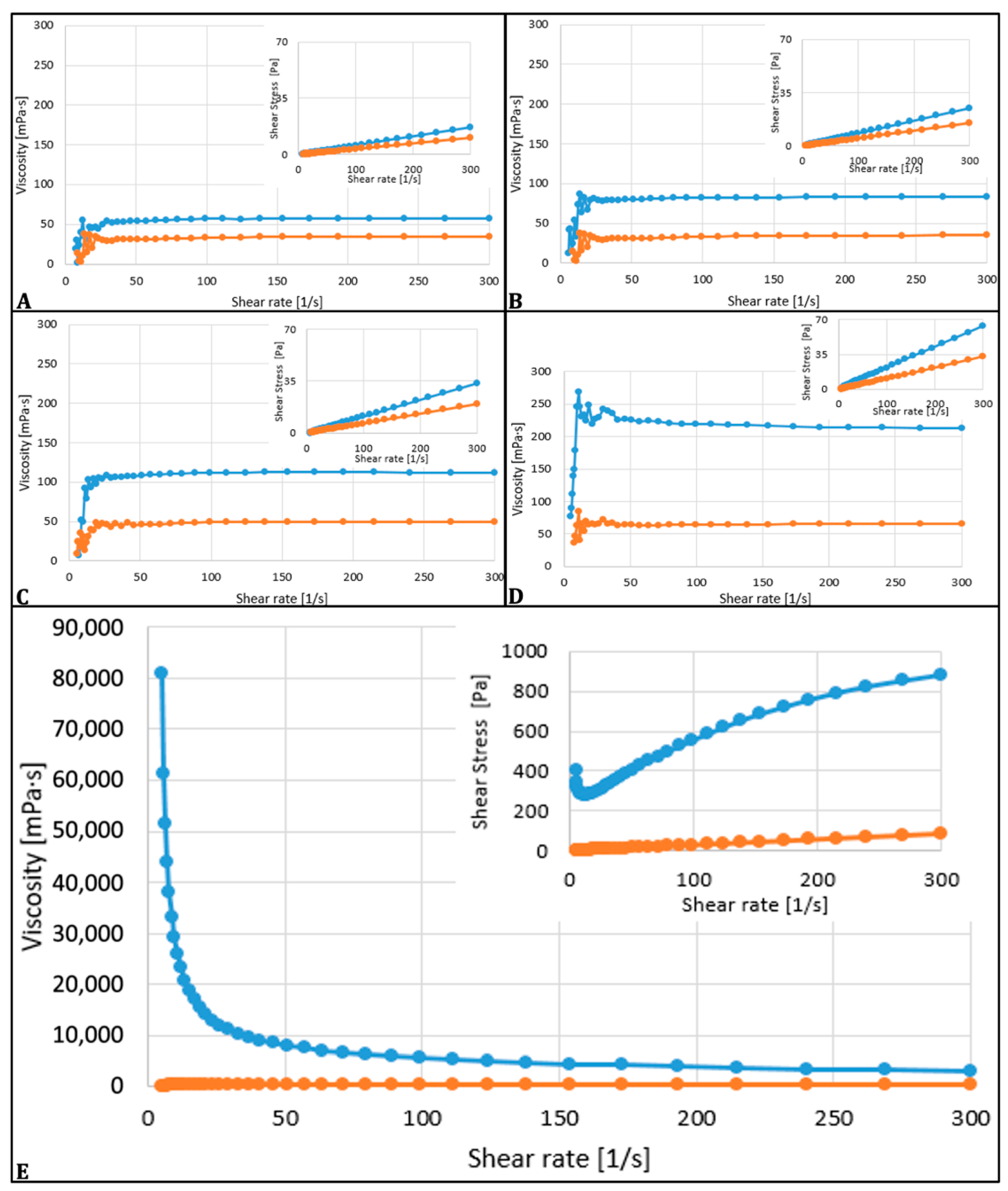
2.2.4. Viscosity Determination
2.2.5. Antimicrobial Activity
2.2.6. Diffusimetric Method Protocol
2.2.7. Minimum Inhibitory Concentration (MIC)
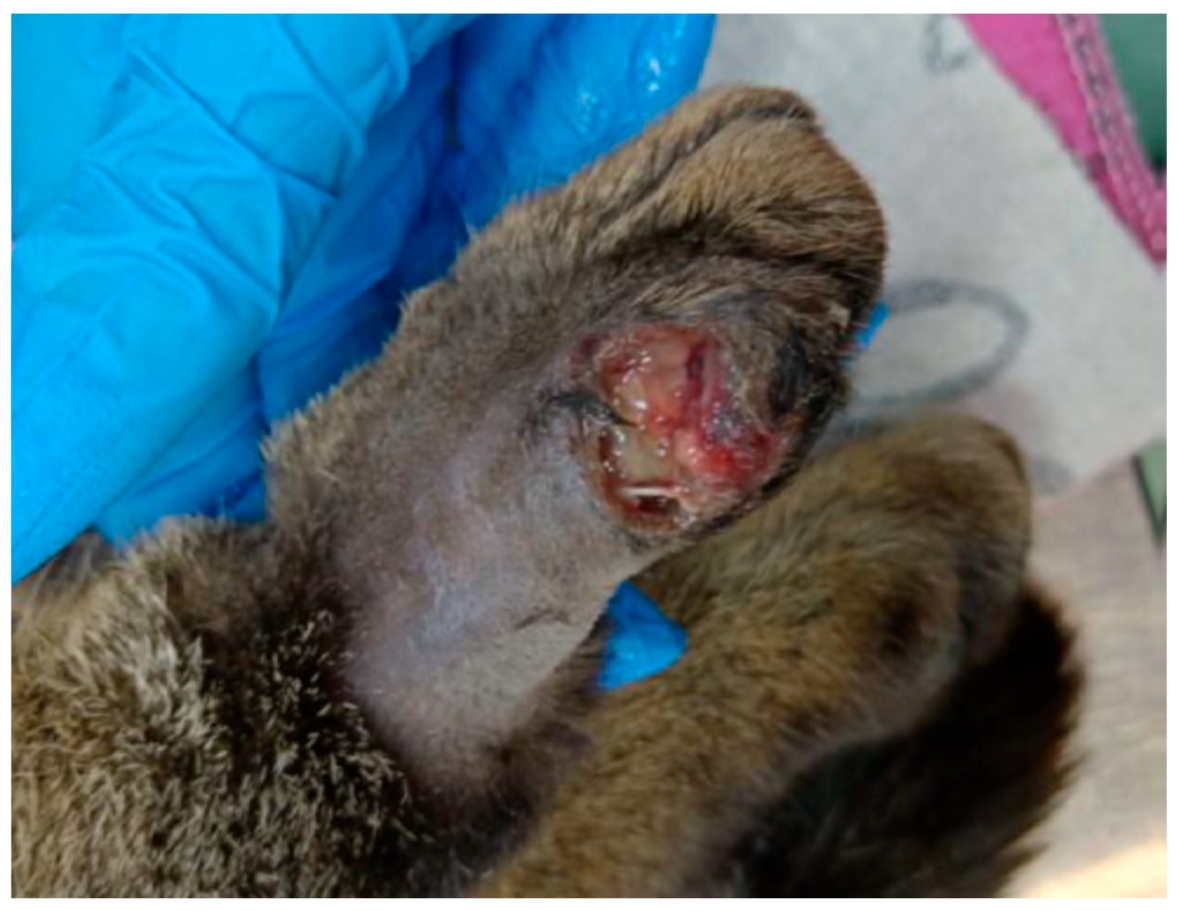
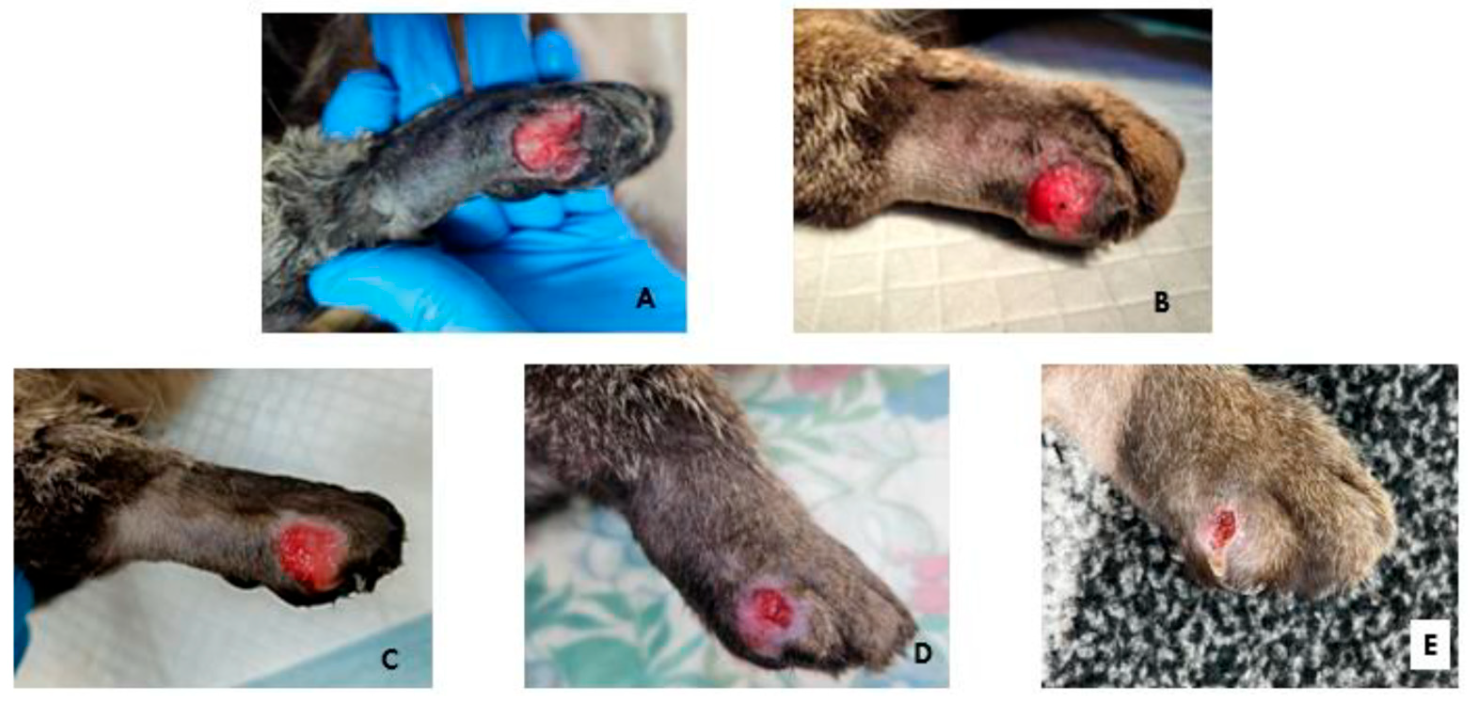
2.2.8. Clinical Case
2.2.9. Statistical Analysis
3. Results
3.1. Physico-Chemical Analysis of Ozonated Oil
3.2. Antimicrobial Activity of Ozonated Oil
3.2.1. Diffusimetric Method
3.2.2. Minimum Inhibitory Concentration
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PUFA | Polyunsaturated fatty acids |
| KI | Potassium iodide |
| KOH | Potassium hydroxide |
| NA | Nutrient Agar |
| SAB | Sabouraud Dextrose medium |
| MIC | Minimum Inhibitory Concentration |
| NFkB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| EOO | Extra virgin olive oil |
| MDR | Resistant to multiple antibiotics |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| UFS | Sunflower oil |
| UM | Olive oil |
| AOAC | Standardized iodometric assay |
| PV | Peroxide value |
| MIR | Mid infrared spectroscopy |
| NIR | Near infrared spectroscopy |
| HHV | Human herpetic virus |
| Fe-SOD | Iron superoxide dismutase |
| Mn-SOD | Manganese superoxide dismutase |
| Cu-SOD | Copper superoxide dismutase |
| Zn-SOD | Zinc superoxide dismutase |
| O2 | Superoxide radical |
| DNA | Desoxyribonucleic acid |
| KatG | Catalase G |
| KatE | Catalase E |
| H2O2 | Hydrogen peroxide |
| ROS | Reactive oxygen species |
| LPS | Lipopolysaccharides |
References
- Sanchez, G.M. Ozonized water, background, general use in medicine and preclinic support. Rev. Esp. Ozonoterapia 2019, 9, 33–60. [Google Scholar]
- Anzolin, A.P.; Da Silveira-Karros, N.L.; Bertol, C.D. Ozonated oil in wound healing: What has already been proven? Med. Gas Res. 2020, 10, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Lim, Y.; Belmonte, G.; Miracco, C.; Zanardi, I.; Bocci, V. Ozonated sesame oil enhances cutaneous wound healing in SKH1 mice. Wound Repair Regen. 2011, 19, 107–115. [Google Scholar] [CrossRef]
- Travagli, V.; Zanardi, I.; Bocci, V. Topical applications of ozone and ozonated oils as anti-infective agents: An insight into the patent claims. Recent Pat. Anti-Infect. Drug Discov. 2009, 4, 130–142. [Google Scholar] [CrossRef]
- Günaydın, Y.; Sevim, H.; Tanyolaç, D.; Gürpınar, Ö.A. Ozonated olive oil with a high peroxide value for topical applications: In vitro cytotoxicity analysis with L929 cells. Ozone Sci. Eng. 2018, 40, 37–43. [Google Scholar] [CrossRef]
- Ugazio, E.; Tullio, V.; Binello, A.; Tagliapietra, S.; Dosio, F. Ozonated oils as antimicrobial systems in topical applications: Their characterization, current applications, and advances in improved delivery techniques. Molecules 2020, 25, 334. [Google Scholar] [CrossRef] [PubMed]
- Skalska, K.; Ledakowicz, S.; Perkowski, J.; Sencio, B. Germicidal Properties of Ozonated Sunflower Oil. Ozone Sci. Eng. 2009, 31, 232–237. [Google Scholar] [CrossRef]
- Uysal, B. Ozonated olive oils and the troubles. J. Intercult. Ethnopharmacol. 2014, 3, 49–50. [Google Scholar] [CrossRef]
- Gultekin, F.A.; Bakkal, B.H.; Sümer, D.; Köktürk, F.; Bektaş, S. Effects of ozonated olive oil on acute radiation proctitis in rats. Balk. Med. J. 2013, 30, 369–374. [Google Scholar] [CrossRef]
