Effect of Enterobacter bugandensis R-18 on Maize Growth Promotion Under Salt Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Samples
2.2. Medium and Reagent
2.3. Experimental Methods
2.3.1. Isolation and Purification of the Bacterial Strains
2.3.2. Physiochemical Characterization of Bacterial Strain R-18
2.3.3. Characterization of the Growth-Promoting Capacity of Strain R-18
2.3.4. Strain R-18 DNA Sequencing and Phylogenetic Analysis
2.3.5. Pot Experiment
- (1)
- Preparation of the Bacterial Suspension
- (2)
- Different treatments of maize seedlings
- (3)
- Determination of various indexes of maize seedlings
2.4. Data Analysis
3. Results
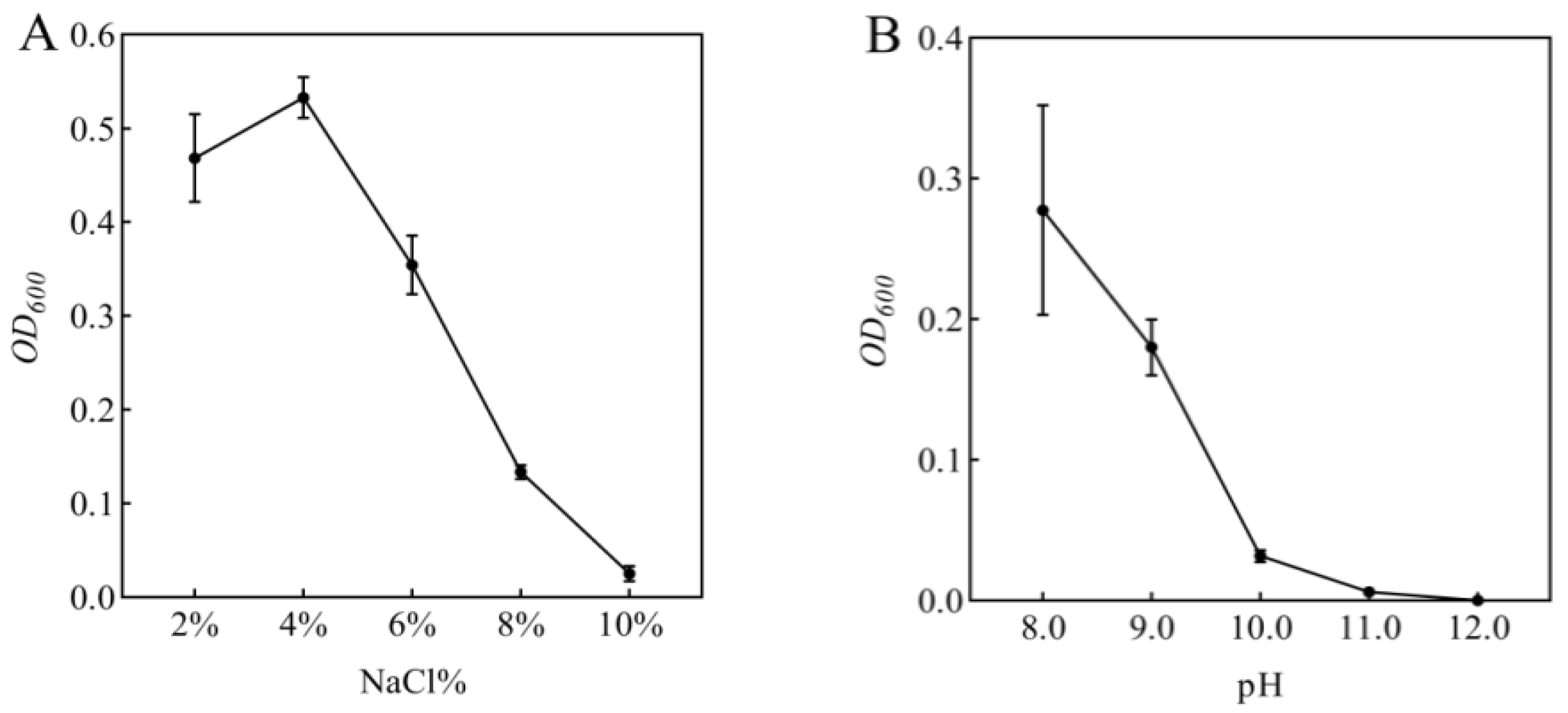
3.1. The Salt and Alkaline Tolerance of Strain R-18
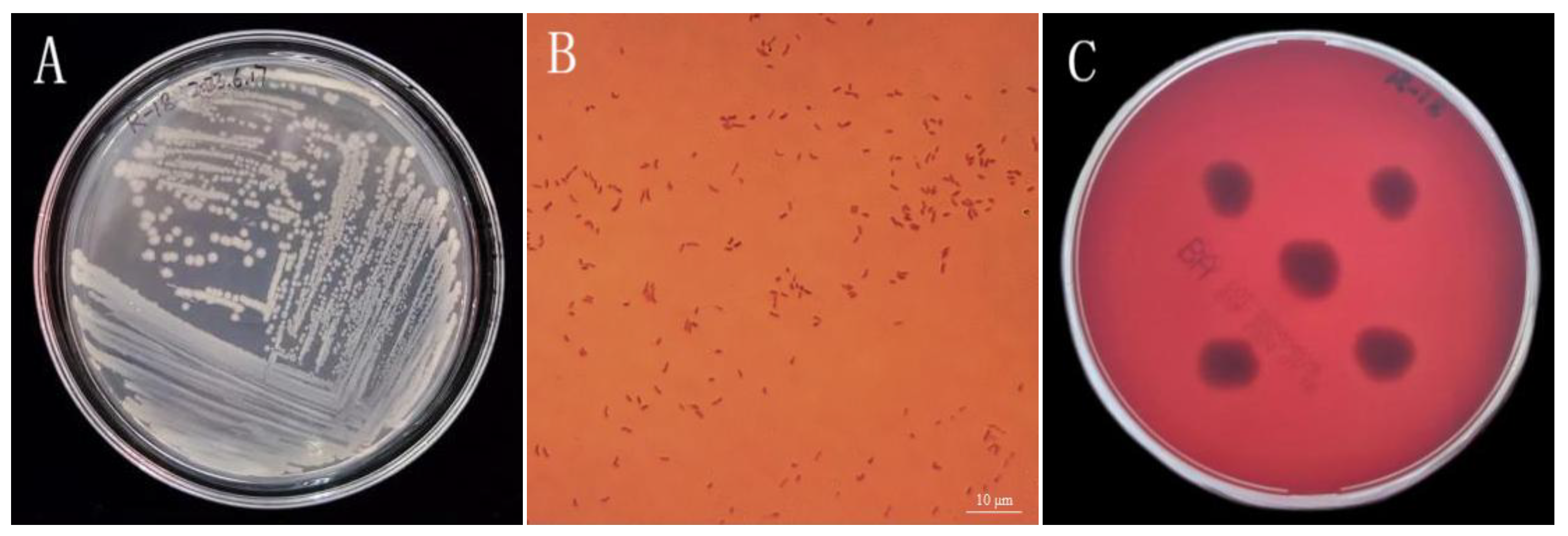
3.2. Assessment of Strain R-18 Morphology and Safety
3.3. Performance Determination of Strain R-18
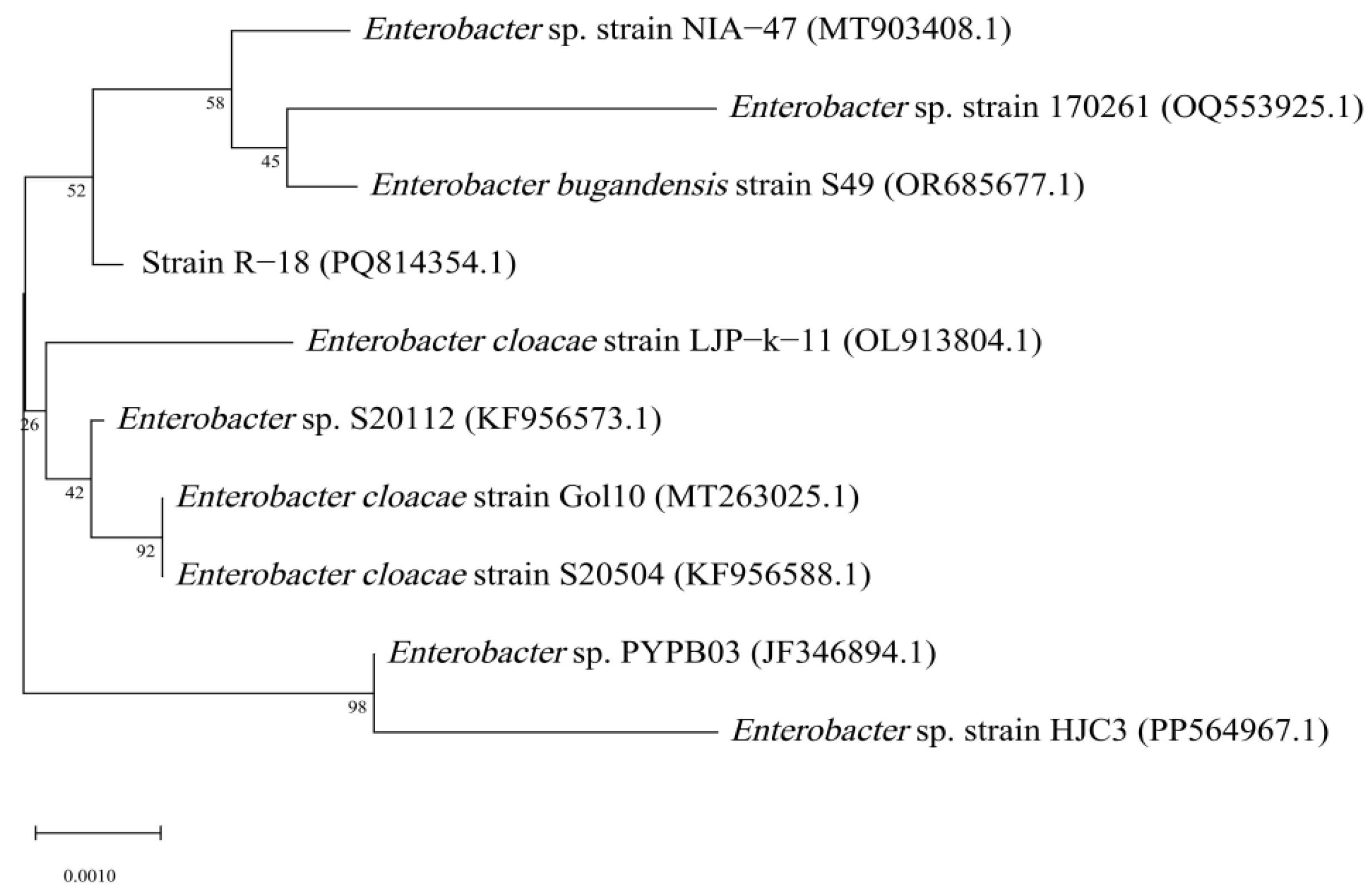
3.4. Molecular Biological Characterization of Strain R-18
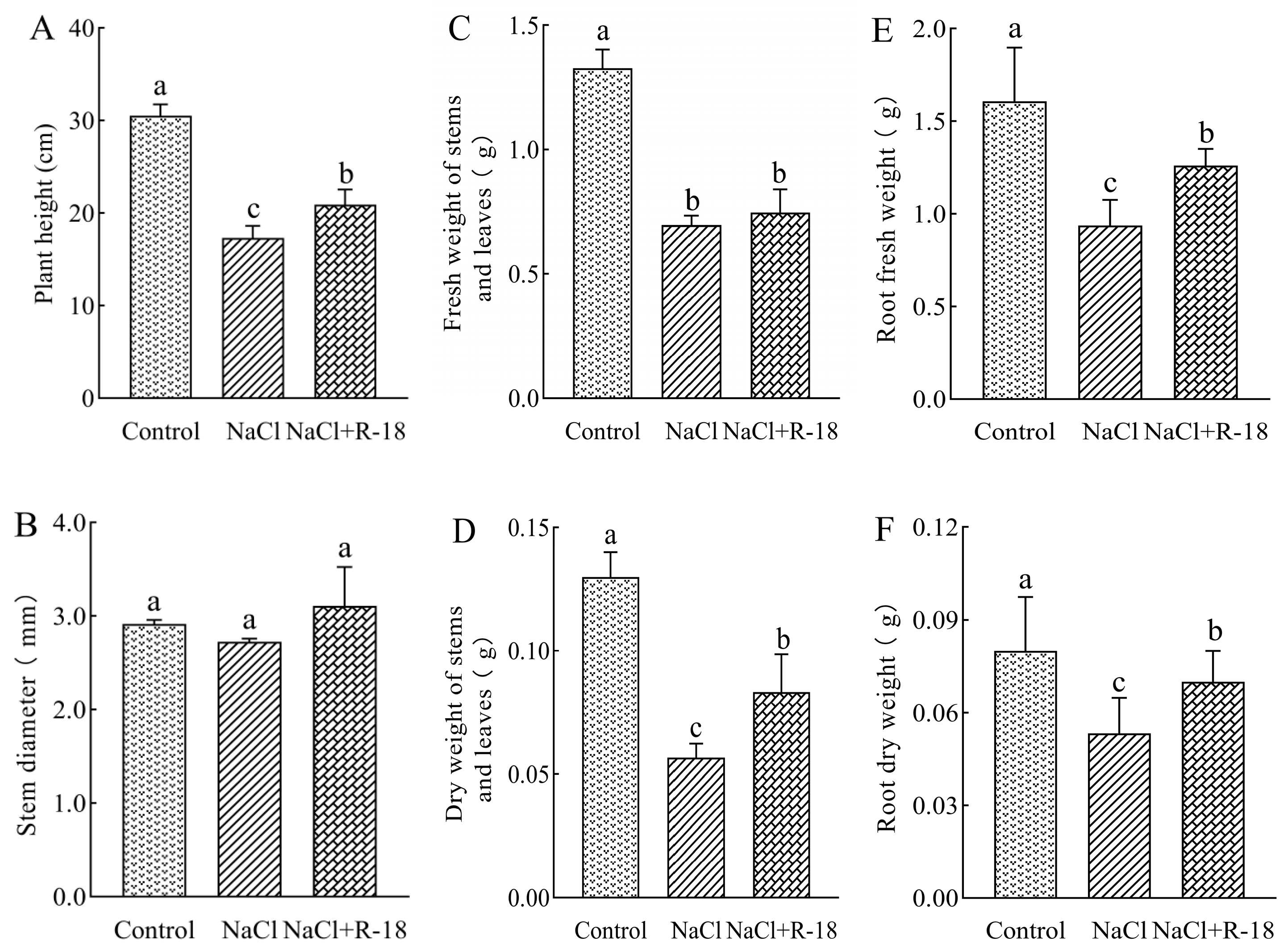
3.5. Effect of Strain R-18 on Maize Seedling Growth Index Under NaCl Stress
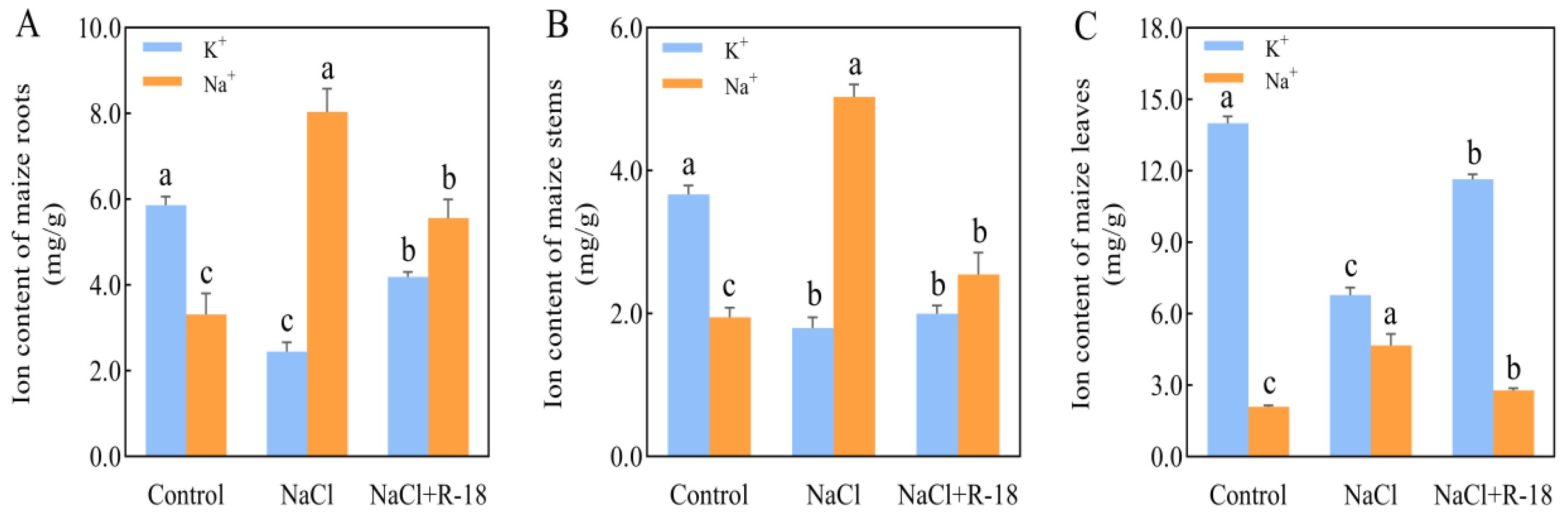
3.6. Effect of Strain R-18 on the Salt Ion Level Under 100 mM NaCl Stress
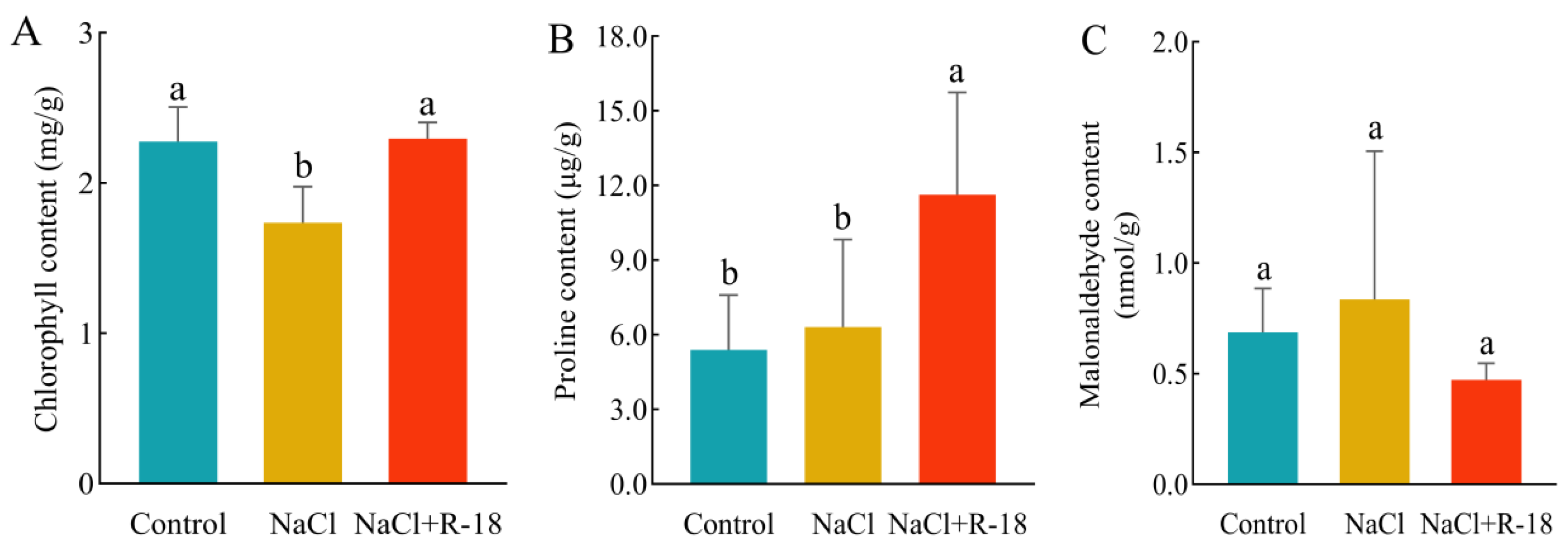
3.7. Effect of Strain R-18 on Chlorophyll, Malondialdehyde, and Proline Content in Maize Seedlings Under 100 mM NaCl Stress
3.8. Effect of Strain R-18 on Antioxidant Enzyme Activity Under 100 mM NaCl Stress in Maize Seedlings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Shang, H.G.; Han, F.; Zhang, M.R.; Li, Q.; Zhou, W.Z. Improvement of nitrogen and phosphorus availability by Pseudoalteromonas sp. during salt-washing in saline-alkali soil. Appl. Soil Ecol. 2021, 168, 9. [Google Scholar] [CrossRef]
