Effect of Multi-Species Probiotic Supplementation on Fecal Microbiota in Pre-Weaned Holstein Dairy Calves in California
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Calf Management and Administration of Probiotic
2.3. Fecal Sample Collection, Sample Storage, and Fecal Scoring
2.4. DNA Extraction, 16S rRNA Sequencing, and Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Descriptive Data, Sequencing Outcomes, and Power Analysis
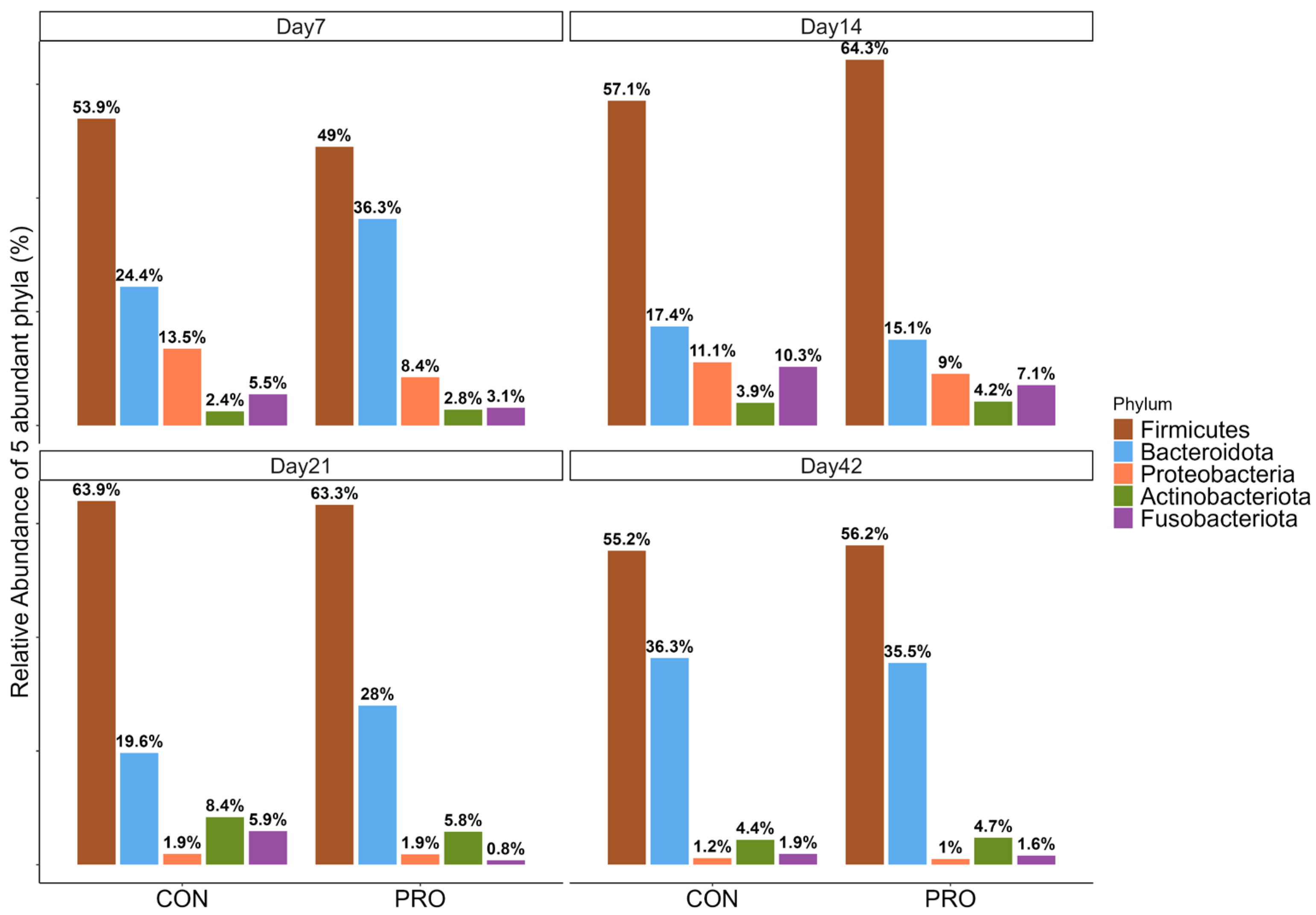
3.2. Relative Abundance
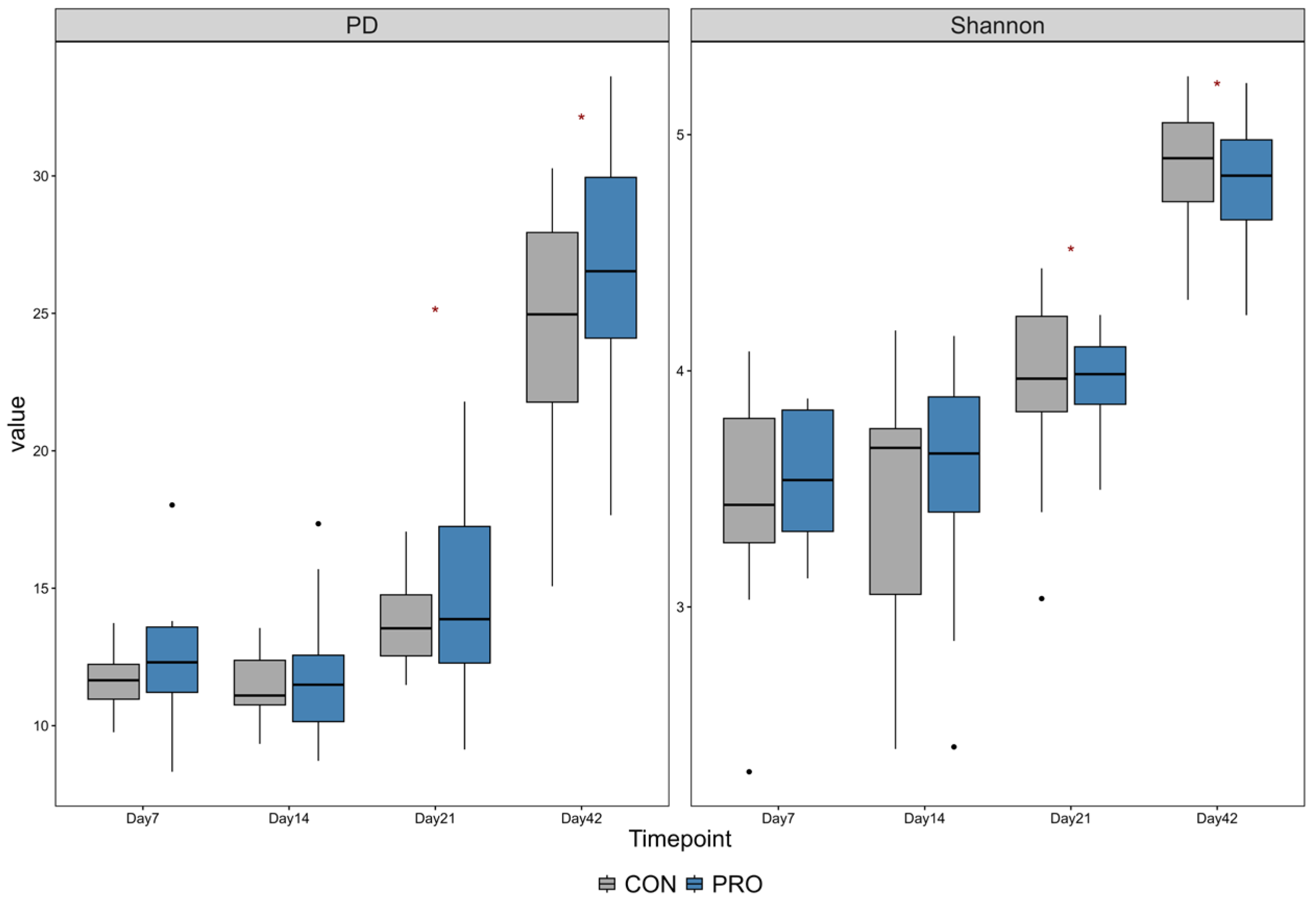
3.3. Alpha and Beta Diversity
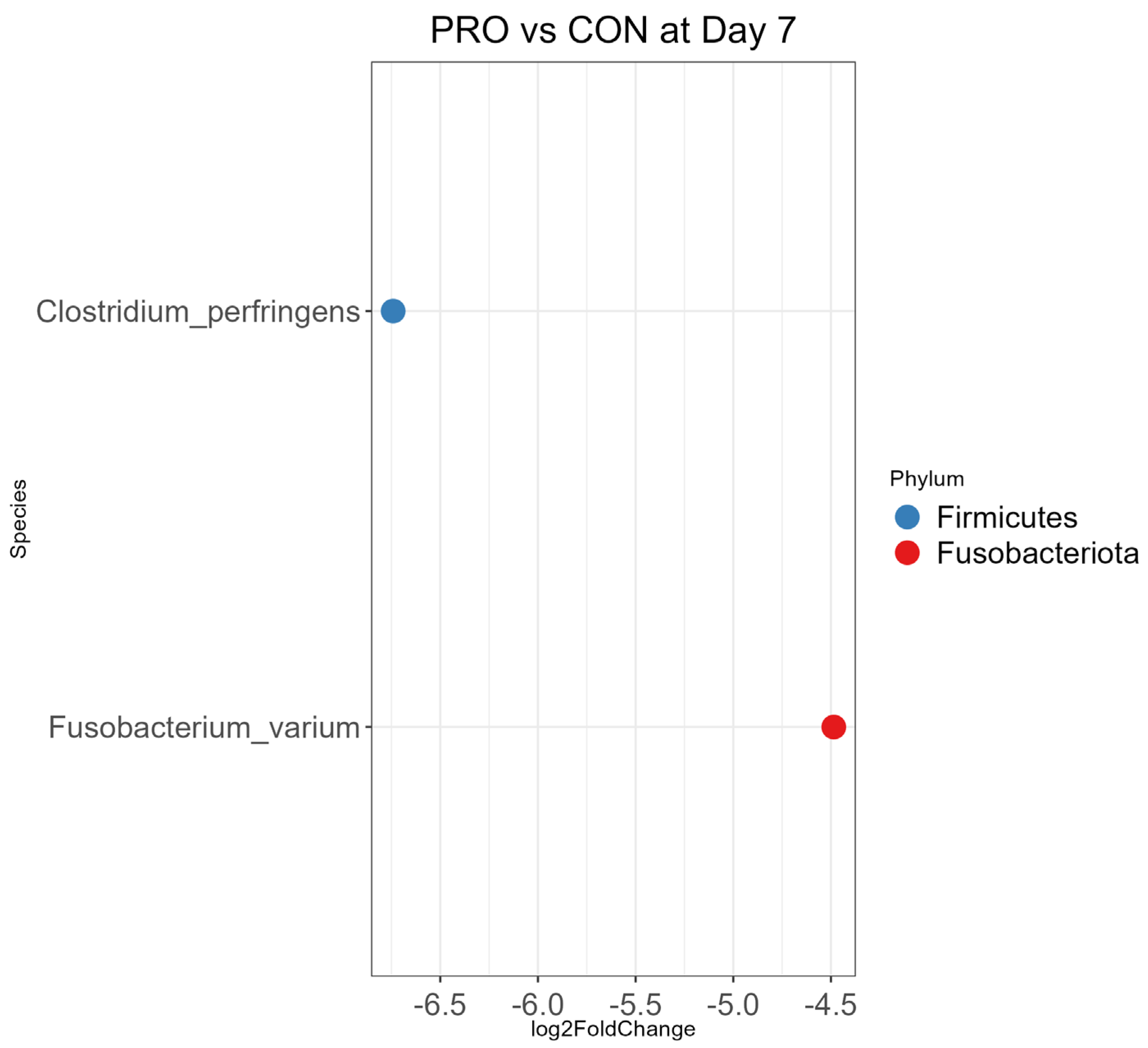
3.4. Differential Abundance Analysis
4. Discussion
4.1. Diarrhea Prevalence, Relative Abundance, and Power Analysis
4.2. Alpha and Beta Diversity
4.3. Differential Abundance Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA-APHIS-VS-CEAH-NAHMS. Dairy 2014 Health and Management Practices on U.S. Dairy Operations; s.l. 2018. 696.0218; USDA: Washington, DC, USA, 2014. [Google Scholar]
- Windeyer, M.C.; Leslie, K.E.; Godden, S.M.; Hodgins, D.C.; Lissemore, K.D.; LeBlanc, S.J. Factors Associated with Morbidity, Mortality, and Growth of Dairy Heifer Calves up to 3 Months of Age. Prev. Vet. Med. 2014, 113, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Svensson, C.; Hultgren, J. Associations between Housing, Management, and Morbidity during Rearing and Subsequent First-Lactation Milk Production of Dairy Cows in Southwest Sweden. J. Dairy Sci. 2008, 91, 1510–1518. [Google Scholar] [CrossRef]
