Abstract
In response to the growing demand for clean-label preservatives, this study investigates the potential of Laetiporus sulphureus, an edible polypore mushroom, as a multifunctional additive in cooked sausages. The ethanolic extract of L. sulphureus (LsEtOH) was evaluated for its chemical composition, antioxidant capacity, and antimicrobial activity. Leucine (12.4 ± 0.31 mg/g d.w.) and linoleic acid (68.6%) were identified as the dominant essential amino acid and fatty acid. LsEtOH exhibited strong antioxidant activity, with IC50 values of 215 ± 0.05 µg/mL (DPPH•), 182 ± 0.40 µg/mL (NO•), and 11.4 ± 0.01 µg/mL (OH•), and showed a selective inhibition of Gram-positive bacteria, particularly Staphylococcus aureus (MIC/MBC: 0.31/0.62 mg/mL). In cooked sausages treated with 0.05 mg/kg of LsEtOH, lipid peroxidation was reduced (TBARS: 0.26 mg MDA/kg compared to 0.36 mg MDA/kg in the control), microbial growth was suppressed (33.3 ± 15.2 CFU/g in the treated sample compared to 43.3 ± 5.7 CFU/g in the control group), and color and pH were stabilized over 30 days. A sensory evaluation revealed minor flavor deviations due to the extract’s inherent aroma. Encapsulation and consumer education are recommended to enhance acceptance. This is the first study to demonstrate the efficacy of L. sulphureus extract as a natural preservative in a meat matrix, supporting its application as a clean-label additive for shelf life and safety improvement.
1. Introduction
The growing consumer demand for healthier food products, combined with a heightened awareness of the potential risks associated with synthetic additives, has intensified the search for natural alternatives capable of preserving food quality and safety. Within this context, the concept of “clean label” has gained prominence, promoting the use of natural, functional, and sensory-acceptable ingredients [1]. The global clean-label ingredient market is projected to exceed USD 60 billion by 2027, reflecting a strong consumer preference for minimally processed and transparent food formulations [2].
Edible mushrooms, beyond their nutritional benefits, have emerged as promising yet underutilized sources of bioactive compounds with potent antioxidant and antimicrobial properties. They are appreciated for their high-quality proteins, balanced amino acid profiles, low fat content, and richness in dietary fiber and essential micronutrients. Moreover, they synthesize a broad range of primary and secondary metabolites—including polysaccharides, phenolic compounds, sterols, flavonoids, and terpenes—that contribute to their health-promoting potential [3,4,5].
Several studies have confirmed that mushroom-derived extracts can effectively improve the oxidative and microbiological stability of meat products, supporting their use as clean-label ingredients in food preservation [6,7,8]. This has further fueled interest in natural antimicrobials as alternatives to synthetic preservatives such as nitrites, particularly in minimally processed meat systems.
Among these species, Laetiporus sulphureus (commonly known as “chicken of the woods”) has attracted attention due to its distinctive flavor profile and its richness in phenolic compounds and fatty acids [9,10]. Although numerous studies have demonstrated the in vitro antioxidant and antimicrobial properties of its bioactive constituents, their efficacy in real food matrices, especially in meat products, remains underexplored [10]. Moreover, limited research has assessed the functional performance of mushroom extracts under realistic processing and storage conditions [11,12].
This study aimed to evaluate the application of an ethanolic extract of L. sulphureus (LsEtOH) as a natural preservative in cooked sausages. The extract was assessed for its ability to inhibit lipid oxidation, reduce microbial proliferation, and maintain key physicochemical and sensory attributes during 30 days of refrigerated storage. A fixed concentration was selected based on preliminary trials, and sensory testing was conducted to assess acceptability. To our knowledge, this is one of the first studies to investigate the multifunctional potential of L. sulphureus extract as a clean-label additive in a real meat system, combining chemical characterization with antioxidant, antimicrobial, and sensory evaluation. These findings may inform the development of sustainable preservation strategies and support the integration of wild mushroom extracts in functional meat products.
2. Materials and Methods
2.1. Chemicals and Reagents
Reference standards for phenolic compounds were obtained from Sigma-Aldrich (Steinheim, Germany), Fluka Chemie GmbH (Buchs, Switzerland), and Chromadex (Santa Ana, CA, USA). Methanol (HPLC grade) was purchased from J.T. Baker (Deventer, The Netherlands), and formic acid (p.a. grade) from Merck (Darmstadt, Germany). A standard FAME mixture (Supelco 37) was supplied by Sigma-Aldrich (Steinheim, Germany). Deionized water was produced using a Millipore purification system (Millipore, Darmstadt, Germany). All other solvents and reagents were of analytical grade and obtained from standard commercial suppliers.
2.2. Mushroom Collection and Sample Preparation
Wild fruiting bodies of the autochthonous Laetiporus sulphureus (Phylum Basidiomycota, Class Agaricomycetes, Order Polyporales, Family Laetiporaceae, Genus Laetiporus) were collected in the autumn season from Sikola, eastern Serbia. Species identification was confirmed by an experienced mycologist, and a voucher specimen (No. 12-00663) was deposited at the ProFungi Laboratory, Department of Biology and Ecology, Faculty of Sciences, University of Novi Sad, and the BUNS Herbarium. The harvested material was initially stored at −20 °C, then freeze-dried to a constant weight using a laboratory lyophilizer (Christ Alpha 1–2 LD; Martin Christ GmbH, Osterode am Harz, Germany). The dried biomass was ground to a fine powder, sealed in polyethylene bags, and kept at −20 °C until further analysis 10.
2.3. Ethanolic Extraction Procedure
A total of 10 grams of lyophilized L. sulphureus powder were extracted with 100 mL of 96% ethanol (EtOH) at room temperature (23 ± 1 °C) for 24 h on a rotary shaker (Thermo Fisher Scientific, Waltham, MA, USA) set at 120 rpm. The extraction conditions (solvent, time, and temperature) were selected based on previously published protocols [4], in order to ensure method reproducibility and to preserve thermolabile bioactive compounds. While further optimization may enhance compound recovery, the selected protocol offered a balanced compromise between efficiency and compound stability. The mixture was filtered through Whatman No. 4 filter paper (Whatman, GE Healthcare, Maidstone, UK), and the solvent was removed by rotary evaporation at 40 °C (Büchi, Flawil, Switzerland). The resulting dry extract was labeled as LsEtOH and stored at −20 °C until further analysis. All measurements were performed in triplicate.
