Rising Threats of MRSA and Carbapenem-Resistant Acinetobacter in Residential Care Homes for the Elderly During COVID-19 in Hong Kong
Abstract
1. Background
2. Methods
2.1. Recruitment and Sampling of RCHE Residents
- (i)
- N is the estimated sample size;
- (ii)
- d is the relative precision (i.e., width of the 95% confidence interval (CI), expressed as the proportion of prevalence), aiming at a relative precision of 10% in the MRSA prevalence estimate being 10%, resulting in a margin of error of ±3.01%;
- (iii)
- The design effect is the multiple by which the sample size might be increased compared with the sample size that would be required if simple random sampling was used.
- (i)
- m is the estimated size of a cluster (RCHE), which is the mean number of residents from RCHEs and is estimated to be 72;
- (ii)
- k is the coefficient of between-cluster variation, which is estimated to be 0.082 on the basis of a previous estimation [15].
2.2. Laboratory Tests for MRSA and CRA
2.3. Data Analysis
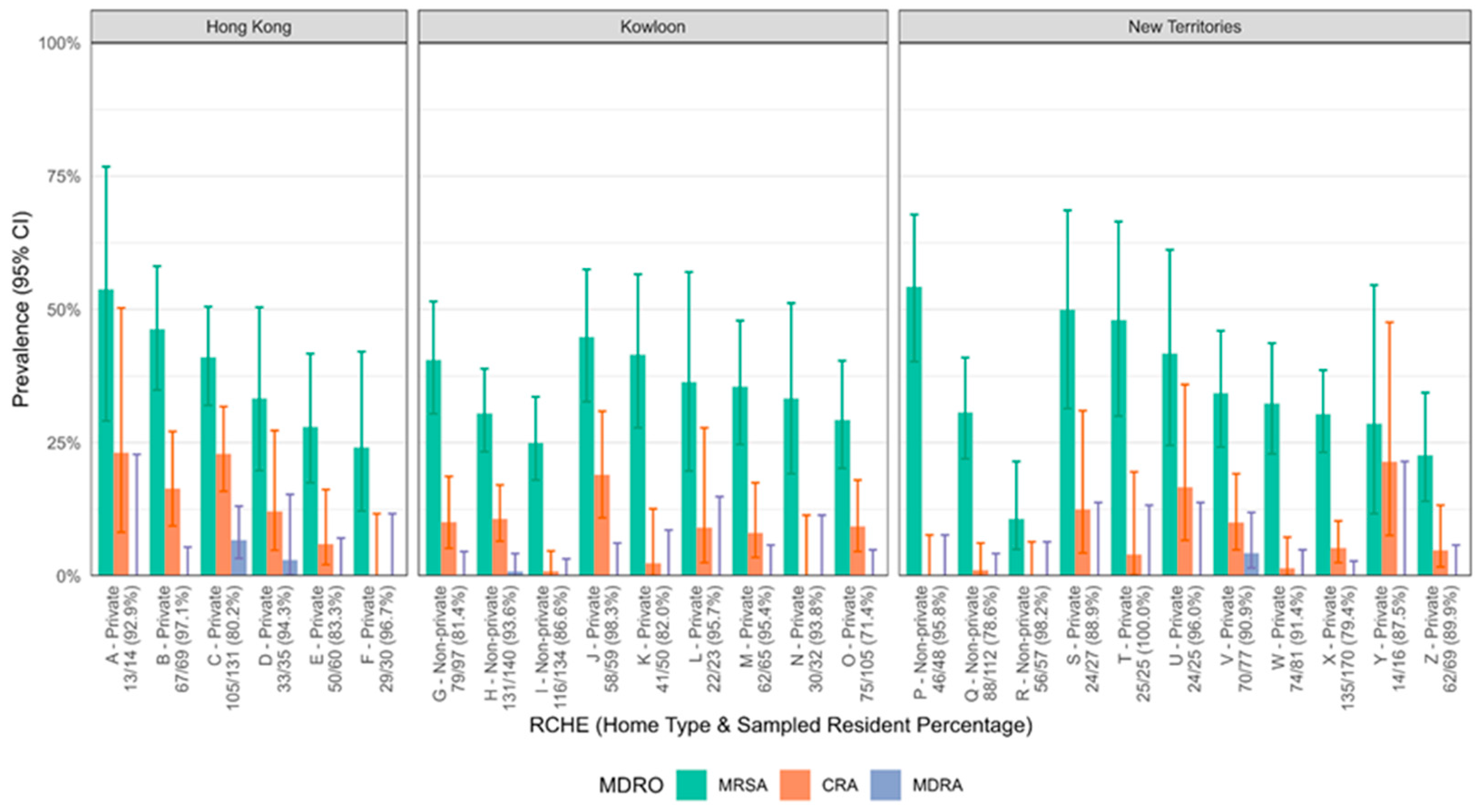
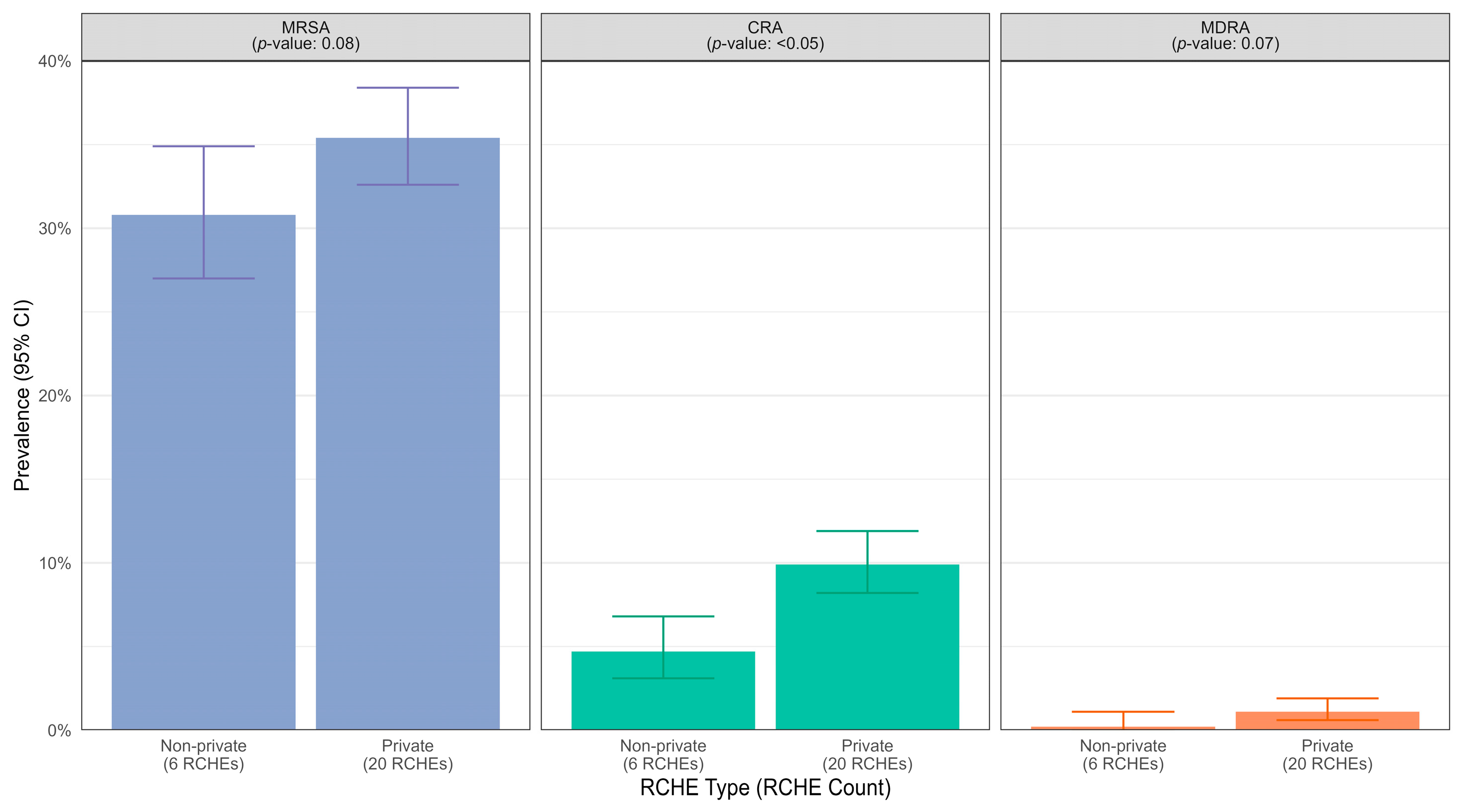
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- World Health Organisation. Antimicrobial Resistance; Published Online November 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 11 April 2025).