- Yang, L.J.; Wang, Y.F.; Yang, K.J. Klebsiella variicola improves the antioxidant ability of maize seedlings under saline-alkali stress. PeerJ 2021, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Nan, L.L.; Guo, Q.N.; Cao, S.Y.; Zhan, Z.B. Diversity of bacterium communities in saline-alkali soil in arid regions of Northwest China. BMC Microbiol. 2022, 22, 12. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.F.; Qi, Y.X.; Zhang, X.; Yang, G.P. Transcriptomics and Metabolomics Analysis Revealed the Ability of Microbacterium ginsengiterrae S4 to Enhance the Saline-Alkali Tolerance of Rice (Oryza sativa L.) Seedlings. Agronomy 2024, 14, 17. [Google Scholar] [CrossRef]
- Xu, B.; Cao, L.N.; Zhang, Z.X.; Li, X.Y.; Zhao, X.Y.; Wang, X.Y.; Wang, Y.N.; Wu, B.C.; Zhou, W.H.; Lin, C.L.; et al. Physiological effects of combined NaCl and NaHCO3 stress on the seedlings of two maple species. Front. Plant Sci. 2023, 14, 9. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef]
- Bao, Y.; Zhao, R.; Li, F.; Tang, W.; Han, L. Simultaneous expression of Spinacia oleracea chloroplast choline monooxygenase (CMO) and betaine aldehyde dehydrogenase (BADH) genes contribute to dwarfism in transgenic Lolium perenne. Plant Mol. Biol. Rep. 2011, 29, 379–388. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, J.P. The role of microbes to improve crop productivity and soil health. In Ecological Wisdom Inspired Restoration Engineering; Springer: Singapore, 2019; pp. 249–265. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-tolerant plant growth-promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress. Microbiol. Res. 2021, 242, 13. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Yan, M.K.; Xue, C.W.; Xiong, Y.; Meng, X.X.; Li, B.J.; Shen, R.F.; Lan, P. Proteomic dissection of the similar and different responses of wheat to drought, salinity and submergence during seed germination. J. Proteom. 2020, 220, 14. [Google Scholar] [CrossRef]
- Yue, H.T.; Mo, W.P.; Li, C.; Zheng, Y.Y.; Li, H. The salt stress relief and growth promotion effect of Rs-5 on cotton. Plant Soil 2007, 297, 139–145. [Google Scholar] [CrossRef]