2.4. Crude Protein Determination
Total protein content was determined by the macro-Kjeldahl method according to AOAC procedures (16th edition), using a nitrogen-to-protein conversion factor of 4.38 [13].
2.5. Protein Profiling
Protein profiling was conducted using a modified method based on Tidona et al. [14]. Briefly, approximately 15 mg of lyophilized L. sulphureus powder was suspended in 100 µL of extraction buffer (0.125 M Tris-HCl, 4% SDS, 2% glycerol, 2% β-mercaptoethanol; pH 6.8) and incubated at 100 °C for 5 min. Protein separation and analysis were performed with a chip-based Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) using the Protein 80 Plus Lab Chip kit (Agilent Technologies, Santa Clara, CA, USA), according to the manufacturer’s instructions. Bovine serum albumin (BSA) served as the molecular weight standard, and protein quantification was based on size distribution profiles [15]. All measurements were performed in triplicate.
2.6. Amino Acid Composition
Amino acid profiling was carried out after acid hydrolysis of the mushroom samples, following an established protocol [16]. The analysis was performed using high-performance liquid chromatography (HPLC) on an Agilent 1200 Series instrument (Agilent Technologies, Santa Clara, CA, USA) equipped with a fluorescence detector (excitation at 340 nm and emission at 450 nm) and an Agilent Eclipse Plus C18 column (5.0 μm, 3.0 × 250 mm). Concentrations were calculated using calibration curves derived from standard amino acid solutions (Sigma-Aldrich, Steinheim, Germany), and the final results are expressed in mg per g dry weight (mg/g d.w.). All measurements were performed in triplicate.
Concentrations were calculated using calibration curves derived from standard amino acid solutions, and the results are expressed in mg per g dry weight (mg/g d.w.).
2.7. Fatty Acid Analysis
Total lipid content was extracted using the Folch method [17], followed by transesterification with 14% boron trifluoride (BF3) in methanol [18]. The resulting fatty acid methyl esters (FAMEs) were analyzed using an Agilent 7890A gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with a flame ionization detector (FID) and SP-2560 capillary column (100 m × 0.25 mm × 0.20 µm, Supelco, Sigma-Aldrich, Steinheim, Germany). Fatty acids were identified by comparison with a standard mixture of 37 FAMEs and quantified as percentages of total identified fatty acids.
2.8. Mineral Element Determination
Macroelements (K, Ca, Mg) and microelements (Fe, Zn, Mn, Cu) were quantified using flame atomic absorption spectrometry (AAS) following dry-ash digestion of the samples, as described in a previously validated method for mushroom matrices [16]. Mineral concentrations were expressed as mg/g dry weight (d.w.) for macroelements and mg/kg d.w. for microelements. All measurements were performed in triplicate.
2.9. Phenolic Profile Analysis
The qualitative and quantitative compositions of phenolic compounds in the ethanolic extract were analyzed using liquid chromatography–tandem mass spectrometry (LC–MS/MS), following the method described by Orčić et al. [19]. Compound identification was based on retention times and fragmentation patterns compared with authenticated standards. The results are expressed as micrograms per gram of dry extract (µg/g d.w.).
2.10. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
Total phenolic content (TPC) was determined using the Folin–Ciocalteu reagent, following the method of Singleton et al. [20], adapted to a 96-well microplate format. Absorbance was measured at 765 nm using a Multiskan Ascent microplate reader (Thermo Electron Corporation, Waltham, MA, USA). Gallic acid was used to generate the standard calibration curve, and the results are expressed as milligrams of gallic acid equivalents (mg GAE) per 100 g of extract.
Total flavonoid content (TFC) was determined according to the aluminum chloride colorimetric method modified for microplate use [21]. Absorbance was read at 415 nm. Quercetin was used as the calibration standard, and the results are expressed as mg quercetin equivalents (QE) per 100 g of extract. All measurements were performed in triplicate
2.11. Antioxidant Activity Assays
The antioxidant capacity of the extract was assessed using three in vitro assays, DPPH•, NO•, and OH• radical scavenging activities, following the protocols described by Espin et al. [22], Green et al. [23], and Cheeseman et al. [24], respectively. Absorbance of the reaction mixtures was measured with a microplate reader. The radical scavenging capacity (RSC) was calculated using the following formula:
RSC (DPPH, NO, OH) (%) = (1 − A sample/A control) × 100%
The IC50 values (µg/mL), defined as the concentration required to inhibit 50% of radical activity, were calculated from dose–response curves. For this purpose, a series of LsEtOH concentrations ranging from 1.15 to 800 µg/mL was tested in all antioxidant assays (DPPH•, NO•, and OH•). Absorbance was measured spectrophotometrically, and solvent controls (5% DMSO without extract) were included to correct for background absorbance. The dried ethanolic extract (LsEtOH) was redissolved in 5% dimethyl sulfoxide (DMSO; Fluka Chimie) prior to analysis. All measurements were performed in triplicate.
2.12. Antibacterial Activity
Antibacterial effects were evaluated against five ATCC reference strains: Staphylococcus aureus ATCC 25923, Bacillus subtilis ATCC 6633, Enterococcus faecalis ATCC 19433, Escherichia coli ATCC 11229, and Salmonella enteritidis ATCC 13076. The broth microdilution method in 96-well plates was used to determine minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC), following Karaman et al. [25]. The tested concentrations of LsEtOH ranged from 0.15 to 10 mg/mL. MIC was defined as the lowest concentration at which no visible bacterial growth was observed, confirmed by staining with TTC solution (2,3,5-triphenyltetrazolium chloride), which enhances the visualization of bacterial growth. MBC was determined by subculturing the contents of wells without visible growth onto nutrient agar. The extract was diluted in 5% DMSO; ampicillin and gentamicin served as positive controls.
2.13. Sausage Formulation and Processing
Cooked sausages were produced in a certified small-scale local meat processing facility using a standardized formulation consisting of chicken breast (25.0%), pork shoulder (16.7%), mechanically deboned chicken meat (16.7%), pork fat (16.7%), ice (16.7%), maize starch (3.3%), textured soy protein (2.2%), curing salt containing sodium nitrite (1.8%), spice blend (0.6%), polyphosphates (0.3%), and dextrose (0.1%). Due to food safety requirements and facility protocols, all sausage formulations included a minimal dose of curing salt (1.8%) to simulate realistic production conditions. All ingredients were homogenized using a bowl cutter (Taifun 200, Nowicki, Skwierzyna, Poland). The treatment batch was supplemented with L. sulphureus ethanolic extract (LsEtOH) at a final concentration of 0.05 mg/kg, while the control batch was prepared without the addition of the extract.