- Puxeddu, S.; Scano, A.; Scorciapino, M.A.; Delogu, I.; Vascellari, S.; Ennas, G.; Manzin, A.; Angius, F. Physico-chemical investigation and antimicrobial efficacy of ozonated oils: The case study of commercial ozonated olive and sunflower seed refined oils. Molecules 2024, 29, 679. [Google Scholar] [CrossRef]
- Silva, V.; Peirone, C.; Amaral, J.S.; Capita, R.; Alonso-Calleja, C.; Marques-Magallanes, J.A.; Martins, Â.; Carvalho, Á.; Maltez, L.; Pereira, J.E.; et al. High Efficacy of Ozonated Oils on the Removal of Biofilms Produced by Methicillin-Resistant Staphylococcus aureus (MRSA) from Infected Diabetic Foot Ulcers. Molecules 2020, 25, 3601. [Google Scholar] [CrossRef]
- Augello, S.; Cameli, V.; Montanari, A.; Tacconi, S.; Uccelletti, D.; Dini, L.; Schifano, E. The antifungal potential of ozonated extra-virgin olive oil against Candida albicans: Mechanisms and efficacy. Biomolecules 2024, 14, 1472. [Google Scholar] [CrossRef] [PubMed]
- Travagli, V.; Zanardi, I.; Valacchi, G.; Bocci, V. Ozone and ozonated oils in skin diseases: A review. Mediat. Inflamm. 2010, 2010, 610418. [Google Scholar] [CrossRef]
- Moureu, S.; Violleau, F.; Haimoud-Lekhal, D.A.; Calmon, A. Ozonation of sunflower oils: Impact of experimental conditions on the composition and the antibacterial activity of ozonized oils. Chem. Phys. Lipids 2015, 186, 79–85. [Google Scholar] [CrossRef]
- Song, M.; Zeng, Q.; Xiang, Y.; Gao, L.; Huang, J.; Huang, J.; Wu, K.; Lu, J. The antibacterial effect of topical ozone on the treatment of MRSA skin infection. Mol. Med. Rep. 2018, 17, 2449–2455. [Google Scholar] [CrossRef]
- Grandi, G.; Cavallo, R.; Zanotto, E.; Cipriani, R.; Panico, C.; Protti, R.; Scapagnini, G.; Davinelli, S.; Costagliola, C. In vitro antimicrobial activity of ozonated oil in liposome eyedrop against multidrug-resistant bacteria. Open Med. 2022, 17, 1057–1063. [Google Scholar] [CrossRef]
- Montevecchi, M.; Dorigo, A.; Cricca, M.; Checchi, L. Comparison of the antibacterial activity of an ozonated oil with chlorhexidine digluconate and povidone-iodine: A disk diffusion test. N. Microbiol. 2013, 36, 289–302. [Google Scholar]
- Pietrocola, G.; Ceci, M.; Preda, F.; Poggio, C.; Colombo, M. Evaluation of the Antibacterial Activity of a new Ozonized Olive Oil against Oral and Periodontal Pathogens. J. Clin. Exp. Dent. 2018, 10, e1103–e1108. [Google Scholar] [CrossRef]
- Zanardi, I.; Burgassi, S.; Paccagnini, E.; Gentile, M.; Bocci, V.; Travagli, V. What is the best strategy for enhancing the effects of topically applied ozonated oils in cutaneous infections? Biomed. Res. Int. 2013, 2013, 702949. [Google Scholar] [CrossRef]
- Serio, F.; Pizzolante, G.; Cozzolino, G.; D’aLba, M.; Bagordo, F.; De Giorgi, M.; Grassi, T.; Idolo, A.; Guido, M.; De Donno, A. A new formulation based on ozonated sunflower seed oil: In vitro antibacterial and safety evaluation. Ozone Sci. Eng. 2017, 39, 139–147. [Google Scholar] [CrossRef]
- Ouf, S.A.; Moussa, T.A.; Abd-Elmegeed, A.M.; Eltahlawy, S.R. Anti-Fungal Potential of Ozone Against some Dermatophytes. Braz. J. Microbiol. 2016, 47, 697–702. [Google Scholar] [CrossRef]
- Rangel, K.; Cabral, F.O.; Lechuga, G.C.; Carvalho, J.P.R.S.; Villas-Bôas, M.H.S.; Midlej, V.; De-Simone, S.G. Detrimental effect of ozone on pathogenic bacteria. Microorganisms 2022, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Z.; Meyrick, B.; Heath, E.; Macleod, J.; Hooper, S.; Prince, M.; Blaxland, J. Standardization of gaseous ozone disinfectant efficacy: Recommendations of methods and conditions for future comparative studies. Ozone Sci. Eng. 2025, 47, 1–20. [Google Scholar] [CrossRef]
