Abstract
Fungal pericarditis is a rare disease but its incidence has risen in parallel with the global increase in invasive fungal infections. This systematic review analyzes data from previously reported cases of fungal pericarditis to provide an improved understanding of the etiology, clinical presentation, management, and outcomes of this rare disease. We reviewed Medline and Scopus databases from 1 January 1990 to 29 January 2024 for case reports that documented the isolation of a fungal pathogen from pericardial fluid or tissue. Of the 2330 articles screened, 101 cases met the inclusion criteria. Patients with fungal pericarditis and the involvement of at least one other organ—usually the lungs, brain, or kidney—had worse outcomes than patients with isolated pericardial disease. Immunosuppression was reported in 50% of cases and was associated with worse outcomes in adults. Patients who presented with chest pain, received adequate empiric antifungal therapy, and underwent pericardiocentesis and pericardiectomy had improved survival. The most common isolated pathogens were Candida spp., followed by Aspergillus spp. and Mucor spp., with the latter two linked to worse outcomes. Only 35% of patients received empiric antifungal medications before the causative pathogen was identified, and mortality was associated with a delay in appropriate therapy. Immunosuppression, disseminated disease, and presence of shock/multiorgan failure were additional risk factors associated with death. Fungal pericarditis carries a mortality rate of up to 50%, with nearly half of patients being immunocompromised. Clinicians frequently do not consider fungal pericarditis in the differential diagnoses, which leads to delays in treatment and poorer outcomes. Further prospective multicenter studies are urgently needed to better understand the epidemiology, improve diagnostic testing and management, and decrease unacceptably high mortality in patients with fungal pericarditis.
Keywords:
pericarditis; fungal; Candida; Aspergillus; Mucor; Cryptococcus; fungemia; immunosuppression 1. Introduction
Pericarditis, defined as inflammation of the pericardial sac, is the most common disease of the pericardium [1,2,3]. It can be characterized as non-infectious or infectious, with viruses being the most common infectious etiology [1]. Bacterial and fungal pathogens can also cause pericarditis, with Mycobacterium tuberculosis isolated more frequently in under-resourced countries [4]. Fungal pericarditis occurs when yeasts or molds invade the pericardial space and is indicative of invasive fungal infection. The pericardium can be involved directly through injury or surgery, or by continuous spread from the surrounding structures or through hematogenous spread. The global incidence of invasive fungal infections has risen significantly over the past decades, largely driven by immunosuppression as a major contributing factor [5,6,7,8,9]. The most common invasive infections identified are those caused by Candida spp., Aspergillus spp., Cryptococcus spp., and Mucor [10]. Furthermore, atypical fungal species, mostly environmental fungi that are historically not associated with human infection, have been on the rise in recent years, significantly contributing to the changing epidemiology of fungal infections [11].
Despite advancements in awareness and non-invasive diagnostic tests, diagnosing invasive fungal infections remains challenging. Timely and accurate diagnosis is crucial, as delays are strongly associated with poor outcomes [12,13]. Treatment follows multisociety guidelines and relies on antifungal drugs; however, rising resistance threatens efficacy and cure rates [11,14,15,16,17,18]. Moreover, the optimal duration of treatment for fungal pericarditis remains undefined due to a lack of clinical trials. Invasive fungal infections are more common in immunocompromised patients who are at a particular risk; however, infections have also been reported in immunocompetent patients [19,20]. The precise incidence of fungal pericarditis remains unknown. This study aims to systematically analyze data from previously published cases of fungal pericarditis to provide a more comprehensive understanding of the clinical presentation, management, and outcomes of this rare disease.
2. Materials and Methods
We performed a systematic review of the literature according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using Medline (National Library of Medicine, Bethesda, MD, USA) via the PubMed search engine, and the SCOPUS database, from 1 January 1990 to 29 January 2024. All included cases had documented isolation of a fungal pathogen from pericardial fluid, biopsy, or autopsy.
The justification behind the timeframe after the year of 1990 is twofold: firstly, case reports dating before 1990 were hard to find in their full-text form, and secondly, the timeframe was chosen to reflect the therapeutic and diagnostic modalities used today, making any statistically significant findings more likely to be relevant today.
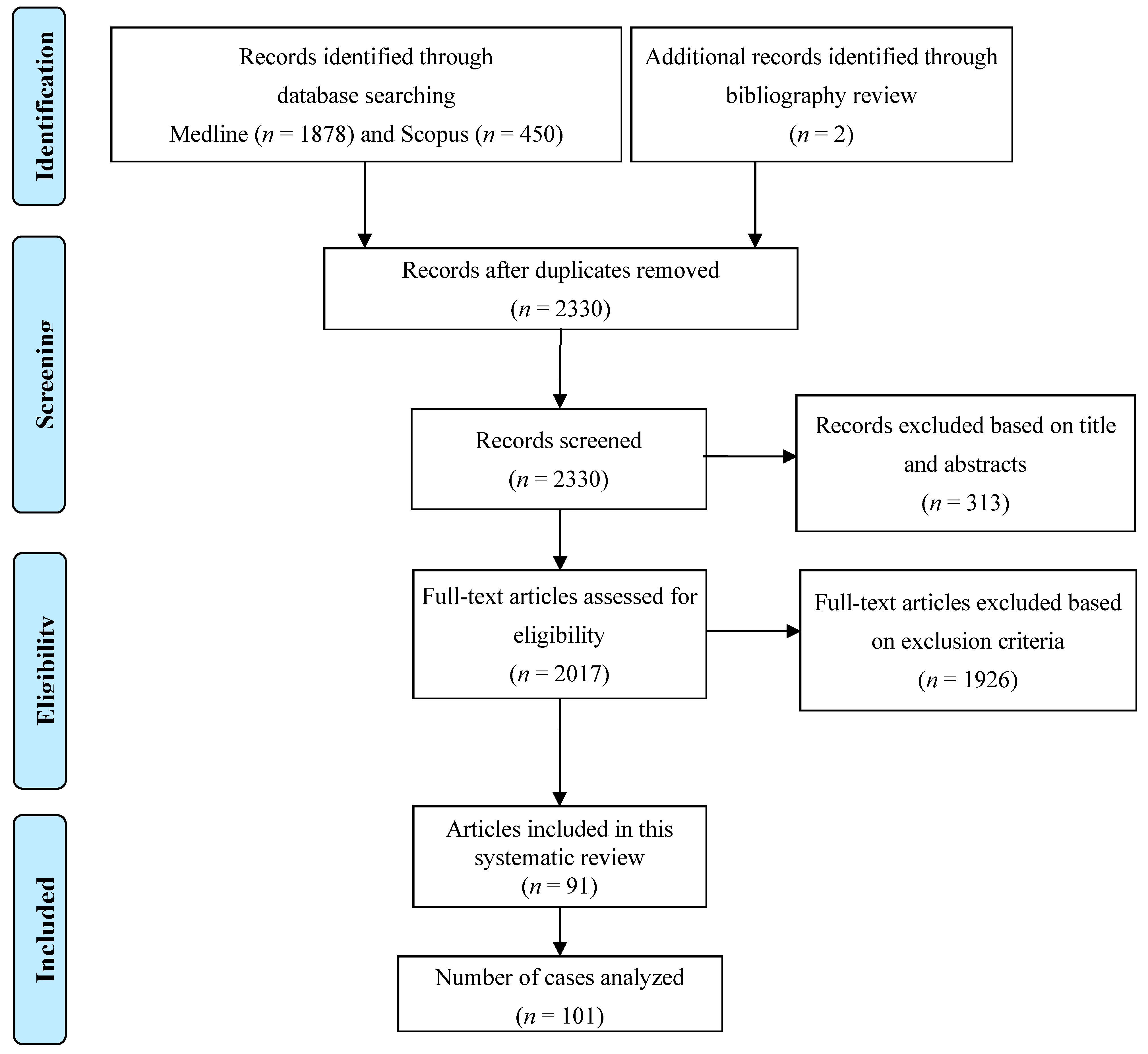
This study is registered with the Research Registry, and the unique identifying number is registry1947. A total of 1878 original articles from Medline and 450 articles from Scopus were identified that mention MeSH terms “pericarditis OR myopericarditis OR pericardium OR pancarditis” AND “fungal OR fungus OR fungi OR candida OR aspergillus OR aspergillosis OR histoplasma OR histoplasmosis OR blastomyces OR blastomycosis OR coccidioidomycosis OR paracoccidioidomycosis OR basidiobolomycosis OR cryptococcus OR cryptococcal OR mucormycosis OR sporothrix”. We excluded cases where the diagnosis was uncertain either because pericardiocentesis, pericardial biopsy, or autopsy was not performed, or pericardial fluid was sterile. We also excluded duplicate articles, articles in languages other than English, abstracts without comprehensive case descriptions, and narrative reviews. Ultimately, our review included 101 cases that fulfilled the criteria. Figure 1 shows the detailed process of article selection and the final cases included in the analysis.
Figure 1.
PRISMA flow diagram of the study selection process.
Four authors (M.A, M.R, S.M., and P.J.) independently and blindly identified and selected titles, abstracts, and full texts in the database search. Discrepancies in the selected articles were resolved by the senior author (I.D.). Subsequently, the reference list of selected articles was searched to identify any additional articles for inclusion in accordance with previously established selection criteria. The following data were extracted: patients’ demographic data, co-morbid conditions, presence of immunosuppression, presenting symptoms, treatment strategies, complications, outcomes, and laboratory and imaging findings including electrocardiography, echocardiography, and computerized tomography scans. Immunosuppression was reported as one of three categories: absent, presence of cancer and/or cancer treatment, and history of organ transplantation and other (autoimmune disease, HIV infection, congenital disease, etc.). Comorbid conditions were categorized into nine categories: absent, cancer diagnosis, history of organ transplantation, liver cirrhosis, history of non-thoracic surgery, presence of hypertension and/or coronary artery disease, autoimmune and/or rheumatic conditions, genetic conditions, and presence of renal failure and/or chronic kidney disease and other.
