The Different Spatial Distribution Patterns of Nitrifying and Denitrifying Microbiome in the Biofilters of the Recirculating Aquaculture System
Abstract
1. Introduction
2. Material and Methods
2.1. RAS System Description
2.2. Sampling Design
2.3. DNA Extraction and Sequencing
2.4. Sequencing Data Processing
2.5. Statistical Analysis
3. Results
3.1. Water Quality Characteristics in Different Positions of the Biofilter
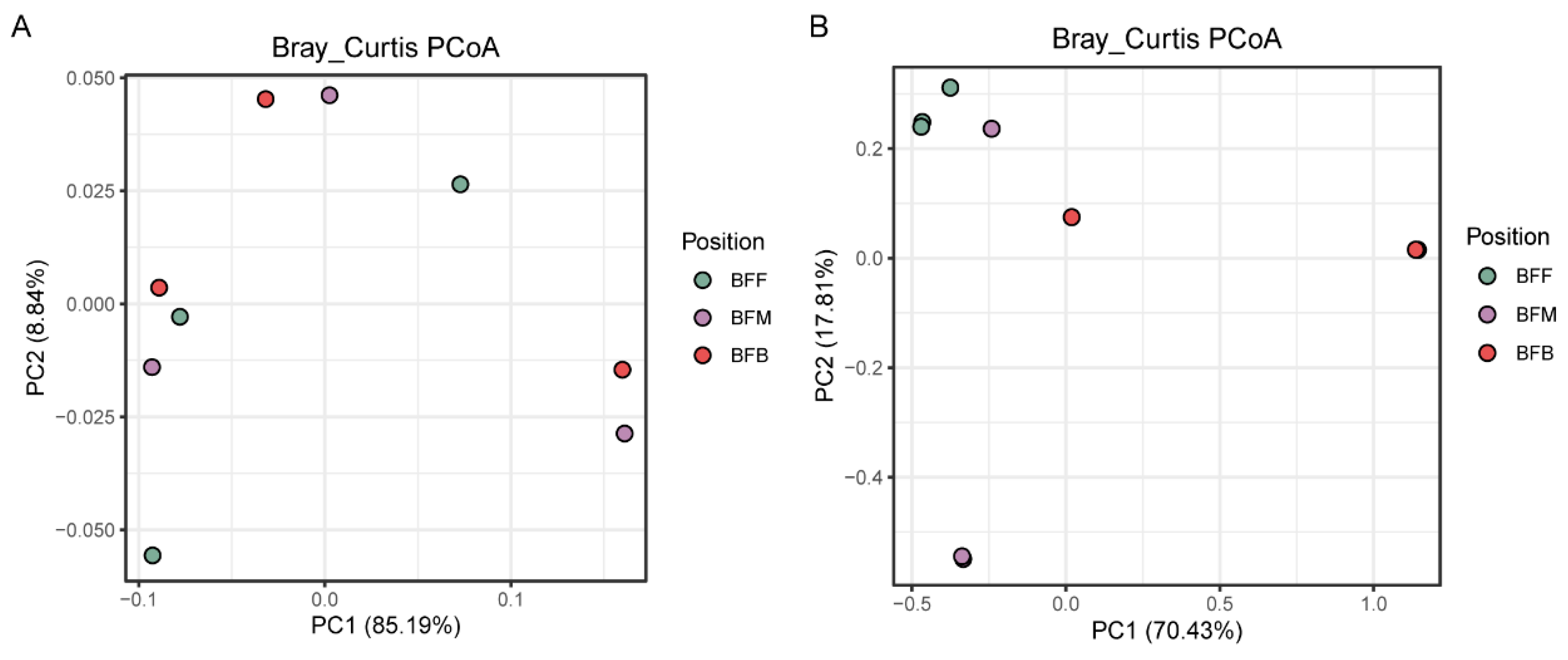
3.2. Microbial Diversity Characteristics of Functional Microbiomes in Different Positions of the Biofilter
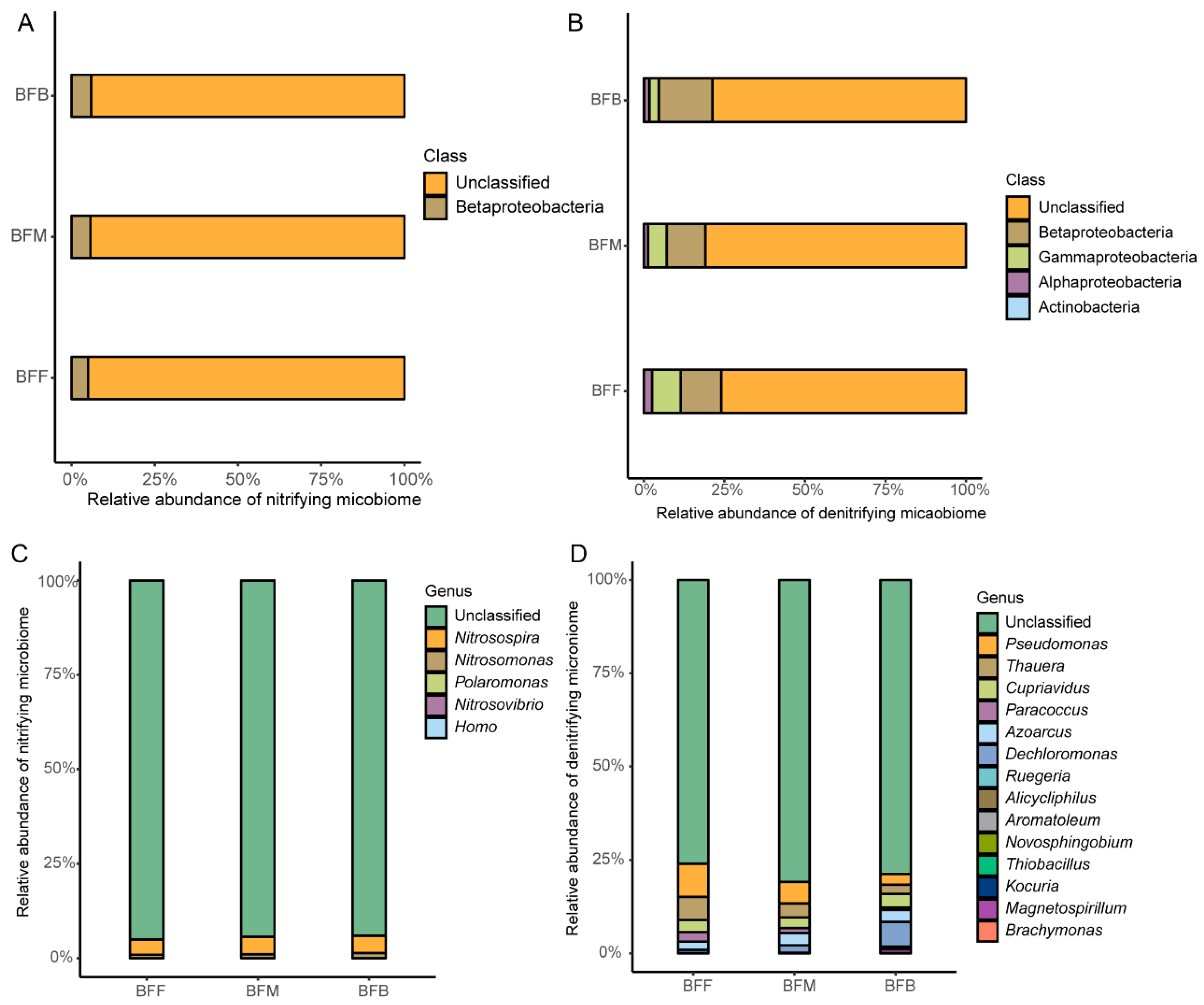
3.3. Functional Community Structure in Different Positions of the Biofilter
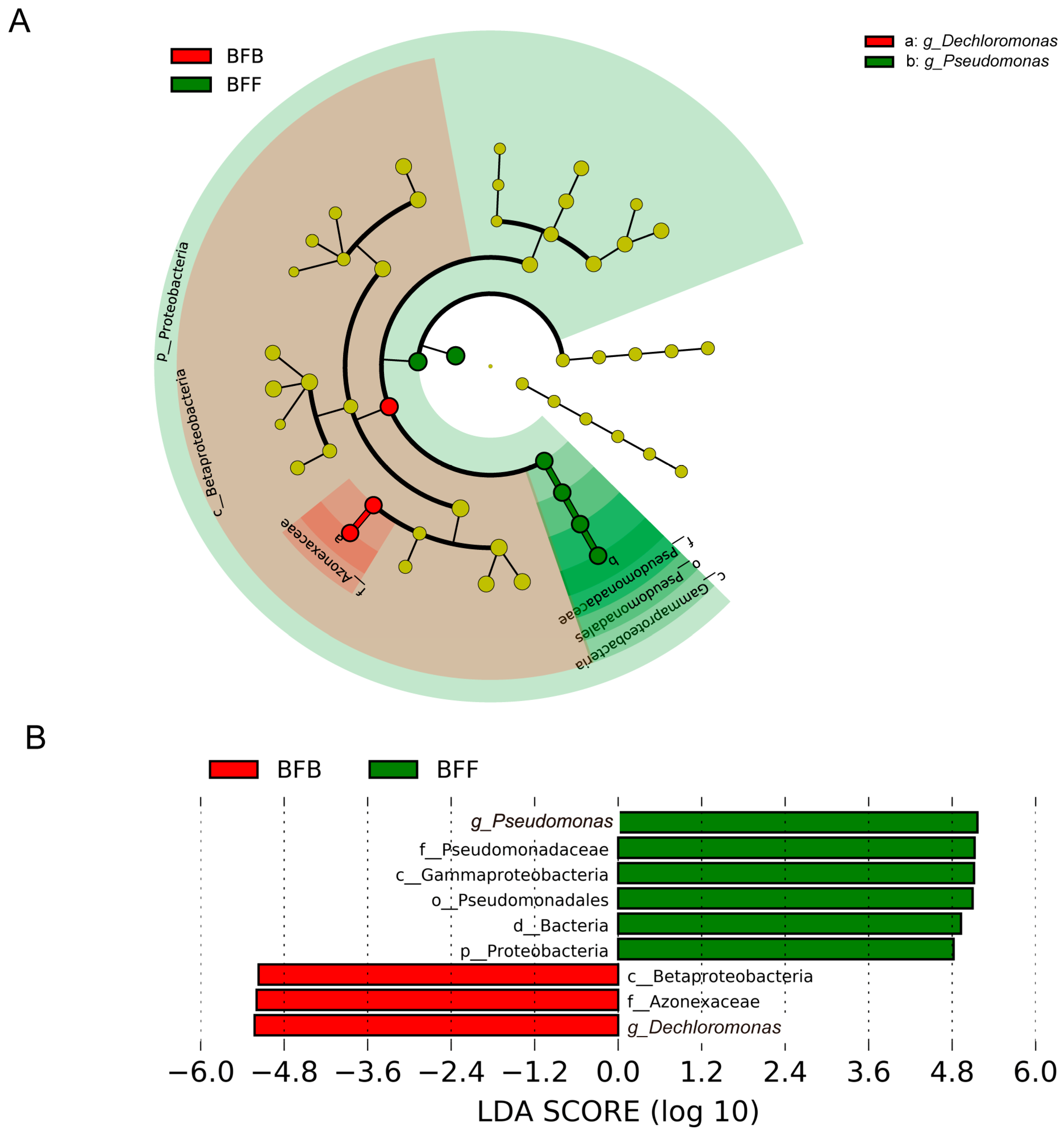
3.4. Functional Biomarkers in Different Positions of the Biofilter
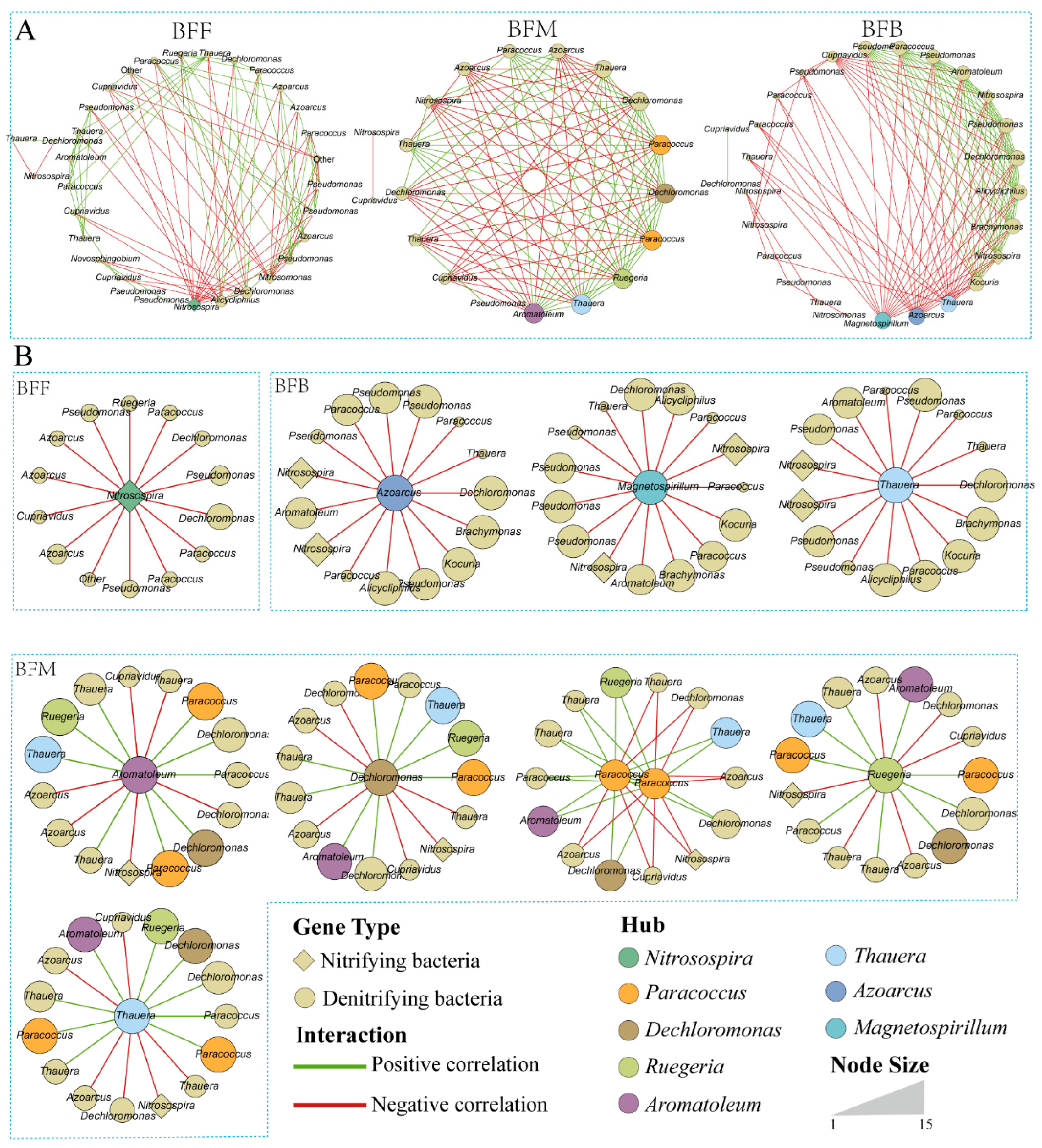
3.5. Microbial Network Analysis in Different Positions of the Biofilter
3.6. Effect of Environmental Conditions on Distribution Pattern of the Functional Microbiome
4. Discussion
4.1. Diversity of Nitrifying and Denitrifying Microbiome in RAS
4.2. Network Characteristics of the Different Functional Microbiomes
4.3. Environmental Factors Effect on the Distribution Pattern of Functional Microbiome in the Biofilter
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Widiasa, I.N.; Susanto, H.; Ting, Y.P.; Suantika, G.; Steven, S.; Khoiruddin, K.; Wenten, I.G. Membrane-based recirculating aquaculture system: Opportunities and challenges in shrimp farming. Aquaculture 2024, 579, 740224. [Google Scholar] [CrossRef]
- Rud, I.; Kolarevic, J.; Holan, A.B.; Berget, I.; Calabrese, S.; Terjesen, B.F. Deep-sequencing of the bacterial microbiota in commercial-scale recirculating and semi-closed aquaculture systems for Atlantic salmon post-smolt production. Aquac. Eng. 2017, 78, 50–62. [Google Scholar] [CrossRef]