- Yue, H.T.; Sun, S.W.; Wang, R.Q.; Ma, X.Y.; Shen, S.W.; Luo, Y.Q.; Ma, X.L.; Wu, T.; Li, S.; Yang, Z.Y.; et al. Study on the mechanism of salt relief and growth promotion of Enterobacter cloacae on cotton. BMC Plant Biol. 2023, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Bezdan, D.; Checinska Sielaff, A.; Wheeler, K.; Mason, C.E.; Venkateswaran, K. Multi-drug resistant Enterobacter bugandensis species isolated from the International Space Station and comparative genomic analyses with human pathogenic strains. BMC Microbiol. 2018, 18, 175. [Google Scholar] [CrossRef]
- Cardoso, P.; Freitas, R.; Figueira, E. Salt tolerance of rhizobial populations from contrasting environmental conditions: Understanding the implications of climate change. Ecotoxicology 2015, 24, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liang, H.B.; Zhang, J.H.; Chen, Y.; Dhital, Y.P.; Zhao, T.; Wang, Z.H. Isolation and Characterization of Potassium-Solubilizing Rhizobacteria (KSR) Promoting Cotton Growth in Saline-Sodic Regions. Microorganisms 2024, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Cai, M. Common Bacterial System Identification Manual, 1st ed.; Beijing Scientific Press: Beijing, China, 2001; pp. 354–357. ISBN 7-03-008460-8. [Google Scholar]
- Chen, L.; Liu, Y.P.; Wu, G.W.; Njeri, K.V.; Shen, Q.R.; Zhang, N.; Zhang, R.F. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol. Plant. 2016, 158, 34–44. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, M.S.; Sun, Y.; Dowd, S.E.; Shi, H.Z.; Paré, P.W. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol. Plant-Microbe Interact. 2008, 21, 737–744. [Google Scholar] [CrossRef]
- Wang, Y.N.; Liang, C.Z.; Meng, Z.G.; Li, Y.Y.; Abid, M.A.; Askari, M.; Wang, P.L.; Wang, Y.; Sun, G.Q.; Cai, Y.P.; et al. Leveraging Atriplex hortensis choline monooxygenase to improve chilling tolerance in cotton. Environ. Exp. Bot. 2019, 162, 364–373. [Google Scholar] [CrossRef]