Statistical Analysis
Data were analyzed by descriptive and analytical statistics using SPSS statistical software (IBM SPSS statistics, Version 21.0, SPSS Inc., Chicago, IL, USA), and are expressed as the mean ± standard deviation for normally distributed data, or as frequency and percentages for categorical data. The Student’s test and chi-square test were used to compare data between the patients regarding survival. The logistic regression analysis (univariate) was used to assess predictors of survival. A p-value <0.05 was considered statistically significant.
We considered the initial diagnosis and treatment as appropriate if the authors suspected fungal infection on admission and if the appropriate empiric therapy was started within 48 h from admission.
3. Results
3.1. Demographics, Comorbidities, and Risk Factors for Invasive Infection
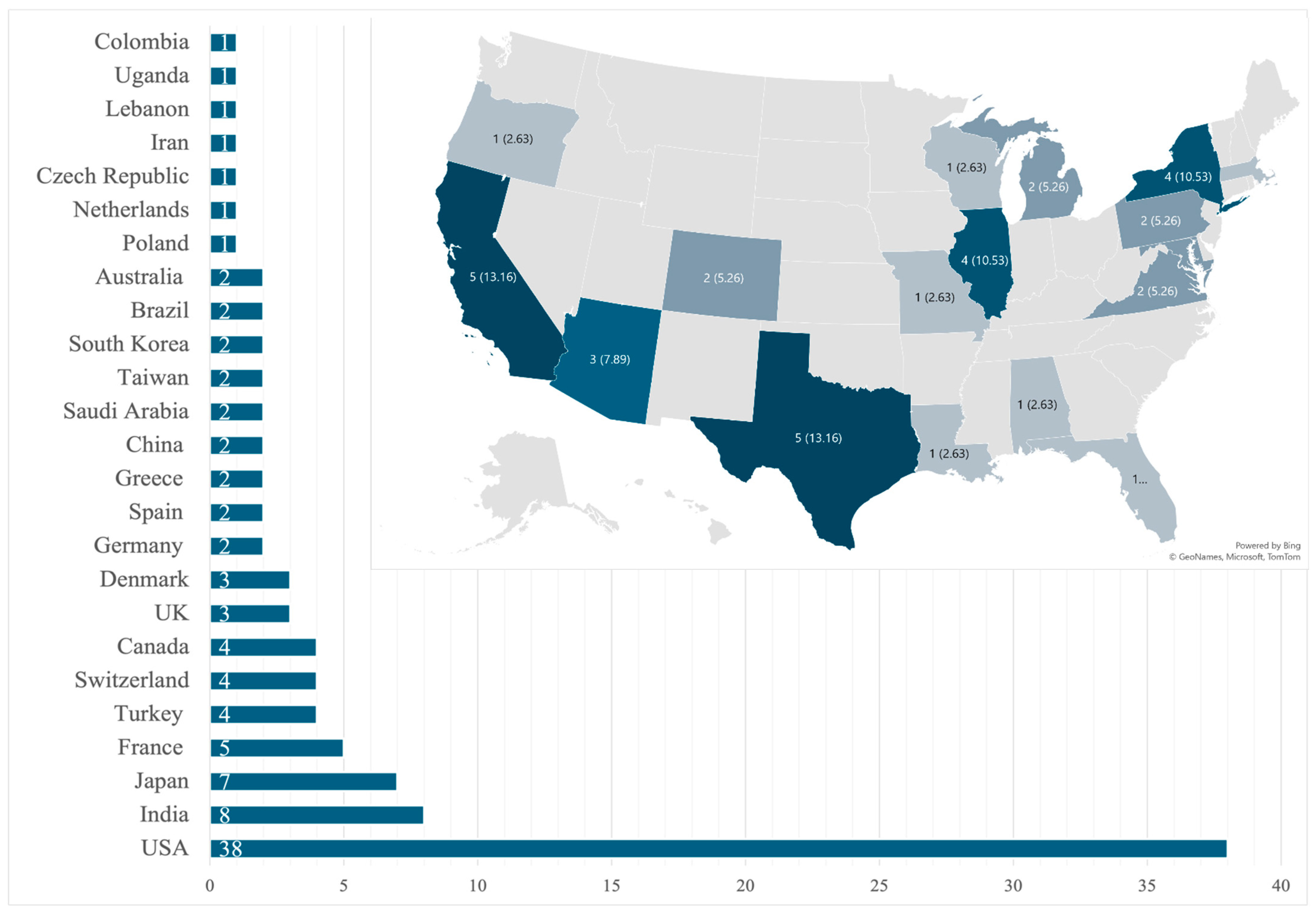
Our review identified 101 unique patients that fulfilled the inclusion criteria from 91 case reports describing a single patient and 5 case series that described two patients each [12,13,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]. A total of 12 cases were pediatric (0 to 18 years) and 89 were adults (18 and older) with an adult mean age of 50.3 ± 16.7 years, with that for pediatrics being 5.8 ± 5.5 years. Most patients were male (n = 68, 67.3%) and no statistical differences were seen in sex-related survival (p = 0.337 for adults; p = 0.224 for the pediatric population). By region, North America reported the most cases (n = 42, 41.6%), of which the USA had 38 cases (Figure 2).
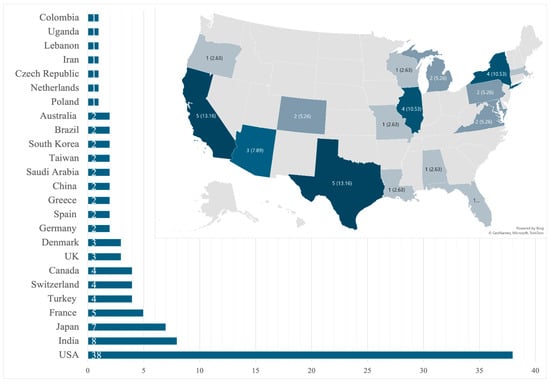
Figure 2.
Global and state-by-state distribution of cases within the United States.
A history of immunosuppression was reported in 51 (50.5%) patients (Table 1). In adults, 44 (49.4%) cases reported immunosuppression, which was more frequently observed in deceased patients compared to survivors (27 vs. 17; p = 0.02). Among the pediatric population, there was no statistically significant difference in survival associated with immunosuppression. Adult patients who underwent pericardiocentesis, underwent pericardiectomy, and received the appropriate initial diagnosis and treatment had better outcomes (p < 0.05), while disseminated disease, presence of complications, and isolation of Aspergillus spp. and Mucor spp. were associated with worse outcomes (p < 0.05). Patients’ demographic data are presented in Table 1, while variables related to the outcome are presented in Table 2.

Table 1.
Demographic characteristics and comorbidities.

Table 2.
Patient characteristics associated with outcome in univariate analysis. Symptoms of chest pain, appropriate initial diagnosis, pericardiocentesis, and pericardiectomy (presented in italics in the table) were associated with improved survival. Immunosuppression, infection caused by Aspergillus spp. and Mucor spp., and development of complications (bolded in the table) were associated with death.
Risk factors for invasive fungal infection include a history of hematological malignancies, bone marrow or solid organ transplantations, prolonged intensive care unit (ICU) stay, and prolonged neutropenia, among others [113,114]. Half of the patients with fungal pericarditis were immunosuppressed, with the most common causes being a history of bone marrow and solid organ transplantation (n = 16, 31.4%) and cancer (n = 14, 27.4%). The majority of cancer patients had hematological malignancy (n = 11, 57.9%), while the other three cases had either gastric and esophageal cancer and Candida spp. that spread contiguously to the pericardium [34,61,94]. All but three adult cases had active cancer treated with chemotherapy at the time of diagnosis, while of the 12 pediatric cases, 3 (25%) had immunosuppression due to cancer treatment, while 2 (16.7%) had a primary immunodeficiency due to chronic granulomatous disease.
In this review, 5 cases of rare fungi (Trichosporon japonicum, Trichosporon inkin, Trichoderma longibrachiatum, Scedosporium prolificans, Curvularia) were seen exclusively in patients with lung or heart transplantation (4 and 1 cases, respectively) [23,32,44,53,62].
3.2. Presenting Signs and Symptoms
Chest pain was reported in 36 (44.4%) adult and 2 (16.7%) pediatric cases, fever in 45 (50.7%) adult and 11 (91.7%) pediatric cases, and dyspnea or tachypnea in 45 (50.7%) adult and 8 (66.7%) pediatric cases. Other commonly reported symptoms included epigastric discomfort and pain, nausea, and vomiting.
