Antibiotics and Antibiotic Resistance Genes in the Environment: Dissemination, Ecological Risks, and Remediation Approaches
Abstract
1. Introduction
2. Overview of Antibiotic Contamination in the Environment
2.1. Current Status of Using Antibiotics
2.2. Main Pollution Sources of Antibiotics
2.2.1. Aquaculture
2.2.2. Livestock and Poultry Field
2.3. Hazards of Antibiotic Overuse
2.3.1. Generation and Spread of ARGs
2.3.2. Microbial Community Imbalance
2.3.3. Destruction of Ecological Systems
3. Overview of ARGs Pollution in the Environment
3.1. Pollution Sources of ARGs
3.1.1. Aquaculture
3.1.2. Wastewater
3.1.3. Animal Husbandry
3.1.4. Soil
3.2. Transmission Mechanisms of ARGs
3.2.1. Conjugation
3.2.2. Transformation
3.2.3. Transduction
4. Removals for Antibiotics and Degradation ARGs in Environments
4.1. Adsorption Method
4.1.1. Adsorbent Materials
- Biochar
- Activated Carbons (ACs)
- Carbon Nanotubes (CNTs)
- Graphene
4.1.2. Adsorption Mechanisms
- Electrostatic Interactions
- Hydrophobic Interactions
- Pore Filling
- Hydrogen Bonding
- π-π Interactions
4.2. Chemical Methods (AOPs)
4.2.1. Photolysis
4.2.2. Electrochemical Oxidation (EO)
4.2.3. Fenton/Fenton-like Technology
4.3. Biological Methods
4.3.1. Microbial Degradation
4.3.2. Constructed Wetlands
5. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, E.Y.; Impalli, I.; Poleon, S.; Denoel, P.; Cipriano, M.; Boeckel, T.P.V.; Pecetta, S.; Bloom, D.E.; Nandi, A. Global trends in antibiotic consumption during 2016-2023 and future projections through 2030. Proc. Natl. Acad. Sci. USA 2024, 121, e2411919121. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.K.; Yu, J.P.; Li, C.; Zhu, Q.J.; Zhang, Y.S.; Lichtfouse, E.; Marmier, N. The Effect Review of Various Biological, Physical and Chemical Methods on the Removal of Antibiotics. Water 2022, 14, 3138. [Google Scholar] [CrossRef]
- Bondad-Reantaso, M.G.; MacKinnon, B.; Karunasagar, I.; Fridman, S.; Alday-Sanz, V.; Brun, E.; Le Groumellec, M.; Li, A.H.; Surachetpong, W.; Karunasagar, I.; et al. Review of alternatives to antibiotic use in aquaculture. Rev. Aquac. 2023, 15, 1421–1451. [Google Scholar] [CrossRef]
- Sazykin, I.S.; Khmelevtsova, L.E.; Seliverstova, E.Y.; Sazykina, M.A. Effect of Antibiotics Used in Animal Husbandry on the Distribution of Bacterial Drug Resistance (Review). Appl. Biochem. Microbiol. 2021, 57, 20–30. [Google Scholar] [CrossRef]
- Jiang, C.X.; Zhao, Z.L.; Zhu, D.; Pan, X.; Yang, Y.Y. Rare resistome rather than core resistome exhibited higher diversity and risk along the Yangtze River. Water Res. 2024, 249, 210911. [Google Scholar] [CrossRef]
- Huang, F.Y.; Zou, S.Z.; Deng, D.D.; Lang, H.; Liu, F. Antibiotics in a typical karst river system in China: Spatiotemporal variation and environmental risks. Sci. Total Environ. 2019, 650, 1348–1355. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Zhou, X.Q.; Cuasquer, G.J.P.; Li, Z.F.; Mang, H.P.; Lv, Y.P. Occurrence of typical antibiotics, representative antibiotic-resistant bacteria, and genes in fresh and stored source-separated human urine. Environ. Int. 2021, 146, 106280. [Google Scholar] [CrossRef]
- MacLauchlin, C.; Schneider, S.E.; Keedy, K.; Fernandes, P.; Jamieson, B.D. Metabolism, Excretion, and Mass Balance of Solithromycin in Humans. Antimicrob. Agents Chemother. 2018, 62, e01474-17. [Google Scholar] [CrossRef]
