Development of a Bacterial Lysate from Antibiotic-Resistant Pathogens Causing Hospital Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Objects
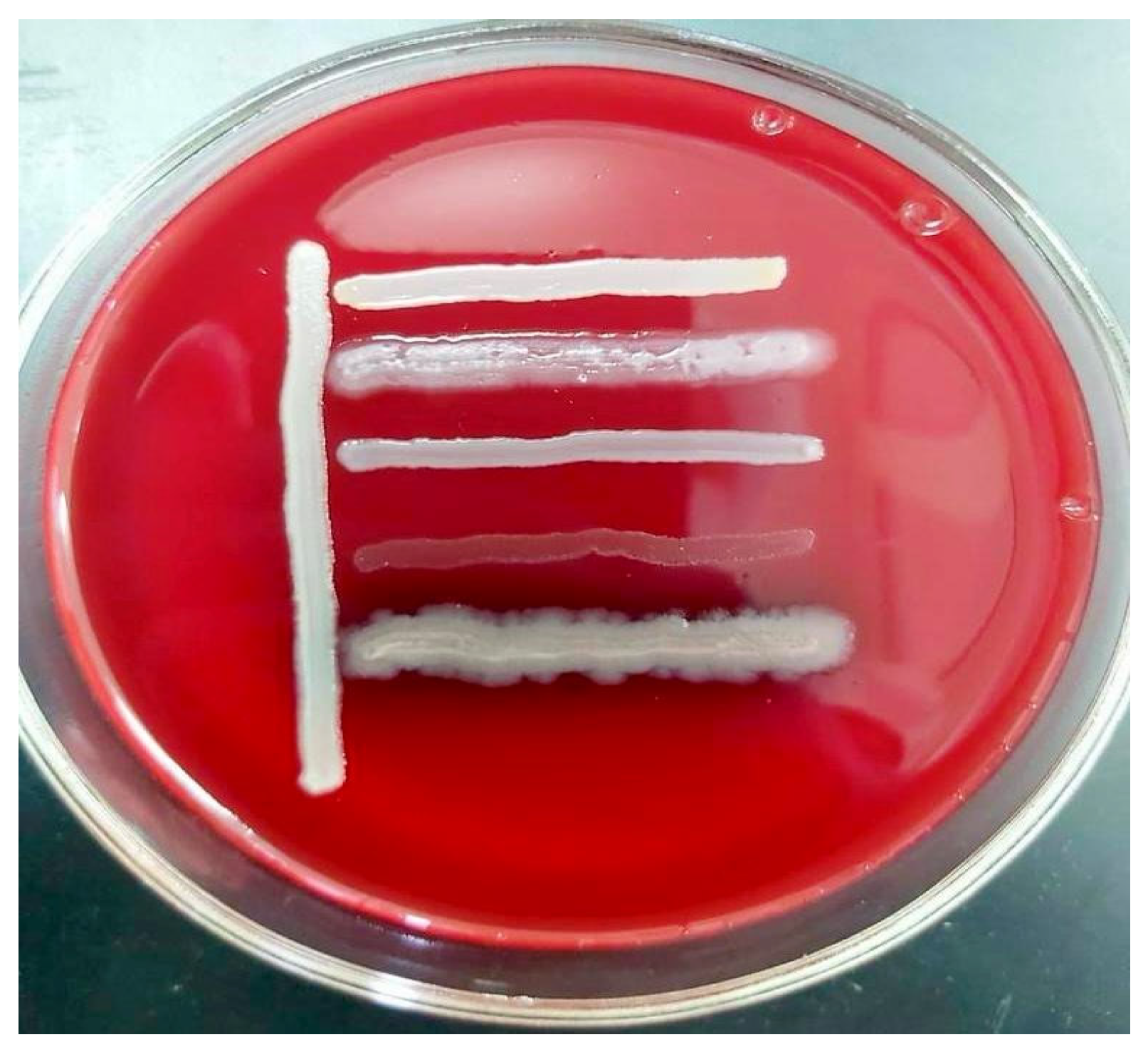
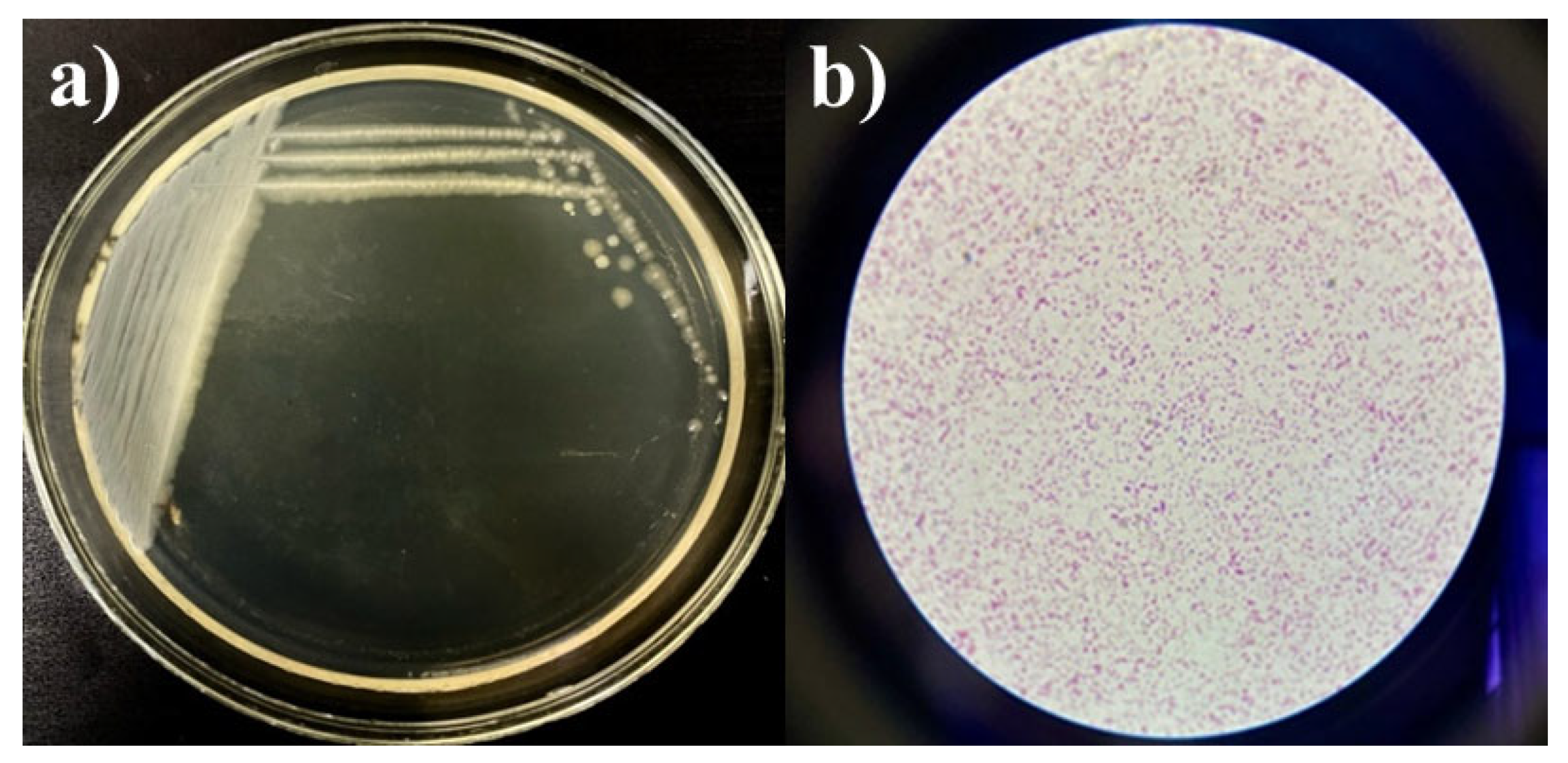
2.2. Determining Microbial Viability
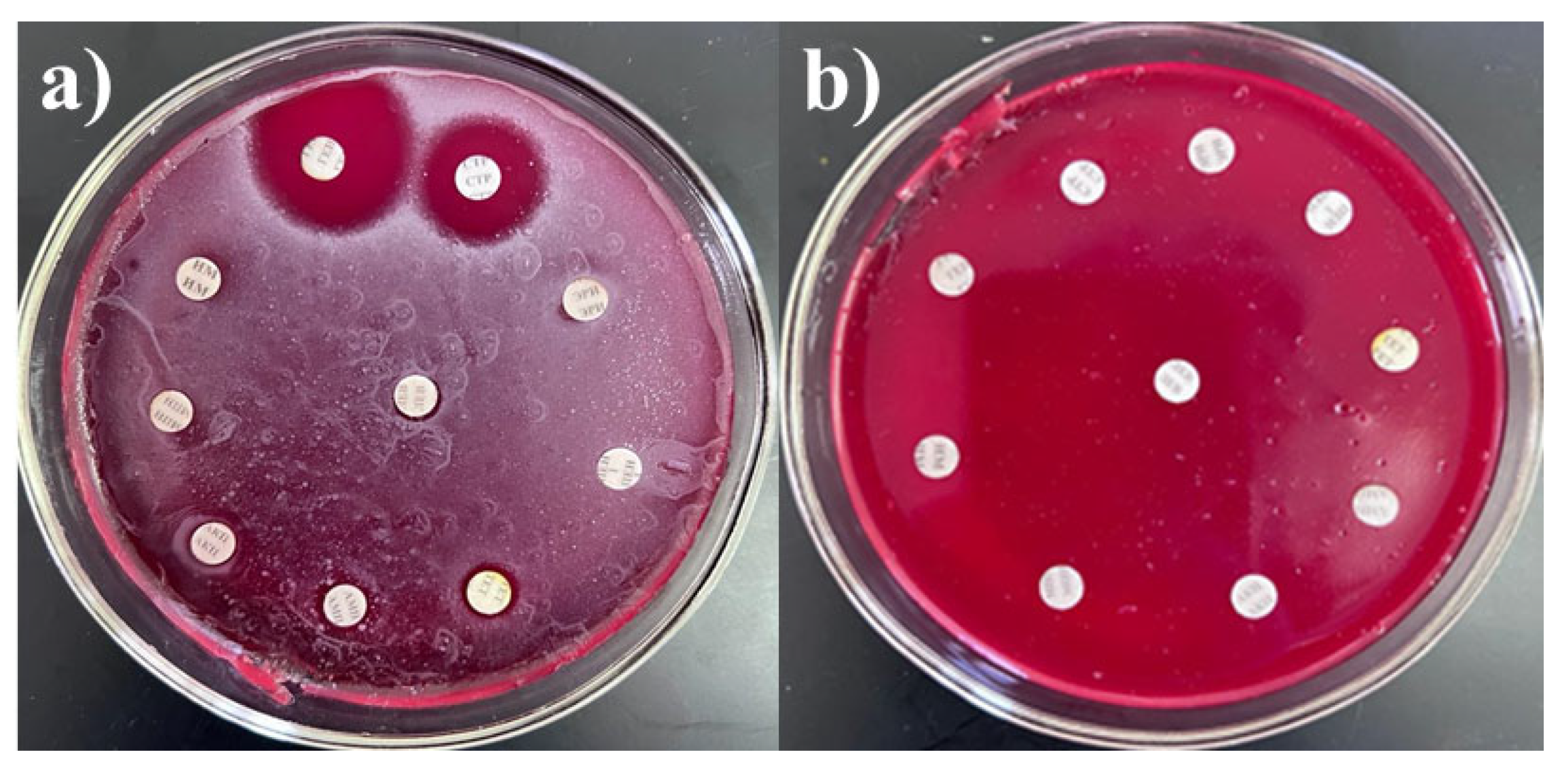
2.3. Determination of Antibacterial Drug Resistance
- -
- SRCPh (Russia): imipenem (10 µg), meropenem (10 µg), gentamicin (10 µg), benzylpenicillin (1 unit), erythromycin (15 µg), amoxicillin (20 