- Xiao, S.L.; Song, W.; Xing, J.F.; Su, A.G.; Zhao, Y.X.; Li, C.H.; Shi, Z.; Li, Z.Y.; Wang, S.; Zhang, R.Y.; et al. ORF355 confers enhanced salinity stress adaptability to S-type cytoplasmic male sterility maize by modulating the mitochondrial metabolic homeostasis. J. Integr. Plant Biol. 2023, 65, 656–673. [Google Scholar] [CrossRef]
- Yin, Y.J.; Chen, C.J.; Guo, S.W.; Li, K.M.; Ma, Y.N.; Sun, W.M.; Xu, F.R.; Cheng, Y.X.; Dong, X. The Fight Against Panax notoginseng Root-Rot Disease Using Zingiberaceae Essential Oils as Potential Weapons. Front. Plant Sci. 2018, 9, 14. [Google Scholar] [CrossRef]
- Li, P.S.; Kong, W.L.; Wu, X.Q. Salt Tolerance Mechanism of the Rhizosphere Bacterium JZ-GX1 and Its Effects on Tomato Seed Germination and Seedling Growth. Front. Microbiol. 2021, 12, 12. [Google Scholar] [CrossRef]
- Weinisch, L.; Kühner, S.; Roth, R.; Grimm, M.; Roth, T.; Netz, D.J.A.; Pierik, A.J.; Filker, S. Identification of osmoadaptive strategies in the halophile, heterotrophic ciliate Schmidingerothrix salinarum. PLoS. Biol. 2018, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Walia, H.; Wilson, C.; Condamine, P.; Liu, X.; Ismail, A.M.; Zeng, L.H.; Wanamaker, S.I.; Mandal, J.; Xu, J.; Cui, X.P.; et al. Comparative transcriptional profiling of two contrasting rice genotypes under salinity stress during the vegetative growth stage. Plant Physiol. 2005, 139, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Jha, P.N. Drought-tolerant Enterobacter bugandensis WRS7 induces systemic tolerance in Triticum aestivum L.(wheat) under drought conditions. J. Plant Growth Regul. 2023, 42, 7715–7730. [Google Scholar] [CrossRef]
- Chen, Y.F.; Ye, J.R.; Kong, Q.Q. Potassium-Solubilizing Activity of Bacillus aryabhattai SK1-7 and Its Growth-Promoting Effect on Populus alba L. Forests 2020, 11, 9. Forests 2020, 11, 9. [Google Scholar] [CrossRef]
- Jin, F.Y.; Hu, Q.L.; Zhao, Y.X.; Lin, X.Y.; Zhang, J.F.; Zhang, J.J. Enhancing quinoa growth under severe saline-alkali stress by phosphate solubilizing microorganism Penicillium funicuiosum P1. PLoS ONE 2022, 17, 16. [Google Scholar] [CrossRef]
