Effectiveness of Feed-Based Monovalent Aeromonas Vaccine in Farmed Carp
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Bacterial Recovery
2.3. Vaccine Preparation
2.4. Development of Vaccinated Feed
2.5. Fish Sampling
2.6. Quality Tests of Vaccinated Feed
2.7. Growth Parameters
2.8. Serum Collection
2.9. Agglutination Antibody Titer Test
2.10. Serum Lysozyme Activity
2.11. Total IgM Contents and Total Protein
2.12. Challenge Test
2.13. Histopathology
2.14. Statistical Analysis
3. Results
3.1. Vaccinated Feed Quality Tests
3.2. Proximate Analysis of Vaccinated and Control Feed
3.3. Proximate Analysis of Vaccinated and Control Fish
3.4. Growth Parameters of Fish
3.5. Agglutination Antibody Titer Test
3.6. Serum Lysozyme Activity
3.7. Total IgM Contents
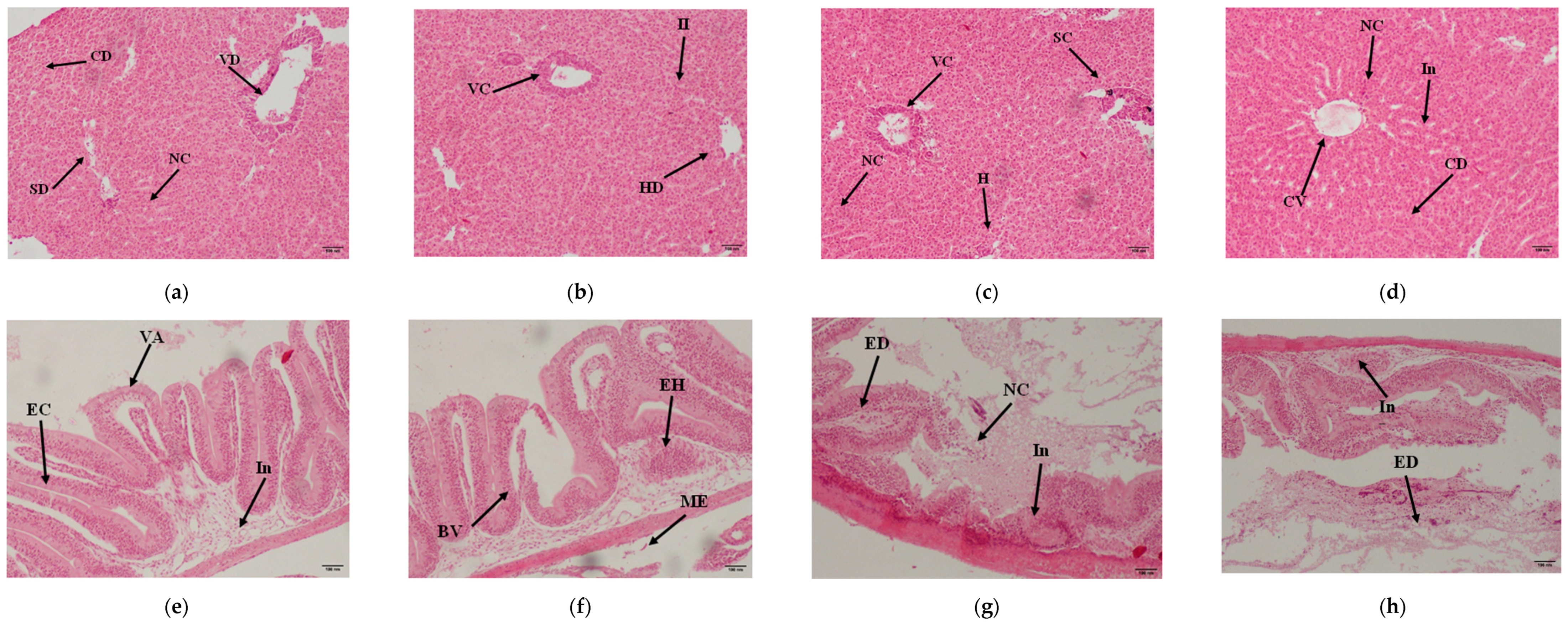
3.8. Histopathology
3.9. Challenge Test and Relative Percentage Survival Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Global Aquaculture Production 1950–2019 (FishStatJ); Food and Agriculture Organization of the United Nations: Rome, Italy. Available online: https://www.fao.org/fishery/statistics/software/fishstatj/ (accessed on 22 July 2025).