µg), streptomycin (10 µg), tetracycline (30 µg), chloramphenicol (levomycetin, 30 µg), and ampicillin (10 µg).
- -
- HiMedia (India): cefepime (30 µg), ceftriaxone (10 µg), levofloxacin (5 µg), and pefloxacin (5 µg).
2.4. In Vitro Biocompatibility Determination
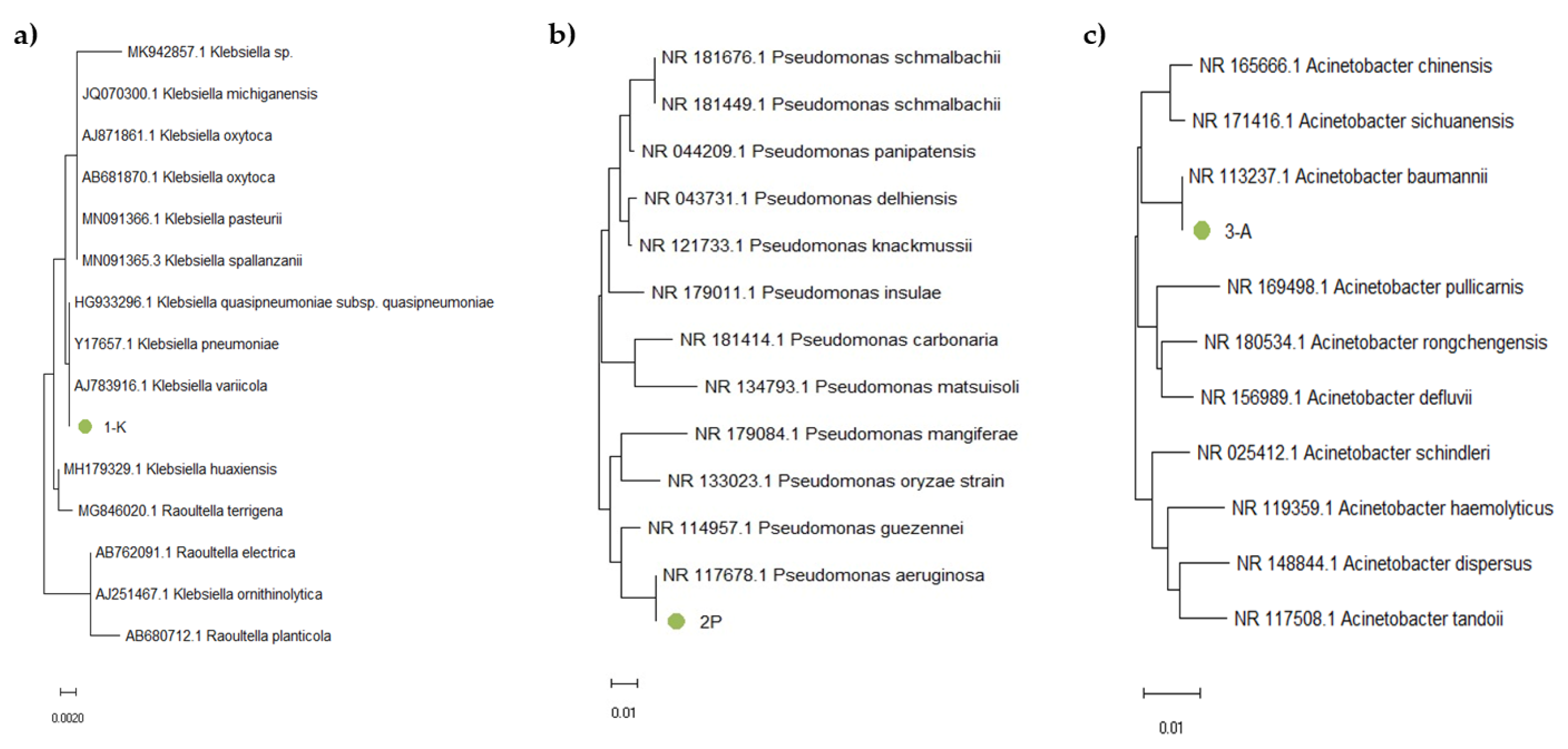
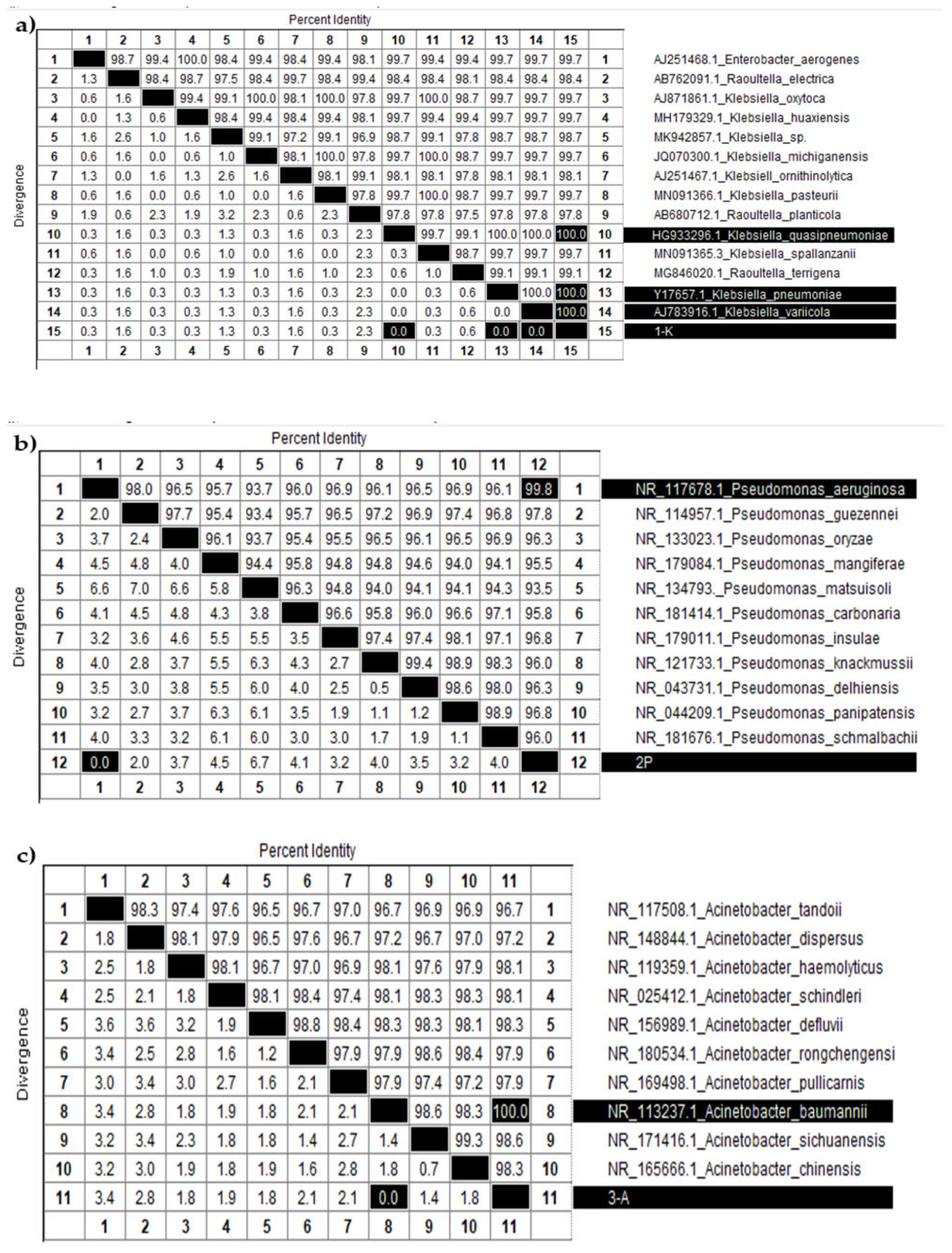
2.5. Identification of Bacterial Isolates Using 16S rRNA Gene Sequencing
2.5.1. Amplification of the 16S rRNA Gene Fragment
2.5.2. Electrophoretic Analysis of Amplification Products
2.5.3. Nucleotide Sequencing and Analysis
2.6. Bacterial Lysate Preparation
2.7. Cell Disintegrator Operation
- -
- 60—moderate power, suitable for delicate cell processing, minimizing damage to the biomaterial;
- -
- 70—medium, balanced for effective cell disintegration with minimal risk of component breakdown;
- -
- 80—high, which accelerates the destruction of cell walls but may increase the risk of damaging more sensitive structures. The pressure was not to exceed 12.9 kPa.