- Gao, X.Y.; Xu, Y.; Liu, Y.; Liu, Y.; Liu, Z.P. Bacterial diversity, community structure and function associated with biofilm development in a biological aerated filter in a recirculating marine aquaculture system. Mar. Biodivers. 2012, 42, 1–11. [Google Scholar] [CrossRef]
- Ren, W.J.; Li, L.; Dong, S.L.; Tian, X.L.; Xue, Y.M. Effects of C/N ratio and light on ammonia nitrogen uptake in culture tanks. Aquaculture 2019, 498, 123–131. [Google Scholar] [CrossRef]
- Siripong, S.; Rittmann, B.E. Diversity study of nitrifying bacteria in full-scale municipal wastewater treatment plants. Water Res. 2007, 41, 1110–1120. [Google Scholar] [CrossRef]
- Blancheton, J.P.; Attramadal, K.J.K.; Michaud, L.; d’Orbcastel, E.R.; Vadstein, O. Insight into bacterial population in aquaculture systems and its implication. Aquac. Eng. 2013, 53, 30–39. [Google Scholar] [CrossRef]
- Gutierrez-Wing, M.T.; Malone, R.F.; Rusch, K.A. Evaluation of polyhydroxybutyrate as a carbon source for recirculating aquaculture water denitrification. Aquac. Eng. 2012, 51, 36–43. [Google Scholar] [CrossRef]
- Yao, R.J.; Li, H.Q.; Yang, J.S.; Wang, X.P.; Xie, W.P.; Zhang, X. Biochar addition inhibits nitrification by shifting community structure of ammonia-oxidizing microorganisms in salt-affected irrigation-silting soil. Microorganisms 2022, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Remmas, N.; Melidis, P.; Katsioupi, E.; Ntougias, S. Effects of high organic load on and gene diversity of an intermittently aerated and fed membrane bioreactor treating landfill leachate. Bioresour. Technol. 2016, 220, 557–565. [Google Scholar] [CrossRef]