- Orlandin, J.R.; Machado, L.C.; Ambrósio, C.E.; Travagli, V. Ozone and its Derivatives in Veterinary Medicine: A Careful Appraisal. Vet. Anim. Sci. 2021, 13, 100191. [Google Scholar] [CrossRef] [PubMed]
- Balea, A.; Ciotlăuș, I.; Pojar-Feneșan, M.; Carpa, R. Comparative chemical and antimicrobial characterization of non-ozonated and ozonated vegetable oils. Stud. Univ. Babes-Bolyai Chem. 2023, 1, 285–301. [Google Scholar] [CrossRef]
- Zerillo, L.; Polvere, I.; Varricchio, R.; Madera, J.R.; D’Andrea, S.; Voccola, S.; Franchini, I.; Stilo, R.; Vito, P.; Zotti, T. Antibiofilm and Repair Activity of Ozonated Oil in Liposome Eye Drops. Microb. Biotechnol. 2022, 15, 1422–1433. [Google Scholar] [CrossRef]
- Firestone, D. (Ed.) Official Methods and Recommended Practices of AOCS, 5th ed.; American Oil Chemists’ Society: Champaign, IL, USA, 1998; pp. 8–87. [Google Scholar]
- Matuschek, E.; Brown, D.F.; Kahlmeter, G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef]
- Hood, J.R.; Wilkinson, J.M.; Cavanagh, H.M.A. Evaluation of common antibacterial screening methods utilized in essential oil research. J. Essent. Oil Res. 2003, 15, 428–433. [Google Scholar] [CrossRef]
- Criegee, R. Mechanism of Ozonolysis. Angew. Chem. Int. Ed. Engl. 1975, 14, 745–752. [Google Scholar] [CrossRef]
- Díaz, M.F.; Hernández, R.; Martínez, G.; Vidal, G.; Gómez, M.; Fernández, H.; Garcés, R. Comparative Study of Ozonized Olive Oil and Ozonized Sunflower Oil. J. Braz. Chem. Soc. 2006, 17, 403–407. [Google Scholar] [CrossRef]
- Vinet, J.; Tréguier, S.; Levasseur-Garcia, C.; Calmon, A.; Violleau, F. Iodine and Peroxide Index Rapid Determination by Mid- and Near-infrared Spectroscopy in Ozonated Sunflower Oil and Ozonated Fats. Ozone: Sci. Eng. 2022, 44, 337–350. [Google Scholar] [CrossRef]
- Jacinto, G.S.; Benvenutti, L.; Santin, J.R.; de Freitas, R.A.; Bella Cruz, A.; Corrêa, R.; Malheiros, A.; Klein-Junior, L.C.; Bresolin, T.M.B. The Effect of Ozone Dosage of Sunflower and Olive Oils on Their Biological Activities and Chemical Properties. Ozone: Sci. Eng. 2024, 46, 197–216. [Google Scholar] [CrossRef]
- Frankel, E.N. Chapter 5—Methods to determine extent of oxidation. In Oily Press Lipid Library Series, Lipid Oxidation, 2nd ed.; Frankel, E.N., Ed.; Woodhead Publishing: Cambridge, UK, 2012. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical Methods for Lipid Oxidation and Antioxidant Capacity in Food Systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef]
- Frankel, E.N. Lipid Oxidation; Woodhead Publishing Ltd.: Cambridge, UK, 2005; ISBN -13 978-0-9531949-8-8. [Google Scholar]
- Tarapoulouzi, M.; Agriopoulou, S.; Koidis, A.; Proestos, C.; Enshasy, H.A.E.; Varzakas, T. Recent Advances in Analytical Methods for the Detection of Olive Oil Oxidation Status during Storage along with Chemometrics, Authenticity and Fraud Studies. Biomolecules 2022, 12, 1180. [Google Scholar] [CrossRef]
- Mitrea, L.; Teleky, B.-E.; Leopold, L.-F.; Nemes, S.-A.; Plamada, D.; Dulf, F.V.; Pop, I.-D.; Vodnar, D.C. The Physicochemical Properties of Five Vegetable Oils Exposed at High Temperature for a Short-Time Interval. J. Food Compos. Anal. 2022, 106, 104305. [Google Scholar] [CrossRef]