3. Results
3.1. Isolation of Microbial Strains and Determination of the Maximum Viability Index
3.2. Development of Bacterial Lysates Based on Drug-Resistant Pathogens
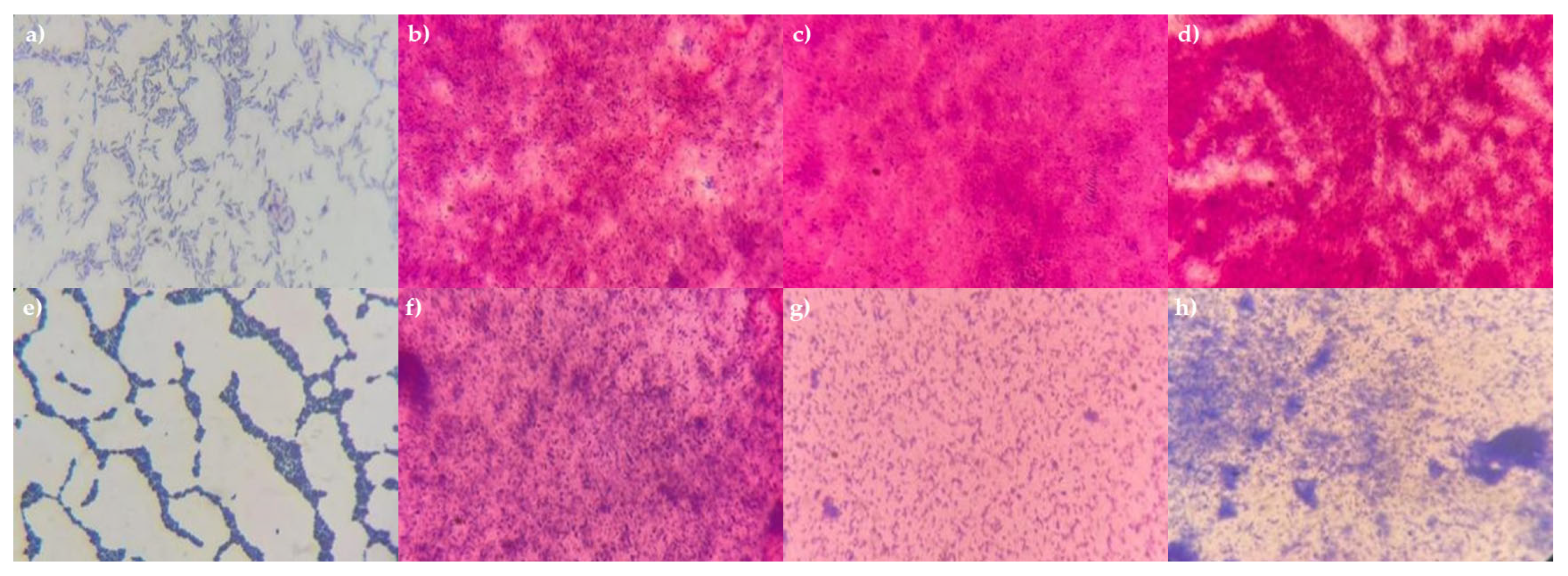
3.3. Identification of the Consortium Cultures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Environmental Factors Influencing the Development and Spread of Antibiotic Resistance. FEMS Microbiol. Rev. 2018, 42, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Cantas, L.; Shah, S.Q.A.; Cavaco, L.M.; Manaia, C.M.; Walsh, F.; Popowska, M.; Garelick, H.; Bürgmann, H.; Sørum, H. A Brief Multi-Disciplinary Review on Antimicrobial Resistance in Medicine and Its Linkage to the Global Environmental Microbiota. Front. Microbiol. 2013, 4, 96. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antimicrobial Resistance; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Eladli, M.G.; Alharbi, N.S.; Khaled, J.M.; Kadaikunnan, S.; Alobaidi, A.S.; Alyahya, S.A. Antibiotic-Resistant Staphylococcus Epidermidis Isolated from Patients and Healthy Students Comparing with Antibiotic-Resistant Bacteria Isolated from Pasteurized Milk. Saudi J. Biol. Sci. 2019, 26, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells Are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef] [PubMed]
- Ruslanuly, K.; Urakova, A.D.; Israilova, V.K. Awareness of the Population of Almaty City about the Threat of Antibiotic Resistance. Vestn. Kazn. 2018, 3, 297–301. [Google Scholar]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Microbiology: Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Yang, X.; Chan, E.W.C.; Zhang, R.; Chen, S. Klebsiella Species: Taxonomy, Hypervirulence and Multidrug Resistance. eBioMedicine 2022, 79, 103998. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.I.; Kim, S.H.; Bang, J.W.; Kim, H.B.; Kim, N.J.; Kim, E.C.; Oh, M.D.; Choe, K.W. Community-Acquired versus Nosocomial Klebsiella Pneumoniae Bacteremia: Clinical Features, Treatment Outcomes, and Clinical Implication of Antimicrobial Resistance. J. Korean Med. Sci. 2006, 21, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, P.; Finch, R. Tigecycline: In-Vitro Performance as a Predictor of Clinical Efficacy. Clin. Microbiol. Infect. 2007, 13, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Makalkina, L.G.; Zhusupova, G.K.; Esbatyrova, L.M.; Sagyndykova, M.; Zhemtimkarynova, G. Antibiotic Resistance—A Serious Threat to Society. Pharm. Bull. Dr. 2015, 9, 12. (In Russian) [Google Scholar]
- Treglia, M.; Pallocci, M.; Passalacqua, P.; Sabatelli, G.; De Luca, L.; Zanovello, C.; Messineo, A.; Quintavalle, G.; Cisterna, A.M.; Marsella, L.T. Medico-Legal Aspects of Hospital-Acquired Infections: 5-Years of Judgements of the Civil Court of Rome. Healthcare 2022, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Danilov, A.I.; Zharkova, L.P. Antimicrobial Resistance: Arguments and Facts. Clin. Pharmacol. Ther. 2017, 26, 6–9. (In Russian) [Google Scholar]
- Zemlyanko, O.M.; Rogoza, T.M.; Zhouravleva, G.A. Mechanisms of Bacterial Multiresistance to Antibiotics. Ecol. Genet. 2018, 16, 4–17. [Google Scholar] [CrossRef]
- Kanafin, Y.N.; Abdirova, P.; Kanafina, D.; Arkhangelsky, E.; Kyzas, G.Z.; Poulopoulos, S.G. UV and Zero-Valent Iron (ZVI) Activated Continuous Flow Persulfate Oxidation of Municipal Wastewater. Catalysts 2022, 13, 25. [Google Scholar] [CrossRef]
- Kanafin, Y.N.; Abdirova, P.; Arkhangelsky, E.; Dionysiou, D.D.; Poulopoulos, S.G. UVA and Goethite Activated Persulfate Oxidation of Landfill Leachate. Chem. Eng. J. Adv. 2023, 14, 100452. [Google Scholar] [CrossRef]
- Edelstein, M.V.; Skleenova, E.Y.; Shevchenko, O.V.; Tapalski, D.V.; Azizov, I.S.; D’souza, J.W.; Timokhova, A.V.; Sukhorukova, M.V.; Kozyreva, V.K.; Safronova, E.V.; et al. Prevalence and Molecular Epidemiology of Gram-Negative Bacteria Producing Metallo-b-Lactamases (MBLs) in Russia, Belarus and Kazakhstan. Clin. Microbiol. Antimicrob. Chemother. 2012, 14, 132–152. (In Russian) [Google Scholar]
- Reshetko, O.V.; Yakimova, Y.N. New Systemic Antimicrobials. Clin. Microbiol. Antimicrob. Chemother. 2015, 17, 272–285. [Google Scholar]
- Banerjee, D.; Puja, G.; Raichaudhuri, A. Development of Antibiotic Resistant Strains in Bacteria. EC Microbiol. 2018, 14, 796–798. [Google Scholar]
- Pikuza, O.I.; Faizullina, R.A.; Zakirova, A.M.; Moroz, T.B.; Volyanyuk, E.V. Bacterial Lysate in the Therapy of Acute and Recurrent Respiratory Infections in Children. Doctor.Ru 2021, 20, 11–16. [Google Scholar] [CrossRef]
- Ancuţa, D.L.; Alexandru, D.M.; Ţucureanu, C.; Coman, C. A Comparative Analysis of the Efficacy of Bacterial Lysate versus Antibiotic Therapy in the Treatment of Experimental Peri-Implantitis in Rats. Microorganisms 2024, 12, 1537. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Soto-Martinez, M.E.; Feleszko, W.; Jones, M.H.; Shen, K.L.; Schaad, U.B. Nonspecific Immunomodulators for Recurrent Respiratory Tract Infections, Wheezing and Asthma in Children: A Systematic Review of Mechanistic and Clinical Evidence. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Kalyuzhin, O.V. Topical Bacterial Lysates in the Prevention and Treatment of Respiratory Infections. Pract. Med. 2016, 2, 69–74. [Google Scholar]