- Abubakar, U.; Al-Anazi, M.; Alanazi, Z.; Rodríguez-Baño, J. Impact of COVID-19 pandemic on multidrug resistant gram-positive and gram-negative pathogens: A systematic review. J. Infect. Public Health 2023, 16, 320–331. [Google Scholar] [CrossRef]
- Wong, S.C.; Chau, P.H.; So, S.Y.; Chiu, K.H.; Yuen, L.L.; AuYeung, C.H.; Lam, G.K.; Chan, V.W.; Chen, J.H.; Chen, H.; et al. Epidemiology of multidrug-resistant organisms before and during COVID-19 in Hong Kong. Infect. Prev. Pract. 2023, 5, 100286. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.S.K.; Kung, K.H.; Chen, H. Combating antimicrobial resistance during the COVID-19 pandemic. Hong Kong Med. J. 2021, 27, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.-C.; Wong, S.-C.; Ma, E.S.-K.; Chen, H.; Chiu, K.H.-Y.; Chen, J.H.-K.; So, S.Y.-C.; Lung, D.C.; Ho, P.-L.; Yuen, K.-Y. Antimicrobial Resistance Situation and Control Measures in Hong Kong: From a One Health Perspective. J. Hosp. Infect. 2025, 162, 174–185. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. WHO Bacterial Priority Pathogens List, 2024. Available online: https://www.who.int/publications/b/64088 (accessed on 11 April 2025).
- World Health Organisation. Health and Economic Impacts of Antimicrobial Resistance in the Western Pacific Region, 2020–2030. Available online: https://www.who.int/publications/i/item/9789290620112 (accessed on 11 April 2025).
- You, J.H.S.; Choi, K.W.; Wong, T.Y.; Ip, M.; Ming, W.K.; Wong, R.Y.; Chan, S.; Tse, S.; Chau, C.T.S.; Lee, N.L.S. Disease Burden, Characteristics, and Outcomes of Methicillin-Resistant Staphylococcus aureus Bloodstream Infection in Hong Kong. Asia Pac. J. Public Health 2017, 29, 451–461. [Google Scholar] [CrossRef]
- Cheng, V.C.C.; Chen, J.H.K.; Ng, W.C.; Wong, J.Y.H.; Chow, D.M.K.; Law, T.C.; So, S.Y.C.; Wong, S.C.Y.; Chan, T.C.; Chan, F.H.W.; et al. Emergence of Carbapenem-Resistant Acinetobacter baumannii in Nursing Homes with High Background Rates of MRSA Colonization. Infect. Control Hosp. Epidemiol. 2016, 37, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Hübner, N.; Dittmann, K.; Begunk, R.; Kramer, A. Infection control measures and prevalence of multidrug-resistant organisms in nonhospital care settings in northeastern Germany: Results from a one-day point prevalence study. J. Hosp. Infect. 2017, 97, 234–240. [Google Scholar] [CrossRef]
- Eveillard, M.; LaFargue, S.; Guet, L.; Mangeol, A.; Piquet, J.; Quenon, J.-L.; Fauvelle, F. Association between Institutionalisation and Carriage of Multiresistant Bacteria in the Elderly at the Time of Admission to a General Hospital. Eur. J. Clin. Microbiol. Infect. Dis. 1999, 18, 133–136. [Google Scholar] [CrossRef]
- Ho, P.L.; Lai, E.L.; Chow, K.H.; Chow, L.S.; Yuen, K.Y.; Yung, R.W. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in residential care homes for the elderly in Hong Kong. Diagn. Microbiol. Infect. Dis. 2008, 61, 135–142. [Google Scholar] [CrossRef]
- Cheng, V.C.; Tai, J.W.; Wong, Z.S.; Chen, J.H.; Pan, K.B.; Hai, Y.; Ng, W.-C.; Chow, D.M.K.; Yau, M.C.Y.; Cham, J.W.F.; et al. Transmission of methicillin-resistant Staphylococcus aureus in the long term care facilities in Hong Kong. BMC Infect. Dis. 2013, 13, 205. [Google Scholar] [CrossRef]
- Chen, H.; Au, K.M.; Hsu, K.E.; Lai, C.K.; Myint, J.; Mak, Y.F.; Lee, S.Y.; Wong, T.Y.; Tsang, N.C. Multidrug-resistant organism carriage among residents from residential care homes for the elderly in Hong Kong: A prevalence survey with stratified cluster sampling. Hong Kong Med. J. 2018, 24, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Chen, J.H.; Yuen, L.L.; Chan, V.W.; AuYeung, C.H.; Leung, S.S.; So, S.Y.-C.; Chan, B.W.-K.; Li, X.; Leung, J.O.-Y.; et al. Air dispersal of meticillin-resistant Staphylococcus aureus in residential care homes for elderly individuals: Implications for transmission during the COVID-19 pandemic. J Hosp. Infect. 2022, 123, 52–60. [Google Scholar] [CrossRef]