- Monir, M.S.; Yusoff, S.M.; Mohamad, A.; Ina-Salwany, M.Y. Vaccination of tilapia against motile Aeromonas septicemia: A review. J. Aquat. Anim. Health 2020, 32, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Dien, L.T.; Ngo, T.P.; Nguyen, T.V.; Kayansamruaj, P.; Salin, K.R.; Mohan, C.V.; Rodkhum, C.; Dong, H.T. Non-antibiotic approaches to combat motile Aeromonas infections in aquaculture: Current state of knowledge and future perspectives. Rev. Aquac. 2023, 15, 333–366. [Google Scholar] [CrossRef]
- Pridgeon, J.W.; Yildirim-Aksoy, M.; Klesius, P.H.; Srivastava, K.K.; Reddy, P.G. Attenuation of a virulent Aeromonas hydrophila with novobiocin and pathogenic characterization of the novobiocin-resistant strain. J. Appl. Microbiol. 2012, 113, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen-Ivey, C.R.; Hossain, M.J.; Odom, S.E.; Terhune, J.S.; Hemstreet, W.G.; Shoemaker, C.A.; Zhang, D.; Xu, D.H.; Griffin, M.J.; Liu, Y.J.; et al. Classification of a hypervirulent Aeromonas hydrophila pathotype responsible for epidemic outbreaks in warm-water fishes. Front. Microbiol. 2016, 7, 1615. [Google Scholar] [CrossRef]
- Patil, H.J.; Benet-Perelberg, A.; Naor, A.; Smirnov, M.; Ofek, T.; Nasser, A.; Minz, D.; Cytryn, E. Evidence of increased antibiotic resistance in phylogenetically-diverse Aeromonas isolates from semi-intensive fish ponds treated with antibiotics. Front. Microbiol. 2016, 7, 1875. [Google Scholar] [CrossRef]
- Awad, E.; Awaad, A. Role of medicinal plants on growth performance and immune status in fish. Fish Shellfish Immunol. 2017, 67, 40–54. [Google Scholar] [CrossRef]
- Romero, J.; Gloria, C.; Navarrete, P. Antibiotics in aquaculture-use, abuse and alternatives. Health Environ. Aquac. 2012, 159, 159–198. [Google Scholar]
- Sapkota, A.; Sapkota, A.R.; Kucharski, M.; Burke, J.; McKenzie, S.; Walker, P.; Lawrence, R. Aquaculture practices and potential human health risks: Current knowledge and future priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef]
- Cheng, Z.X.; Ma, Y.M.; Li, H.; Peng, X.X. N-acetylglucosamine enhances survival ability of tilapias infected by Streptococcus iniae. Fish Shellfish Immunol. 2014, 40, 524–530. [Google Scholar] [CrossRef]
- Pridgeon, J.W.; Klesius, P.H. Major bacterial diseases in aquaculture and their vaccine development. CAB Rev. 2012, 7, 1–16. [Google Scholar] [CrossRef]
- Assefa, A.; Abunna, F. Maintenance of fish health in aquaculture: Review of epidemiological approaches for prevention and control of infectious disease of fish. Vet. Med. Int. 2018, 2018, 5432497. [Google Scholar] [CrossRef]
- Ismail, M.S.; Siti-Zahrah, A.; Syafiq, M.R.; Amal, M.N.; Firdaus-Nawi, M.; Zamri-Saad, M. Feed-based vaccination regime against streptococcosis in red tilapia, Oreochromis niloticus x Oreochromis mossambicus. BMC Vet. Res. 2016, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Areechon, N. Efficacy of formalin-killed Aeromonas hydrophila and Streptococcus sp. vaccine in red tilapia. Our Nat. 2010, 8, 231–240. [Google Scholar] [CrossRef]