3.3. Etiology, Other Organ Involvement, and Diagnosis
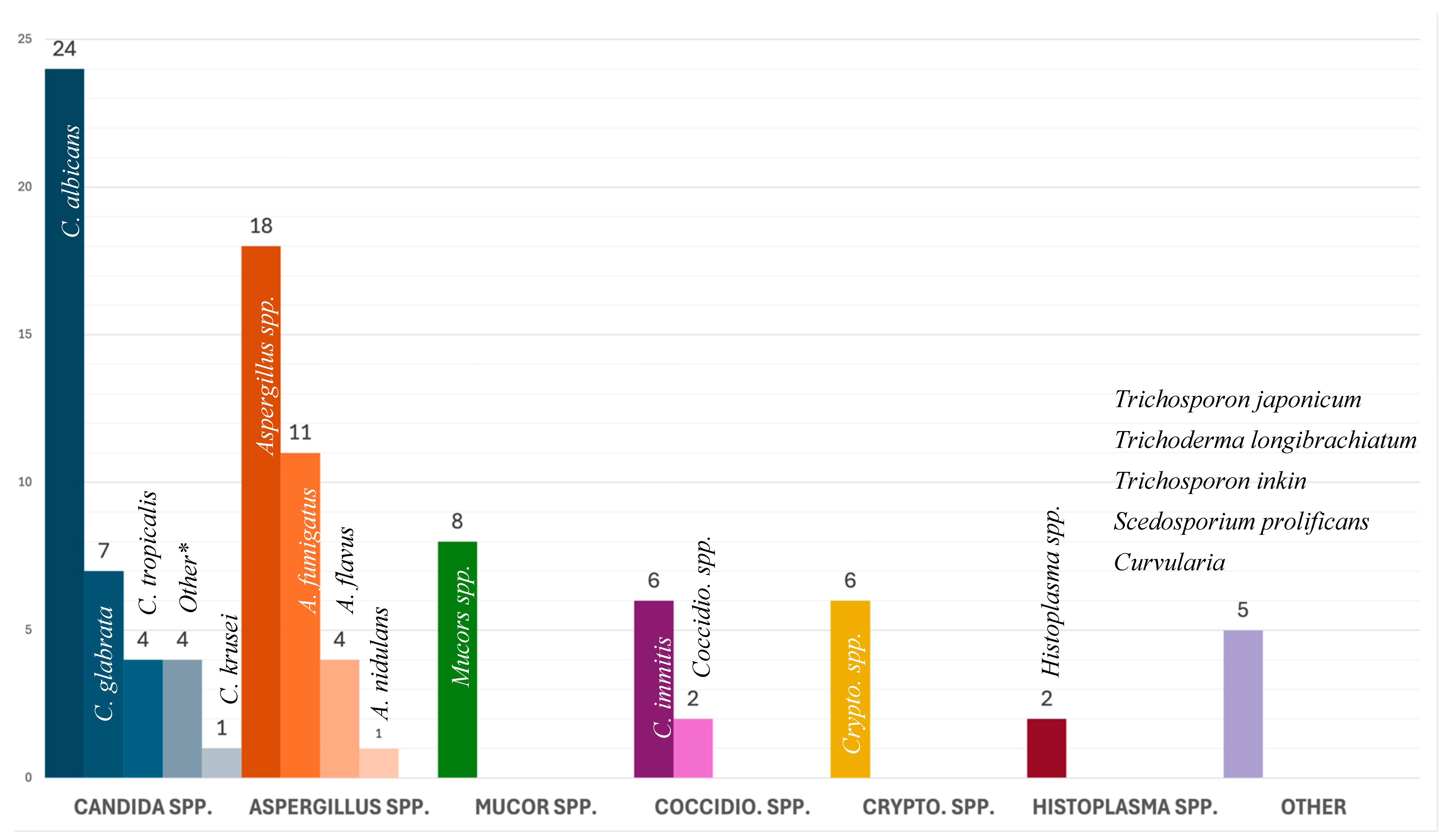
In adults, Candida spp. (n = 34, 38.2%) were the most common fungal isolate with C. albicans as the dominant species (n = 20, 22.5%). In pediatric cases, Aspergillus spp. (n = 7, 58.3%) were the most common fungal isolate, followed by Candida spp. (n = 4, 33.3%). Two cases reported a co-infection, one with C. albicans and C. galbrata, and another with C. albicans and C. tropicalis. Infection with Aspergillus spp. and Mucor spp. was statistically associated with lower survival rates (p = 0.008). All isolated fungi are presented in Figure 3.
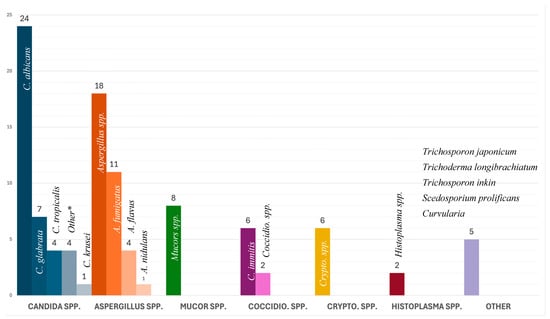
Figure 3.
Causative agent by group and number. * Other Candida: C. parapsilosis (n = 2), C. novrvegensis (n = 1), and C. guilliermondii (n = 1).
Isolated fungal pericarditis was found in 31 (30.7%) cases, while 70 (69.3%) cases had at least another organ involved: most commonly the lungs (n = 43, 42.6%), brain (n = 16, 15.8%), and kidney (n = 11, 10.9%). Involvement of the other organs was reported in 61 of the 89 adult cases (68.5%) and was associated with death (recovered vs. deceased: 27 vs. 34; p = 0.002). Time to diagnosis was inconsistently reported in adults and never in pediatric cases; however, in the cases where patients were promptly diagnosed with fungal pericarditis (14.6%), there was an associated lower mortality (84.6% vs. 15.4%; p = 0.011).
A concomitant presence of endocarditis was reported in 4 (4.5%) adult and 2 (16.7%) pediatric cases, and myocarditis in 12 (13.5%) adults and 1 (8.3%) pediatric case. Combined inflammation of the endocardium, myocardium, and pericardium was diagnosed in 10 (11.2%) adults and 1 (8.3%) pediatric case. Blood cultures were reported in 34 (33.7%) cases, of which 8 yielded a positive result (5 Candida, 2 Cryptococcus neoformans, and 1 Aspergillus case). There was no observed association of the presence of fungemia and patient survival rates (alive vs. deceased: 0.0% vs. 3.4%, p = 0.249 for adult cases; 16.7% vs. 8.3%, p = 1.00 for pediatric cases).
3.4. Treatment and Interventions
The most commonly prescribed antifungal was amphotericin B (n = 29, 28.7%), followed by fluconazole (n = 21, 20.8%), voriconazole (n = 17, 16.8%), capsofungin (n = 7, 6.9%), itraconazole (n = 5, 4.9%), flucytosine (n = 4, 4.0%), and other (posaconazole, anidulafungin, micafungin, terbinafine, 5-fluorocytosine, isavuconazole). Of the 68 cases that reported administering antifungal therapy, 44 (64.7%) reported monotherapy and 24 (35.3%) reported either combined therapy or switched between different medications. Procedural interventions included pericardiocentesis in 38 adult (42.7%) and 7 pediatric cases (58.3%), and pericardiectomy in 25 adult (28.0%) and 4 pediatric (33.3%) cases. In adults, both pericardiocentesis (27 vs. 11, p = 0.002) and pericardiectomy (22 vs. 3, p < 0.001, Table 3) were associated with improved survival. In pediatric cases, this statistical difference was not observed (p > 0.05). Recurrence of the pericardial effusion after initial evacuation was observed in 15 cases (14.9%).

Table 3.
Comparing selected variables based on pericarditis etiology.
3.5. Risk Factors for Death, and Complications
In the univariate analysis, pericardiocentesis, pericardiectomy, symptoms of chest pain, and appropriate initial diagnosis were independent predictors of survival in patients with fungal pericarditis, while in multivariate analysis, pericardiocentesis was the only variable that was associated with survival (p < 0.05).
Statistically significant risk factors for death included immunosuppression, disseminated disease, development of complication, and infection caused by Aspergillus spp. and Mucor spp.
Complications were reported in 56 cases (55.5%), with the most common being fungal embolization (n = 14 13.9%), followed by shock (n = 13, 12.9%), heart failure (n = 11, 10.9%), pneumonia/respiratory failure (n = 9, 8.9%), multiorgan failure (n = 6, 5.9%), and stroke (n = 4, 4.0%). Shock, multiorgan failure, stroke from infective emboli, and respiratory failure were significantly associated with death (p < 0.001), with 84% of patients dying (Table 2).
4. Discussion
4.1. Epidemiology, Demographics, and Risk Factors for Fungal Infection
The rising incidence of fungal infections is closely linked to the growing population of older and immunosuppressed individuals [5,6,7,8,9]. The two most common fungi associated with pericarditis in this review are common commensal microorganisms (Candida spp.), or ubiquitous in nature (Aspergillus spp.) [118,119,120,121,122]. These infections have become a public health emergency due to a limited choice of antifungal medication, an increase in antimicrobial resistance, a lack of vaccines, and a growing at-risk population (older and immunosuppressed) [14,15,16,17,18].
Large-scale epidemiological data on fungal pericarditis are lacking in the available literature [2,123]. The disease is rare enough that even retrospective single-center studies have not been conducted, and the current understanding of this disease is based on case reports and case series. The literature from Sub-Saharan Africa and Asia reports on the more prevalent tuberculosis pericarditis [124], with fungal cases seldom reported. One review from 2009 showed that of 660 cases of purulent pericarditis, only 1.4% were caused by fungi [36]. Our review shows a predominance of fungal pericarditis case reports from high-income countries (Figure 2), which may reflect the lack of diagnostic testing in under-resourced nations, leading to underreporting and publication bias [125,126,127,128,129,130].
Invasive fungal infections have a high mortality rate with approximately 1.5 million deaths annually, over 80% of which are caused by three main fungal species, Candida spp., Aspergillus spp., and Cryptococcus spp. [113,131], findings that were consistent in this review on fungal pericarditis as well. The highest mortality in patients with fungal pericarditis is due to Mucor (88%), followed by Aspergillus spp. (71%), Cryptococcus spp. (33%), and Candida spp. (24%) [131].
Interestingly, unlike other invasive fungal infections that primarily affect immunocompromised individuals, 50% of the patients with fungal pericarditis in this review did not present any of the conditions typically associated with immunosuppression. This finding is significant, as it may lead clinicians to overlook fungal pericarditis in immunocompetent populations.
Other risk factors uniquely associated with invasive candidiasis were skin and mucosal barrier defects, gastrointestinal perforations or surgery, and the presence of indwelling intravenous catheters [114]. Surgery, specifically cardiac, gastric and esophageal, is a known risk factor for the formation of gastro-pericardial or esophago-pericardial fistulas which promote contiguous fungal spread and increase the risk for development of fungal pericarditis [132]. Additionally, manipulations of the pericardial and mediastinal space through thoracotomies and pericardiotomies have previously been linked to the fungal invasion [68] of Candida spp. into the pericardial space [12,68]. In our review, almost a third (n = 12) of pericarditis cases secondary to Candida spp. reported some form of thoracic surgery or intervention, while nine cases (23.7%) had a gastrointestinal pathology such as gastric cancer or esophageal fistulas.