- Mureșan, V.; Danthine, S.; Racolta, E.; Muste, S.; Blecker, C. The Influence of Particle Size Distribution on Sunflower Tahini rheology and Structure. J. Food Process Eng. 2014, 37, 411–426. [Google Scholar] [CrossRef]
- Songkram, C.; Changcharoensuk, C.; Amnuaikit, T. Effect of ozonation on physicochemical properties of olive oil based sunscreen product. Ozone Sci. Eng. 2024, 47, 51–63. [Google Scholar] [CrossRef]
- Sagai, M.; Bocci, V. Mechanisms of action involved in ozone therapy: Is healing induced via a mild oxidative stress? Med. Gas Res. 2011, 1, 29. [Google Scholar] [CrossRef] [PubMed]
- Zanardi, I.; Borrelli, E.; Valacchi, G.; Travagli, V.; Bocci, V. Ozone: A Multifaceted Molecule with Unexpected Therapeutic Activity. Curr. Med. Chem. 2016, 23, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Serio, F.; Pizzolante, G.; Cozzolino, G.; D’aLba, M.; Bagordo, F.; De Giorgi, M.; Grassi, T.; Idolo, A.; Guido, M.; De Donno, A. Microbiological aspects of ozone: Bactericidal activity and antibiotic/antimicrobial resistance in bacterial strains treated with ozone. Ozone Ther. 2018, 3, 48–59. [Google Scholar] [CrossRef]
- Sadowska, J.; Johansson, B.; Johannessen, E.; Friman, R.; Broniarz-Press, L.; Rosenholm, J.B. Characterization of ozonated vegetable oils by spectroscopic and chromatographic methods. Chem. Phys. Lipids 2008, 151, 85–91. [Google Scholar] [CrossRef]
- De Almeida Kogawa, N.R.; de Fatima Cepa Matos, M.; Silva de Oliveira, L.C.; Pires de Lima, D.; Pereira Carvalho, N.C.; Dias de Oliveira, P.; de Castro Cunha, M.; Ojeda, M.; Beatriz, A. Synthesis, characterization, thermal behavior, and biological activity of ozonides from vegetable oils. RSC Adv. 2015, 5, 65427–65436. [Google Scholar] [CrossRef]
- Calienni, M.N.; Martínez, L.M.; Izquierdo, M.C.; Alonso, S.d.V.; Montanari, J. Rheological and viscoelastic analysis of hybrid formulations for topical application. Pharmaceutics 2021, 13, 2392. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.F.; Sánchez, Y.; Gómez, M.; Hernández, F.; Veloso, M.C.D.C.; Pereira, P.A.D.P.; Mangrich, A.S.; De Andrade, J.B. Physicochemical characteristics of ozonated sunflower oils obtained by different procedures. Grasas Aceites 2012, 63, 466–474. [Google Scholar] [CrossRef]
- da Cruz Nizer, W.S.; Inkovskiy, V.; Versey, Z.; Strempel, N.; Cassol, E.; Overhage, J. Oxidative stress response in Pseudomonas aeruginosa. Pathogens 2021, 10, 1187. [Google Scholar] [CrossRef] [PubMed]
- Slavinskienė, G.; Grigonis, A.; Ivaškienė, M.; Sinkevičienė, I.; Andrulevičiūtė, V.; Ivanauskas, L.; Juodžentė, D.; Ramanauskienė, K.; Daunoras, G. A comparative study of the chemical properties and antibacterial activity of four different ozonated oils for veterinary purposes. Vet. Sci. 2024, 11, 161. [Google Scholar] [CrossRef]
- Seixas, A.F.; Quendera, A.P.; Sousa, J.P.; Silva, A.F.Q.; Arraiano, C.M.; Andrade, J.M. Bacterial response to oxidative stress and RNA oxidation. Front. Genet. 2022, 12, 821535. [Google Scholar] [CrossRef]
- Dominguez Lacueva, P.; Corella Guillamón, P.; Cantalejo Díez, M.J. Changes in the quality parameters and antimicrobial activity of ozonated virgin and pomace olive oils under different storage conditions. Foods 2025, 14, 999. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Cozzolino, R.; Martignetti, A.; Malorni, L.; De Feo, V.; Cruz, A.G.; d’Acierno, A. Antibacterial Activity of Three Extra Virgin Olive Oils of the Campania Region, Southern Italy, Related to Their Polyphenol Content and Composition. Microorganisms 2019, 7, 321. [Google Scholar] [CrossRef]