Both sausage batches were stuffed into 40 mm diameter polyamide casings and thermally processed in steam until a core temperature of 72 °C was achieved. Pasteurization was followed by rapid cooling using a water/air system to 25 °C. All samples were vacuum-packed and stored at 4 °C for 30 days. Three independent production batches were prepared for each treatment group.
2.14. pH and Color Measurement
The pH of the sausages was measured using a portable penetration-type pH meter (Testo SE & Co. KGaA, Lenzkirch, Germany). Measurements were performed in duplicate on three randomly selected sausages from each batch.
Color parameters—lightness (L), redness (a), and yellowness (b*)—were assessed on freshly cut surfaces using a CR-400 Chroma Meter (Konica Minolta, Tokyo, Japan) under D65 illumination. The instrument was calibrated with a standard white plate prior to measurement. Each value represents the mean of 15 readings obtained from three sausages per batch [26,27,28].
2.15. Lipid Oxidation Assay (TBARS)
Lipid oxidation was evaluated using the thiobarbituric acid reactive substances (TBARS) assay, following the method of Botsoglou et al. [29] with minor modifications [30]. The absorbance of the pink MDA–TBA complex was measured at 532 nm using a Jenway 6300 spectrophotometer (Cole-Parmer Ltd., Stone, Staffordshire, UK). The results are expressed as milligrams of malondialdehyde per kilogram of sausage sample (mg MDA/kg). Analyses were conducted in duplicate on three sausages per treatment group.
2.16. Microbiological Evaluation
Microbial load was evaluated by quantifying total aerobic mesophilic bacteria (AMB), Escherichia coli, Clostridium spp., Enterobacteriaceae, and yeasts/molds, following the method described by Šojić et al. [31]. The results are expressed as colony-forming units per gram (CFU/g). For samples marked as “not detected” (Nd), no colony growth was observed on the corresponding plates. The detection limit of the applied plating method was 10 CFU/g, corresponding to the lowest quantifiable count under the experimental conditions. Microbial counts were expressed as colony-forming units per gram (cfu/g). Analyses were conducted on three samples per batch on day 1 and day 30 of refrigerated storage.
2.17. Sensory Evaluation
Sensory attributes, specifically color and taste, were evaluated by a trained panel of seven assessors in accordance with international ISO standards [32,33,34]. Panelists were selected and trained following the guidelines of ISO 8586:2012, which define general principles for the selection, training, and monitoring of sensory assessors.
The training program included:
- Familiarization with key sensory characteristics relevant to cooked sausages (color and taste);
- Use of standardized terminology in accordance with ISO 5492:2008 to ensure consistency in the description and interpretation of sensory attributes;
- Application of structured quantitative sensory scales based on ISO 4121:2003, including calibration with reference samples to ensure reliable and repeatable scoring.
All evaluations were conducted in individual sensory booths under standardized environmental conditions [35]. Data were recorded using pre-designed scoring sheets. A six-point hedonic scale was used to assess overall sensory quality, where:
5 = excellent/typical, 4 = minor deviation, 3 = moderate defect, 2 = distinct defect, 1 = unacceptable, and 0 = spoilage/contamination.
Sensory evaluations were performed on days 1 and 30 of refrigerated storage in a controlled sensory analysis laboratory. The evaluation procedure, including the timing of assessments and sample handling, was based on previously published studies that assessed the effects of natural additives on the oxidative, microbial, and sensory stability of cooked sausages during chilled storage [31].
2.18. Statistical Analysis
All data are expressed as mean ± standard deviation (SD). Differences between control and treatment groups were analyzed using one-way ANOVA, followed by Duncan’s post hoc test for multiple comparisons. Statistical significance was set at p < 0.05. Analyses were performed using STATISTICA software (version 12.0, StatSoft, Inc., Tulsa, OK, USA).
3. Results and Discussion
3.1. Chemical Composition of L. sulphureus Extract
Mushroom-derived proteins are known for their favorable amino acid profiles, often containing all essential amino acids (EAAs) required for human nutrition [3,36] In our study, the protein content of L. sulphureus was determined to be 14.5%, with electrophoretic analysis identifying 24 distinct fractions (6.4–59.7 kDa), including a prominent band at 51.8 kDa (Figure 1). The amino acids most commonly detected in fungi include lysine, methionine, tryptophan, threonine, valine, leucine, isoleucine, histidine, and phenylalanine [3]. The amino acid composition of L. sulphureus determined in this study (Table 1) revealed particularly high concentrations of non-essential amino acids, such as cysteine (26.8 ± 0.20 mg/g d.w.), glutamic acid (26.2 ± 0.06 mg/g d.w.), arginine (23.3 ± 0.16 mg/g d.w.), and aspartic acid (16.1 ± 0.22 mg/g d.w.). Among the essential amino acids, leucine was the most abundant (12.4 ± 0.31 mg/g d.w.), followed by valine (9.80 ± 0.22 mg/g d.w.) and threonine (8.50 ± 0.17 mg/g d.w.). The total content of essential and non-essential amino acids was calculated as 53.98 mg/g and 131.51 mg/g d.w., respectively. These results are consistent with previous findings reported by Agafonova et al. for two L. sulphureus strains isolated from Siberia [37].
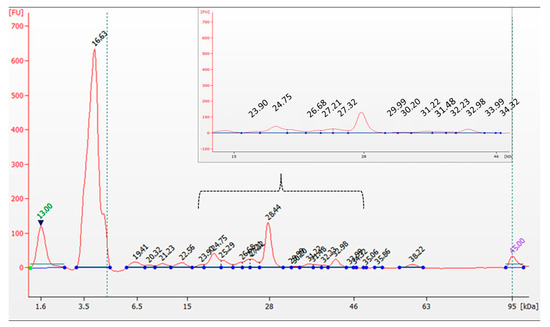
Figure 1.
Electropherogram of analyzed L. sulphureus.

Table 1.
Amino acid composition of L. sulphureus (mg/g d.w.).