- Ma, E.S.K. Combating antimicrobial resistance in Hong Kong: Where are we and where should we go? Hong Kong Med. J. 2022, 28, 424–426. [Google Scholar] [CrossRef]
- Wong, S.C.; Lam, G.K.; Chen, J.H.; Li, X.; Ip, F.T.; Yuen, L.L.; Chan, V.W.; AuYeung, C.H.; So, S.Y.; Ho, P.L.; et al. Air dispersal of multidrug-resistant Acinetobacter baumannii: Implications for nosocomial transmission during the COVID-19 pandemic. J. Hosp. Infect. 2021, 116, 78–86. [Google Scholar] [CrossRef]
- Ma, E.S.; Wong, S.C.; Cheng, V.C.; Wu, P. Global trends and projections in antimicrobial resistance. Lancet 2025, 405, 1904–1905. [Google Scholar] [CrossRef]
- O’Fallon, E.; Schreiber, R.; Kandel, R.; D’Agata, E.M. Multidrug-resistant gram-negative bacteria at a long-term care facility: Assessment of residents, healthcare workers, and inanimate surfaces. Infect. Control Hosp. Epidemiol. 2009, 30, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Jans, B.; Schoevaerdts, D.; Huang, T.D.; Berhin, C.; Latour, K.; Bogaerts, P.; Nonhoff, C.; Denis, O.; Catry, B.; Glupczynski, Y. Epidemiology of multidrug-resistant microorganisms among nursing home residents in Belgium. PLoS ONE 2013, 8, e64908. [Google Scholar] [CrossRef] [PubMed]
- Hogardt, M.; Proba, P.; Mischler, D.; Cuny, C.; Kempf, V.A.; Heudorf, U. Current prevalence of multidrug-resistant organisms in long-term care facilities in the Rhine-Main district, Germany, 2013. Euro Surveill. 2015, 20, 21171. [Google Scholar] [CrossRef]
- Giufrè, M.; Ricchizzi, E.; Accogli, M.; Barbanti, F.; Monaco, M.; de Araujo, F.P.; Farina, C.; Fazii, P.; Mattei, R.; Sarti, M.; et al. Colonisation by multidrug-resistant organisms in long-term care facilities in Italy: A point-prevalence study. Clin. Microbiol. Infect. 2017, 23, 961–967. [Google Scholar] [CrossRef]
- March, A.; Aschbacher, R.; Sleghel, F.; Soelva, G.; Kaczor, M.; Migliavacca, R.; Piazza, A.; Mattioni Marchetti, V.; Pagani, L.; Scalzo, K.; et al. Colonisation of residents and staff of an Italian long-term care facility and an adjacent acute care hospital geriatric unit by multidrug-resistant bacteria. New Microbiol. 2017, 40, 258–263. [Google Scholar] [PubMed]
- McKinnell, J.A.; Singh, R.D.; Miller, L.G.; Kleinman, K.; Gussin, G.; He, J.; Saavedra, R.; Dutciuc, T.C.; Estevez, M.; Chang, J.; et al. The SHIELD Orange County Project: Multidrug-resistant Organism Prevalence in 21 Nursing Homes and Long-term Acute Care Facilities in Southern California. Clin. Infect. Dis. 2019, 69, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- McKinnell, J.A.; Miller, L.G.; Singh, R.D.; Gussin, G.; Kleinman, K.; Mendez, J.; Laurner, B.; Catuna, T.D.; Heim, L.; Saavedra, R.; et al. High Prevalence of Multidrug-Resistant Organism Colonisation in 28 Nursing Homes: An “Iceberg Effect”. J. Am. Med. Dir. Assoc. 2020, 21, 1937–1943.e2. [Google Scholar] [CrossRef]
- Callejón Fernández, M.; Madueño Alonso, A.; Abreu Rodríguez, R.; Aguirre-Jaime, A.; Castro Hernández, M.B.; Ramos-Real, M.J.; Pedroso-Fernandez, Y.; Lecuona Fernandez, M. Risk factors for colonisation by carbapenemase-producing bacteria in Spanish long-term care facilities: A multicentre point-prevalence study. Antimicrob. Resist. Infect. Control 2022, 11, 163. [Google Scholar] [CrossRef]
- Wong, S.C.; Au, A.K.; Lo, J.Y.; Ho, P.L.; Hung, I.F.; To, K.K.; Yuen, K.Y.; Cheng, V.C. Evolution and Control of COVID-19 Epidemic in Hong Kong. Viruses 2022, 14, 2519. [Google Scholar] [CrossRef] [PubMed]
- Centre for Health Protection, Department of Health, Hong Kong Special Administrative Region. Guidelines on Prevention of Communicable. Diseases in Residential Care Homes for the Elderly. Available online: https://www.chp.gov.hk/files/pdf/guidelines_on_prevention_of_communicable_diseases_in_rche_eng.pdf (accessed on 5 August 2025).