- Goberna, M.; Donat, S.; Pérez-Valera, E.; Hallin, S.; Verdú, M. Gene-based co-occurrence patterns reveal assembly mechanisms of soil denitrifiers in response to fire. Environ. Microbiol. 2021, 23, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L. Denitrifying genes in bacterial and archaeal genomes. Biochim. Biophys. Acta 2002, 1577, 355–376. [Google Scholar] [CrossRef]
- Li, P.P.; Zhang, S.Q.; Li, F.; Zhang, Y.T.; Han, Y.L. Long term combined fertilization and soil aggregate size on the denitrification and community of denitrifiers. Appl. Soil Ecol. 2020, 156, 103718. [Google Scholar] [CrossRef]
- Yu, Z.H.; Liu, J.J.; Li, Y.S.; Jin, J.; Liu, X.B.; Wang, G.H. Impact of land use, fertilization and seasonal variation on the abundance and diversity of nirS-type denitrifying bacterial communities in a Mollisol in Northeast China. Eur. J. Soil Biol. 2018, 85, 4–11. [Google Scholar] [CrossRef]
- Sun, H.M.; Yang, Z.C.; Wei, C.J.; Wu, W.Z. Nitrogen removal performance and functional genes distribution patterns in solid-phase denitrification sub-surface constructed wetland with micro aeration. Bioresour. Technol. 2018, 263, 223–231. [Google Scholar] [CrossRef]
- Lu, Z.B.; Lin, W.C.; Li, Q.; Wu, Q.Y.; Ren, Z.M.; Mu, C.K.; Wang, C.L.; Shi, C.; Ye, Y.F. Recirculating aquaculture system as microbial community and water quality management strategy in the larviculture of Scylla paramamosain. Water Res. 2024, 252, 121218. [Google Scholar] [CrossRef] [PubMed]
- Saengsuk, N.; Boonanuntanasarn, S.; Boonchuen, P.; Phonsiri, K.; Kingwascharapong, P.; Petsong, K.; Pongsetkul, J. Microbiota differences of giant river prawn (Macrobrachium rosenbergii) cultured in a recirculating aquaculture system (RAS)—A prototype vertical farming and traditional pond cultured system and their impact on autolysis rate and textural characteristics. Aquaculture 2024, 588, 740959. [Google Scholar] [CrossRef]