- Jurkiewicz, D.; Zielnik-Jurkiewicz, B. Bacterial Lysates in the Prevention of Respiratory Tract Infections. Otolaryngol. Pol. 2018, 72, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kirilochev, O.; Sibiryakova, N. Effect of Immune Stimulator Ismigen on Immunological Indicators of Children with Ob-Structive Bronchitis. Russ. Bull. Perinatol. Pediatr. (Ross. Vestn. Perinatol. Pediatr.) 2018, 63, 96–99. [Google Scholar]
- Rahman, M.M.; Grice, I.D.; Ulett, G.C.; Wei, M.Q. Advances in Bacterial Lysate Immunotherapy for Infectious Diseases and Cancer. J. Immunol. Res. 2024, 2024, 4312908. [Google Scholar] [CrossRef] [PubMed]
- Suárez, N.; Ferrara, F.; Rial, A.; Dee, V.; Chabalgoity, J.A. Bacterial Lysates as Immunotherapies for Respiratory Infections: Methods of Preparation. Front. Bioeng. Biotechnol. 2020, 8, 545. [Google Scholar] [CrossRef] [PubMed]
- Ballarini, S.; Ardusso, L.; Ortega Martell, J.A.; Sacco, O.; Feleszko, W.; Rossi, G.A. Can Bacterial Lysates Be Useful in Prevention of Viral Respiratory Infections in Childhood? The Results of Experimental OM-85 Studies. Front. Pediatr. 2022, 10, 1051079. [Google Scholar] [CrossRef] [PubMed]
- Kearney, S.C.; Dziekiewicz, M.; Feleszko, W. Immunoregulatory and Immunostimulatory Responses of Bacterial Lysates in Respiratory Infections and Asthma. Ann. Allergy Asthma Immunol. 2015, 114, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Bianchini, S.; Bosis, S.; Tagliabue, C.; Coro, I.; Argentiero, A.; Principi, N. A Randomized, Placebo-Controlled, Double-Blinded, Single-Centre, Phase IV Trial to Assess the Efficacy and Safety of OM-85 in Children Suffering from Recurrent Respiratory Tract Infections. J. Transl. Med. 2019, 17, 284. [Google Scholar] [CrossRef] [PubMed]
- Di Gioacchino, M.; Santilli, F.; Pession, A. Is There a Role for Immunostimulant Bacterial Lysates in the Management of Respiratory Tract Infection? Biomolecules 2024, 14, 1249. [Google Scholar] [CrossRef] [PubMed]
- Schleifer, K. Phylum XIII. Firmicutes Gibbons and Murray 1978, 5 (Firmacutes [Sic] Gibbons and Murray 1978, 5). In Bergey’s Manual® of Systematic Bacteriology; Vos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A., Schleifer, K.-H., Whitman, W.B., Eds.; Springer: New York, NY, USA, 2009; Volume 3, ISBN 978-0-387-68489-5. [Google Scholar]
- Yegorov, N.S. Guide to Practical Microbiology Classes, 3rd ed.; Moscow University: Moscow, Russia, 1995. (In Russian) [Google Scholar]
- Anuarbekova, S.; Bekshin, Z.; Shaikhin, S.; Alzhanova, G.; Sadykov, A.; Temirkhanov, A.; Sarmurzina, Z.; Kanafin, Y. Exploring the Antimicrobial and Probiotic Potential of Microorganisms Derived from Kazakh Dairy Products. Microbiol. Res. 2024, 15, 1298–1318. [Google Scholar] [CrossRef]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The Estimation of the Bactericidal Power of Blood. J. Hyg. 1938, 38, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Bavelaar, H.; Justesen, U.S.; Morris, T.E.; Anderson, B.; Copsey-Mawer, S.; Stubhaug, T.T.; Kahlmeter, G.; Matuschek, E. Development of a EUCAST Disk Diffusion Method for the Susceptibility Testing of Rapidly Growing Anaerobic Bacteria Using Fastidious Anaerobe Agar (FAA): A Development Study Using Bacteroides Species. Clin. Microbiol. Infect. 2021, 27, 1695.e1–1695.e6. [Google Scholar] [CrossRef] [PubMed]
- Irkitova, A.N.; Kagan, J.R.; Sokolova, G.G. Comparative Analysis of the Methods to Define Antagonistic Activity of Lactic Bacteria. Izv. Altai State Univ. 2012, 3, 41–44. (In Russian) [Google Scholar]
- De Vegas, E.Z.S.; Nieves, B.; Araque, M.; Velasco, E.; Ruíz, J.; Vila, J. Outbreak of Infection With Acinetobacter Strain RUH 1139 in an Intensive Care Unit. Infect. Control Hosp. Epidemiol. 2006, 27, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Berdimuratova, K.T.; Amirgazin, A.; Kuibagarov, M.A.; Lutsay, V.B.; Mukanov, K.K.; Shevtsov, A.B. Optimization of PCR Purification Using Silica-Coated Magnetic Beads. Eurasian J. Appl. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Levin, R.; Löhr, F.; Karakoc, B.; Lichtenecker, R.; Dötsch, V.; Bernhard, F.E. Coli “Stablelabel” S30 Lysate for Optimized Cell-Free NMR Sample Preparation. J. Biomol. NMR 2023, 77, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Thapa, S. Evaluation of WGS-Subtyping Methods for Epidemiological Surveillance of Foodborne Salmonellosis. One Health Outlook 2020, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Chattaway, M.A.; Dallman, T.J.; Larkin, L.; Nair, S.; McCormick, J.; Mikhail, A.; Hartman, H.; Godbole, G.; Powell, D.; Day, M.; et al. The Transformation of Reference Microbiology Methods and Surveillance for Salmonella With the Use of Whole Genome Sequencing in England and Wales. Front. Public Health 2019, 7, 317. [Google Scholar] [CrossRef] [PubMed]
- Khokhlova, O.E.; Larionova, I.A.; Peryanova, O.V.; Kozlov, R.S.; Eidelshtein, M.V.; Modestov, A.A.; Eremeeva, O.G.; Lazareva, I.V.; Akusheva, D.N.; Lobova, T.I.; et al. The Mechanisms of Antibiotic Resistance in Major Pathogens of Purulent-Inflammatory Complications in Cancer Patients. Russ. J. Infect. Immun. 2021, 11, 324–336. [Google Scholar] [CrossRef]