- Centre for Health Protection, Department of Health, Hong Kong Special Administrative Region. Infection Control Advice on Multi-Drug Resistant Organisms (MDROs) for Residential Care Homes for the Elderly (RCHEs). Available online: https://www.chp.gov.hk/files/pdf/infection_control_advice_on_mdro_for_rche_eng.pdf (accessed on 5 August 2025).
- Centre for Health Protection, Department of Health, Hong Kong Special Administrative Region. Statistics on Antimicrobial Resistance Control. Available online: https://www.chp.gov.hk/en/statistics/data/10/100044/6864.html (accessed on 25 June 2025).
- Batra, R.; Eziefula, A.C.; Wyncoll, D.; Edgeworth, J. Throat and rectal swabs may have an important role in MRSA screening of critically ill patients. Intensive Care Med. 2008, 34, 1703–1706. [Google Scholar] [CrossRef]
- Lim, C.J.; Cheng, A.; Kennon, J.; Spelman, D.; Hale, D.; Melican, G.; Sidjabat, H.E.; Paterson, D.L.; Kong, D.C.M.; Peleg, A.Y. Prevalence of multidrug-resistant organisms and risk factors for carriage in long-term care facilities: A nested case-control study. J. Antimicrob. Chemother. 2014, 69, 1972–1980. [Google Scholar] [CrossRef]
- Denis, O.; Jans, B.; Deplano, A.; Nonhoff, C.; De Ryck, R.; Suetens, C.; Struelens, M.J. Epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) among residents of nursing homes in Belgium. J. Antimicrob. Chemother. 2009, 64, 1299–1306. [Google Scholar] [CrossRef]
- Rodríguez-Villodres, Á.; Martín-Gandul, C.; Peñalva, G.; Guisado-Gil, A.B.; Crespo-Rivas, J.C.; Pachón-Ibáñez, M.E.; Lepe, J.A.; Cisneros, J.M. Prevalence and Risk Factors for Multidrug-Resistant Organisms Colonisation in Long-Term Care Facilities Around the World: A Review. Antibiotics 2021, 10, 680. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, E.S.-K.; Wong, S.-C.; Cheng, V.C.-C.; Hsu, E.; Chen, H.; Tsui, E.L.-K. Rising Threats of MRSA and Carbapenem-Resistant Acinetobacter in Residential Care Homes for the Elderly During COVID-19 in Hong Kong. Microorganisms 2025, 13, 1912. https://doi.org/10.3390/microorganisms13081912
Ma ES-K, Wong S-C, Cheng VC-C, Hsu E, Chen H, Tsui EL-K. Rising Threats of MRSA and Carbapenem-Resistant Acinetobacter in Residential Care Homes for the Elderly During COVID-19 in Hong Kong. Microorganisms. 2025; 13(8):1912. https://doi.org/10.3390/microorganisms13081912
Chicago/Turabian StyleMa, Edmond Siu-Keung, Shuk-Ching Wong, Vincent Chi-Chung Cheng, Enoch Hsu, Hong Chen, and Edwin Lok-Kin Tsui. 2025. "Rising Threats of MRSA and Carbapenem-Resistant Acinetobacter in Residential Care Homes for the Elderly During COVID-19 in Hong Kong" Microorganisms 13, no. 8: 1912. https://doi.org/10.3390/microorganisms13081912
APA StyleMa, E. S.-K., Wong, S.-C., Cheng, V. C.-C., Hsu, E., Chen, H., & Tsui, E. L.-K. (2025). Rising Threats of MRSA and Carbapenem-Resistant Acinetobacter in Residential Care Homes for the Elderly During COVID-19 in Hong Kong. Microorganisms, 13(8), 1912. https://doi.org/10.3390/microorganisms13081912