- Prittesh, P.; Avnika, P.; Kinjal, P.; Jinal, H.N.; Sakthivel, K.; Amaresan, N. Amelioration effect of salt-tolerant plant growth-promoting bacteria on growth and physiological properties of rice (Oryza sativa) under salt-stressed conditions. Arch. Microbiol. 2020, 202, 2419–2428. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Toward a Sustainable Agriculture Through Plant Biostimulants: From Experimental Data to Practical Applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Sanders, W.E., Jr.; Sanders, C.C. Enterobacter spp.: Pathogens poised to flourish at the turn of the century. Clin. Microbiol. Rev. 1997, 10, 220–241. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Raimi, A.; Horn, S.; Pieters, R.; Adeleke, R. Evaluation of the Virulence and Plant Growth-Promoting Potential of Endophytic Bacteria for Improving Vegetable Production. Curr. Microbiol. 2025, 82, 374. [Google Scholar] [CrossRef]
- Meng, Y.F.; Yin, Q.; Yan, Z.J.; Wang, Y.Q.; Niu, J.M.; Zhang, J.; Fan, K. Exogenous Silicon Enhanced Salt Resistance by Maintaining K+/Na+ Homeostasis and Antioxidant Performance in Alfalfa Leaves. Front. Plant Sci. 2020, 11, 1183. [Google Scholar] [CrossRef]
- Li, L.; Ward, D.M. Iron toxicity in yeast: Transcriptional regulation of the vacuolar iron importer Ccc1. Curr. Genet. 2018, 64, 413–416. [Google Scholar] [CrossRef]
- Chen, Z.; Pottosin, I.I.; Cuin, T.A.; Fuglsang, A.T.; Tester, M.; Jha, D.; Zepeda-Jazo, I.; Zhou, M.; Palmgren, M.G.; Newman, I.A.; et al. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiol. 2007, 145, 1714–1725. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, N.; Pang, Q.; Khan, R.A.A.; Xu, Q.; Wu, C.; Liu, T. A Salt-Tolerant Strain of Trichoderma longibrachiatum HL167 Is Effective in Alleviating Salt Stress, Promoting Plant Growth, and Managing Fusarium Wilt Disease in Cowpea. J. Fungi. 2023, 9, 304. [Google Scholar] [CrossRef]