- Shome, R.; Shome, B. Evaluation of three types of Aeromonas hydrophila vaccines against acute infectious dropsy disease in Indian major carps. Indian J. Fish 2005, 52, 405–412. [Google Scholar]
- Shamsuzzaman, M.M.; Islam, M.M.; Tania, N.J.; Abdullah, A.M.; Barman, P.P.; Xu, X. Fisheries resources of Bangladesh: Present status and future direction. Aquac. Fish 2017, 2, 145–156. [Google Scholar] [CrossRef]
- Alishahi, M.; Tollabi, M.; Ghorbanpour, M. Comparison of the adjuvant effect of propolis and Freund on the efficacy of Aeromonas hydrophila vaccine in common carp (Cyprinus carpio). Iran. J. Fish Sci. 2019, 18, 428–444. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, W.; Xu, Z. Current use and development of fish vaccines in China. Fish Shellfish Immunol. 2020, 96, 223–234. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Mudryk, Z.J.; Perlinski, P. Abundance and antibiotic resistance of Aeromonas isolated from the water of three carp ponds. Vet. Res. Commun. 2020, 44, 9–18. [Google Scholar] [CrossRef]
- Firdaus-Nawi, M.; Yusoff, S.M.; Yusoff, H.; Abdullah, S.Z.; Zamri-Saad, M. Efficacy of feed-based adjuvant vaccine against Streptococcus agalactiae in Oreochromis spp. in Malaysia. Aquac. Res. 2014, 45, 87–96. [Google Scholar] [CrossRef]
- Han, B.; Xu, K.; Liu, Z.; Ge, W.; Shao, S.; Li, P.; Yan, N.; Li, X.; Zhang, Z. Oral yeast-based DNA vaccine confers effective protection from Aeromonas hydrophila infection on Carassius auratus. Fish Shellfish Immunol. 2019, 84, 948–954. [Google Scholar] [CrossRef]
- Sughra, F.; Rahman, M.; Abbas, F.; Altaf, I. Evaluation of three alum-precipitated Aeromonas hydrophila vaccines administered to Labeo rohita, Cirrhinus mrigala and Ctenopharyngodon idella: Immunokinetics, immersion challenge and histopathology. Braz. J. Biol. 2021, 83, e249913. [Google Scholar] [CrossRef]
- Yazdanpanah-Goharrizi, L.; Rokhbakhsh-Zamin, F.; Zorriehzahra, M.J.; Kazemipour, N.; Kheirkhah, B. Isolation, biochemical and molecular detection of Aeromonas hydrophila from cultured Oncorhynchus mykiss. Iran. J. Fish Sci. 2020, 19, 2422–2436. [Google Scholar] [CrossRef]
- Legario, F.S.; Choresca-Jr, C.H.; Turnbull, J.F.; Crumlish, M. Isolation and molecular characterization of streptococcal species recovered from clinical infections in farmed Nile tilapia (Oreochromis niloticus) in the Philippines. J. Fish Dis. 2020, 43, 1431–1442. [Google Scholar] [CrossRef]
- Monir, M.S.; Yusoff, S.B.; Zulperi, Z.B.; Hassim, H.B.; Mohamad, A.; Ngoo, M.S.; Ina-Salwany, M.Y. Haemato-immunological responses and effectiveness of feed-based bivalent vaccine against Streptococcus iniae and Aeromonas hydrophila infections in hybrid red tilapia (Oreochromis mossambicus × O. niloticus). BMC Vet. Res. 2020, 16, 226. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 19th ed.; Association of Official Analytical Chemist: Washington, DC, USA, 2012. [Google Scholar]