- Kadri, S.S. Key Takeaways from the U.S. Cdc’s 2019 Antibiotic Resistance Threats Report for Frontline Providers. Crit. Care Med. 2020, 48, 939–945. [Google Scholar] [CrossRef]
- Singer, R.S.; Finch, R.; Wegener, H.C.; Bywater, R.; Walters, J.; Lipsitch, M. Antibiotic Resistance—the Interplay between Antibiotic Use in Animals and Human Beings. Lancet Infect. Dis. 2003, 3, 47–51. [Google Scholar] [CrossRef]
- Davidson, K.E.; Byrne, B.A.; Pires, A.F.A.; Magdesian, K.G.; Pereira, R.V.; Guccione, J. Antimicrobial Resistance Trends in Fecal Salmonella Isolates from Northern California Dairy Cattle Admitted to a Veterinary Teaching Hospital, 2002–2016. PLoS ONE 2018, 13, e0199928. [Google Scholar] [CrossRef]
- Cangiano, L.; Yohe, T.; Steele, M.; Renaud, D. Invited Review: Strategic use of microbial-based probiotics and probiotics in dairy calf rearing. Appl. Anim. Sci. 2020, 36, 630–651. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An Immunomodulatory Molecule of Symbiotic Bacteria Directs Maturation of the Host Immune System. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef]
- Górka, P.; Budzińska, K.; Budziński, W.; Jankowiak, T.; Kehoe, S.; Kański, J. Effect of Probiotic and Nucleotide Supplementation in Milk Replacer on Growth Performance and Fecal Bacteria in Calves. Livest. Sci. 2021, 250, 104556. [Google Scholar] [CrossRef]