- Luo, G.Z.; Li, L.; Liu, Q.; Xu, G.M.; Tan, H.X. Effect of dissolved oxygen on heterotrophic denitrification using poly(butylene succinate) as the carbon source and biofilm carrier. Bioresour. Technol. 2014, 171, 152–158. [Google Scholar] [CrossRef]
- Yu, M.J.; Meng, J.; Yu, L.; Su, W.Q.; Afzal, M.; Li, Y.; Brookes, P.C.; Redmile-Gordon, M.; Luo, Y.; Xu, J.M. Changes in nitrogen related functional genes along soil pH, C and nutrient gradients in the charosphere. Sci. Total Environ. 2019, 650, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Wei, Y.; An, D.; Li, D.; Ta, X.; Wu, Y.; Ren, Q. A review on the research status and development trend of equipment in water treatment processes of recirculating aquaculture systems. Rev. Aquac. 2018, 11, 1–33. [Google Scholar] [CrossRef]
- Bakke, I.; Åm, A.L.; Kolarevic, J.; Ytrestoyl, T.; Vadstein, O.; Attramadal, K.J.K.; Terjesen, B.F. Microbial community dynamics in semi-commercial RAS for production of Atlantic salmon post-smolts at different salinities. Aquac. Eng. 2017, 78, 42–49. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Guo, H.; Kong, X.; Lan, L.; Xu, H.; Zhu, S.; Ye, Z. Start-up evaluations and biocarriers transfer from a trickling filter to a moving bed bioreactor for synthetic mariculture wastewater treatment. Chemosphere 2019, 218, 696–704. [Google Scholar] [CrossRef]
- Jiang, W.; Tian, X.; Li, L.; Dong, S.; Zhao, K.; Li, H.; Cai, Y. Temporal bacterial community succession during the start-up process of biofilters in a cold-freshwater recirculating aquaculture system. Bioresour. Technol. 2019, 287, 121441. [Google Scholar] [CrossRef]