- Guo, L.; Gong, S.; Wang, Y.; Sun, Q.; Duo, K.; Fei, P. Antibacterial Activity of Olive Oil Polyphenol Extract Against Salmonella Typhimurium and Staphylococcus aureus: Possible Mechanisms. Foodborne Pathog. Dis. 2020, 17, 396–403. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; Illescas-Montes, R.; Costela-Ruiz, V.J.; Ramos-Torrecillas, J.; de Luna-Bertos, E.; García-Martínez, O.; Ruiz, C. Antimicrobial Properties of Olive Oil Phenolic Compounds and Their Regenerative Capacity towards Fibroblast Cells. J. Tissue Viability 2021, 30, 372–378. [Google Scholar] [CrossRef]
- Ciafardini, G.; Cioccia, G.; Zullo, B.A. Survival of Candida parapsilosis yeast in olive oil. Ann. Microbiol. 2013, 63, 1645–1648. [Google Scholar] [CrossRef]
- Zullo, B.A.; Maiuro, L.; Ciafardini, G. Survival of coliform bacteria in virgin olive oil. BioMed Res. Int. 2018, 2018, 8490614. [Google Scholar] [CrossRef]
- Sciorsci, R.L.; Lillo, E.; Occhiogrosso, L.; Rizzo, A. Ozone therapy in veterinary medicine: A review. Res. Vet. Sci. 2020, 130, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Spadea, L.; Tonti, E.; Spaterna, A.; Marchegiani, A. Use of ozone-based eye drops: A series of cases in veterinary and human spontaneous ocular pathologies. Case Rep. Ophthalmol. 2018, 9, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Marchegiani, A.; Magagnini, M.; Cerquetella, M.; Troiano, P.; Franchini, I.; Franchini, A.; Scapagnini, G.; Spaterna, A. Preoperative topical liposomal ozone dispersion to reduce bacterial colonization in conjunctival sac and periocular skin: Preliminary study in dogs. Exp. Eye Res. 2019, 189, 107848. [Google Scholar] [CrossRef]
- Hugues-Hernandorena, B.; Torres, M.; Acosta, I.; Masdeu, V.; Zamora, Z. Efectividad del aceite de girasol ozonizado (colirio) en el tratamiento de la queratoconjuntivitis seca en perros. AMMVEPE 2016, 27, 102–107. [Google Scholar]
- Abdelhamid, A.I. The use of oleozon gel in the treatment of surgically induced two-wall osseous defects in mongrel dogs (histological study). J. Am. Sci. 2012, 8, 1017–1023. [Google Scholar]
- Lenart-Boroń, A.; Stankiewicz, K.; Bulanda, K.; Czernecka, N.; Heliasz, M.; Hunter, W.; Ratajewicz, A.; Khachatryan, K.; Khachatryan, G. In Vitro Antibacterial Activity of Ozonated Olive Oil against Bacteria of Various Antimicrobial Resistance Profiles Isolated from Wounds of Companion Animals. Int. J. Mol. Sci. 2024, 25, 3557. [Google Scholar] [CrossRef]
- Augello, S. Enhancement of Ozonated Oil for Veterinary Applications. Ph.D. Thesis, University of Rome, Rome, Italy, 2025. [Google Scholar]
- Szponder, T.; Zdziennicka, J.; Nowakiewicz, A.; Świeca, M.; Sobczyńska-Rak, A.; Żylińska, B.; Patkowski, K.; Junkuszew, A.; Wessely-Szponder, J. Effects of topical treatment of foot rot in sheep using ozonated olive ointment. J. Vet. Res. 2021, 65, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Aydın, O.; Kumandaş, A. Evaluation of the effect of ozone therapy on the treatment of cutaneous wounds with tissue-loss in dogs and cats. J. Adv. VetBio Sci. Tech. 2022, 7, 313–320. [Google Scholar] [CrossRef]