- Sun, P.; Wang, J.; Zhang, H. Effects of Bacillus Subtilis Natto on Performance and Immune Function of Preweaning Calves. J. Dairy Sci. 2010, 93, 5851–5855. [Google Scholar] [CrossRef]
- Lucey, P.; Lean, I.; Aly, S.; Golder, H.; Block, E.; Thompson, J.; Rossow, H. Effects of Mannan-Oligosaccharide and Bacillus Subtilis Supplementation to Preweaning Holstein Dairy Heifers on Body Weight Gain, Diarrhea, and Shedding of Fecal Pathogens. J. Dairy Sci. 2021, 104, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.; Polukis, S.; Barnard, A.; Miller, M.; Kung, L.; Gressley, T. Evaluating the Effects of Lactobacillus Animalis and Propionibacterium Freudenreichii on Performance and Rumen and Fecal Measures in Lactating Dairy Cows. J. Dairy Sci. 2021, 104, 4119–4133. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, H.; Mulder, L.; Everts, H.; van Espen, D.; van der Wal, E.; Klaassen, G.; Rouwers, S.; Hartemink, R.; Rombouts, F.; Beynen, A. Health and Growth of Veal Calves Fed Milk Replacers with or without Probiotics. J. Dairy Sci. 2005, 88, 2154–2165. [Google Scholar] [CrossRef]
- Wang, L.; Sun, H.; Gao, H.; Xia, Y.; Zan, L.; Zhao, C. A meta-analysis on the effects of probiotics on the performance of pre-weaning dairy calves. J. Anim. Sci. Biotechnol. 2023, 14, 3. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Cao, D.; Yang, J.; Mao, H.; Sun, L.; Wang, C. Integrated multi-omics reveals the relationship between growth performance, rumen microbes and metabolic status of Hu sheep with different residual feed intakes. Anim. Nutr. 2024, 18, 284–295. [Google Scholar] [CrossRef]
- Wei, X.; Wu, H.; Wang, Z.; Zhu, J.; Wang, W.; Wang, J.; Wang, Y.; Wang, C. Rumen-protected lysine supplementation improved amino acid balance, nitrogen utilization and altered hindgut microbiota of dairy cows. Anim. Nutr. 2023, 15, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Pollock, J.; Glendinning, L.; Wisedchanwet, T.; Watson, M.; Liu, S.-J. The Madness of Microbiome: Attempting to Find Consensus ‘Best Practice’ for 16S Microbiome Studies. Appl. Environ. Microbiol. 2018, 84, e02627-17. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Rani, A.; Metwally, A.; McGee, H.S.; Perkins, D.L. Analysis of the microbiome: Advantages of whole genome shotgun versus 16S amplicon sequencing. Biochem. Biophys. Res. Commun. 2017, 469, 967–977. [Google Scholar] [CrossRef]
- Durazzi, F.; Sala, C.; Castellani, G.; Manfreda, G.; Remondini, D.; De Cesare, A. Comparison between 16S rRNA and shotgun sequencing data for the taxonomic characterization of the gut microbiota. Sci. Rep. 2021, 11, 3030. [Google Scholar] [CrossRef]