- Anwar, N.; Jiang, Y.; Ma, W.; Yao, Y.; Li, J.; Ababaikeli, G.; Li, G.; Ma, T. Culturable bacteria diversity in stem liquid and resina from Populus euphratica and screening of plant growth-promoting bacteria. BMC Microbiol. 2022, 22, 322. [Google Scholar] [CrossRef]
- Yue, H.; Zhao, L.; Yang, D.; Zhang, M.; Wu, J.; Zhao, Z.; Xing, X.; Zhang, L.; Qin, Y.; Guo, F.; et al. Comparative Analysis of the Endophytic Bacterial Diversity of Populus euphratica Oliv. in Environments of Different Salinity Intensities. Microbiol. Spectr. 2022, 10, e0050022. [Google Scholar] [CrossRef]
- Khan, V.; Umar, S.; Iqbal, N. Palliating Salt Stress in Mustard Through Plant-Growth-Promoting Rhizobacteria: Regulation of Secondary Metabolites, Osmolytes, Antioxidative Enzymes and Stress Ethylene. Plants 2023, 12, 705. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Druzhinina, I.S.; Pan, X.Y.; Yuan, Z.L. Microbially Mediated Plant Salt Tolerance and Microbiome-Based Solutions for Saline Agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.Q.; Lü, X.P.; Bai, J.P.; Qiao, Y.; Paré, P.W.; Wang, S.M.; Zhang, J.L.; Wu, Y.N.; Pang, X.P.; Xu, W.B.; et al. Beneficial soil bacterium Bacillus subtilis (GB03) augments salt tolerance of white clover. Front. Plant Sci. 2014, 5, 525. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Khan, M.A.; Sahile, A.A.; Jan, R.; Asaf, S.; Hamayun, M.; Imran, M.; Adhikari, A.; Kang, S.M.; Kim, K.M.; Lee, I.J. Halotolerant bacteria mitigate the effects of salinity stress on soybean growth by regulating secondary metabolites and molecular responses. BMC Plant Biol. 2021, 21, 15. [Google Scholar] [CrossRef]
- Cruz, C.; Cardoso, P.; Santos, J.; Matos, D.; Sa, C.; Figueira, E. Application of Plant Growth-Promoting Bacteria from Cape Verde to Increase Maize Tolerance to Salinity. Antioxidants 2023, 12, 21. [Google Scholar] [CrossRef]