Beyond their nutritional role, several amino acids abundant in mushrooms, such as arginine, cysteine, and glutamic acid, are involved in immunomodulation, antioxidative defense, and neuroprotection, further enhancing the functional value of mushroom-derived proteins [38,39]. The presence of bioactive peptides and lectin-like proteins within mushroom matrices may also contribute not only to nutritional quality but to health promoting properties relevant for functional food applications [40,41].
The fatty acid composition was assessed via gas chromatography-mass spectrometry (GC-MS), identifying 15 individual fatty acids (Table 2). While mushrooms are generally characterized by their low lipid content (2.96–4.50 g/100 g d.w.) [11], they are appreciated for their high proportion of polyunsaturated fatty acids (PUFAs), known for promoting cardiovascular health [42].

Table 2.
The fatty acid composition of L. sulphureus relative %.
In the sample studied, linoleic acid was predominant (68.6%), followed by oleic (11.0%) and palmitic acid (9.68%). PUFAs accounted for 70.54% of the total fatty acids, while saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs) constituted 16.83% and 12.62%, respectively. Compared to the previously reported data for L. sulphureus from Serbia [10], this profile suggests a favorable fatty acid composition, likely influenced by the local habitat environmental impacts.
LsEtOH exhibited a total phenolic content of 78.1 ± 0.40 mg GAE/100 g (d.w.) and a flavonoid content of 6.40 ± 0.10 mg QE/100 g d.w. LC–MS/MS analysis identified p-coumaric acid at 0.30 ± 0.01 µg/g d.w., suggesting its contribution to the extract’s antioxidant activity. This mycochemical profile underscores the species’ potential for developing nutraceuticals targeting oxidative stress management and metabolic health support [10].
Mineral analysis revealed potassium (K) as the predominant microelement (21.5 ± 0.30 mg/g d.w.), followed by magnesium (Mg) and calcium (Ca) (Table 3). Among microelements, zinc (Zn) was most abundant (58.3 ± 0.50 mg/kg d.w.), followed by copper (Cu), manganese (Mn), and iron (Fe). These findings align with previous reports that highlight potassium as the major microelement in mushrooms [43,44]. Elevated K and Zn levels may reflect adaptive mineral uptake related to environmental factors [3].

Table 3.
Mineral composition of L. sulphureus.
3.2. In Vitro Antioxidant and Antimicrobial Activities of L. sulphureus Extract
The ethanolic extract of L. sulphureus (LsEtOH) exhibited pronounced antioxidant activity, with IC50 values of 215 ± 0.05 µg/mL for DPPH•, 182 ± 0.40 µg/mL for NO•, and 11.4 ± 0.01 µg/mL for OH• radicals (Table 4). These results confirm the extract’s ability to effectively neutralize both oxygen and nitrogenderived reactive species.

Table 4.
Total phenolic/flavonoid content and IC50 (µg/mL) for radical scavenging DPPH•, NO• and •OH activity.
In comparison to previous studies, the LsEtOH extract demonstrated a stronger OH• scavenging capacity than the methanolic extract reported by Karaman et al., which achieved 57.06% inhibition at a concentration of 400 µg/mL [45]. Conversely, the ethanolic extract of Meripilus giganteus showed even greater efficacy, with an IC50 of 1.74 ± 0.2 µg/mL for OH• and an IC25 of 148.04 ± 4.98 µg/mL for NO• radicals [46]. The NO• scavenging effect of our LsEtOH extract (IC50 = 182 ± 0.40 µg/mL) was comparable, indicating its relevance in modulating nitrogen-based radical species.
Regarding the DPPH• assay, the LsEtOH extract exhibited superior activity compared to polysaccharide-rich fractions of L. sulphureus studied by Klaus et al. Their most active alkaline-extracted fraction displayed an EC50 value of 500 ± 200 µg/mL [47], more than twice the concentration required to achieve a similar effect in our ethanolic extract.
These comparisons highlight the broad spectrum and potent radical scavenging potential of the LsEtOH extract, particularly its high efficacy against hydroxyl radicals.
The antioxidant potential of L. sulphureus extract can be primarily attributed to its rich phenolic profile, notably including compounds such as p-coumaric acid. Phenolic constituents are well known for their capacity to donate hydrogen atoms or electrons, thereby neutralizing free radicals and preventing oxidative damage in both biological and food matrices [48]. The activity of p-coumaric acid has been validated in situ, where it effectively scavenged reactive oxygen and nitrogen species and contributed to oxidative stability in food systems [49]. Its functional role has also been linked to dual mechanisms of microbial and oxidative inhibition [50], further supporting its contribution to the multifunctional properties of the extract.
In addition, the presence of polyunsaturated fatty acids, particularly linoleic and oleic acids, may play a synergistic role in enhancing antioxidant defenses. These fatty acids can contribute to membrane stabilization and reduce lipid peroxidation, thereby complementing the radical scavenging effects of phenolic compounds [51].
The ethanolic extract of Laetiporus sulphureus (LsEtOH) demonstrated selective inhibitory activity against Gram-positive bacteria (Table 5), with the strongest effect on Staphylococcus aureus (MIC/MBC: 0.31/0.62 mg/mL). This level of activity is comparable to that of thyme and oregano essential oils, which typically exhibit MIC values ranging from 0.25 to 0.5 mg/mL against S. aureus [52,53].

Table 5.
MIC and MBC (mg/mL) of LsEtOH tested strains of Gram-positive and Gram-negative bacteria.
Moderate inhibition was observed against Enterococcus faecalis (2.50/10.0 mg/mL), which is in line with previously reported MIC values for rosemary and sage extracts (2–8 mg/mL) [54]. In contrast, the extract showed weak activity against Bacillus subtilis (10.0/10.0 mg/mL), whereas most plant-based extracts report MIC values below 2 mg/mL for this strain [54].
Compared to widely used plant-derived antimicrobials, LsEtOH exhibits promising efficacy against S. aureus, a major foodborne pathogen. This activity is likely due to the synergistic effects of phenolic acids and fatty acids present in the extract [49,51].
These findings support the potential application of Laetiporus sulphureus as a natural preservative, particularly in formulations targeting Gram-positive spoilage organisms. The selective activity of the LsEtOH extract aligns with previous observations that Gram-negative bacteria are generally more resistant to phenolic compounds. This resistance is largely attributed to the structural properties of their outer membrane, which limits permeability and restricts access of antimicrobial agents to intracellular targets [55].