- Dong, C.; He, G.; Mai, K.; Zhou, H.; Xu, W. Palatability of water-soluble extracts of protein sources and replacement of fishmeal by a selected mixture of protein sources for juvenile turbot (Scophthalmus maximus). J. Ocean. Univ. China 2016, 15, 561–567. [Google Scholar] [CrossRef]
- Obaldo, L.G.; Divakaran, S.; Tacon, A.G. Method for determining the physical stability of shrimp feeds in water. Aquac. Res. 2002, 33, 369–377. [Google Scholar] [CrossRef]
- Kaur, A.; Holeyappa, S.A.; Bansal, N.; Kaur, V.I. Ameliorative effect of turmeric supplementation in feed of Labeo rohita challenged with pathogenic Aeromonas veronii. Aquac. Int. 2020, 28, 1169–1182. [Google Scholar] [CrossRef]
- Biller-Takahashi, J.D.; Montassier, H.J.; Takahashi, L.S.; Urbinati, E.C. Proposed method for agglutinating antibody titer analysis and its use as indicator of acquired immunity in pacu, Piaractus mesopotamicus. Braz. J. Biol. 2014, 74, 238–242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruckenstein, E.; Zeng, X. Macroporous chitin affinity membranes for lysozyme separation. Biotechnol. Bioeng. 1997, 56, 610–617. [Google Scholar] [CrossRef]
- Anderson, D.P.; Siwicki, A.K. Basic Haematology and Serology for Fish Health Programs. Disease in Asian Aquaculture II; Shariff, M., Arthur, J.R., Subangsinghe, R.P., Eds.; Fish Health Section Asian Fisheries Society: Quezon City, Philippines, 1995; pp. 185–202. [Google Scholar]
- Amend, D.F. Potency testing of fish vaccines. Dev. Biol. Stand. 1981, 49, 447–454. [Google Scholar]
- Yang, H.; Zhang, M.; Ji, T.; Zhang, Y.; Wei, W.; Liu, Q. Bacillus subtilis CK3 used as an aquatic additive probiotics enhanced the immune response of crayfish Procambarus clarkii against newly identified Aeromonas veronii pathogen. Aquac. Res. 2022, 5, 255–264. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Ayisi, C.L.; Zhao, J.; Rupia, E.J. Growth performance, feed utilization, body and fatty acid composition of Nile tilapia (Oreochromis niloticus) fed diets containing elevated levels of palm oil. Aquac. Fish 2017, 2, 67–77. [Google Scholar] [CrossRef]
- Thompson, B.; Subasinghe, R. Aquaculture’s role in improving food and nutrition security. In Combating Micronutrient Deficiencies: Food-Based Approaches; CABI: Wallingford, UK, 2020; pp. 150–162. [Google Scholar] [CrossRef]
- Calder, P.C. Immunoregulatory and anti-inflammatory effects of n-3 polyunsaturated fatty acids. Braz. J. Med. Biol. Res. 1998, 31, 4. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, A.; Zamri-Saad, M.; Amal, M.N.; Al-Saari, N.; Monir, M.S.; Chin, Y.K.; Md Yasin, I.S. Vaccine efficacy of a newly developed feed-based whole-cell polyvalent vaccine against vibriosis, streptococcosis and motile aeromonad septicemia in asian seabass, Lates calcarifer. Vaccines 2021, 9, 368. [Google Scholar] [CrossRef]
- Terpstra, A.H.M. The Composition and Production of Fish Feeds. Ph. D. Thesis, Universitate Vadensi, Orando, The Netherlands, 2015. [Google Scholar]