- Lei, Y. Waters Environmental Chemistry for Aquaculture Experiment; Agriculture Press: Beijing, China, 2006. [Google Scholar]
- Zhang, K.; Tian, X.L.; Dong, S.L.; Feng, J.; He, R.P. An experimental study on the budget of organic carbon in polyculture systems of swimming crab with white shrimp and short-necked clam. Aquaculture 2016, 451, 58–64. [Google Scholar] [CrossRef]
- Bai, Y.H.; Sun, Q.H.; Wen, D.H.; Tang, X.Y. Abundance of ammonia-oxidizing bacteria and archaea in industrial and domestic wastewater treatment systems. FEMS Microbiol. Ecol. 2012, 80, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Qu, Y.; Shen, W.; Zhang, Z.; Wang, J.; Liu, Z.; Li, D.; Li, H.; Zhou, J. Bacterial community compositions of coking wastewater treatment plants in steel industry revealed by Illumina high-throughput sequencing. Bioresour. Technol. 2015, 179, 436–443. [Google Scholar] [CrossRef]
- Huang, Z.T.; Wan, R.; Song, X.F.; Liu, Y.; Hallerman, E.; Dong, D.P.; Zhai, J.M.; Zhang, H.S.; Sun, L.Y. Metagenomic analysis shows diverse, distinct bacterial communities in biofilters among different marine recirculating aquaculture systems. Aquac. Int. 2016, 24, 1393–1408. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.X.; Lu, X.; Liu, B.; Li, Y.; Long, C.; Li, A.M. Abundance and diversity of bacterial nitrifiers and denitrifiers and their functional genes in Tannery wastewater treatment plants revealed by high-throughput sequencing. PLoS ONE 2014, 9, e113603. [Google Scholar] [CrossRef]
- Winkler, M.K.H.; Kleerebezem, R.; de Bruin, L.M.M.; Verheijen, P.J.T.; Abbas, B.; Habermacher, J.; van Loosdrecht, M.C.M. Microbial diversity differences within aerobic granular sludge and activated sludge flocs. Appl. Microbiol. Biot. 2013, 97, 7447–7458. [Google Scholar] [CrossRef]