4.2. Diagnostic and Treatment Challenges
Diagnosing pericardial fungal infections is often challenging. Pericardial fluid is a sterile space, and the gold standard for diagnosis is documenting positive fungal culture from the pericardial fluid or fungal visualization on a biopsy or autopsy specimen. Imaging, such as Computed Tomography (CT) and Magnetic Resonance Imaging (MRI), can aid in diagnosis, but alone, it is not sufficient in establishing the diagnosis [133,134,135]. Blood cultures for fungemia have low sensitivity as noted in a recent review of Aspergillus spp. liver abscesses [136,137]. In this review, we found that fungal blood cultures were conducted in 36 (35.6%) cases, out of which 8 (22.2%) were positive. In comparison, patients with bacterial pericarditis, such as Methicillin-resistant Staphylococcus aureus (MRSA) pericarditis, had bacteremia in 64% of cases [116]. While the sensitivity of bacterial cultures for diagnosing bacterial pericarditis is higher than in cases of fungal pericarditis, it is still considered low, and molecular techniques of pathogen identifications are preferrable due to the higher diagnostic yield [138,139]. The exact reason for a low blood culture sensitivity for fungal pathogens is unclear but might be due to the test itself, and depends on a variety of factors, including the amount of blood taken and whether the patient was on antifungal medication at the time of culture [140,141,142]. Nonetheless, blood cultures in purulent pericarditis are still considered a key diagnostic tool, belonging to the first class of the level of evidence in the European Society of Cardiology (ESC) guidelines for management of pericardial diseases [123]. In patients with fungal pericarditis, the absence of fungemia does not rule out disease and negative blood cultures should be interpreted cautiously.
Interestingly, the rates of disseminated disease (fungal infection confirmed at least two organs) were similar in patients who had fungemia and those without (25.0% vs. 21.4%). These numbers further show that blood cultures may not be as reliable when assessing the dissemination of fungal infection as they are for bacterial infections.
Serum markers such as galactomannan (GM) and beta-D-glucan (BDG) have different sensitivities and specificities in diagnosing fungal infections, depending on the patient population. Galactomannan has the highest sensitivity in neutropenic patients and those who received allogeneic stem cell transplantation [143]. In non-neutropenic patients, GM tends to have a much lower sensitivity; this also includes solid-organ-transplant recipients [143,144]. Beta-D-glucan follows a similar pattern, with the test exhibiting poor sensitivity and specificity and being used only as an accessory diagnostic tool in some cases [32,144,145,146,147].
In this review, only 4 (4.0%) reported galactomannan and 1 (1.0%) reported beta-D-glucan positivity. All five tests were seen in pericarditis due to Aspergillus spp. Many cases omitted serologic testing in their case description altogether. Given these low numbers, we have not been able to make any conclusion on sensitivities of these markers.
4.3. Clinical Presentation
Compared to other causes of pericarditis, symptoms seem to vary as displayed in Table 3. Fever was the most common presenting symptom in 56 (55.4%) cases. It was frequent in pediatric patients (n = 11, 91.7%) compared to adults (n = 45, 50.7%). In comparison, a literature review of influenza myopericarditis that analyzed a similar number of cases showed that 94.7% of patients presented with fever [115]. Chest pain in our review was reported more commonly in the adult (n = 36, 44.4%) compared to the pediatric (n = 2, 16.7%) population [115]. In the mentioned influenza myopericarditis review [115], 77.3% of the cases had influenza myocarditis accompanying pericarditis, while in our review, only 25 (24.7%) cases reported myocardial involvement, possibly explaining the lack of other common signs of myopericarditis such as tachycardia, hypotension, and shock. As illustrated in Table 3, patients with fungal pericarditis tend to have fever and chest pain less often compared to viral, bacterial, and mycobacterial causes, which might contribute to delayed diagnosis and poorer outcomes.
The lack of pathognomonic signs and symptoms specific to fungal infection might lead to an increase in morbidity and mortality due to a delay in appropriate diagnosis and timely treatment [148,149]. This review showed that the most common causative agent, Candida spp., presented with symptoms such as chest pain, fever, and dyspnea in 47.4%, 55.3%, and 55.3% of the cases, respectively, leading to the conclusion that half of the cases had unspecific symptoms to guide diagnosis. Such a delay in timely diagnosis is evidenced in this review, by only 16 (15.84%) of cases correctly assessing the infection as fungal within 48 h of presentation. Additionally, only 36 (35.6%) cases included antifungal medication in their initial empirical treatment, before isolating the causative agent. These numbers suggest that clinicians had a low suspicion of fungal infection. This report also showed that patients had a significantly better survival if there was a high suspicion of fungal infection on presentation and if they were treated timely (p = 0.011), highlighting the importance of raised awareness for fungal infections, particularly in immunocompromised patients.
4.4. Outcome
Fungal pericarditis has a high rate of mortality of 50% (n = 49), which is likely related to the fact that 50.5% of patients who develop fungal pericarditis are immunosuppressed [92,150]. The low index of suspicion, delay in diagnosis, and lack of empiric treatment are other factors that influence high mortality in this group. Despite possessing fewer complications (such as shock and tamponade), patients with fungal pericarditis have markedly higher mortality rates compared to other infectious etiologies (Table 3).
5. Conclusions
Fungal pericarditis is a rare but life-threatening condition associated with a 50% mortality rate. Notably, in this review, half of the affected individuals were immunocompetent, underscoring the need for heightened clinical suspicion beyond immunosuppressed populations. Delayed diagnosis and suboptimal empirical treatment contribute to poor outcomes, as fungal pericarditis is often overlooked in differential diagnosis. Patients with fungal pericarditis tend to have a fever and chest pain less often compared to viral, bacterial, and mycobacterial causes, which might contribute to delayed diagnosis and poorer outcomes. Patients with disseminated disease, caused by Aspergillus spp. and Mucor, and those with complications such as disseminated disease, multiorgan failure, and shock experience significantly worse outcomes. Despite the high mortality, fungemia was rarely reported and the majority of cases did not report galactomannan and beta-D-glucan testing. Given these challenges, there is an urgent need for multicenter studies to enhance our understanding of the epidemiology, clinical presentation, and management of this condition. Future research should prioritize incorporating prospective case registries, recruit larger multicenter datasets, and develop more sensitive and specific biomarkers to facilitate timely diagnosis by clinicians and improve outcomes.
Limitations of This Study
This systematic review has several limitations. First, we included only English language publications indexed in the MEDLINE and SCOPUS databases. Although stringent criteria were established to exclude low-quality case reports, this approach may have inadvertently omitted high-quality cases that did not meet our pre-selection criteria. Second, due to ongoing updates in microbial nomenclature, multiple names for the same microorganism may appear in the literature. Since this review pooled articles from a wide timeframe, some fungal names may be outdated and relevant articles might have been unintentionally missed. Third, as a systematic review of case reports and case series, our findings are inherently susceptible to publication bias and variability in data reporting. Fourth, the rarity of fungal pericarditis resulted in a small dataset, which limits the broad generalizability of our findings. Lastly, the limited number of pediatric case reports reduces the statistical power of the results and complicates the direct comparison of adult and pediatric cases.
Author Contributions