- Chalmers, L.; Thompson, K.D.; Taylor, J.F.; Black, S.; Migaud, H.; North, B.; Adams, A. A comparison of the response of diploid and triploid Atlantic salmon (Salmo salar) siblings to a commercial furunculosis vaccine and subsequent experimental infection with Aeromonas salmonicida. Fish Shellfish Immunol. 2016, 57, 301–308. [Google Scholar] [CrossRef][Green Version]
- Reyes, M.; Ramírez, C.; Nancucheo, I.; Villegas, R.; Schaffeld, G.; Kriman, L.; Gonzalez, J.; Oyarzun, P. A Novel “In-Feed” Delivery platform applied for oral DNA vaccination against IPNV enables high protection in atlantic salmon (Salmon salar). Vaccine 2017, 35, 626–632. [Google Scholar] [CrossRef]
- Fraser, T.W.K.; Hansen, T.; Mayer, I.; Skjæraasen, J.E.; Glover, K.A.; Sambraus, F.; Fjelldal, P.G. The effect of triploidy on vaccine side-effects in Atlantic salmon. Aquaculture 2014, 433, 481–490. [Google Scholar] [CrossRef]
- Beck, B.R.; Lee, S.H.; Kim, D.; Park, J.H.; Lee, H.K.; Kwon, S.S.; Lee, K.H.; Lee, J.I.; Song, S.K. A Lactococcus lactis BFE920 feed vaccine expressing a fusion protein composed of the OmpA and FlgD antigens from Edwardsiella tarda was significantly better at protecting olive flounder (Paralichthys olivaceus) from edwardsiellosis than single antigen vaccines. Fish Shellfish Immunol. 2017, 68, 19–28. [Google Scholar] [CrossRef]
- Ismail, M.S.; Syafiq, M.R.; Siti-Zahrah, A.; Fahmi, S.; Shahidan, H.; Hanan, Y.; Amal, M.N.; Saad, M.Z. The effect of feed-based vaccination on tilapia farm endemic for streptococcosis. Fish Shellfish Immunol. 2017, 60, 21–24. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Z.H.; Wang, B.; Lu, Y.S.; Huang, Y.C.; Jian, J.C. Immune protective effect of Streptococcus agalactiae inactivated vaccine on Tilapia (Oreochromis niloticus). J. Guangdong Ocean. Univ. 2012, 32, 50–56. [Google Scholar]
- Wei, L.L.; Liu, Y.; Zhou, Q.B.; Xiong, L.F.; Wu, H.D. Immune protective effects of inactivated Edwardsiella ictaluri vaccine on yellow catfish (Pelteobagrus fulvidraco). J. Nanchang Univ. 2014, 38, 263–267. (In Chinese) [Google Scholar]
- Purwanti, C.S.; Suminto, S.A. The description of blood profile catfish Clarias gariepinus that is fed with a combination of artificial feed and earthworm Lumbricus rubellus. JAMT 2014, 3, 53–60. [Google Scholar]
- Pasaribu, W.; Sukenda, S.; Nuryati, S. The efficacy of Nile tilapia (Oreochromis niloticus) Broodstock and larval immunization against Streptococcus agalactiae and Aeromonas hydrophila. Fishes 2018, 3, 16. [Google Scholar] [CrossRef]
- Secombes, C.J.; Wang, T. The Innate and Adaptive Immune System of Fish; Woodhead Publishing Limited: Cambridge, UK, 2012. [Google Scholar] [CrossRef]