- Jha, Y.; Subramanian, R.B. PGPR regulate caspase-like activity, programmed cell death, and antioxidant enzyme activity in paddy under salinity. Physiol. Mol. Biol. Plants 2014, 20, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Ouhaddou, R.; Ben-Laouane, R.; Lahlali, R.; Anli, M.; Ikan, C.; Boutasknit, A.; Slimani, A.; Oufdou, K.; Baslam, M.; Barka, E.A.; et al. Application of Indigenous Rhizospheric Microorganisms and Local Compost as Enhancers of Lettuce Growth, Development, and Salt Stress Tolerance. Microorganisms 2022, 10, 23. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Li, L.; Lindström, K.; Räsänen, L.A. A synergistic interaction between salt-tolerant Pseudomonas and Mesorhizobium strains improves growth and symbiotic performance of liquorice (Glycyrrhiza uralensis Fish.) under salt stress. Appl. Microbiol. Biotechnol. 2016, 100, 2829–2841. [Google Scholar] [CrossRef]
- Wang, M.Q.; Gong, S.C.; Fu, L.X.; Hu, G.H.; Li, G.L.; Hu, S.X.; Yang, J.F. The Involvement of Antioxidant Enzyme System, Nitrogen Metabolism and Osmoregulatory Substances in Alleviating Salt Stress in Inbred Maize Lines and Hormone Regulation Mechanisms. Plants 2022, 11, 21. [Google Scholar] [CrossRef]
- Rashedy, A.A.; Abd-ElNafea, M.H.; Khedr, E.H. Co-application of proline or calcium and humic acid enhances productivity of salt stressed pomegranate by improving nutritional status and osmoregulation mechanisms. Sci. Rep. 2022, 12, 10. [Google Scholar] [CrossRef]
- Abbas, R.; Rasul, S.; Aslam, K.; Baber, M.; Shahid, M.; Mubeen, F.; Naqqash, T. Halotolerant PGPR: A hope for cultivation of saline soils. J. King Saud Univ. Sci. 2019, 31, 1195–1201. [Google Scholar] [CrossRef]
- Li, H.Q.; Jiang, X.W. Inoculation with plant growth-promoting bacteria (PGPB) improves salt tolerance of maize seedling. Russ. J. Plant Physiol. 2017, 64, 235–241. [Google Scholar] [CrossRef]