- Koops, H.-P.; Pommerening-Röser, A. Distribution and ecophysiology of the nitrifying bacteria emphasizing cultured species. FEMS Microbiol. Ecol. 2001, 37, 1–9. [Google Scholar] [CrossRef]
- Schreier, H.J.; Mirzoyan, N.; Saito, K. Microbial diversity of biological filters in recirculating aquaculture systems. Curr. Opin. Biotechnol. 2010, 21, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Sagastume, F.; Nielsen, J.L.; Nielsen, P.H. Substrate-dependent denitrification of abundant probe-defined denitrifying bacteria in activated sludge. FEMS Microbiol. Ecol. 2008, 66, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Sun, B.; Li, Q.; Deng, X.Y.; Hu, J.; Wu, W.X. Pilot-scale nitrogen removal from leachate by nitrification and denitrification in a landfill bioreactor. Chemosphere 2014, 101, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.J.; Wang, C.; Sun, F.Q.; Zhao, L.C.; Dou, W.J.; Mao, Z.H.; Wu, W.X. Overall bacterial community composition and abundance of nitrifiers and denitrifiers in a typical macrotidal estuary. Mar. Pollut. Bull. 2018, 126, 540–548. [Google Scholar] [CrossRef]
- Xu, W.W.; Wang, L.M.; Peng, F.Q.; Zhang, A.G.; Xie, X.G.; Wang, Z.B.; Wang, X.; Lian, J.J.; Ni, L.X.; Cui, Y.B.; et al. Spatiotemporal distribution and interaction of denitrifying functional genes in a novel DAS-NUA biofilter used for groundwater nitrate treatment. Sci. Total Environ. 2020, 712, 136595. [Google Scholar] [CrossRef]
- Liu, T.; Li, L.; Jiang, W.; Cai, Y.; Dong, S. Performance evaluation of a six-stage bio-filter in recirculating aquaculture system. Chin. J. Environ. Eng. 2019, 13, 902–909. [Google Scholar]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, P.; Qin, Y.J.; Tu, Q.C.; Yang, Y.F.; He, Z.L.; Schadt, C.W.; Zhou, J.Z. Network succession reveals the importance of competition in response to emulsified vegetable oil amendment for uranium bioremediation. Environ. Microbiol. 2016, 18, 205–218. [Google Scholar] [CrossRef]