- Giri, S.S.; Sen, S.S.; Jun, J.W.; Sukumaran, V.; Park, S.C. Role of Bacillus subtilis VSG4-derived biosurfactant in mediating immune responses in Labeo rohita. Fish Shellfish Immunol. 2016, 54, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Sirimanapong, W.; Thompson, K.D.; Kledmanee, K.; Thaijongrak, P.; Collet, B.; Ooi, E.L.; Adams, A. Optimisation and standardisation of functional immune assays for striped catfish (Pangasianodon hypophthalmus) to compare their immune response to live and heat killed Aeromonas hydrophila as models of infection and vaccination. Fish Shellfish Immunol. 2014, 40, 374–383. [Google Scholar] [CrossRef] [PubMed]
- do Vale Pereira, G.; da Silva, B.C.; do Nascimento Vieira, F.; Seiffert, W.Q.; Ushizima, T.T.; Mourino, J.L.P.; Martins, M.L. Vaccination strategies with oral booster for Surubim hybrid (Pseudoplatystoma corruscans × P. reticulatum) against haemorrhagic septicaemia. Aquac. Res. 2015, 46, 1831–1841. [Google Scholar] [CrossRef]
- Adelmann, M.; Köllner, B.; Bergmann, S.M.; Fischer, U.; Lange, B.; Weitschies, W.; Enzmann, P.J.; Fichtner, D. Development of an oral vaccine for immunisation of rainbow trout (Oncorhynchus mykiss) against viral haemorrhagic septicaemia. Vaccine 2008, 26, 837–844. [Google Scholar] [CrossRef]
- Chideroli, R.T.; Amoroso, N.; Mainardi, R.M.; Suphoronski, S.A.; de Padua, S.B.; Alfieri, A.F.; Alfieri, A.A.; Mosela, M.; Moralez, A.T.; de Oliveira, A.G.; et al. Emergence of a new multidrug-resistant and highly virulent serotype of Streptococcus agalactiae in fish farms from Brazil. Aquaculture 2017, 479, 45–51. [Google Scholar] [CrossRef]
- Song, X.; Zhao, J.; Bo, Y.; Liu, Z.; Wu, K.; Gong, C. Aeromonas hydrophila induces intestinal inflammation in grass carp (Ctenopharyngodon idella): An experimental model. Aquaculture 2014, 434, 171–178. [Google Scholar] [CrossRef]
- Mamun, M.A.; Nasren, S.; Abhiman, P.B.; Rathore, S.S.; Sowndarya, N.S.; Ramesh, K.S.; Shankar, K.M. Effect of biofilm of Aeromonas hydrophila oral vaccine on growth performance and histopathological changes in various tissues of Striped Catfish, Pangasianodon Hypophthalmus (Sauvage 1878). Indian J. Anim. Res. 2020, 54, 563–569. [Google Scholar] [CrossRef]
- Chettri, J.K.; Jaafar, R.M.; Skov, J.; Kania, P.W.; Dalsgaard, I.; Buchmann, K. Booster immersion vaccination using diluted Yersinia ruckeri bacterin confers protection against ERM in rainbow trout. Aquaculture 2015, 440, 1–5. [Google Scholar] [CrossRef]
- Gravningen, K.; Sakai, M.; Mishiba, T.; Fujimoto, T. The efficacy and safety of an oil-based vaccine against Photobacterium damsela subsp. piscicida in yellowtail (Seriola quinqueradiata): A field study. Fish Shellfish Immunol. 2008, 24, 523–529. [Google Scholar] [CrossRef]
- Villumsen, K.R.; Koppang, E.O.; Raida, M.K. Adverse and long-term protective effects following oil-adjuvanted vaccination against Aeromonas salmonicida in rainbow trout. Fish Shellfish Immunol. 2015, 42, 193–203. [Google Scholar] [CrossRef]
- Fredriksen, B.N.; Olsen, R.H.; Furevik, A.; Souhoka, R.A.; Gauthier, D.; Brudeseth, B. Efficacy of a divalent and a multivalent water-in-oil formulated vaccine against a highly virulent strain of Flavobacterium psychrophilum after intramuscular challenge of rainbow trout (Oncorhynchus mykiss). Vaccine 2013, 31, 1994–1998. [Google Scholar] [CrossRef]