Phenolic acids, such as p-coumaric acid, are known to exert a dual antimicrobial mode of action. Recent studies have shown that p-coumaric acid compromises bacterial cell membrane integrity, leading to leakage of intracellular contents and membrane hyperpolarization. Additionally, it interacts with genomic DNA, thereby interfering with replication and transcription processes [55].
Despite these potent mechanisms, the efficacy of p-coumaric acid and related phenolics is notably reduced against Gram-negative bacteria. This is due to the combined effect of their protective outer membrane, active efflux pumps, and enzymatic detoxification systems, all of which contribute to their diminished susceptibility to natural antimicrobials [56,57,58].
These findings support the potential application of L. sulphureus extracts in the development of multifunctional natural preservatives or biobased formulations targeting oxidative and microbial degradation in food and agricultural systems. Studies have demonstrated the species’ rich composition of phenolic compounds and its efficacy in prolonging shelf life [9,10], while broader investigations into wild edible mushrooms also support the role of fungal metabolites in food preservation and functional applications [59].
While the extraction parameters in this study were adapted from previous protocols [4], there is still room for further optimization. Future research could benefit from the application of multifactorial experimental designs, such as Response Surface Methodology (RSM), to systematically explore the influence of extraction variables on both yield and bioactivity. Building on the current methodological framework, such approaches may lead to more efficient and targeted extraction strategies in future studies.
3.3. Preserving Properties of Sausages
This section presents the influence of L. sulphureus ethanolic extract (LsEtOH) on key physicochemical, oxidative, microbiological, and sensory parameters of cooked sausages during 30 days of refrigerated storage. The results offer an integrative perspective on its preservative capacity.
Physicochemical Parameters: Initial pH values were comparable between the control (6.24 ± 0.1) and LsEtOH-treated sausages (6.23 ± 0.1). After 30 days of storage, a slight increase was recorded in both groups (6.26 ± 0.1 in control and 6.28 ± 0.1 in treated), with no statistically significant differences (p > 0.05) (Table 6). This suggests minimal proteolysis and stability of the protein matrix in the presence of fungal extract. Regarding color attributes (CIE L*, a*, b*), a marginal reduction in lightness and increase in redness was observed in treated samples. These changes are likely attributed to pigment myoglobin interactions or to direct coloration from L. sulphureus’s naturally vivid pigments, which may act as natural coloring agents [60]. Though subtle, such modifications could be advantageous in formulations targeting clean label status or seeking naturally enhanced visual appeal.

Table 6.
Effect of LsEtOH on pH and color (CIE L*a*b*) of cooked sausages (SLs) during storage.
Lipid Oxidation: TBARS values increased in both groups over the storage period; however, sausages supplemented with LsEtOH showed significantly lower TBARS levels (0.26 ± 0.02 mg MDA/kg) compared to the control group (0.36 ± 0.02 mg MDA/kg) by day 30 (p < 0.05) (Table 7). Notably, all values remained below the sensory threshold of 1.0 mg MDA/kg, above which rancidity becomes organoleptically detectable [61]. These results highlight the antioxidant capacity of the extract, attributed mainly to phenolic acids, such as p-coumaric acid and membrane stabilizing polyunsaturated fatty acids. Similar antioxidant effects have also been reported for extracts derived from other medicinal mushrooms [49,51].

Table 7.
Effect of LsEtOH on TBARS values (mg MDA/kg) of SLs during storage.
Microbiological Stability: During 30 days of refrigerated storage, sausages treated with LsEtOH consistently exhibited lower total aerobic mesophilic bacterial (AMB) counts compared to the untreated control. On day 1, bacterial counts reached 26.6 ± 11.5 cfu/g in the control samples, whereas treated sausages showed significantly lower counts of 16.6 ± 5.7 cfu/g. This difference persisted through day 30, with 43.3 ± 5.7 cfu/g in control versus 33.3 ± 15.2 cfu/g in the LsEtOH group (p < 0.05) (Table 8). Pathogens, such as Escherichia coli, Clostridium spp., Salmonella enterica, and molds were absent in all samples, likely reflecting the effectiveness of thermal processing and stringent hygienic production practices. The antimicrobial activity of the fungal extract is likely attributable to its secondary metabolites, particularly phenolic compounds and fatty acids, which are known to disrupt microbial membrane integrity and metabolic functions [10,11,12].

Table 8.
Effect of LsEtOH on microbiological profile (cfu/g) of cooked sausages during storage.
These findings support the potential application of L. sulphureus extracts in the development of multifunctional natural preservatives or bio-based formulations targeting oxidative and microbial degradation in food systems. Previous studies confirmed the rich phenolic profile and antioxidant activity of L. sulphureus primarily via in vitro analyses [10,11,12]. However, to the best of our knowledge, this is the first study to evaluate the preservative efficacy of L. sulphureus extract in a real meat matrix specifically, cooked sausages.
In comparison, Ganoderma lucidum extract has been added to pork products at 200–1000 mg/kg to enhance oxidative stability and reduce heterocyclic amines, and formulations using Pleurotus ostreatus puree (10–40%) in pork sausages achieved 24–37% lipid oxidation reduction over 15 days without sensory drawbacks. Additionally, Agaricus brasiliensis extract (0.5–1%) demonstrated antioxidant and antimicrobial results in pork patties like BHT (0.02%) over 9 days of chilled storage [62,63,64,65].
Based on the MIC determined for S. aureus, a sub-inhibitory concentration of 0.05 mg/kg was selected for incorporation into cooked sausages. This approach aimed to ensure sufficient antimicrobial and antioxidant functionality while avoiding potential sensory drawbacks associated with higher extract levels. Although the applied dose was lower than the typical concentrations used for plant-derived preservatives (e.g., 500–2000 mg/kg for thyme or rosemary extracts), it yielded measurable effects and aligns with clean-label formulation goals. Similar low-dose efficacy has been observed with essential oils, such as nutmeg (Myristica fragrans), which showed significant preservative potential in cooked sausages at only 10–20 ppm [31].
The novelty and practical relevance of our work positions L. sulphureus extract as a promising clean-label preservative with low-dose efficacy and multifunctional bioac-tivity.