I.D. and M.R.—conceptualization; P.J., S.M., M.W., M.R. and I.D.—wrote and edited the main manuscript text; N.J., M.A., N.N., D.J. and A.A.—collected all data; M.P.—statistical analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are publically available and all case reports used in this research have been appropriately cited and can be accessed through appropriate databases.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dababneh, E.; Siddique, M.S. Pericarditis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Imazio, M.; Gaita, F.; LeWinter, M. Evaluation and Treatment of Pericarditis: A Systematic Review. JAMA 2015, 314, 1498–1506. [Google Scholar] [CrossRef]
- Kytö, V.; Sipilä, J.; Rautava, P. Clinical Profile and Influences on Outcomes in Patients Hospitalized for Acute Pericarditis. Circulation 2014, 130, 1601–1606. [Google Scholar] [CrossRef]
- Lucero, O.D.; Bustos, M.M.; Ariza Rodríguez, D.J.; Perez, J.C. Tuberculous Pericarditis-a Silent and Challenging Disease: A Case Report. World J. Clin. Cases 2022, 10, 1869–1875. [Google Scholar] [CrossRef]
- Crum, N.F.; Lederman, E.R.; Wallace, M.R. Infections Associated with Tumor Necrosis Factor-Alpha Antagonists. Medicine 2005, 84, 291–302. [Google Scholar] [CrossRef]
- Bergstrom, L.; Yocum, D.E.; Ampel, N.M.; Villanueva, I.; Lisse, J.; Gluck, O.; Tesser, J.; Posever, J.; Miller, M.; Araujo, J.; et al. Increased Risk of Coccidioidomycosis in Patients Treated with Tumor Necrosis Factor Alpha Antagonists. Arthritis Rheum. 2004, 50, 1959–1966. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Iliev, I.D.; Hohl, T.M. Immunity Against Fungi. J. Clin. Investig. 2017, 2, e93156. [Google Scholar] [CrossRef]
- McCarty, T.P.; White, C.M.; Pappas, P.G. Candidemia and Invasive Candidiasis. Infect. Dis. Clin. N. Am. 2021, 35, 389–413. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive Candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Xess, I.; Pagano, L.; Dabas, Y. Invasive Fungal Infections 2021. J. Fungi 2022, 8, 760. [Google Scholar] [CrossRef] [PubMed]
- Cosio, T.; Pica, F.; Fontana, C.; Pistoia, E.S.; Favaro, M.; Valsecchi, I.; Zarabian, N.; Campione, E.; Botterel, F.; Gaziano, R. Stephanoascus ciferrii Complex: The Current State of Infections and Drug Resistance in Humans. J Fungi 2024, 10, 294. [Google Scholar] [CrossRef]
- Schrank, J.H.; Dooley, D.P. Purulent Pericarditis Caused by Candida Species: Case Report and Review. Clin. Infect. Dis. 1995, 21, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mouhayar, E.; Tarrand, J.J.; Kontoyiannis, D.P. Fulminant Cryptococcus Neoformans Infection with Fatal Pericardial Tamponade in a Patient with Chronic Myelomonocytic Leukaemia Who Was Treated with Ruxolitinib: Case Report and Review of Fungal Pericarditis. Mycoses 2018, 61, 245–255. [Google Scholar] [CrossRef]
- Fortún, J.; Martín-Dávila, P.; Gómez-García de la Pedrosa, E.; Pintado, V.; Cobo, J.; Fresco, G.; Meije, Y.; Ros, L.; Alvarez, M.E.; Luengo, J.; et al. Emerging Trends in Candidemia: A Higher Incidence but a Similar Outcome. J. Infect. 2012, 65, 64–70. [Google Scholar] [CrossRef]
- Posteraro, B.; De Carolis, E.; Criscuolo, M.; Ballanti, S.; De Angelis, G.; Del Principe, M.I.; Delia, M.; Fracchiolla, N.; Marchesi, F.; Nadali, G.; et al. Candidaemia in Haematological Malignancy Patients from a SEIFEM Study: Epidemiological Patterns According to Antifungal Prophylaxis. Mycoses 2020, 63, 900–910. [Google Scholar] [CrossRef]
- Scudeller, L.; Bassetti, M.; Concia, E.; Corrao, S.; Cristini, F.; De Rosa, F.G.; Del Bono, V.; Durante-Mangoni, E.; Falcone, M.; Menichetti, F.; et al. MEDical Wards Invasive Candidiasis ALgorithms (MEDICAL):Consensus Proposal for Management. Eur. J. Intern. Med. 2016, 34, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Luzzati, R.; Cavinato, S.; Deiana, M.L.; Rosin, C.; Maurel, C.; Borelli, M. Epidemiology and Outcome of Nosocomial Candidemia in Elderly Patients Admitted Prevalently in Medical Wards. Aging Clin. Exp. Res. 2015, 27, 131–137. [Google Scholar] [CrossRef]
- Tischler, B.Y.; Hohl, T.M. Menacing Mold: Recent Advances in Aspergillus Pathogenesis and Host Defense. J. Mol. Biol. 2019, 431, 4229–4246. [Google Scholar] [CrossRef]
- Lazarou, E.; Tsioufis, P.; Vlachopoulos, C.; Tsioufis, C.; Lazaros, G. Acute Pericarditis: Update. Curr. Cardiol. Rep. 2022, 24, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Neughebauer, B.; Alvarez, V.; Harb, T.; Keefer, M. Constrictive Pericarditis Caused by Candida Glabrata in an Immunocompetent Patient: Case Report and Review of Literature. Scand. J. Infect. Dis. 2002, 34, 615–619. [Google Scholar] [CrossRef]
- Tomita, T.; Ho, H.; Allen, M.; Diaz, J. Zygomycosis Involving Lungs, Heart and Brain, Superimposed on Pulmonary Edema. Pathol. Int. 2005, 55, 202–205. [Google Scholar] [CrossRef]
- Alkuwaiti, F.A.; Elghoneimy, Y.; Alabdrabalrasol, E.A.; Alshreadah, S.T. Unusual Presentation of Aspergillus Pericarditis: A Case Report. Saudi J. Med. Med. Sci. 2019, 7, 175–178. [Google Scholar] [CrossRef]
- Recio, R.; Meléndez-Carmona, M.Á.; Martín-Higuera, M.C.; Pérez, V.; López, E.; López-Medrano, F.; Pérez-Ayala, A. Trichoderma Longibrachiatum: An Unusual Pathogen of Fungal Pericarditis. Clin. Microbiol. Infect. 2019, 25, 586–587. [Google Scholar] [CrossRef]
- Puius, Y.A.; Scully, B. Treatment of Candida Albicans Pericarditis in a Heart Transplant Patient. Transpl. Infect. Dis. 2007, 9, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.; Chatterjee, A.; Brott, B.C. Transdiaphragmatic Rupture of Hepatic Abscess Producing Purulent Pericarditis and Pericardial Tamponade. Circulation 2015, 131, e1–e2. [Google Scholar] [CrossRef]
- Panchanatheeswaran, K.; Ram, D.; Prasad, S.; Srinivas, B.H.; Rath, D.; SaiChandran, B.V.; Munuswamy, H. Thoracic Mucormycosis in Immunocompetent Patients. J. Card. Surg. 2021, 36, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.D.; Simonsen, R.L. The Clinical Manifestations of Cardiac Mucormycosis. Chest 1992, 101, 1733–1736. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Omo, A.; Chen, T.; Zheng, Z.; Pan, T. Tension Pneumopericardium Caused by Pericarditis. Ann. Thorac. Surg. 2007, 83, 321. [Google Scholar] [CrossRef]
- Kok, V.K.; Chu, M.Y.; Wong, Y.K.; Lee, F.C. Successful Treatment of Candida Pericarditis Following Sternotomy. Asian Cardiovasc. Thorac. Ann. 2000, 8, 384–386. [Google Scholar] [CrossRef]
- Mortensen, K.L.; Knudsen, J.B.; Jensen-Fangel, S.; Stausbøl-Grøn, B.; Arendrup, M.C.; Petersen, E. Successful Management of Invasive Aspergillosis Presenting as Pericarditis in an Adult Patient with Chronic Granulomatous Disease. Mycoses 2011, 54, e233–e236. [Google Scholar] [CrossRef]
- Azhar, A. Successful Management of Fungal Pericarditis and Endocarditis in a Neonate: A Case Report. J. Saudi Heart Assoc. 2012, 24, 195–199. [Google Scholar] [CrossRef]
- Sayah, D.M.; Schwartz, B.S.; Kukreja, J.; Singer, J.P.; Golden, J.A.; Leard, L.E. Scedosporium Prolificans Pericarditis and Mycotic Aortic Aneurysm in a Lung Transplant Recipient Receiving Voriconazole Prophylaxis. Transpl. Infect. Dis. 2013, 15, E70–E74. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, C.D.; McKinsey, D.S. Recurrent Massive Pleural Effusion Due to Pleural, Pericardial, and Epicardial Fibrosis in Histoplasmosis. Chest 1991, 100, 1715–1717. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Takama, N.; Ohtaki, Y.; Koitabashi, N.; Kurabayashi, M.; Ishii, H. Pyothorax and Constrictive Pericarditis after Chemoradiotherapy for Esophageal Cancer. Intern. Med. 2024, 63, 1387–1393. [Google Scholar] [CrossRef]
- Lee, J.; Ramkumar, S.; Ha, P.; Raghunath, A.; Dundon, B. Pyopneumopericarditis from a Gastropericardial Fistula: A Case Report. Eur. Heart J. Case Rep. 2021, 5, ytab408. [Google Scholar] [CrossRef]
- Parikh, S.V.; Memon, N.; Echols, M.; Shah, J.; McGuire, D.K.; Keeley, E.C. Purulent Pericarditis: Report of 2 Cases and Review of the Literature. Medicine 2009, 88, 52–65. [Google Scholar] [CrossRef]
- Kertmen, Ö.; Gök, G.; Akçay, M. Purulent Pericarditis with Cardiac Tamponade Secondary to Candida Albicans after Total Parenteral Nutrition: A Case Report. J. Tehran Heart Cent. 2020, 15, 128–130. [Google Scholar] [CrossRef]
- McNamee, C.J.; Wang, S.; Modry, D. Purulent Pericarditis Secondary to Candida Parapsilosis and Peptostreptococcus Species. Can. J. Cardiol. 1998, 14, 85–86. [Google Scholar]
- Yeh, J.; Culbertson, C.; Petru, A.M. Purulent Pericardial Effusion in a 14-Year-Old Girl. Pediatr. Infect. Dis. J. 2009, 28, 1140, 1147–1148. [Google Scholar] [CrossRef]