- Ginel, P.J.; Negrini, J.; Guerra, R.; Lucena, R.; Ruiz-Campillo, M.T.; Mozos, E. Effect of topical ozonated sunflower oil on second intention wound healing in turtles: A randomised experimental study. J. Vet. Sci. 2021, 22, e27. [Google Scholar] [CrossRef]
- Prządka, P.; Kuberka, M.; Skrzypczak, P.; Kiełbowicz, Z. Healing of a large skin defect in a dog with concurrent ozonated olive oil application. J. Small Anim. Pract. 2022, 63, 492. [Google Scholar] [CrossRef]
- Diniz, M.; Brandão, A.M.H. Óleo de girassol ozonizado na cicatrização de ferida em gato: Relato de caso. Pubvet 2023, 17, e1406. [Google Scholar] [CrossRef]
- de Souza, R.C.; Dias, R.A.G.; de Santos Jesus, M.D.L.; de Araújo Barreto, C.O.; de Andrade, E.S.; de Araújo Machado, M.C. Effects of ozonized sunflower oil in the treatment of surgical wound in cats submitted to elective ovariohysterectomy. Vet. Zootec. 2022, 29, 20230305151. [Google Scholar]
- Di Filippo, P.A.; Ribeiro, L.M.F.; Gobbi, F.P.; Lemos, G.B.; Rodrigues, R.B.R.; Jerdy, H.; da Silva, L.C.; Viana, I.S.; Quirino, C.R. Effects of pure and ozonated sunflower seed oil (Helianthus annuus) on hypergranulation tissue formation, infection and healing of equine lower limb wounds. Braz. J. Vet. Med. 2020, 42, e113520. [Google Scholar] [CrossRef]
- Ekren-Aşıcı, G.S.; Kal, U.; Berberoğlu, S.; Kıral, F. The therapeutic effect of ozone-enriched propolis oil extraction in cats with gingivitis. Rev. Cient. Fac. Vet. 2025, 35, 1–8. [Google Scholar] [CrossRef]
- Carrijo, B.N.; Pires, R.H.; Costa, G.B.; Guiotto, F.G.; Rodrigues, V.S.; Ferreira, J.C. Ozone gas and ozonized sunflower oil as alternative therapies against Pythium insidiosum isolated from dogs. Ozone Sci. Eng. 2022, 44, 398–406. [Google Scholar] [CrossRef]
- Júnior, J.I.D.S.S.; dos Santos, C.S.F.; Da Silva, B.M.; dos Santos, I.F.C.; Ferro, B.S.; Barros, T.I.S.; Tomacheuski, R.M.; Simões-Mattos, L. Topical ozone therapy in the treatment of pharmacodermia in a dog (Canis lupus familiaris). Acta Sci. Vet. 2019, 47, 1–5. [Google Scholar] [CrossRef]
- Yipel, F.A.; Acar, A.; Yipel, M. Effect of some essential oils (Allium sativum L., Origanum majorana L.) and ozonated olive oil on the treatment of ear mites (Otodectes cynotis) in cats. Turk. J. Vet. Anim. Sci. 2016, 40, 782–787. [Google Scholar] [CrossRef]
- Rodríguez, Z.Z.; Teste, I.S.; Gómez, D.; Lozano, O.E.L. Efectividad y eficacia del aceite de girasol ozonizado (AGO) de uso oral como tratamiento de la giardiasis en perros Beagles. REDVET Rev. Electr. Vet. 2016, 17, 1–13. [Google Scholar]
- Rodriguez, Z.Z.; Lemus, M.; González, E.F.; Lozano, O.E.L. Efficacy of ozonized sunflower oil as treatment of canine generalized demodicosis. Insights Vet. Sci. 2021, 5, 015–021. [Google Scholar]
- Da Vinha, E.C.; de Pierro, E.F.; Alemán Gainza, Y.; da Silva, O.; Camargo-Mathias, M.I. Effects of Sarolaner and Ozonated Sunflower Oil on the Morphology of Salivary Glands in Rhipicephalus linnaei and Liver of Hosts. Ozone Sci. Eng. 2025, ahead of print. [Google Scholar] [CrossRef]
- Daud, F.V.; Ueda, S.M.Y.; Navarini, A.; Mímica, L.M.J. The use of ozonized oil in the treatment of dermatophitosis caused by Microsporum canis in rabbits. Braz. J. Microbiol. 2011, 42, 274–281. [Google Scholar] [CrossRef]
| Sample | Peroxide Value (mEq O2/Kg) | Acidity Value (mg KOH/g) | Iodine Value (g/100 g) | Viscosity (mPas) | |
|---|---|---|---|---|---|