- Bircher, L.; Geirnaert, A.; Hammes, F.; Lacroix, C.; Schwab, C. Effect of Cryopreservation and Lyophilization on Viability and Growth of Strict Anaerobic Human Gut Microbes. Microb. Biotechnol. 2018, 11, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Bissenova, N.M.; Yergaliyeva, A.S. Antibiotic Resistance Surveillance in a Pediatric Cardiac Surgery. Sci. Healthc. (Nauka Zdr.) 2020, 3, 105–110. [Google Scholar] [CrossRef]
- Palagin, I.S.; Sukhorukova, M.V.; Dekhnich, A.V.; Eidelstein, M.V.; Perepanova, T.S.; Kozlov, R.S. Antimicrobial Resistance of Pathogens Causing Community-Acquired Urinary Tract Infections in Russia, Belarus, and Kazakhstan: Results of the Multicenter International Study “DARMIS-2018”. Urologiia 2020. (In Russian) [Google Scholar] [CrossRef]
- Bissenova, N.; Yergaliyeva, A.; Mitus, N. Resistance of Gram-Negative Bacilli Isolated from Patients in Intensive Care Unit. J. Clin. Med. Kazakhstan 2016, 4, 46–51. [Google Scholar] [CrossRef]
- Bissenova, N. Patterns of Antimicrobial Resistance in a Pediatric Cardiac Intensive Care Unit. J. Clin. Med. Kazakhstan 2018, 2, 27–32. [Google Scholar] [CrossRef]
- Bissenova, N.M.; Yergaliyeva, A.S. Resistance of Main Pathogens of Respiratory Tract Infections in the Pediatric Cardiac Surgery Department. In Proceedings of the Current Issues in Medical Microbiology: Materials of the International Scientific and Practical Conference Dedicated to the Memory of the Founders of the Department of Microbiology A.L. Kotova and Sh.I. Sarbasova and the 60th Anniversary of the NJSC “Astana Medical University”, Astana, Kazakhstan, 21 June 2024; Astana Medical University: Astana, Kazakhstan, 2024; pp. 34–36. [Google Scholar]
- Bissenova, N.M.; Yergaliyeva, A.S. Antibiotic Resistance of Acinetobacter Baumannii Strains in the Pediatric Cardiac Surgery Center. In Proceedings of the Current Issues in Medical Microbiology: Materials of the International Scientific and Practical Conference dedicated to the memory of the founders of the Department of Microbiology A.L. Kotova and Sh.I. Sarbasova and the 60th anniversary of the NJSC “Astana Medical University”, Astana, Kazakhstan, 21 June 2024; Astana Medical University: Astana, Kazakhstan, 2024; pp. 38–39. [Google Scholar]
- Sutyimbekova, N.S.; Bissenova, N.M.; Yergaliyeva, A.S.; Dusmagambetov, M.U. Monitoring the Dynamic Level of Acinetobacter Baumannii Infection in a Multidisciplinary Hospital. In Proceedings of the Current Issues in Medical Microbiology: Materials of the International Scientific and Practical Conference dedicated to the memory of the founders of the Department of Microbiology A.L. Kotova and Sh.I. Sarbasova and the 60th anniversary of the NJSC “Astana Medical University”, Astana, Kazakhstan, 21 June 2024; Astana Medical University: Astana, Kazakhstan, 2024; p. 70. [Google Scholar]
- Sarsenova, A.G.-A.; Duisebekova, G.P.; Amanova, Z.Z. Microbiological Monitoring of Blood Samples from Hematological Patients between 2020 and 2023. In Proceedings of the Current Issues in Medical Microbiology: Materials of the International Scientific and Practical Conference dedicated to the memory of the founders of the Department of Microbiology A.L. Kotova and Sh.I. Sarbasova and the 60th anniversary of the NJSC “Astana Medical University”, Astana, Kazakhstan, 21 June 2024; Astana Medical University: Astana, Kazakhstan, 2024; pp. 68–69. [Google Scholar]
- Shahriar, A.; Akter, T.; Kobra, A.T.; Emran, T.B.; Mallick, J.; Dutta, M. Isolation of Pathogenic and Non-Pathogenic Microbial Stains from Different Types of Sea Fish Samples and Their Quality Assessment with Antibiogram Properties. J. Adv. Microbiol. 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Kotov, S.V.; Pulbere, S.A.; Alesina, N.V.; Boyarkin, V.S.; Guspanov, R.I.; Belomytsev, S.V.; Kotova, D.P. The Problem of Antibiotic Resistance in Patients with Urinary Tract Infection. Urology 2021, 1, 5–12. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Michalik-Provasek, J.; Parker, H.; Lessor, L.; Gill, J.J. Solvent Extraction of Klebsiella Pneumoniae Bacteriophage Lysates with 1-Dodecanol Results in Endotoxin Reduction with Low Risk of Solvent Contamination. PHAGE Ther. Appl. Res. 2021, 2, 112–119. [Google Scholar] [CrossRef]
- Sharma, S.; Datta, S.; Chatterjee, S.; Dutta, M.; Samanta, J.; Vairale, M.G.; Gupta, R.; Veer, V.; Dwivedi, S.K. Isolation and Characterization of a Lytic Bacteriophage against Pseudomonas Aeruginosa. Sci. Rep. 2021, 11, 19393. [Google Scholar] [CrossRef] [PubMed]
- Khalilov, R.; Mammadova, M.; Abdullayeva, S. Mechanism of Resistance to Beta-Lactam Antibiotics. Bull. L.N. Gumilyov Eurasian Natl. Univ. Biosci. Ser. 2023, 143, 115–127. [Google Scholar] [CrossRef]
- Leungtongkam, U.; Thummeepak, R.; Tasanapak, K.; Sitthisak, S. Acquisition and Transfer of Antibiotic Resistance Genes in Association with Conjugative Plasmid or Class 1 Integrons of Acinetobacter Baumannii. PLoS ONE 2018, 13, e0208468. [Google Scholar] [CrossRef] [PubMed]
- Ryazantsev, S.V.; Konoplev, O.I.; Sapova, K.I. Bacterial Lysates in the Treatment of Respiratory Tract and ENT Diseases. Russ. Med. J. 2015, 23, 1387–1390. [Google Scholar]
- Wang, Q.; Qi, Z.; Fu, W.; Pan, M.; Ren, X.; Zhang, X.; Rao, Z. Research and Prospects of Enzymatic Hydrolysis and Microbial Fermentation Technologies in Protein Raw Materials for Aquatic Feed. Fermentation 2024, 10, 648. [Google Scholar] [CrossRef]