| (Control) | T1 | T2 | T3 | |
|---|---|---|---|---|
| Sprayed vaccinated feed | Commercial feed | Sprayed vaccinated feed (SVF) | Sprayed vaccinated feed with mineral oil (SVFM) | Sprayed vaccinated feed with fish oil (SVFF) |
| Incorporated vaccinated feed | Incorporated vaccinated feed (IVF) | Incorporated vaccinated feed with mineral oil (IVFM) | Incorporated vaccinated feed with fish oil (IVFF) |
| Treatments | Feed Stability (%) | Feed Palatability |
|---|---|---|
| C | 70.0 ± 2.82 c | 0.50 ± 0.14 c |
| SVF | 72.3 ± 6.14 b | 0.51 ± 0.28 c |
| IVF | 73.2 ± 4.51 b | 0.53 ± 0.42 c |
| SVFM | 72.5 ± 1.61 b | 0.65 ± 0.07 b |
| IVFM | 74.2 ± 5.97 b | 0.61 ± 0.01 b |
| SVFF | 75.7 ± 8.01 b | 0.60 ± 0.28 b |
| IVFF | 80.9 ± 0.95 a | 0.73 ± 0.17 a |
| Treatments | Crude Protein (%) | Crude Lipid (%) | Ash (%) | Moisture (%) |
|---|---|---|---|---|
| C | 30.0 ± 2.82 b | 4.5 ± 0.14 c | 8.5 ± 0.70 ab | 10.0 ± 2.82 b |
| SVF | 31.2 ± 0.25 ab | 3.4 ± 0.09 d | 8.3 ± 0.28 b | 10.3 ± 0.42 b |
| IVF | 31.5 ± 1.41 ab | 5.9 ± 0.05 ab | 8.8 ± 1.13 a | 12.2 ± 3.11 ab |
| SVFM | 32.0 ± 2.82 a | 5.5 ± 0.14 bc | 9.5 ± 0.05 a | 16.0 ± 1.41 a |
| IVFM | 32.4 ± 3.45 a | 6.7 ± 0.42 ab | 8.0 ± 1.41 c | 11.0 ± 2.82 ab |
| SVFF | 32.7 ± 5.30 a | 5.8 ± 1.13 ab | 8.5 ± 2.12 ab | 14.7 ± 0.14 ab |
| IVFF | 33.2 ± 3.88 a | 6.9 ± 0.04 a | 8.2 ± 0.28 b | 10.4 ± 0.56 b |
| Treatments | Crude Protein (%) | Crude Lipid (%) | Ash (%) | Moisture (%) |
|---|---|---|---|---|
| C | 16.5 ± 0.42 c | 2.2 ± 0.74 ab | 1.2 ± 0.03 a | 77.0 ± 0.67 b |
| SVF | 19.7 ± 3.29 bc | 2.2 ± 0.11 ab | 1.1 ± 0.06 a | 79.0 ± 0.45 ab |
| IVF | 18.3 ± 2.38 bc | 1.8 ± 0.48 ab | 1.2 ± 0.02 a | 78.0 ± 1.07 b |
| SVFM | 21.0 ± 2.56 abc | 2.3 ± 0.31 ab | 1.2 ± 0.07 a | 76.4 ± 0.78 c |
| IVFM | 21.2 ± 0.58 abc | 1.3 ± 0.84 b | 1.1 ± 0.11 a | 78.6 ± 0.49 ab |
| SVFF | 23.5 ± 0.92 ab | 2.4 ± 0.87 ab | 1.2 ± 0.11 a | 77.6 ± 0.56 bc |
| IVFF | 26.1 ± 2.96 a | 2.7 ± 0.14 a | 1.1 ± 0.05 a | 80.2 ± 0.41 a |
| Treatments | NWG (g) | SGR (%/day) | FCR (g/g) |
|---|---|---|---|
| C | 4.9 ± 0.81 c | 0.59 ± 0.01 ab | 2.0 ± 0.71 a |
| SVF | 8.3 ± 0.43 ab | 0.64 ± 0.02 b | 1.2 ± 0.28 ab |
| IVF | 6.2 ± 0.35 bc | 0.72 ± 0.07 ab | 1.4 ± 0.42 ab |
| SVFM | 7.7 ± 0.86 abc | 0.72 ± 0.01 ab | 1.2 ± 0.28 ab |
| IVFM | 7.1 ± 2.89 abc | 0.88 ± 0.09 a | 1.0 ± 0.03 b |
| SVFF | 7.9 ± 0.09 abc | 0.80 ± 0.24 ab | 1.1 ± 0.01 b |
| IVFF | 9.8 ± 0.82 a | 0.85 ± 0.05 ab | 1.1 ± 0.14 ab |
| Treatments | Day 14 | Day 28 | Day 42 | Day 56 |
|---|---|---|---|---|
| C | 0.24 ± 0.03 b | 0.23 ± 0.01 d | 0.23 ± 0.01 d | 0.23 ± 0.01 e |
| SVF | 0.27 ± 0.04 b | 0.28 ± 0.05 c | 0.34 ± 0.06 cd | 0.38 ± 0.02 d |
| IVF | 0.29 ± 0.01 b | 0.29 ± 0.04 c | 0.36 ± 0.04 cd | 0.39 ± 0.01 d |
| SVFM | 0.32 ± 0.03 ab | 0.35 ± 0.03 bc | 0.44 ± 0.06 bcd | 0.48 ± 0.06 cd |