| UN | 15.25 ± 0.02 | 0.53 ± 0.14 | 39.51 ± 0.89 | 22 °C | 56.6 |
| 35 °C | 34.9 | ||||
| UO1 | 83.21 ± 0.06 | 2.55 ± 0.33 | 35.59 ± 1.1 | 22 °C | 83.1 |
| 35 °C | 49.6 | ||||
| UO3 | 118.95 ± 0.10 | 2.90 ± 0.12 | 30.45 ± 0.7 | 22 °C | 112.1 |
| 35 °C | 65.7 | ||||
| UO6 | 183.86 ± 0.20 | 4.54 ± 0.32 | 13.39 ± 0.3 | 22 °C | 212.6 |
| 35 °C | 109 | ||||
| UO12 | 224.22 ± 0.22 | 8.31 ± 0.01 | 1.5 ± 0.01 | 22 °C | 2937.3 |
| 35 °C | 296.8 | ||||
| p = 0.011 | p = 0.0012 | p = 0.0033 | - | ||
| - | |||||
| Microorganism | Tween 80 Concentration | The Diffusimetric Method | Inhibition Zone Diameter (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Untreated Oil | Oil O3 1 h | Oil O3 3 h | Oil O3 6 h | Oil O3 12 h | Antibiotic/ Antimicotic | Negativ Sample | ||||
| Gram-positive strains | Staphylococcus aureus ATCC 6538P | 0.5% | Filter paper | 0 | 0 | 0 | 6.5 ± 0.19 | 9.1 ± 0.27 | 13.3 ± 0.24 | 0 |
| well | 0 | 0 | 4.1 ± 0.21 | 7.3 ± 0.32 | 10.4 ± 0.41 | 13.4 ± 0.12 | 0 | |||
| 2.5% | Filter paper | 0 | 0 | 0 | 0 | 10.9 ± 0.37 | 14 ± 0.32 | 0 | ||
| well | 0 | 0 | 5.3 ± 0.13 | 7.9 ± 0.41 | 12.3 ± 0.52 | 13.9 ± 0.21 | 0 | |||
| Enterococcus faecalis ATCC 29212 | 0.5% | Filter paper | 0 | 0 | 0 | 0 | 0 | 13.7 ± 0.12 | 0 | |
| well | 0 | 0 | 0 | 0 | 2.6 ± 0.11 | 13.2 ± 0.38 | 0 | |||
| 2.5% | Filter paper | 0 | 0 | 0 | 0 | 0 | 13.9 ± 0.26 | 0 | ||
| well | 0 | 0 | 0 | 0 | 3.5 ± 0.17 | 13.5 ± 0.15 | 0 | |||
| Gram-negative strains | Escherichia coli ATCC 13076 | 0.5% | Filter paper | 0 | 0 | 0 | 0 | 0 | 19.7 ± 0.18 | 0 |
| well | 0 | 0 | 0 | 0 | 7.5 ± 0.37 | 19.3 ± 0.23 | 0 | |||
| 2.5% | Filter paper | 0 | 0 | 0 | 0 | 0 | 20.7 ± 0.33 | 0 | ||
| well | 0 | 0 | 0 | 0 | 9.5 ± 0.32 | 20.7 ± 0.13 | 0 | |||
| Pseudomonas aeruginosa ATCC 27853 | 0.5% | Filter paper | 0 | 0 | 0 | 0 | 3.3 ± 0.14 | 25.2 ± 0.21 | 0 | |
| well | 0 | 0 | 0 | 0 | 0 | 24.9 ± 0.31 | 0 | |||
| 2.5% | Filter paper | 0 | 0 | 0 | 0 | 0 | 24.9 ± 0.17 | 0 | ||
| well | 0 | 0 | 0 | 0 | 0 | 24.6 ± 0.14 | 0 | |||
| Klebsiella pneumoniae NCTC 13438 | 0.5% | Filter paper | 0 | 0 | 0 | 0 | 0 | 16.7 ± 0.26 | 0 | |
| well | 0 | 0 | 0 | 0 | 7.9 ± 0.34 | 15.8 ± 0.32 | 0 | |||
| 2.5% | Filter paper | 0 | 0 | 0 | 0 | 0 | 16.7 ± 0.11 | 0 | ||
| well | 0 | 0 | 2.8 ± 0.16 | 3.6 ± 0.14 | 8.6 ± 0.39 | 16.1 ± 0.16 | 0 | |||
| Fungi | Candida albicans DMSZ 1386 | 0.5% | Filter paper | 0 | 0 | 0 | 14.8 ± 0.48 | 16.6 ± 0.51 | 19 ± 0.32 | 0 |
| well | 0 | 0 | 0 | 15.9 ± 0.36 | 23.3 ± 0.44 | 20.2 ± 0.19 | 0 | |||
| 2.5% | Filter paper | 0 | 0 | 0 | 0 | 17.6 ± 0.36 | 19.4 ± 0.24 | 0 | ||
| well | 0 | 0 | 0 | 13.9 ± 0.13 | 23.9 ± 0.53 | 19.6 ± 0.36 | 0 | |||
| Micro-Organism | Dilution | |||||
|---|---|---|---|---|---|---|
| 1/4 | 1/8 | 1/16 | ||||
| 1/10 | 1/100 | 1/1000 | ||||
| Gram-positive strains | Staphylococcus aureus ATCC 6538P | + | + | + | − | − |
| Enterococcus faecalis ATCC 29212 | − | − | − | − | − | |
| Gram-negative strains | Escherichia coli ATCC 13076 | − | − | − | − | − |
| Pseudomonas aeruginosa ATCC 27 | − | − | − | − | − | |
| Klebsiella pneumoniae NCTC 13438 | + | + | + | − | − | |
| Fungi | Candida albicans DMSZ 1386 | + | + | + | + | − |
| Study | Oil Type | Ozonating Method | PV (mEq O2/kg) | Antibacterial/Antifungic Effect | Observations |
|---|---|---|---|---|---|