- Buczinski, S.; Gicquel, E.; Fecteau, G.; Takwoingi, Y.; Chigerwe, M.; Vandeweerd, J. Systematic review and meta—Analysis of diagnostic accuracy of serum refractometry and brix refractometry for the diagnosis of inadequate transfer of passive immunity in calves. J. Vet. Intern. Med. 2018, 32, 474–483. [Google Scholar] [CrossRef]
- Foditsch, C.; Pereira, R.V.V.; Ganda, E.K.; Gomez, M.S.; Marques, E.C.; Santin, T.; Bicalho, R.C.; Suchodolski, J.S. Oral Administration of Faecalibacterium Prausnitzii Decreased the Incidence of Severe Diarrhea and Related Mortality Rate and Increased Weight Gain in Preweaned Dairy Heifers. PLoS ONE 2015, 10, e0145485. [Google Scholar] [CrossRef]
- Feldmann, H.R.; Williams, D.R.; Champagne, J.D.; Lehenbauer, T.W.; Aly, S.S.; Raboisson, D. Effectiveness of Zinc Supplementation on Diarrhea and Average Daily Gain in Pre-Weaned Dairy Calves: A Double-Blind, Block-Randomized, Placebo-Controlled Clinical Trial. PLoS ONE 2019, 14, e0219321. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Jordan, E.B. qiime2R: Importing QIIME2 Artifacts and Associated Data into R Sessions, GitHub, version 0.99. 2018. Available online: https://github.com/jbisanz/qiime2R (accessed on 21 July 2023).
- Jari, O. Vegan: Ecological diversity. R Proj. 2013, 368, 1–11. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Champely, S. pwr: Basic Functions for Power Analysis, R package version 1.3-0. 2020. Available online: https://github.com/heliosdrm/pwr (accessed on 27 September 2024).