- Yachi, S.; Loreau, M. Biodiversity and ecosystem productivity in a fluctuating environment: The insurance hypothesis. Proc. Natl. Acad. Sci. USA 1999, 96, 1463–1468. [Google Scholar] [CrossRef]
- Jiang, W.W.; Ren, W.J.; Li, L.; Dong, S.L.; Tian, X.L. Light and carbon sources addition alter microbial community in biofloc-based culture systems. Aquaculture 2020, 515, 734572. [Google Scholar] [CrossRef]
- Li, B.X.; Chen, J.F.; Wu, Z.; Wu, S.F.; Xie, S.G.; Liu, Y. Seasonal and spatial dynamics of denitrification rate and denitrifier community in constructed wetland treating polluted river water. Int. Biodeter. Biodegr. 2018, 126, 143–151. [Google Scholar] [CrossRef]
- Liu, H.Y.; Li, J.; Zhao, Y.; Xie, K.X.; Tang, X.J.; Wang, S.X.; Li, Z.P.; Liao, Y.L.; Xu, J.M.; Di, H.J.; et al. Ammonia oxidizers and nitrite-oxidizing bacteria respond differently to long-term manure application in four paddy soils of south of China. Sci. Total Environ. 2018, 633, 641–648. [Google Scholar] [CrossRef] [PubMed]
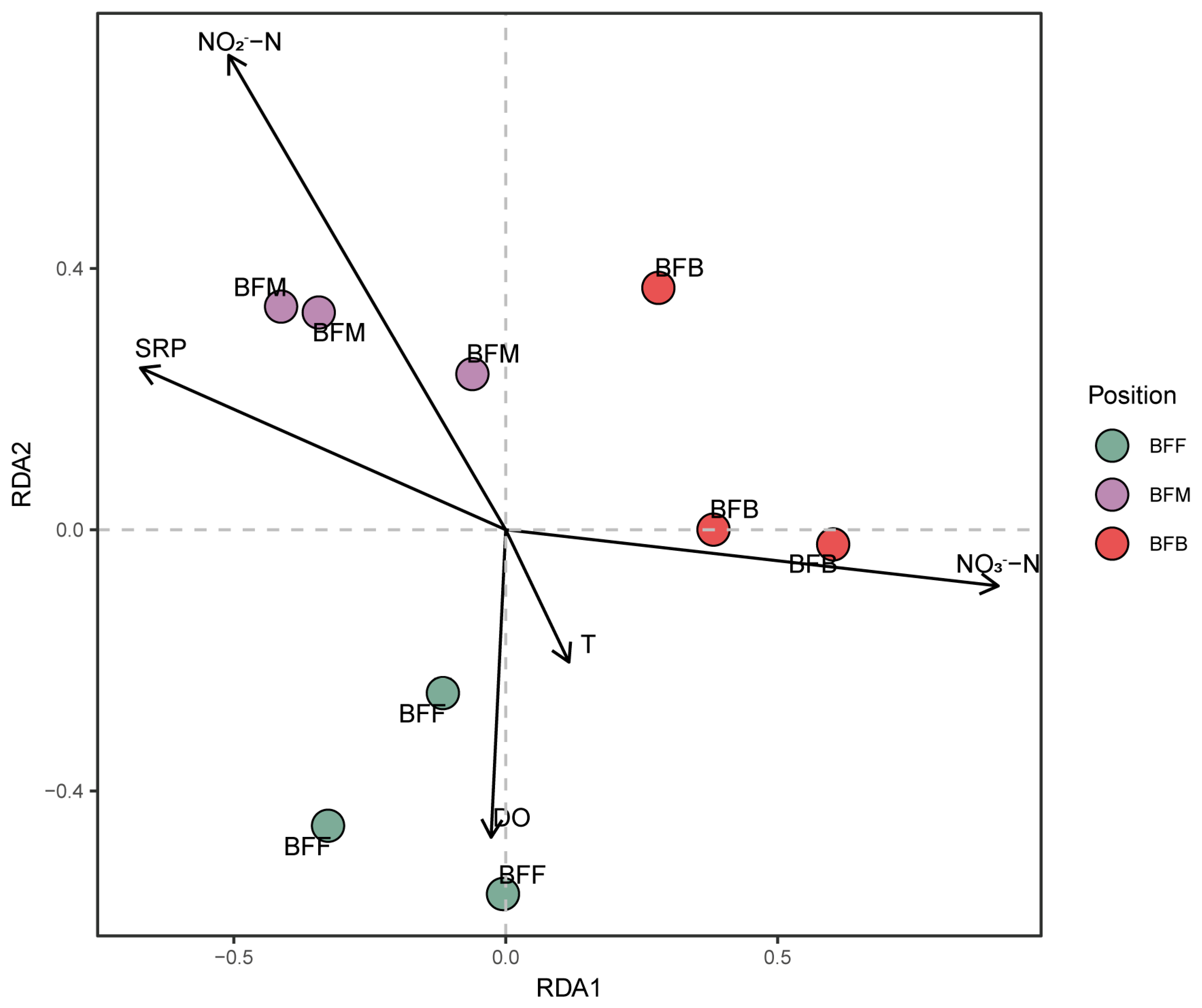
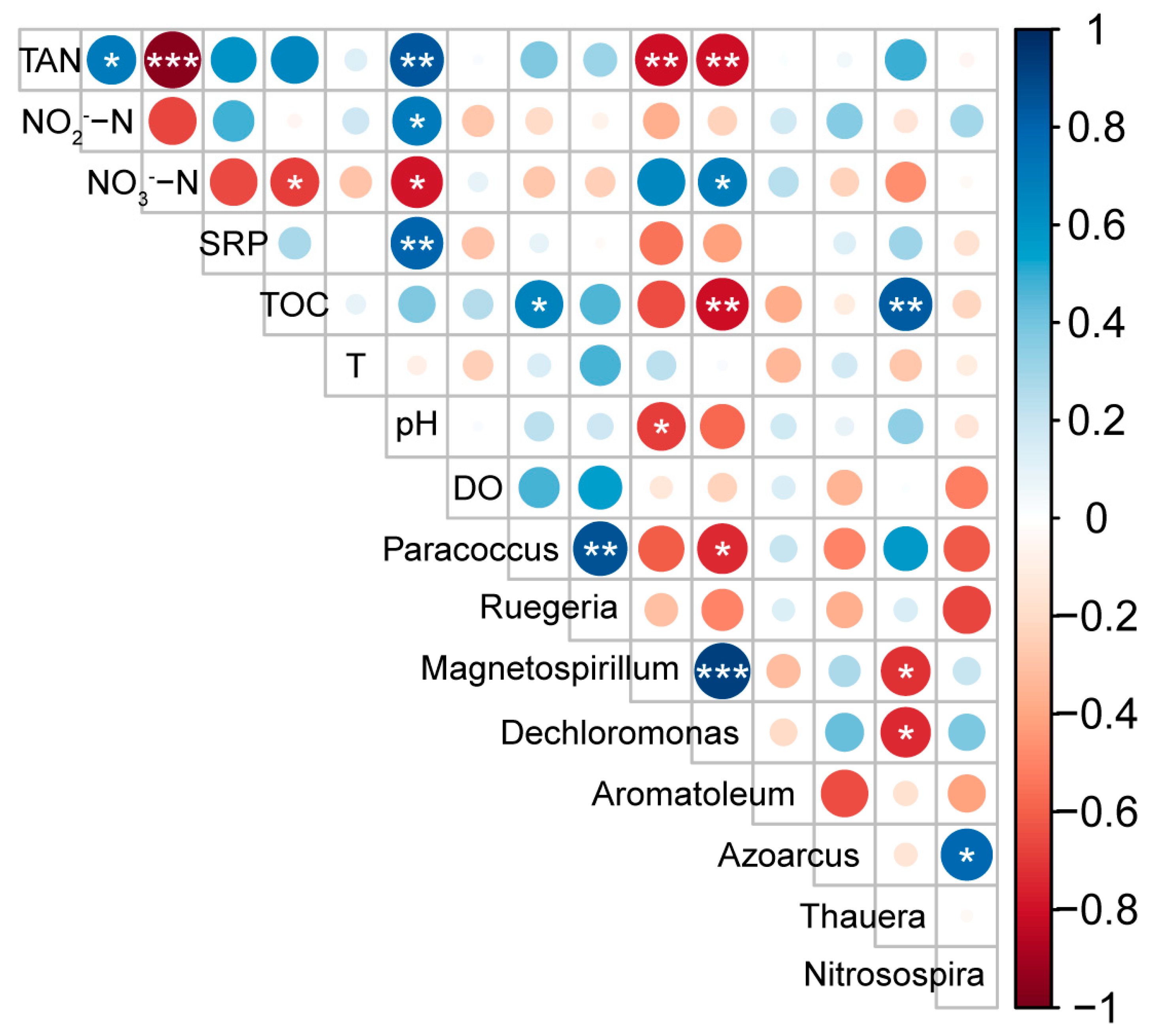
| Parameters | T/℃ | pH | DO (mg·L−1) | TAN (mg·L−1) | NO2−-N (mg·L−1) | NO3−-N (mg·L−1) | SRP (mg·L−1) | TOC (mg·L−1) |
|---|---|---|---|---|---|---|---|---|
| BFF | 17.50 ± 0.10 a | 7.35 ± 0.00 ab | 7.77 ± 0.64 a | 0.16 ± 0.00 b | 0.11 ± 0.00 b | 7.69 ± 0.15 b | 0.08 ± 0.00 a | 34.54 ± 0.07 a |
| BFM | 17.53 ± 0.12 a | 7.40 ± 0.00 a | 7.38 ± 0.55 a | 0.19 ± 0.00 a | 0.21 ± 0.00 a | 6.43 ± 0.60 c | 0.09 ± 0.00 a | 24.71 ± 2.17 b |