- Mullane, K.; Toor, A.A.; Kalnicky, C.; Rodriguez, T.; Klein, J.; Stiff, P. Posaconazole Salvage Therapy Allows Successful Allogeneic Hematopoietic Stem Cell Transplantation in Patients with Refractory Invasive Mold Infections. Transpl. Infect. Dis. 2007, 9, 89–96. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.; Ko, J.K.; Park, J.; Yu, M.Y.; Oh, C.K.; Hong, S.P.; Kim, Y.; Lim, Y.; Kim, H.; et al. Polymicrobial Purulent Pericarditis Probably Caused by a Broncho-Lymph Node-Pericardial Fistula in a Patient with Tuberculous Lymphadenitis. Infect. Chemother. 2015, 47, 261–267. [Google Scholar] [CrossRef]
- van Ede, A.E.; Meis, J.F.; Koot, R.A.; Heystraten, F.M.; de Pauw, B.E. Pneumopericardium Complicating Invasive Pulmonary Aspergillosis: Case Report and Review. Infection 1994, 22, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Vecchié, A.; Raybould, J.E.; Sangal, K.; Bonaventura, A.; Gillen, M.; Abbate, A.; Sastry, S.; Bhardwaj, H. Pericarditis and Sacroiliitis in a World Traveler. JACC Case Rep. 2021, 3, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Menu, E.; Kabtani, J.; Roubin, J.; Ranque, S.; L’Ollivier, C. Pericardial Effusion Due to Trichosporon japonicum: A Case Report and Review of the Literature. Pathogens 2022, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.Y.; Habib, G.; Reynaud-Gaubert, M.; Raoult, D.; Rolain, J.M. Pericardial Effusion due to Cryptococcus Neoformans in a Patient with Cystic Fibrosis Following Lung Transplantation. Int. J. Infect. Dis. 2008, 12, 452. [Google Scholar] [CrossRef]
- Benedík, J.; Cerný, J.; Benedík, J.; Dendis, M. Pericardial Constriction Caused by Candida Albicans. Eur. J. Cardiothorac. Surg. 2002, 21, 591–592. [Google Scholar] [CrossRef]
- Poupelin, J.C.; Philit, F.; Richard, J.C.; Badet, M.; Lemasson, S.; Bayle, F.; Guérin, C. Pericardial and Pleural Diffusion of Voriconazole During Disseminated Invasive Aspergillosis: Report of a Case with Successful Outcome. Intensive Care Med. 2006, 32, 939–940. [Google Scholar] [CrossRef]
- Knee, G.; Quirke, T.; Lau, J.O.; Fenech, A.; Teall, A.J. Penetrating Gastric Ulcer as a Cause of Mixed Bacterial and Fungal Pericarditis. Mycoses 1991, 34, 129–132. [Google Scholar] [CrossRef]
- Eren Akarcan, S.; Karaca, N.; Aksu, G.; Bozkaya, H.; Ayik, M.F.; Ozdemir Sahan, Y.; Kilinc, M.A.; Dokumcu, Z.; Eraslan, C.; Divarci, E.; et al. Necrotizing Liver Granuloma/Abscess and Constrictive Aspergillosis Pericarditis with Central Nervous System Involvement: Different Remarkable Phenotypes in Different Chronic Granulomatous Disease Genotypes. Case Rep. Immunol. 2017, 2017, 2676403. [Google Scholar] [CrossRef]
- Itoh, M.; Takahashi, M.; Mori, M.; Tamekiyo, H.; Yoshida, H.; Yago, K.; Shimada, H.; Arai, K. Myocardial Infarction Caused by Aspergillus Embolization in a Patient with Aplastic Anemia. Intern. Med. 2006, 45, 547–550. [Google Scholar] [CrossRef]
- Tobón, A.M.; Arango, M.; Fernández, D.; Restrepo, A. Mucormycosis (Zygomycosis) in a Heart-Kidney Transplant Recipient: Recovery After Posaconazole Therapy. Clin. Infect. Dis. 2003, 36, 1488–1491. [Google Scholar] [CrossRef]
- Salanitri, G.C.; Huo, E.; Miller, F.H.; Gupta, A.; Pereles, F.S. MRI of Mycotic Sinus of Valsalva Pseudoaneurysm Secondary to Aspergillus Pericarditis. AJR Am. J. Roentgenol. 2005, 184, S25–S27. [Google Scholar] [CrossRef] [PubMed]
- Almeida Júnior, J.N.; Song, A.T.W.; Campos, S.V.; Strabelli, T.M.V.; Del Negro, G.M.; Figueiredo, D.S.Y.; Motta, A.L.; Rossi, F.; Guitard, J.; Benard, G.; et al. Invasive Trichosporon Infection in Solid Organ Transplant Patients: A Report of Two Cases Identified Using IGS1 Ribosomal DNA Sequencing and a Review of the Literature. Transpl. Infect. Dis. 2014, 16, 135–140. [Google Scholar] [CrossRef]
- Nielsen, H.; Stenderup, J. Invasive Candida Norvegensis Infection in Immunocompromised Patients. Scand. J. Infect. Dis. 1996, 28, 311–312. [Google Scholar] [CrossRef]
- Vazquez, J.A.; Lundstrom, T.; Dembry, L.; Chandrasekar, P.; Boikov, D.; Parri, M.B.; Zervos, M.J. Invasive Candida Guilliermondii Infection: In Vitro Susceptibility Studies and Molecular Analysis. Bone Marrow Transplant. 1995, 16, 849–853. [Google Scholar]
- Bhat, M.; Ghali, P.; Wong, P.; Marcus, V.; Michel, R.; Cantarovich, M.; Metrakos, P.; Deschenes, M. Immunosuppression with Budesonide for Liver Transplant Recipients with Severe Infections. Liver Transpl. 2012, 18, 262–263. [Google Scholar] [CrossRef]
- Soriano, P.K.; Iqbal, M.; Kandaswamy, S.; Akram, S.; Kulkarni, A.; Hudali, T. H. capsulatum: A Not-So-Benign Cause of Pericarditis. Case Rep. Cardiol. 2017, 2017, 3626917. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk, A.E.; Chapman, C.G.; Ferguson, M.K.; Gelrud, A. Gastropericardial Fistula: An Unusual Case of “Heart Burn”. VideoGIE 2016, 1, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, J.; Grodzki, T.; Kubisa, B.; Pieróg, J.; Kozak, A.; Wójcik, N. Gastropericardial Fistula: A Case Report. Kardiochirurgia Torakochirurgia Pol. Pol. J. Thorac. Cardiovasc. Surg. 2011, 8, 497–499. [Google Scholar]
- Farjah, F.; Komanapalli, C.B.; Shen, I.; Sukumar, M.S. Gastropericardial Fistula and Candida Kruzei Pericarditis Following Laparoscopic Nissen Fundoplication (Gastropericardial Fistula). Thorac. Cardiovasc. Surg. 2005, 53, 365–367. [Google Scholar] [CrossRef]
- Tang, C.-P.; Wang, Y.-W.; Shiau, Y.-T.; Lee, R.-C.; Lan, K.-H.; Chao, Y. Gastropericardial Fistula and Candida Albicans Pericarditis: A Rare Complication of Gastric Adenocarcinoma Treated with Radiation and Chemotherapy. J. Chin. Med. Assoc. 2009, 72, 374–378. [Google Scholar] [CrossRef]
- Levi, M.; Basgoz, N. Fungal Wound Infection in a Lung Transplant Recipient. Transpl. Infect. Dis. 2000, 2, 36–43. [Google Scholar] [CrossRef]
- Canver, C.C.; Patel, A.K.; Kosolcharoen, P.; Voytovich, M.C. Fungal Purulent Constrictive Pericarditis in a Heart Transplant Patient. Ann. Thorac. Surg. 1998, 65, 1792–1794. [Google Scholar] [CrossRef]
- Kombade, S.P.; Abhishek, K.S.; Nag, V.L. Fungal Pericarditis due to Aspergillus nidulans: A Rare Case Report. J. Clin. Diagn. Res. 2018, 12, DD5–DD6. [Google Scholar] [CrossRef]
- Miyoshi, I.; Saito, T.; Machida, H.; Uemura, Y.; Kuroda, N.; Taguchi, H. Fungal Effusions Associated with Invasive Pulmonary Aspergillosis. Intern. Med. 2006, 45, 1019–1020. [Google Scholar] [CrossRef]
- Chatterjee, D.; Bal, A.; Singhal, M.; Vijayvergiya, R.; Das, A. Fibrosing Mediastinitis due to Aspergillus with Dominant Cardiac Involvement: Report of Two Autopsy Cases with Review of Literature. Cardiovasc. Pathol. 2014, 23, 354–357. [Google Scholar] [CrossRef]
- Ozsahin, H.; Wacker, P.; Brundler, M.A.; Starobinski, M.; Helg, C.; Pastore, Y.; Miralbell, R.; Hanquinet, S.; Gervaix, A.; Chapuis, B.; et al. Fatal Myocardial Aspergillosis in an Immunosuppressed Child. J. Pediatr. Hematol. Oncol. 2001, 23, 456–459. [Google Scholar] [CrossRef]
- Carrel, T.P.; Schaffner, A.; Schmid, E.R.; Schneider, J.; Bauer, E.P.; Laske, A.; von Segesser, L.K.; Turina, M.I. Fatal Fungal Pericarditis After Cardiac Surgery and Immunosuppression. J. Thorac. Cardiovasc. Surg. 1991, 101, 161–164. [Google Scholar] [CrossRef]
- Basti, A.; Taylor, S.; Tschopp, M.; Sztajzel, J. Fatal Fulminant Myocarditis Caused by Disseminated Mucormycosis. Heart 2004, 90, e60. [Google Scholar] [CrossRef]
- Dalhoff, K.; Braun, J.; Gatermann, S.; Djonlagic, H. Fatal Aspergillus Pericarditis in Acquired Immunodeficiency Syndrome. Intensive Care Med. 1996, 22, 831–833. [Google Scholar] [CrossRef]
- Ohya, I.; Bunai, Y.; Tsujinaka, M.; Akaza, K.; Nakamura, I. Fatal Aspergillus Pancarditis after Incompatible Blood Transfusion Intended to Be an Autologous Blood Transfusion. Leg. Med. 2001, 3, 246–251. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Frantzeskaki, F.; Kosmopoulos, M.; Taccone, F.S.; Van den Abeele, A.-M.; Bulpa, P.; Forêt, F.; Vogelaers, D.; Blot, S. AspICU investigators Endomyocardial and Pericardial Aspergillosis in Critically Ill Patients. Mycoses 2017, 60, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Hashimoto, T.; Sakamoto, K.; Matsushima, S.; Higo, T.; Sonoda, H.; Kimura, Y.; Mori, M.; Shiose, A.; Tsutsui, H. Effusive-Constrictive Pericarditis Secondary to Pneumopericardium Associated with Gastropericardial Fistula. ESC Heart Fail. 2021, 8, 778–781. [Google Scholar] [CrossRef]
- Margoles, L.; DeNofrio, D.; Patel, A.R.; Golan, Y.; Vest, A.R.; Arkun, K.; Boucher, H.W.; Kiernan, M.S.; Upshaw, J.N. Disseminated Mucormycosis Masquerading as Rejection Early after Orthotopic Heart Transplantation. Transpl. Infect. Dis. 2018, 20, e12820. [Google Scholar] [CrossRef]