- Cull, C.; Singu, V.K.; Cull, B.J.; Lechtenberg, K.F.; Amachawadi, R.G.; Schutz, J.S.; Bryan, K.A. Efficacy of Lactobacillus Animalis and Propionibacterium Freudenreichii-Based Feed Additives in Reducing Salmonella-Associated Health and Performance Effects in Commercial Beef Calves. Antibiotics 2022, 11, 1328. [Google Scholar] [CrossRef] [PubMed]
- Frizzo, L.S.; Soto, L.P.; Zbrun, M.V.; Signorini, M.L.; Bertozzi, E.; Sequeira, G.; Armesto, R.R.; Rosmini, M. Effect of Lactic Acid Bacteria and Lactose on Growth Performance and Intestinal Microbial Balance of Artificially Reared Calves. Livest. Sci. 2011, 140, 246–252. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, L.; Luo, R.; Chen, H.; Nie, C.; Niu, J.; Chen, C.; Xu, Y.; Li, X.; Zhang, W. Effect of a Multispecies Probiotic Mixture on the Growth and Incidence of Diarrhea, Immune Function, and Fecal Microbiota of Pre-Weaning Dairy Calves. Front. Microbiol. 2021, 12, 681014. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Arroyo, L.; Costa, M.; Viel, L.; Weese, J. Characterization of the Fecal Bacterial Microbiota of Healthy and Diarrheic Dairy Calves. J. Vet. Intern. Med. 2017, 31, 928–939. [Google Scholar] [CrossRef]
- Fan, P.; Kim, M.; Liu, G.; Zhai, Y.; Liu, T.; Driver, J.D.; Jeong, K.C. The Gut Microbiota of Newborn Calves and Influence of Potential Probiotics on Reducing Diarrheic Disease by Inhibition of Pathogen Colonization. Front. Microbiol. 2021, 12, 772863. [Google Scholar] [CrossRef]
- Kim, E.-T.; Lee, S.-J.; Kim, T.-Y.; Lee, H.-G.; Atikur, R.M.; Gu, B.-H.; Kim, D.-H.; Park, B.-Y.; Son, J.-K.; Kim, M.-H. Dynamic Changes in Fecal Microbial Communities of Neonatal Dairy Calves by Aging and Diarrhea. Animals 2021, 11, 1113. [Google Scholar] [CrossRef]
- Júnior, G.F.V.; Coelho, M.G.; de Toledo, A.F.; Montenegro, H.; Coutinho, L.L.; Bittar, C.M.M. The Liquid Diet Composition Affects the Fecal Bacterial Community in Pre-Weaning Dairy Calves. Front. Anim. Sci. 2021, 2, 649468. [Google Scholar] [CrossRef]
- Cangiano, L.R.; Ipharraguerre, I.R.; Guan, L.L.; Buss, L.N.; Amorin-Hegedus, R.; Chirivi, M.; Contreras, G.A.; Steele, M.A. Prophylactic feeding of neomycin to Holstein calves alters gut microbiota, bile acid metabolism, and expression of genes involved in immunometabolic regulation. Front. Microbiol. 2013, 14, 1210142. [Google Scholar] [CrossRef]
- Park, T.; Cersosimo, L.M.; Li, W.; Radloff, W.; Zanton, G.I. Pre-Weaning Ruminal Administration of Differentially-Enriched, Rumen-Derived Inocula Shaped Rumen Bacterial Communities and Co-Occurrence Networks of Post-Weaned Dairy Calves. Front. Microbiol. 2021, 12, 625488. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Z.; Deng, M.; Li, Y.; Liu, G.; Liu, D.; Liu, Q.; Liu, Q.; Sun, B. Effects of a Multi-Strain Probiotic on Growth, Health, and Fecal Bacterial Flora of Neonatal Dairy Calves. Anim. Biosci. 2022, 35, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.; Marcondes, M.I.; de Souza, S.M.; Silva, B.C.d.M.e.; Noronha, M.F.; Resende, R.T.; Machado, F.S.; Mantovani, H.C.; Dill-McFarland, K.A.; Suen, G.; et al. Bacterial Community Dynamics across the Gastrointestinal Tracts of Dairy Calves during Preweaning Development. Appl. Environ. Microbiol. 2018, 84, e02675-17. [Google Scholar] [CrossRef]