| Puxeddu et al., 2024 [10] | OO/SFO OS Srl (Pesaro, Italy) | - | 3110–3520 | C. albicans (+++/+++) E. faecalis (+/++) E. coli (−/++) S. aureus (++/+) K. pneumoniae (−) P. aeruginosa (−) | Diffusion method |
| Moureu et al., 2015 [14] | SFO ± water | 50 g oil ±5 g water ≈60 µg/mL, 30 L/h 1–7 h | 397 (560–2680) | S. aureus (++) E. coli (+) S. uberis (+) | MIC—Only oil samples ozonated in the presence of water had visible effects |
| Silva et al., 2020 [11] | OO + SFO (50:50) | 100 mL oil 75 µg/mL, 4 L/min 160 min | 113.5 ± 3.7 | MRSA (++) | In vivo: Cutaneous ulceras in mice with diabetes |
| Song et al., 2018 [15] | Green tea (Camellia oil) + ozonated water | - | 2000–2200 | S. aureus (+++) MRSA (+++) | In vivo: cutaneous infections in humans |
| S. aureus (+++) MRSA (+++) | In vitro: 400 µL ozonated oil + 50 µL DMSO + 50 µL cult. | ||||
| S. aureus (17 cm) MRSA (13 cm) | Diffusion method (Kirby Bauer) | ||||
| Grandi et al., 2022 [16] | Liposomal SFO LipozonEye® 10.5% | - | - | P. aeruginosa MDR MRSA S. epidermidis Streptococcus spp. | Diffusion method inhibition present at 6–8 h after incubation, but not after 24 h |
| Montevecchi et al., 2013 [17] | OO Novox® | - | - | S. aureus (dil. 1:128 = 20.67 ± 0.58 mm) Porphyromonas gingivalis (dil. 1:128 = 19 mm) | Diffusion method Oil dilutions 1:2, 1:4, 1:8, 1:16, 1:32, 1:64 si 1:128 |
| Pietrocola et al., 2018 [18] | OO O-zone Gel® | - | - | P. intermedia (4.5 mm ± 0.38), A. actinomycetemcomitans (3.5 mm ± 0.14) S. mutans (3.5 mm ± 0.2) | Diffusion method |
| P. intermedia (10%), A. actinomycetemcomitans (10%) S. mutans (−) | MIC | ||||
| Skalska et al., 2009 [7] | SFO | 150 mL 20–30 µg/mL 1–50 h | 1187 | B. subtillis (200 mg O3/g) E. coli (>50 mg O3/g) C. albicans (>50 mg O3/g) | MIC |
| Zanardi et al., 2013 [19] | Sesame Oil | 40 mL oil 45 µg/mL, 1.5 L/min | low: 949 ± 33, medium: 1631 ± 64, high: 3170 ± 101 | S. aureus E. faecalis P. aeruginosa E.coli C. albicans | - |
| Serio et al., 2017 [20] | SFO | (30% UFS) | 335 | E. coli (22.5 ± 0.07) P. aeruginosa (20.75 ± 0.1) M. luteus (21 ± 0.28) S. aureus (23 ± 0.14) | Diffusion method |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Repciuc, C.C.; Vișan, G.-A.-M.; Teleky, B.-E.; Pintea, A.; Novac, C.Ș.; Oros, N.V. Physico-Chemical and Antimicrobial Evaluation of Ozonated Olive Oil Produced with a Medical-Grade Generator for Veterinary Purposes. Microorganisms 2025, 13, 1932. https://doi.org/10.3390/microorganisms13081932
Repciuc CC, Vișan G-A-M, Teleky B-E, Pintea A, Novac CȘ, Oros NV. Physico-Chemical and Antimicrobial Evaluation of Ozonated Olive Oil Produced with a Medical-Grade Generator for Veterinary Purposes. Microorganisms. 2025; 13(8):1932. https://doi.org/10.3390/microorganisms13081932
Chicago/Turabian StyleRepciuc, Călin Cosmin, Giulia-Ana-Maria Vișan, Bernadette-Emoke Teleky, Adela Pintea, Cristiana Ștefania Novac, and Nicușor Valentin Oros. 2025. "Physico-Chemical and Antimicrobial Evaluation of Ozonated Olive Oil Produced with a Medical-Grade Generator for Veterinary Purposes" Microorganisms 13, no. 8: 1932. https://doi.org/10.3390/microorganisms13081932
APA StyleRepciuc, C. C., Vișan, G.-A.-M., Teleky, B.-E., Pintea, A., Novac, C. Ș., & Oros, N. V. (2025). Physico-Chemical and Antimicrobial Evaluation of Ozonated Olive Oil Produced with a Medical-Grade Generator for Veterinary Purposes. Microorganisms, 13(8), 1932. https://doi.org/10.3390/microorganisms13081932