Sensory Evaluation: A trained sensory panel comprising seven individuals evaluated the organoleptic properties of the sausage samples. Sausages formulated with 0.05 mg/kg LsEtOH received significantly lower flavor acceptability scores compared to the control group (p < 0.05), likely attributable to the extract’s inherent taste and aroma. Sensory evaluations indicated noticeable differences in flavor and aroma, while color remained within acceptable sensory thresholds (Figure 2). To mitigate these flavor-related drawbacks, future product development could explore advanced formulation strategies, such as microencapsulation or nanoemulsion systems, which allow the controlled release of bioactive compounds and can effectively mask off-notes while preserving antimicrobial and antioxidant efficacy [66].
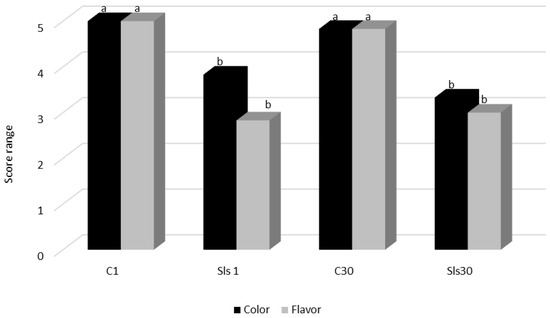
Figure 2.
Effect of LsEtOH on the flavor and color of cooked sausages during storage. Color and flavor scores (mean ± SD) of cooked sausage samples evaluated by a sensory panel. Different letters (a, b) above the bars indicate statistically significant differences (p < 0.05) between samples within the same sensory attribute.
The concentration of 0.05 mg/kg was selected based on preliminary internal tests that indicated good product stability during refrigerated storage. Although a sensory evaluation was not conducted at that stage, the results support its use in this study. The low dose was chosen to avoid sensory alteration, commonly seen with higher concentrations of natural extracts. Despite its low level, this dose produced measurable antioxidant and antimicrobial effects and is markedly lower than typical concentrations of plant-based preservatives (500–2000 mg/kg) or synthetic additives, like sodium nitrite (100–150 mg/kg) [31].
Importantly, the distinctive aroma noted in LsEtOH-treated sausages reflects a well-recognized sensory compromise frequently observed in functional food products. Previous studies indicate that consumer receptivity to novel flavor profiles may improve when the health benefits are clearly communicated. One comprehensive review highlighted perceived health value as a key determinant of consumer acceptance of functional foods [67]. Accordingly, strategic marketing and consumer education focused on the scientifically supported antioxidant and antimicrobial benefits of L. sulphureus may enhance market acceptance and shift perception toward its unique sensory attributes.
4. Conclusions
This study provides a comprehensive characterization of Laetiporus sulphureus collected from eastern Serbia, emphasizing its potential as a multifunctional, clean-label ingredient for meat preservation. The ethanolic extract (LsEtOH) exhibited notable antioxidant and antimicrobial activity in cooked sausages, effectively reducing lipid oxidation and microbial proliferation over 30 days of refrigerated storage. These findings support the practical application of wild edible fungi-derived extracts in functional food systems. This study’s limitations include the use of a single extract concentration, limited sampling intervals, and a sensory analysis focused solely on flavor. Nevertheless, this work represents the first report to validate the preservative efficacy of L. sulphureus in a real meat matrix, offering novel insights beyond previous in vitro studies. Future research should focus on dose optimization, extended sensory profiling (including texture and appearance), time-resolved stability studies, and the isolation of individual bioactive compounds. Overall, LsEtOH emerges as a promising candidate for natural food preservation, aligning with current consumer trends for safety, sustainability, and transparency.
Author Contributions
Conceptualization, A.N. and M.K.; Methodology, A.N., B.Š., J.T., P.I., T.P. and M.Š.; Validation, P.I., T.P., A.N. and M.K.; Writing—original draft preparation, A.N.; Writing—review and editing, A.N., M.K. and P.I.; Visualization, A.N., T.P. and M.Š.; Supervision, M.K.; Project administration, A.N.; Funding acquisition, A.N. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Scientific and Technological Development and Higher Education of the Republic of Srpska (Contract No. 19032/961-102/24) and the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Grants No. 451-03-137/2025-03/200125 and 451-03-136/2025-03/200125). The APC was funded by the Ministry of Scientific and Technological Development and Higher Education of the Republic of Srpska (Contract No. 19032/961-102/24).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Institute of Food Technology in Novi Sad (protocol code 175/I/27-3, date of approval: 27 October 2022).
Informed Consent Statement
Informed consent was obtained from all panelists involved in the sensory evaluation.
Data Availability Statement
All data supporting the findings of this study are contained within the article.
Acknowledgments
During the preparation of this manuscript, the authors used ChatGPT (GPT-4.0, OpenAI, 2025) for the purposes of language editing and improving the clarity of expression. The tool was not used for generating or interpreting scientific content. The authors have carefully reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Acknowledgments. This change does not affect the scientific content of the article.
References
- Inguglia, E.S.; Song, Z.; Kerry, J.P.; O’Sullivan, M.G.; Hamill, R.M. Addressing Clean Label Trends in Commercial Meat Processing: Strategies, Challenges and Insights from Consumer Perspectives. Foods 2023, 12, 2062. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research. Clean Label Ingredients Market Size, Share & Trends Analysis Report, 2020–2027. Available online: https://www.grandviewresearch.com/industry-analysis/clean-label-ingredients-market (accessed on 14 July 2025).