- Islam, M.S.; Aryasomayajula, A.; Selvaganapathy, P.R. A Review on Macroscale and Microscale Cell Lysis Methods. Micromachines 2017, 8, 83. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Z.; Huang, H. Physical Cell Disruption Technologies for Intracellular Compound Extraction from Microorganisms. Processes 2024, 12, 2059. [Google Scholar] [CrossRef]
- Brouchkov, A.V.; Averin, M.V.; Callender, T.; Griva, G.I.; Gapeev, A.B.; Ignatov, S.G.; Khramov, R.N.; Man, Y.G.; Marshall, P.; Shcherbatyuk, T.G.; et al. Bacterial Cell Lysates of Bacillus Sp. F Isolated From Permafrost Diminish DNA Damage by Hydrogen Peroxide in Murine Blood Cells Ex Vivo. Adv. Gut Microbiome Res. 2024, 2024, 4559054. [Google Scholar] [CrossRef]
- Ricci, R.; Palmero, C.; Bazurro, G.; Riccio, A.M.; Garelli, V.; Di Marco, E.; Cirillo, C.; Braido, F.; Canonica, G.W.; Melioli, G. The Administration of a Polyvalent Mechanical Bacterial Lysate in Elderly Patients with COPD Results in Serological Signs of an Efficient Immune Response Associated with a Reduced Number of Acute Episodes. Pulm. Pharmacol. Ther. 2014, 27, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Braido, F.; Melioli, G.; Cazzola, M.; Fabbri, L.; Blasi, F.; Moretta, L.; Canonica, G.W. Sub-Lingual Administration of a Polyvalent Mechanical Bacterial Lysate (PMBL) in Patients with Moderate, Severe, or Very Severe Chronic Obstructive Pulmonary Disease (COPD) According to the GOLD Spirometric Classification: A Multicentre, Double-Blind, Ran. Pulm. Pharmacol. Ther. 2015, 33, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Kryshen, K.L.; Kukharenko, A.E.; Vichare, A.S.; Gaidai, E.A.; Kryshen, A.A.; Gushchin, Y.A.; Kalyuzhin, O.V.; Makarova, M.N.; Makarov, V.G.; Mahadevan, B. Anti-Inflammatory and Immunomodulating Effects of the Bacterial Lysate in the Invivomodels of Aseptic Lymphadenitis and Pneumococcal Pneumonia. Med. Immunol. 2020, 22, 111–122. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Nie, J.; Wang, Y.; Zhang, L.; Shi, Q.; Tan, H.; Kong, W. Bacterial Lysate for the Prevention of Chronic Rhinosinusitis Recurrence in Children. J. Laryngol. Otol. 2017, 131, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Fang, Z.; Saborío, G.P.; Xiu, Q. Efficacy and Safety of OM-85 in Patients with Chronic Bronchitis and/or Chronic Obstructive Pulmonary Disease. Lung 2015, 193, 513–519. [Google Scholar] [CrossRef] [PubMed]

| Strains | Source Separation | Viability Index | |
|---|---|---|---|
| ×109, CFU/mL | lg CFU/mL | ||
| Escherichia coli 1 BL | Wound washout | 200 ± 0 | 11.3 |
| Staphylococcus haemolyticus 2 BL | Oropharyngeal mucosa | 16 ± 0.94 | 10.2 |
| Pseudomonas aeruginosa 3 BL | Oropharyngeal mucosa | 82 ± 0.94 | 10.91 |
| Staphylococcus haemolyticus 4 BL | Urine | 180 ± 9.4 | 11.25 |
| Streptococcus pneumoniae 5 BL | Oropharyngeal mucosa | 55.3 ± 0.27 | 10.74 |
| Escherichia coli 6 BL | Urine | 68 ± 0.94 | 10.83 |
| Staphylococcus epidermidis 7 BL | Ear discharge swab | 17.3 ± 0.27 | 10.23 |
| Citrobacter koseri 8 BL | Urine | 36 ± 0.94 | 10.56 |
| Citrobacter freundii 9 BL | Oropharyngeal mucosa | 30 ± 0.94 | 10.48 |
| Klebsiella pneumoniae 10 BL | Oropharyngeal mucosa | 11 ± 0.58 | 10.04 |
| Klebsiella pneumoniae 11 BL | Oropharyngeal mucosa | 14 ± 0.94 | 11.15 |
| Klebsiella pneumoniae 12 BL | Purulent wound smear | 54 ± 0.94 | 10.73 |
| Pseudomonas aeruginosa 13 BL | Purulent wound smear | 7 ± 1.41 | 9.84 |
| Pseudomonas aeruginosa 14 BL | Tracheostomy tube flushing | 19 ± 0.81 | 10.23 |
| Pseudomonas aeruginosa 15 BL | Wound washout | 36 ± 0.67 | 10.56 |
| Pseudomonas aeruginosa 16 BL | Pleural cavity contents | 96 ± 0.94 | 10.98 |
| Pseudomonas aeruginosa 17 BL | Tracheostomy tube flushing | 39 ± 4.24 | 10.55 |
| Staphylococcus aureus 18 BL | Ear discharge swab | 33.3 ± 0.38 | 10.48 |
| Staphylococcus aureus 19 BL | Ear discharge swab | 91 ± 0.58 | 10.96 |
| Staphylococcus aureus 20 BL | Wound washout | 9 ± 0.99 | 9.95 |
| Escherichia coli 21 BL | Bile ducts | 81 ± 0.47 | 10.91 |
| Escherichia coli 22 BL | Wound washout | 17 ± 0.47 | 10.23 |
| Escherichia coli 23 BL | Urine | 75 ± 0.47 | 10.87 |
| Acinetobacter sp. 24 BL | Wound washout | 76.6 ± 1.44 | 10.89 |
| Haemophilus influenzae 25 BL | Pleural cavity contents | 1 | 9.0 |
| Strains | Inhibition Zone, mm | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mp | Ip | Axc | Amp | Bpc | Tc | Gm | Stm | Em | Lc | Cf | Ctr | Lf | Pf | |
| E. coli 1 BL | 11 | 12 | 0 | 0 | 0 | 6 | 7 | 10 | 0 | 15 | 16 | 0 | 10 | 0 |
| S. haemolyticus 2 BL | 18 | 23 | 0 | 6 | 0 | 0 | 9 | 14 | 8 | 16 | 16 | 0 | 14 | 6 |
| P. aeruginosa 3 BL | 16 | 15 | 0 | 0 | 0 | 0 | 14 | 7 | 0 | 0 | 15 | 0 | 7 | 0 |
| S. haemolyticus 4 BL | 0 | 0 | 0 | 6 | 0 | 6 | 23 | 18 | 0 | 7 | 21 | 0 | 0 | 0 |
| Str. pneumoniae 5 BL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| E. coli 6 BL | 13 | 21 | 10 | 0 | 0 | 11 | 17 | 14 | 8 | 13 | 16 | 0 | 10 | 0 |
| S. epidermidis 7 BL | 15 | 0 | 0 | 0 | 0 | 16 | 14 | 8 | 0 | 8 | 16 | 0 | 14 | 6 |
| C. koseri 8 BL | 8 | 10 | 0 | 0 | 0 | 14 | 14 | 12 | 0 | 17 | 0 | 7 | 3 | 3 |
| C. freundii 9 BL | 18 | 17 | 11 | 8 | 0 | 7 | 7 | 16 | 0 | 0 | 8 | 7 | 6 | 10 |
| Kl. pneumoniae 10 BL | 18 | 0 | 0 | 0 | 0 | 7 | 20 | 16 | 0 | 21 | 12 | 0 | 2 | 3 |