- Mundo, W.; Berning, A.; Koullias, Y.; Chastain, D.B.; Stone, N.; Franco-Paredes, C.; Henao-Martínez, A.F.; Vargas Barahona, L. Disseminated Cryptococcal Disease in A Patient with Chronic Chylothorax and a Pleurovenous Catheter, a Case Report With Autopsy Findings. Open Forum Infect. Dis. 2021, 8, ofab258. [Google Scholar] [CrossRef]
- Oudiz, R.; Mahaisavariya, P.; Peng, S.K.; Shane-Yospur, L.; Smith, C.; Baumgartner, F.; Shapiro, S. Disseminated Coccidioidomycosis with Rapid Progression to Effusive-Constrictive Pericarditis. J. Am. Soc. Echocardiogr. 1995, 8, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Orem, J.; Mpanga, L.; Habyara, E.; Nambuya, A.; Aisu, T.; Wamukota, W.; Otim, M.A. Disseminated Aspergillus Fumigatus Infection: Case Report. East. Afr. Med. J. 1998, 75, 436–438. [Google Scholar] [PubMed]
- Yu, G.; Zhong, F.; Ye, B. Destroyed Lung with Constrictive Pericarditis. Med. Clin. 2020, 155, 471. [Google Scholar] [CrossRef]
- El Helou, G.; Hellinger, W. Cryptococcus Neoformans Pericarditis in a Lung Transplant Recipient: Case Report, Literature Review and Pearls. Transpl. Infect. Dis. 2019, 21, e13137. [Google Scholar] [CrossRef]
- Mann, S.; Tobolowsky, F.; Purohit, S.; Henao-Martínez, A.; Bajrovic, V.; Ramanan, P.; Wolfel, E.; Khazanie, P.; Barron, M.; Madinger, N.; et al. Cryptococcal Pericarditis in a Heart Transplant Recipient. Transpl. Infect. Dis. 2020, 22, e13366. [Google Scholar] [CrossRef] [PubMed]
- Kurahara, Y. Cryptococcal Pericarditis. QJM 2022, 115, 541–542. [Google Scholar] [CrossRef]
- Faul, J.L.; Hoang, K.; Schmoker, J.; Vagelos, R.H.; Berry, G.J. Constrictive Pericarditis due to Coccidiomycosis. Ann. Thorac. Surg. 1999, 68, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cano, M.J.; Fernández-Ruiz, M.; Sánchez, V.; López-Medrano, F. Constrictive Pericarditis due to Candida Albicans: An Unexpected Cause of Pericardial Effusion after Heart Transplantation. Rev. Clin. Esp. 2012, 212, 551–552. [Google Scholar] [CrossRef]
- Visbal, A.L.; DeValeria, P.A.; Blair, J.E.; Zarka, M.A.; Lanza, L.A. Coccidioidal Pericarditis: Implications of Surgical Treatment in the Elderly. Ann. Thorac. Surg. 2003, 75, 1328–1330. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, R.S.N.; Cordeiro, R.A.; Rocha, M.F.G.; Fechine, M.A.B.; Furtado, F.M.; Nagao-Dias, A.T.; Camargo, Z.P.; Sidrim, J.J.C. Coccidioidal Pericarditis: A Rapid Presumptive Diagnosis by an in-House Antigen Confirmed by Mycological and Molecular Methods. J. Med. Microbiol. 2008, 57, 1288–1292. [Google Scholar] [CrossRef]
- Arsura, E.L.; Bobba, R.K.; Reddy, C.M. Coccidioidal Pericarditis: A Case Presentation and Review of the Literature. Int. J. Infect. Dis. 2005, 9, 104–109. [Google Scholar] [CrossRef]
- Roberts, W.C. Case Reports in Cardiology: Cardiovascular Diseases with a Focus on Aorta; CRC Press: Boca Raton, FL, USA, 2023; ISBN 978-1-00-099461-2. [Google Scholar]
- Gökahmetoğlu, S.; Koç, A.N.; Patiroğlu, T. Case Report. Fatal Aspergillus Flavus Pericarditis in a Patient with Acute Myeloblastic Leukaemia. Mycoses 2000, 43, 65–66. [Google Scholar] [CrossRef]
- Khoueiry, Z.; Delseny, D.; Leclercq, F.; Piot, C.; Roubille, F. Cardiac Tamponade Likely due to Candida Infection, in an Immunocompetent Patient. Ann. Cardiol. Angeiol. 2013, 62, 122–123. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, J.B.; Kim, M.H. Cardiac Tamponade due to Purulent Pericarditis. Asian Cardiovasc. Thorac. Ann. 2013, 21, 621. [Google Scholar] [CrossRef]
- Xie, L.; Gebre, W.; Szabo, K.; Lin, J.H. Cardiac Aspergillosis in Patients with Acquired Immunodeficiency Syndrome: A Case Report and Review of the Literature. Arch. Pathol. Lab. Med. 2005, 129, 511–515. [Google Scholar] [CrossRef]
- Rabinovici, R.; Szewczyk, D.; Ovadia, P.; Greenspan, J.R.; Sivalingam, J.J. Candida Pericarditis: Clinical Profile and Treatment. Ann. Thorac. Surg. 1997, 63, 1200–1204. [Google Scholar] [CrossRef]
- Pancorvo, C.; Cohen, I. Candida Pericarditis in a Child. J. La. State Med. Soc. 1993, 145, 53–54. [Google Scholar]
- Ghunaim, M.; Someili, A.; Mawardi, M. Candida Cardiac Tamponade Secondary to Oesophageal-Pericardial Fistula: A Rare Presentation of Oesophageal Squamous Cell Carcinoma. Eur. J. Case Rep. Intern. Med. 2022, 9, 003200. [Google Scholar] [CrossRef]
- Karp, R.; Meldahl, R.; McCabe, R. Candida Albicans Purulent Pericarditis Treated Successfully without Surgical Drainage. Chest 1992, 102, 953–954. [Google Scholar] [CrossRef]
- Le Moing, V.; Lortholary, O.; Timsit, J.F.; Couvelard, A.; Bouges-Michel, C.; Wolff, M.; Guillevin, L.; Casassus, P. Aspergillus Pericarditis with Tamponade: Report of a Successfully Treated Case and Review. Clin. Infect. Dis. 1998, 26, 451–460. [Google Scholar] [CrossRef]
- Biso, S.; Lekkham, R.; Climaco, A. Aspergillus Pericarditis with Tamponade in a Renal Transplant Patient. Case Rep. Cardiol. 2017, 2017, 7134586. [Google Scholar] [CrossRef]
- Vaideeswar, P. Aspergillus Pancarditis Manifesting as Hospital-Acquired Infection: Report of Two Cases and Review of Literature. Cardiovasc. Pathol. 2010, 19, e253–e257. [Google Scholar] [CrossRef]
- Mishra, A.K.; Bansal, V.; Roy, G.; Halder, V.; Gupta, P.; Chakrabarti, A. Aspergillus Mediastinitis in a Post-Operative Immunocompetent Child. Indian. J. Med. Microbiol. 2020, 38, 492–495. [Google Scholar] [CrossRef]
- Sergi, C.; Weitz, J.; Hofmann, W.J.; Sinn, P.; Eckart, A.; Otto, G.; Schnabel, P.A.; Otto, H.F. Aspergillus Endocarditis, Myocarditis and Pericarditis Complicating Necrotizing Fasciitis. Case Report and Subject Review. Virchows Arch. 1996, 429, 177–180. [Google Scholar] [CrossRef]
- Azarisman, S.M.; Richardson, J.D.; Chua, S.K.; Cunningham, M.S.; Teo, K.S.; Worthley, S.G. An Ideal Image: Effusive Constrictive Pericarditis. Am. J. Med. 2013, 126, 25–26. [Google Scholar] [CrossRef]
- Şişli, E.; Özdemir Şahan, Y.; Ayık, M.F.; Nart, D.; Atay, Y. A Rare Complication of Chronic Granulomatous Disease in a Child: Constrictive Aspergillus Pericarditis. Turk. Kardiyol. Dern. Ars. 2017, 45, 660–663. [Google Scholar] [CrossRef]
- Patel, M.; Wilson, Z.T.; Seibolt, L.; Cherukuri, M.; Colon, M.; Morris, M.F. A Rare Case of Coccidioidomycosis Constrictive Pericarditis. Circ. Cardiovasc. Imaging 2020, 13, e010825. [Google Scholar] [CrossRef]
- Matta, A.; Elenizi, K.; AlHarthi, R.; Moussallem, N.; Elhajjaj, N.; Lhermusier, T.; Carrie, D. A Rare Case of Candida Pericarditis Associated with Esophagopericardial Fistula. Am. J. Case Rep. 2019, 20, 975–979. [Google Scholar] [CrossRef]
- Kupsky, D.F.; Alaswad, K.; Rabbani, B.T. A Rare Case of Aspergillus Pericarditis with Associated Myocardial Abscess and Echocardiographic Response to Therapy. Echocardiography 2016, 33, 1085–1088. [Google Scholar] [CrossRef]
- Dahl, C.; Fuursted, K.; Schrøder, H. A Paediatric Case of Candida Pericarditis and Eosophagus Stricture During Treatment for Acute Lymphatic Leukaemia. Acta Oncol. 2007, 46, 859–861. [Google Scholar] [CrossRef]
- Hampson, F.G.; Ridgway, E.J.; Feeley, K.; Reilly, J.T. A Fatal Case of Disseminated Zygomycosis Associated with the Use of Blood Glucose Self-Monitoring Equipment. J. Infect. 2005, 51, e269–e272. [Google Scholar] [CrossRef]
- Salimi, M.; Davoodi, L.; Jalalian, R.; Darayee, M.; Moslemi, A.; Faeli, L.; Mirzakhani, R.; Shokohi, T. A fatal Candida Albicans Pericarditis Presenting with Cardiac Tamponade After COVID-19 Infection and Cardiothoracic Surgery. J. Clin. Lab. Anal. 2023, 37, e24968. [Google Scholar] [CrossRef]
- Pal, R.; Hazra, D.; Pichamuthu, K.; Abhilash, K.P.P. A Disastrous Omen—Candidal Pyo Pneumopericardium. J. Glob. Infect. Dis. 2021, 13, 189–191. [Google Scholar] [CrossRef]
- Navaratnam, A.M.; Al-Freah, M.; Cavazza, A.; Auzinger, G. A Case Series of Non-Valvular Cardiac Aspergillosis in Critically Ill Solid Organ Transplant and Non-Transplant Patients and Systematic Review. J. Intensive Care Soc. 2021, 22, 241–247. [Google Scholar] [CrossRef]
- Sung, J.; Perez, I.E.; Feinstein, A.; Stein, D.K. A Case Report of Purulent Pericarditis Caused by Candida Albicans: Delayed Complication Forty-Years After Esophageal Surgery. Medicine 2018, 97, e11286. [Google Scholar] [CrossRef]
- Guimaron, S.; Gervais, P.; Voisine, P. A Case of Aspergillus Pericarditis in the Setting of Invasive Aspergillosis: The Role of Surgery in a Multi-Modal Treatment. CJC Open 2022, 4, 663–666. [Google Scholar] [CrossRef]
- Ledoux, M.-P.; Herbrecht, R. Invasive Pulmonary Aspergillosis. J. Fungi 2023, 9, 131. [Google Scholar] [CrossRef]
- Lass-Flörl, C.; Kanj, S.S.; Govender, N.P.; Thompson, G.R.; Ostrosky-Zeichner, L.; Govrins, M.A. Invasive Candidiasis. Nat. Rev. Dis. Primers 2024, 10, 20. [Google Scholar] [CrossRef]