- Ali, B.; Hafeez, A.; Ahmad, S.; Javed, M.A.; Sumaira, M.S.; Afridi, M.S.M.; Dawoud, T.M.S.; Almaary, K.S.; Muresan, C.C.; Marc, R.A.; et al. Bacillus thuringiensis PM25 ameliorates oxidative damage of salinity stress in maize via regulating growth, leaf pigments, antioxidant defense system, and stress responsive gene expression. Front. Plant Sci. 2022, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.K.; Singh, J.S.; Saxena, A.K.; Singh, D.P. Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol. 2012, 14, 605–611. [Google Scholar] [CrossRef]


| Test Indicators | Results | Test Indicators | Results |
|---|---|---|---|
| Gram stain | − | Cellulose hydrolysis | − |
| Methylic-red test | − | Nitrogen fixation | + |
| V–P test | + | Organophosphorus solubilizing | + |
| Catalase activity | + | Inorganic phosphorus | + |
| Citrate test | + | Potassium dissolving | + |
| Fluorescent pigment | + | Siderophore production | − |
| Hydrolysis of starch | + | ACC deaminase activity | + |
| Gelatin liquefaction | + | IAA production | + |
| Ammonia production test | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Liu, Q.; Song, J.; Zhang, X.; Yang, G.; Li, M.; Qu, H.; Tastanbek, A.; Tan, Y. Effect of Enterobacter bugandensis R-18 on Maize Growth Promotion Under Salt Stress. Microorganisms 2025, 13, 1796. https://doi.org/10.3390/microorganisms13081796
Tian X, Liu Q, Song J, Zhang X, Yang G, Li M, Qu H, Tastanbek A, Tan Y. Effect of Enterobacter bugandensis R-18 on Maize Growth Promotion Under Salt Stress. Microorganisms. 2025; 13(8):1796. https://doi.org/10.3390/microorganisms13081796
Chicago/Turabian StyleTian, Xingguo, Qianru Liu, Jingjing Song, Xiu Zhang, Guoping Yang, Min Li, Huan Qu, Ahejiang Tastanbek, and Yarong Tan. 2025. "Effect of Enterobacter bugandensis R-18 on Maize Growth Promotion Under Salt Stress" Microorganisms 13, no. 8: 1796. https://doi.org/10.3390/microorganisms13081796
APA StyleTian, X., Liu, Q., Song, J., Zhang, X., Yang, G., Li, M., Qu, H., Tastanbek, A., & Tan, Y. (2025). Effect of Enterobacter bugandensis R-18 on Maize Growth Promotion Under Salt Stress. Microorganisms, 13(8), 1796. https://doi.org/10.3390/microorganisms13081796