- Song, Y.; Malmuthuge, N.; A Steele, M.; Guan, L.L. Shift of Hindgut Microbiota and Microbial Short Chain Fatty Acids Profiles in Dairy Calves from Birth to Pre-Weaning. FEMS Microbiol. Ecol. 2018, 94, fix179. [Google Scholar] [CrossRef]
- Cho, Y.-I.; Yoon, K.-J. An Overview of Calf Diarrhea—Infectious Etiology, Diagnosis, and Intervention. J. Vet. Sci. 2014, 15, 1–17. [Google Scholar] [CrossRef]
- Ichikawa-Seki, M.; Motooka, D.; Kinami, A.; Murakoshi, F.; Takahashi, Y.; Aita, J.; Hayashi, K.; Tashibu, A.; Nakamura, S.; Iida, T.; et al. Specific Increase of Fusobacterium in the Faecal Microbiota of Neonatal Calves Infected with Cryptosporidium Parvum. Sci. Rep. 2019, 9, 12517. [Google Scholar] [CrossRef]
- Hennessy, M.; Indugu, N.; Vecchiarelli, B.; Redding, L.; Bender, J.; Pappalardo, C.; Leibstein, M.; Toth, J.; Stefanovski, D.; Katepalli, A.; et al. Short Communication: Comparison of the Fecal Bacterial Communities in Diarrheic and Nondiarrheic Dairy Calves from Multiple Farms in Southeastern Pennsylvania. J. Dairy Sci. 2021, 104, 7225–7232. [Google Scholar] [CrossRef]
- Slanzon, G.S.; Ridenhour, B.J.; Moore, D.A.; Sischo, W.M.; Parrish, L.M.; Trombetta, S.C.; McConnel, C.S.; Abuelo, A. Fecal Microbiome Profiles of Neonatal Dairy Calves with Varying Severities of Gastrointestinal Disease. PLoS ONE 2022, 17, e0262317. [Google Scholar] [CrossRef] [PubMed]
- Fernández, S.; Fraga, M.; Castells, M.; Colina, R.; Zunino, P. Effect of the Administration of Lactobacillus Spp. Strains on Neonatal Diarrhoea, Immune Parameters and Pathogen Abundance in Pre-Weaned Calves. Benef. Microbes 2020, 11, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Zhang, H.; Yang, D.; Zhang, Y.; Xiong, B.; Jiang, L.; Loor, J.J. Illumina Sequencing Analysis of the Ruminal Microbiota in High-Yield and Low-Yield Lactating Dairy Cows. PLoS ONE 2018, 13, e0198225. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.-S.; Lkhagva, E.; Jung, S.; Kim, H.-J.; Chung, H.-J.; Hong, S.-T.; Kolla, J.N. Fecal microbiome does not represent whole gut microbiome. Cell. Microbiol. 2023, 1, 1–14. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Rossow, H.A.; Williams, D.R.; Cheong, S.; Okella, H.; Widmer, L.; Okello, E. Effect of Multi-Species Probiotic Supplementation on Fecal Microbiota in Pre-Weaned Holstein Dairy Calves in California. Microorganisms 2025, 13, 1810. https://doi.org/10.3390/microorganisms13081810
Lee Y, Rossow HA, Williams DR, Cheong S, Okella H, Widmer L, Okello E. Effect of Multi-Species Probiotic Supplementation on Fecal Microbiota in Pre-Weaned Holstein Dairy Calves in California. Microorganisms. 2025; 13(8):1810. https://doi.org/10.3390/microorganisms13081810
Chicago/Turabian StyleLee, Yoonsuk, Heidi A. Rossow, Deniece R. Williams, Sejin Cheong, Hedmon Okella, Logan Widmer, and Emmanuel Okello. 2025. "Effect of Multi-Species Probiotic Supplementation on Fecal Microbiota in Pre-Weaned Holstein Dairy Calves in California" Microorganisms 13, no. 8: 1810. https://doi.org/10.3390/microorganisms13081810
APA StyleLee, Y., Rossow, H. A., Williams, D. R., Cheong, S., Okella, H., Widmer, L., & Okello, E. (2025). Effect of Multi-Species Probiotic Supplementation on Fecal Microbiota in Pre-Weaned Holstein Dairy Calves in California. Microorganisms, 13(8), 1810. https://doi.org/10.3390/microorganisms13081810