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef]
- Rašeta, M.; Karaman, M.; Jakšić, M.; Šibul, F.; Kebert, M.; Novaković, A.; Popović, M. Mineral Composition, Antioxidant and Cytotoxic Biopotentials of Wild-Growing Ganoderma Species (Serbia): G. lucidum (Curtis) P. Karst vs. G. applanatum (Pers.) Pat. Free Radic. Biol. Med. 2023, 198, 129–139. [Google Scholar] [CrossRef]
- Lindequist, U.; Niedermeyer, T.H.J.; Jülich, W.D. The pharmacological potential of mushrooms. Evid.-Based Complement. Altern. Med. 2005, 2, 285–299. [Google Scholar] [CrossRef]
- Tao, Y.; Xiao, S.; Cai, J.; Wang, J.; Li, L. Effects of ergothioneine-enriched mushroom extract on oxidative stability, volatile compounds and sensory quality of emulsified sausage. Anim. Biosci. 2021, 34, 1695–1704. [Google Scholar] [CrossRef]
- Fogarasi, M.; Devai, G.; Hegedűsné, K.; Gálik, B.; Soós, G.; Csapo, J.; Spilási, I. Polyphenol-enrichment of Vienna sausages using microcapsules containing acidic aqueous extract of Boletus edulis mushrooms. Foods 2023, 13, 979. [Google Scholar] [CrossRef]
- Santi, M.; Zambonelli, A.; Venturella, G. Edible mushrooms as sources of bioactive compounds in meat product development: Current knowledge and future perspectives. Appl. Sci. 2021, 11, 3998. [Google Scholar] [CrossRef]
- Kovács, D.; Vetter, J. Chemical composition of the mushroom Laetiporus sulphureus (Bull.) Murill. Acta Alimentaria 2015, 44, 104–110. [Google Scholar] [CrossRef]
- Petrović, J.; Stojković, D.; Reis, F.S.; Barros, L.; Glamočlija, J.; Ćirić, A.; Soković, M. Study on chemical, bioactive and food preserving properties of Laetiporus sulphureus (Bull.: Fr.) Murr. Food Funct. 2014, 5, 1441–1451. [Google Scholar] [CrossRef]
- Bulam, S.; Üstün, N.Ş.; Pekşen, A. Nutraceutical and food preserving importance of Laetiporus sulphureus. Turk. J. Agric. Food Sci. Technol. 2019, 7, 94–100. [Google Scholar] [CrossRef]
- Petrović, J.; Glamočlija, J.; Stojković, D.S.; Ćirić, A.; Nikolić, M.; Bukvički, D.; Soković, M.D. Laetiporus sulphureus, edible mushroom from Serbia: Investigation on volatile compounds, in vitro antimicrobial activity and in situ control of Aspergillus flavus in tomato paste. Food Chem. Toxicol. 2013, 59, 297–302. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Tidona, F.; Sekse, C.; Criscione, A.; Jacobsen, M.; Bordonaro, S.; Marletta, D.; Vegarud, G.E. Antimicrobial Effect of Donkeys’ Milk Digested In Vitro with Human Gastrointestinal Enzymes. Int. Dairy J. 2011, 21, 158–165. [Google Scholar] [CrossRef]
- Torbica, A.M.; Živančev, D.R.; Nikolić, Z.T.; Đorđević, V.B.; Nikolovski, B.G. Advantages of the Lab-on-a-Chip method in the determination of the Kunitz trypsin inhibitor in soybean varieties. J. Agric. Food Chem. 2010, 58, 7980–7985. [Google Scholar] [CrossRef] [PubMed]
- Novaković, A.R.; Karaman, M.A.; Milovanović, I.L.; Torbica, A.M.; Tomić, J.M.; Pejin, B.M.; Sakač, M.B. Nutritional and phenolic profile of small edible fungal species Coprinellus disseminatus (Pers.) J.E. Lange 1938. Food Feed Res. 2018, 45, 119–128. Available online: https://scindeks.ceon.rs/article.aspx?artid=2217-53691807119N (accessed on 14 July 2025). [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Chromatogr. A 1957, 226, 497–509. [Google Scholar]
- Ackman, R.G. Remarks on official methods employing boron trifluoride in the preparation of methyl esters of the fatty acids of fish oils. J. Am. Oil Chem. Soc. 1998, 75, 541–545. [Google Scholar] [CrossRef]
- Orčić, D.; Francišković, M.; Bekvalac, K.; Svirčev, E.; Beara, I.; Lesjak, M.; Mimica-Dukić, N. Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection. Food Chem. 2014, 143, 48–53. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Espín, J.C.; Soler-Rivas, C.; Wichers, H.J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J. Agric. Food Chem. 2000, 48, 648–656. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Cheeseman, K.H.; Beavis, A.; Esterbauer, H. Hydroxyl-radical-induced iron-catalysed degradation of 2-deoxyribose. Quantitative determination of malondialdehyde. Biochem. J. 1988, 252, 649–653. [Google Scholar] [CrossRef]
- Karaman, M.; Mimica-Dukić, N.; Knežević, P.; Svirčev, Z.; Matavulj, M. Antibacterial properties of selected lignicolous mushrooms and fungi from northern Serbia. Int. J. Med. Mushrooms 2009, 11, 269–279. [Google Scholar] [CrossRef]
- CIE. Colorimetry: Official Recommendation of the International Commission on Illumination; CIE Publication No. E-1.31; Bureau Central de la CIE: Paris, France, 1976. [Google Scholar]
- Honikel, K. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Tomović, V.; Jokanović, M.; Petrović, L.; Tomović, M.; Tasić, T.; Ikonić, P.; Šumić, Z.; Šojić, B.; Škaljac, S.; Šošo, M. Sensory, physical and chemical characteristics of cooked ham manufactured from rapidly chilled and earlier deboned M. semimembranosus. Meat Sci. 2013, 93, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Mandić, A. Antioxidant Activities of Grape Seed Extracts from White Grape Varieties. Ph.D. Thesis, University of Novi Sad, Novi Sad, Serbia, 2007. [Google Scholar]
- Šojić, B.; Tomović, V.; Kocić-Tanackov, S.; Škaljac, S.; Ikonić, P.; Džinić, N.; Kravić, S. Effect of nutmeg (Myristica fragrans) essential oil on the oxidative and microbial stability of cooked sausage during refrigerated storage. Food Control 2015, 54, 282–286. [Google Scholar] [CrossRef]
- ISO 8586:2012; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. ISO: Geneva, Switzerland, 2012.
- ISO 5492:2008; Sensory Analysis—Vocabulary. ISO: Geneva, Switzerland, 2008.
- ISO 4121:2003; Sensory Analysis—Guidelines for the Use of Quantitative Response Scales. ISO: Geneva, Switzerland, 2003.