| BFB | 17.47 ± 0.15 a | 7.31 ± 0.06 b | 7.48 ± 0.17 a | 0.11 ± 0.01 c | 0.12 ± 0.01 b | 10.57 ± 0.37 a | 0.08 ± 0.00 a | 13.74 ± 0.51 c |
| Functional Microbiome | Sample | OTUs | Chao1 | Shannon | Simpson | Coverage |
|---|---|---|---|---|---|---|
| Nitrifying | BFF | 20 a | 21 a | 0.99 a | 0.60 a | 0.99 |
| BFM | 22 a | 22 a | 1.09 a | 0.56 a | 0.99 | |
| BFB | 22 a | 25 a | 1.06 a | 0.57 a | 0.99 | |
| Denitrifying | BFF | 189 a | 193 a | 3.50 b | 0.07 a | 0.99 |
| BFM | 94 a | 96 a | 3.28 c | 0.08 a | 0.99 | |
| BFB | 142 a | 144 a | 4.23 a | 0.03 b | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Liu, T.; Li, S.; Li, L.; Xu, K.; Wang, G.; Guo, E. The Different Spatial Distribution Patterns of Nitrifying and Denitrifying Microbiome in the Biofilters of the Recirculating Aquaculture System. Microorganisms 2025, 13, 1833. https://doi.org/10.3390/microorganisms13081833
Jiang W, Liu T, Li S, Li L, Xu K, Wang G, Guo E. The Different Spatial Distribution Patterns of Nitrifying and Denitrifying Microbiome in the Biofilters of the Recirculating Aquaculture System. Microorganisms. 2025; 13(8):1833. https://doi.org/10.3390/microorganisms13081833
Chicago/Turabian StyleJiang, Wenwen, Tingting Liu, Shuting Li, Li Li, Kefeng Xu, Guodong Wang, and Enmian Guo. 2025. "The Different Spatial Distribution Patterns of Nitrifying and Denitrifying Microbiome in the Biofilters of the Recirculating Aquaculture System" Microorganisms 13, no. 8: 1833. https://doi.org/10.3390/microorganisms13081833
APA StyleJiang, W., Liu, T., Li, S., Li, L., Xu, K., Wang, G., & Guo, E. (2025). The Different Spatial Distribution Patterns of Nitrifying and Denitrifying Microbiome in the Biofilters of the Recirculating Aquaculture System. Microorganisms, 13(8), 1833. https://doi.org/10.3390/microorganisms13081833