| IVFM | 0.35 ± 0.04 ab | 0.41 ± 0.06 bc | 0.46 ± 0.08 bc | 0.54 ± 0.06 b |
| SVFF | 0.51 ± 0.14 ab | 0.58 ± 0.16 ab | 0.62 ± 0.11 b | 0.68 ± 0.06 b |
| IVFF | 0.62 ± 0.29 a | 0.76 ± 0.21 a | 0.82 ± 0.15 a | 0.92 ± 0.07 a |
| Treatments | Day 14 | Day 28 | Day 42 | Day 56 |
|---|---|---|---|---|
| C | 1850 ± 70.0 d | 2080 ± 58.6 e | 2269 ± 72.1 e | 2553 ± 77.7 d |
| SVF | 2110 ± 1.55 d | 2269 ± 72.1 e | 2660 ± 56.5 d | 3370 ± 436.9 cd |
| IVF | 1869 ± 98.2 d | 2265 ± 21.2 e | 2800 ± 14.1 d | 3300 ± 424.2 c |
| SVFM | 3220 ± 28.2 c | 3927 ± 68.5 d | 4280 ± 282.8 c | 4450 ± 282.8 b |
| IVFM | 4208 ± 130.1 b | 4186 ± 141.2 c | 4290 ± 282.8 c | 4350 ± 282.8 b |
| SVFF | 4349 ± 167.5 b | 4729 ± 60.8 b | 5067 ± 68.5 b | 5469 ± 67.8 a |
| IVFF | 4834 ± 84.1 a | 5170 ± 141.2 a | 5802 ± 96.8 a | 5960 ± 424.2 a |
| Treatments | Day 14 | Day 28 | Day 42 | Day 56 |
|---|---|---|---|---|
| C | 0.20 ± 0.01 b | 0.20 ± 0.01 d | 0.21 ± 0.01 d | 0.21 ± 0.01 e |
| SVF | 0.22 ± 0.01 b | 0.27 ± 0.02 cd | 0.29 ± 0.01 c | 0.36 ± 0.03 de |
| IVF | 0.22 ± 0.02 b | 0.36 ± 0.04 b | 0.38 ± 0.03 b | 0.43 ± 0.01 cd |
| SVFM | 0.25 ± 0.04 ab | 0.34 ± 0.01 bc | 0.37 ± 0.01 b | 0.42 ± 0.09 cd |
| IVFM | 0.26 ± 0.04 ab | 0.35 ± 0.07 bc | 0.38 ± 0.06 b | 0.52 ± 0.02 bc |
| SVFF | 0.26 ± 0.02 ab | 0.36 ± 0.02 b | 0.46 ± 0.03 a | 0.58 ± 0.02 ab |
| IVFF | 0.33 ± 0.04 a | 0.45 ± 0.01 a | 0.53 ± 0.04 a | 0.68 ± 0.07 a |
| Treatments | Total No. of Fish | No. of Dead | Protection (%) | Mortality (%) | RPS (%) |
|---|---|---|---|---|---|
| C | 15 | 12 | 20 | 80 | ---- |
| SVF | 15 | 10 | 34 | 66 | 18 |
| IVF | 15 | 9 | 40 | 60 | 25 |
| SVFM | 15 | 8 | 47 | 53 | 34 |
| IVFM | 15 | 8 | 47 | 53 | 34 |
| SVFF | 15 | 5 | 67 | 33 | 58 |
| IVFF | 15 | 2 | 87 | 13 | 83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mubeen, N.; Abbas, F.; Hafeez-ur-Rehman, M.; Crumlish, M.; Mahboob, H.; Akmal, M.; Sadiqa, A.; Alam, T.M.; Jalil, S. Effectiveness of Feed-Based Monovalent Aeromonas Vaccine in Farmed Carp. Microorganisms 2025, 13, 1903. https://doi.org/10.3390/microorganisms13081903
Mubeen N, Abbas F, Hafeez-ur-Rehman M, Crumlish M, Mahboob H, Akmal M, Sadiqa A, Alam TM, Jalil S. Effectiveness of Feed-Based Monovalent Aeromonas Vaccine in Farmed Carp. Microorganisms. 2025; 13(8):1903. https://doi.org/10.3390/microorganisms13081903
Chicago/Turabian StyleMubeen, Nimra, Farzana Abbas, Muhammad Hafeez-ur-Rehman, Margaret Crumlish, Haris Mahboob, Muhammad Akmal, Ayesha Sadiqa, Talha Mahboob Alam, and Samama Jalil. 2025. "Effectiveness of Feed-Based Monovalent Aeromonas Vaccine in Farmed Carp" Microorganisms 13, no. 8: 1903. https://doi.org/10.3390/microorganisms13081903
APA StyleMubeen, N., Abbas, F., Hafeez-ur-Rehman, M., Crumlish, M., Mahboob, H., Akmal, M., Sadiqa, A., Alam, T. M., & Jalil, S. (2025). Effectiveness of Feed-Based Monovalent Aeromonas Vaccine in Farmed Carp. Microorganisms, 13(8), 1903. https://doi.org/10.3390/microorganisms13081903