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques, 5th ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- FAO/WHO/UNU. Protein and Amino Acid Requirements in Human Nutrition. Report of a Joint FAO/WHO/UNU Expert Consultation; WHO Technical Report Series No. 935; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Agafonova, S.V.; Olennikov, D.N.; Borovskii, G.B.; Penzina, T.A. Chemical composition of fruiting bodies from two strains of Laetiporus sulphureus. Chem. Nat. Compd. 2007, 6, 687–688. [Google Scholar] [CrossRef]
- Khatua, S.; Ghosh, S.; Acharya, K. Laetiporus sulphureus (Bull.: Fr.) Murr. as food and medicine. Pharmacogn. J. 2017, 9 (Suppl. S6), s1–s15. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Heleno, S.A.; Reis, F.S.; Stojković, D.; Queiroz, M.J.R.P.; Vasconcelos, M.H. Chemical features of edible mushrooms and their bioactive compounds as potential ingredients for functional foods and nutraceuticals. Food Chem. 2015, 179, 25–35. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Wang, H.; Wang, X.; Qi, X. Purification and characterization of a novel lectin from Laetiporus sulphureus with antiproliferative and apoptosis-inducing activities. Biochimie 2011, 93, 1847–1854. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Recent developments in mushrooms as anti-cancer therapeutics: A review. 3 Biotech 2012, 2, 1–15. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the appropriate age for introduction of complementary feeding of infants. EFSA J. 2009, 7, 1423. [Google Scholar] [CrossRef]
- Kalač, P. Trace Element Contents in European Species of Wild Growing Edible Mushrooms: A Review. Food Chem. 2010, 122, 2–15. [Google Scholar] [CrossRef]
- Fonseca, L.; Legua, P.; Zúñiga, M.; Pardo, J.E.; Saavedra, F.; Carreccio, V.; García, J. Quantification of Minerals in Edible Mushrooms via Optimized Microwave-Assisted Digestion and ICP-OES. Foods 2023, 13, 4051. [Google Scholar] [CrossRef]
- Karaman, M.; Jovin, E.; Malbaša, R.; Matavulj, M.; Popović, M. Medicinal and Edible Lignicolous Fungi as Natural Sources of Antioxidative and Antibacterial Agents. Phytother. Res. 2010, 24, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, J.; Tesei, A.; Zambelli, V.; Varchi, G.; Ferroni, L.; Pignedoli, F.; Ferretti, E.; Falconi, M.; Benati, D.; Zweyer, M.; et al. Meripilus giganteus Ethanolic Extract Exhibits Pro-Apoptotic and Anti-Proliferative Effects in Leukemic Cell Lines. BMC Complement. Altern. Med. 2018, 18, 306. [Google Scholar] [CrossRef]
- Klaus, A.; Kozarski, M.; Niksic, M.; Jakovljevic, D.; Todorovic, N.; Vrvic, M.M.; van Griensven, L.J.L.D. The edible mushroom Laetiporus sulphureus as a potential source of natural antioxidants. Int. J. Food Sci. Nutr. 2013, 64, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Sova, D.; Petrović, J.; Soković, M.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Karaman, M.; Atlagić, K.; Novaković, A.; Šibul, F.; Živić, M.; Stevanović, K.; Pejin, B. Fatty acids predominantly affect anti-hydroxyl radical activity and FRAP value: The case study of two edible mushrooms. Antioxidants 2019, 8, 480. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; van Griensven, L.J.L.D. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Bozin, B.; Mimica-Dukić, N.; Simin, N.; Anackov, G. Characterization of the volatile composition and antimicrobial activity of essential oils of some Lamiaceae spices. Food Chem. 2007, 104, 1368–1374. [Google Scholar]
- Li, J.; Zhao, N.; Xu, R.; Li, G.; Dong, H.; Wang, B.; Li, Z.; Fan, M.; Wei, X. Deciphering the antibacterial activity and mechanism of p-coumaric acid against Alicyclobacillus acidoterrestris and its application in apple juice. Int. J. Food Microbiol. 2022, 378, 109822. [Google Scholar] [CrossRef]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Mechanisms of bacterial biocide and antibiotic resistance. J. Appl. Microbiol. 2002, 92, 55S–64S. [Google Scholar] [CrossRef] [PubMed]
- Tegos, G.P.; Stermitz, F.R.; Lomovskaya, O.; Lewis, K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother. 2002, 46, 3133–3141. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Martins, A.; Morales, P.; Fernandez-Ruiz, V.; Glamočlija, J.; Ferreira, I.C.F.R. Nutritional value, bioactive compounds, antimicrobial activity and bioaccessibility studies with wild edible mushrooms. LWT—Food Sci. Technol. 2015, 63, 799–806. [Google Scholar] [CrossRef]
- Davoli, P.; Mucci, A.; Schenetti, L.; Weber, R.W. Laetiporic acids, a family of non-carotenoid polyene pigments from fruit-bodies and liquid cultures of Laetiporus sulphureus. Phytochemistry 2005, 66, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, F.S.; Cavalheiro, C.P.; Ludtke, F.L.; Silva, M.D.S.D.; Fries, L.L.M.; Kubota, E.H. Oxidative and microbiological stability of fresh pork sausage with added sun mushroom powder. Ciênc. Agrotecnol. 2015, 39, 381–389. [Google Scholar] [CrossRef]
- Luangharn, T.; Hyde, K.; Chukeatirote, E. Antibacterial and antioxidant activity of the ethyl acetate extract of two strains of Thai Laetiporus sulphureus mushroom. Food Res. 2024, 8, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Sánchez, R.D.; Torres-Martínez, B.M.; Huerta-Leidenz, N.; Sarturi, J. Effects of Ganoderma lucidum hydroalcoholic extract on the antioxidant status of pork patties during storage and gastro-intestinal digestion. Food Biosci. 2024, 62, 105579. [Google Scholar]
- Wu, X.; Wang, P.; Xu, Q.; Jiang, B.; Li, L.; Ren, L.; Li, X.; Wang, L. Effects of Pleurotus ostreatus on physicochemical properties and residual nitrite of pork sausage. Coatings 2022, 12, 484. [Google Scholar]
- Vargas-Sánchez, R.D.; Torres-Martínez, B.M.; Huerta-Leidenz, N.; Fernandez-Lopez, J.; Torrescano-Urrutia, G.R.; Pérez-Álvarez, J.A.; Sánchez-Escalante, A. Antioxidant and antibacterial effect of Agaricus brasiliensis extract on raw and cooked pork patties during storage. Agriculture 2023, 13, 346. [Google Scholar] [CrossRef]
- Mehta, N.; Kumar, P.; Verma, A.K.; Umaraw, P.; Kumar, Y.; Malav, O.P.; Sazili, A.Q.; Domínguez, R.; Lorenzo, J.M. Microencapsulation as a Noble Technique for the Application of Bioactive Compounds in the Food Industry: A Comprehensive Review. Appl. Sci. 2022, 12, 1424. [Google Scholar] [CrossRef]
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer Acceptance toward Functional Foods: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 1217. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).