| Kl. pneumoniae 11 BL | 17 | 19 | 0 | 0 | 0 | 15 | 18 | 16 | 0 | 22 | 9 | 9 | 16 | 0 |
| Kl. pneumoniae 12 BL | 0 | 0 | 0 | 0 | 0 | 0 | 15 | 15 | 0 | 17 | 17 | 17 | 16 | 0 |
| P. aeruginosa 13 BL | 9 | 9 | 9 | 0 | 11 | 6 | 18 | 21 | 20 | 11 | 13 | 10 | 15 | 0 |
| P. aeruginosa 14 BL | 16 | 15 | 0 | 0 | 11 | 0 | 6 | 0 | 20 | 18 | 7 | 20 | 21 | 0 |
| P. aeruginosa 15 BL | 15 | 18 | 0 | 0 | 7 | 15 | 25 | 14 | 13 | 20 | 12 | 8 | 0 | 0 |
| P. aeruginosa 16 BL | 10 | 0 | 0 | 8 | 0 | 7 | 17 | 7 | 9 | 0 | 6 | 10 | 16 | 0 |
| P. aeruginosa 17 BL | 7 | 16 | 0 | 0 | 0 | 13 | 12 | 9 | 10 | 15 | 7 | 10 | 16 | 0 |
| S. aureus 18 BL | 15 | 10 | 0 | 0 | 0 | 10 | 6 | 10 | 0 | 0 | 6 | 10 | 0 | 7 |
| S. aureus 19 BL | 14 | 16 | 0 | 0 | 0 | 0 | 7 | 10 | 0 | 16 | 7 | 10 | 6 | 10 |
| S. aureus 20 BL | 26 | 18 | 20 | 0 | 0 | 14 | 27 | 11 | 24 | 13 | 20 | 22 | 12 | 10 |
| E. coli 21 BL | 17 | 20 | 0 | 0 | 0 | 0 | 7 | 9 | 7 | 0 | 0 | 0 | 0 | 0 |
| E. coli 22 BL | 9 | 19 | 12 | 7 | 0 | 0 | 21 | 9 | 0 | 14 | 18 | 20 | 0 | 18 |
| E. coli 23 BL | 13 | 15 | 0 | 0 | 0 | 0 | 23 | 16 | 0 | 14 | 10 | 8 | 2 | 0 |
| Acinetobacter sp. 24 BL | 7 | 0 | 0 | 0 | 0 | 16 | 0 | 0 | 9 | 9 | 11 | 10 | 0 | 0 |
| Haem. influenzae 25 BL | 9 | 0 | 8 | 5 | 6 | 0 | 0 | 0 | 9 | 9 | 11 | 7 | 0 | 0 |
| Strains | Viability Index, CFU/mL | ||
|---|---|---|---|
| 1st Cycle | 2nd Cycle | 3rd Cycle | |
| E. coli 1 BL | 108 | 105 | 102 |
| S. haemolyticus 2 BL | 108 | 105 | 102 |
| P. aeruginosa 3 BL | 107 | 105 | 103 |
| S. haemolyticus 4 BL | 108 | 105 | 102 |
| Str. pneumoniae 5 BL | 108 | 105 | 102 |
| E. coli 6 BL | 107 | 105 | 103 |
| S. epidermidis 7 BL | 107 | 105 | 102 |
| C. koseri 8 BL | 108 | 105 | 102 |
| C. freundii 9 BL | 108 | 105 | 102 |
| Kl. pneumoniae 10 BL | 107 | 105 | 102 |
| Kl. pneumoniae 11 BL | 108 | 105 | 102 |
| Kl. pneumoniae 12 BL | 108 | 105 | 102 |
| P. aeruginosa 13 BL | 107 | 105 | 102 |
| P. aeruginosa 14 BL | 108 | 105 | 103 |
| P. aeruginosa 15 BL | 108 | 105 | 102 |
| P. aeruginosa 16 BL | 107 | 105 | 102 |
| P. aeruginosa 17 BL | 108 | 105 | 102 |
| S. aureus 18 BL | 108 | 105 | 102 |
| S. aureus 19 BL | 107 | 105 | 103 |
| S. aureus 20 BL | 108 | 105 | 102 |
| E. coli 21 BL | 108 | 105 | 102 |
| E. coli 22 BL | 107 | 105 | 102 |
| E. coli 23 BL | 108 | 105 | 102 |
| Acinetobacter sp. 24 BL | 108 | 105 | 102 |
| Haem. influenzae 25 BL | 108 | 105 | 103 |
| Test | Glucose (Acid/Gas) | Lactose (Acid) | Dulcite (Acid) | Methyl Red | Voges- Proskauer | Citrate Utilization | Urease | Malonate |
|---|---|---|---|---|---|---|---|---|
| Result | +/+ | + | − | − | + | + | + | + |
| No. | Mode (%Power), Number of Cycles | No. | Mode (%Power), Number of Cycles | No. | Mode (%Power), Number of Cycles |
|---|---|---|---|---|---|
| 1 | 30% power, 1 cycle | 6 | 100% power, 1 cycle | 11 | 100% power, 2 cycles |
| 2 | 55% power, 1 cycle | 7 | 65% power, 2 cycles | 12 | 80%–90%–100% |
| 3 | 65% power, 1 cycle | 8 | 75% power, 2 cycles | 13 | 100%–90%–80% |
| 4 | 75% 1 cycle | 9 | 85% power, 2 cycles | 14 | 90% power, 3 cycles |
| 5 | 80% power, 1 cycle | 10 | 90% power, 2 cycles | 15 | 100% power, 3 cycles |
| Parameters | Ismigen [28,66,67] | Imudon [68] | OM-85 [28,69,70] | BL |
|---|---|---|---|---|
| Type of lysate | Mechanical | Chemical | Chemical | Mechanical |
| Indications | Treatment and prevention of diseases of the upper and lower respiratory tract | Treatment and prevention of diseases of the oral cavity and pharynx | Treatment and prevention of diseases of the upper and lower respiratory tract | Treatment and prevention of diseases of the upper and lower respiratory tract |
| Content | Lysates of 8 pathogenic bacteria | Lysates of 13 pathogenic bacteria | Lysates of 8 pathogenic bacteria | Lysates of 3 pathogenic bacteria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anuarbekova, S.; Sadykov, A.; Amangeldinova, D.; Kanafina, M.; Sharova, D.; Alzhanova, G.; Nurgaliyeva, R.; Jumagaziyeva, A.; Tynybayeva, I.; Zhumakaeva, A.; et al. Development of a Bacterial Lysate from Antibiotic-Resistant Pathogens Causing Hospital Infections. Microorganisms 2025, 13, 1831. https://doi.org/10.3390/microorganisms13081831
Anuarbekova S, Sadykov A, Amangeldinova D, Kanafina M, Sharova D, Alzhanova G, Nurgaliyeva R, Jumagaziyeva A, Tynybayeva I, Zhumakaeva A, et al. Development of a Bacterial Lysate from Antibiotic-Resistant Pathogens Causing Hospital Infections. Microorganisms. 2025; 13(8):1831. https://doi.org/10.3390/microorganisms13081831
Chicago/Turabian StyleAnuarbekova, Sandugash, Azamat Sadykov, Dilnaz Amangeldinova, Marzhan Kanafina, Darya Sharova, Gulzhan Alzhanova, Rimma Nurgaliyeva, Ardak Jumagaziyeva, Indira Tynybayeva, Aikumys Zhumakaeva, and et al. 2025. "Development of a Bacterial Lysate from Antibiotic-Resistant Pathogens Causing Hospital Infections" Microorganisms 13, no. 8: 1831. https://doi.org/10.3390/microorganisms13081831
APA StyleAnuarbekova, S., Sadykov, A., Amangeldinova, D., Kanafina, M., Sharova, D., Alzhanova, G., Nurgaliyeva, R., Jumagaziyeva, A., Tynybayeva, I., Zhumakaeva, A., Rsaliyev, A., Abduraimov, Y., & Kanafin, Y. N. (2025). Development of a Bacterial Lysate from Antibiotic-Resistant Pathogens Causing Hospital Infections. Microorganisms, 13(8), 1831. https://doi.org/10.3390/microorganisms13081831