- Radovanovic, M.; Petrovic, M.; Barsoum, M.K.; Nordstrom, C.W.; Calvin, A.D.; Dumic, I.; Jevtic, D.; Hanna, R.D. Influenza Myopericarditis and Pericarditis: A Literature Review. J. Clin. Med. 2022, 11, 4123. [Google Scholar] [CrossRef]
- Radovanovic, M.; Petrovic, M.; Hanna, R.D.; Nordstrom, C.W.; Calvin, A.D.; Barsoum, M.K.; Milosavljevic, N.; Jevtic, D.; Sokanovic, M.; Dumic, I. Clinical Presentation and Management of Methicillin-Resistant Staphylococcus aureus Pericarditis—Systematic Review. J. Cardiovasc. Dev. Dis. 2022, 9, 103. [Google Scholar] [CrossRef]
- Chang, S.-A. Tuberculous and Infectious Pericarditis. Cardiol. Clin. 2017, 35, 615–622. [Google Scholar] [CrossRef]
- Segal, B.H. Aspergillosis. N. Engl. J. Med. 2009, 360, 1870–1884. [Google Scholar] [CrossRef]
- Kwon-Chung, K.J.; Fraser, J.A.; Doering, T.L.; Wang, Z.; Janbon, G.; Idnurm, A.; Bahn, Y.-S. Cryptococcus Neoformans and Cryptococcus Gattii, the Etiologic Agents of Cryptococcosis. Cold Spring Harb. Perspect. Med. 2014, 4, a019760. [Google Scholar] [CrossRef]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.-H.; et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef]
- Pathakumari, B.; Liang, G.; Liu, W. Immune Defence to Invasive Fungal Infections: A Comprehensive Review. Biomed. Pharmacother. 2020, 130, 110550. [Google Scholar] [CrossRef]
- Siller, R.A.; Skubic, J.J.; Almeda, J.L.; Villarreal, J.F.; Kaplan, A.E. Candida Pericarditis Presenting with Cardiac Tamponade and Multiple Organ Failure After Combined Damage Control Thoracotomy and Laparotomy with Splenectomy in a Trauma Patient: Case Report and Review of Literature. Trauma Case Rep. 2022, 37, 100564. [Google Scholar] [CrossRef]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the Diagnosis and Management of Pericardial Diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC). Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [CrossRef]
- Mayosi, B.M. Contemporary Trends in the Epidemiology and Management of Cardiomyopathy and Pericarditis in Sub-Saharan Africa. Heart 2007, 93, 1176–1183. [Google Scholar] [CrossRef]
- Ashraf, N.; Kubat, R.C.; Poplin, V.; Adenis, A.A.; Denning, D.W.; Wright, L.; McCotter, O.; Schwartz, I.S.; Jackson, B.R.; Chiller, T.; et al. Re-Drawing the Maps for Endemic Mycoses. Mycopathologia 2020, 185, 843–865. [Google Scholar] [CrossRef]
- Park, B.J.; Wannemuehler, K.A.; Marston, B.J.; Govender, N.; Pappas, P.G.; Chiller, T.M. Estimation of the Current Global Burden of Cryptococcal Meningitis Among Persons Living with HIV/AIDS. AIDS 2009, 23, 525–530. [Google Scholar] [CrossRef]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global Burden of Disease of HIV-Associated Cryptococcal Meningitis: An Updated Analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef]
- Rajasingham, R.; Govender, N.P.; Jordan, A.; Loyse, A.; Shroufi, A.; Denning, D.W.; Meya, D.B.; Chiller, T.M.; Boulware, D.R. The Global Burden of HIV-Associated Cryptococcal Infection in Adults in 2020: A Modelling Analysis. Lancet Infect. Dis. 2022, 22, 1748–1755. [Google Scholar] [CrossRef]
- Drummond, R.A.; Desai, J.V.; Ricotta, E.E.; Swamydas, M.; Deming, C.; Conlan, S.; Quinones, M.; Matei-Rascu, V.; Sherif, L.; Lecky, D.; et al. Long-Term Antibiotic Exposure Promotes Mortality After Systemic Fungal Infection by Driving Lymphocyte Dysfunction and Systemic Escape of Commensal Bacteria. Cell Host Microbe 2022, 30, 1020–1033.e6. [Google Scholar] [CrossRef]
- Hou, S.; Wang, X.; Yu, Y.; Ji, H.; Dong, X.; Li, J.; Li, H.; He, H.; Li, Z.; Yang, Z.; et al. Invasive Fungal Infection is Associated with Antibiotic Exposure in Preterm Infants: A Multi-Centre Prospective Case-Control Study. J. Hosp. Infect. 2023, 134, 43–49. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, rv13–rv165. [Google Scholar] [CrossRef]
- Davidson, J.P.; Connelly, T.M.; Libove, E.; Tappouni, R. Gastropericardial Fistula: Radiologic Findings and Literature Review. J. Surg. Res. 2016, 203, 174–182. [Google Scholar] [CrossRef]
- Elzayat, S.; El-Deeb, M.E.; El-Shirbeny, H.A.; El-Shirbiny, H.; Abdel-Maboud, M.; Nasr, K. The Prevalence and Association of Biofilms with Otitis Media with Effusion: A Systematic Review and Meta-Analysis. Ann. Otol. Rhinol. Laryngol. 2024, 133, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Cadena, J.; Thompson, G.R.; Patterson, T.F. Aspergillosis: Epidemiology, Diagnosis, and Treatment. Infect. Dis. Clin. N. Am. 2021, 35, 415–434. [Google Scholar] [CrossRef]
- Maisch, B.; Seferović, P.M.; Ristić, A.D.; Erbel, R.; Rienmüller, R.; Adler, Y.; Tomkowski, W.Z.; Thiene, G.; Yacoub, M.H.; Task Force on the Diagnosis and Management of Pricardial Diseases of the European Society of Cardiology. Guidelines on the Diagnosis and Management of Pericardial Diseases Executive Summary. The Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Eur. Heart J. 2004, 25, 587–610. [Google Scholar] [CrossRef]
- Herrera, L.N.; Khodadadi, R.; Leal, S.; Kulkarni, P.; Pappas, P.; McCarty, T. Clinical Utility of Routine Use of Fungal Blood Cultures. Am. J. Med. 2023, 136, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Dumic, I.; Caetano, E.M.; Domingues, S.M.; Pantic, I.; Radovanovic, M.; Prada, L.R.; Nordstrom, C.W.; Antic, M.; Milovanovic, T.; Kotseva, M.; et al. Clinical Characteristics, Diagnosis, Treatment, and Outcome of Patients with Liver Abscess Due to Aspergillus Spp: A Systematic Review of Published Cases. BMC Infect. Dis. 2024, 24, 345. [Google Scholar] [CrossRef]
- Miller, J.M.; Binnicker, M.J.; Campbell, S.; Carroll, K.C.; Chapin, K.C.; Gilligan, P.H.; Gonzalez, M.D.; Jerris, R.C.; Kehl, S.C.; Patel, R.; et al. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin. Infect. Dis. 2018, 67, e1–e94. [Google Scholar] [CrossRef]
- Levy, P.-Y.; Fournier, P.-E.; Charrel, R.; Metras, D.; Habib, G.; Raoult, D. Molecular Analysis of Pericardial Fluid: A 7-Year Experience. Eur. Heart J. 2006, 27, 1942–1946. [Google Scholar] [CrossRef]
- Whimbey, E.; Gold, J.W.; Polsky, B.; Dryjanski, J.; Hawkins, C.; Blevins, A.; Brannon, P.; Kiehn, T.E.; Brown, A.E.; Armstrong, D. Bacteremia and Fungemia in Patients with the Acquired Immunodeficiency Syndrome. Ann. Intern. Med. 1986, 104, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Archibald, L.K.; McDonald, L.C.; Rheanpumikankit, S.; Tansuphaswadikul, S.; Chaovanich, A.; Eampokalap, B.; Banerjee, S.N.; Reller, L.B.; Jarvis, W.R. Fever and Human Immunodeficiency Virus Infection as Sentinels for Emerging Mycobacterial and Fungal Bloodstream Infections in Hospitalized Patients >/=15 Years Old, Bangkok. J. Infect. Dis. 1999, 180, 87–92. [Google Scholar] [CrossRef]
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The Changing Epidemiology of Invasive Fungal Infections. Methods Mol. Biol. 2017, 1508, 17–65. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and Management of Aspergillus Diseases: Executive Summary of the 2017 ESCMID-ECMM-ERS Guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. S1), e1–e38. [Google Scholar] [CrossRef]
- Husain, S.; Camargo, J.F. Invasive Aspergillosis in Solid-Organ Transplant Recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13544. [Google Scholar] [CrossRef]
- Theel, E.S.; Doern, C.D. Point-Counterpoint: β-d-Glucan Testing Is Important for Diagnosis of Invasive Fungal Infections. J. Clin. Microbiol. 2020, 51, 3478–3483. [Google Scholar] [CrossRef]
- Rodriguez-Tudela, J.L.; Berenguer, J.; Guarro, J.; Kantarcioglu, A.S.; Horre, R.; de Hoog, G.S.; Cuenca-Estrella, M. Epidemiology and Outcome of Scedosporium Prolificans Infection, a Review of 162 Cases. Med. Mycol. 2009, 47, 359–370. [Google Scholar] [CrossRef]
- Wakabayashi, Y.; Okugawa, S.; Tatsuno, K.; Ikeda, M.; Misawa, Y.; Koyano, S.; Tsuji, E.; Yanagimoto, S.; Hatakeyama, S.; Moriya, K.; et al. Scedosporium Prolificans Endocarditis: Case Report and Literature Review. Intern. Med. 2016, 55, 79–82. [Google Scholar] [CrossRef]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global Burden of Allergic Bronchopulmonary Aspergillosis with Asthma and its Complication Chronic Pulmonary Aspergillosis in Adults. Med. Mycol. 2013, 51, 361–370. [Google Scholar] [CrossRef]
- Morgan, J. Global Trends in Candidemia: Review of Reports from 1995-2005. Curr. Infect. Dis. Rep. 2005, 7, 429–439. [Google Scholar] [CrossRef]
- Letoquart, J.P.; Fasquel, J.L.; L’Huillier, J.P.; Babatasi, G.; Gruel, Y.; Lauvin, R.; Mambrini, A. [Gastropericardial fistula. Review of the literature apropos of an original case]. J. Chir. 1990, 127, 6–12. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).