- Malakootian, M.; Yaseri, M.; Faraji, M. Removal of antibiotics from aqueous solutions by nanoparticles: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. 2019, 26, 8444–8458. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Nogrady, B. The fight against antimicrobial resistance. Nature 2023, 624, S30–S32. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, J.J.; Zhu, Y.G.; Chen, Q.L.; Shen, F.X.; Wu, Y.; Xu, S.Y.; Fan, H.; Da, G.; Huang, R.J.; et al. Global Survey of Antibiotic Resistance Genes in Air. Environ. Sci. Technol. 2018, 52, 10975–10984. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, A.; Khiadani, M.; Foroughi, M.; Siuki, H.A.; Mehrfar, H. Wastewater treatment plants: The missing link in global One-Health surveillance and management of antibiotic resistance. J. Infect. Public Health 2023, 16, 217–224. [Google Scholar] [CrossRef]
- Yuan, X.; Lv, Z.Q.; Zhang, Z.Y.; Han, Y.; Liu, Z.Q.; Zhang, H.J. A Review of Antibiotics, Antibiotic Resistant Bacteria, and Resistance Genes in Aquaculture: Occurrence, Contamination, and Transmission. Toxics 2023, 11, 420. [Google Scholar] [CrossRef]
- Millan, A.S.; Maclean, R.C. Fitness Costs of Plasmids: A Limit to Plasmid Transmission. Microbiol. Spectr. 2017, 5, 10–1128. [Google Scholar] [CrossRef]
- Foster, T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef]
- Wang, Q.; Geng, L.L.; Gao, Z.; Sun, Y.; Li, X.L.; Sun, S.J.; Luo, Y. Microalgae Enhances the Adaptability of Epiphytic Bacteria to Sulfamethoxazole Stress and Proliferation of Antibiotic Resistance Genes Mediated by Integron. Environ. Sci. Technol. 2024, 58, 19397–19407. [Google Scholar] [CrossRef]
- Tang, T.T.; Chen, Y.; Du, Y.; Yao, B.; Liu, M. Effects of functional modules and bacterial clusters response on transmission performance of antibiotic resistance genes under antibiotic stress during anaerobic digestion of livestock wastewater. J. Hazard. Mater. 2023, 441, 129870. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.P.; Peng, Y.; Zhang, M.K.; Wen, Y.X.; Lu, X.; Kan, B. Genomic diversity of mcr-carrying plasmids and the role of type IV secretion systems in IncI2 plasmids conjugation. Commun. Biol. 2025, 8, 342. [Google Scholar] [CrossRef]
- King, A.M.; Reid-Yu, S.A.; Wang, W.L.; King, D.T.; De Pascale, G.; Strynadka, N.C.; Walsh, T.R.; Coombes, B.K.; Wright, G.D. Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature 2014, 510, 503. [Google Scholar] [CrossRef] [PubMed]
- Jurcisek, J.A.; Brockman, K.L.; Novotny, L.A.; Goodman, S.D.; Bakaletz, L.O. Nontypeable Haemophilus influenzae releases DNA and DNABII proteins via a T4SS-like complex and ComE of the type IV pilus machinery. Proc. Natl. Acad. Sci. USA 2017, 114, E6632–E6641. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, M.L.; Bao, C.X.; Wu, J.P.; Zhou, J.; He, W.C.; Shi, X.C.; Li, G. Application of Pig Manure Compost with Different Biochar Modifies the Antibiotic Resistome and Bacterial Community in Agriculture Soil. Water Air Soil Pollut. 2022, 233, 108. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Wu, Y.; Hu, J.; Zhang, Y.; Sun, Q.; Sun, W.; Geng, J.; Liu, X.; Jia, D.; et al. Antibiotics in global rivers. Natl. Sci. Open 2022, 1, 20220029. [Google Scholar] [CrossRef]
- Ren, H.; Qin, M.; Zhang, L.; Li, Z.; Li, Y.; He, Q.; Zhong, J.; Zhao, D.; Lian, X.; Jiang, H.; et al. Modular Engineering of a Synthetic Biology-Based Platform for Sustainable Bioremediation of Residual Antibiotics in Aquatic Environments. Engineering 2025, in press. [Google Scholar] [CrossRef]
- Danner, M.C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef]
- Boreen, A.L.; Arnold, W.A.; McNeill, K. Photochemical fate of sulfa drugs in the aquatic environment: Sulfa drugs containing five-membered heterocyclic groups. Environ. Sci. Technol. 2004, 38, 3933–3940. [Google Scholar] [CrossRef]
- Liu, P.X.; Zhang, H.M.; Feng, Y.J.; Shen, C.; Yang, F.L. Influence of spacer on rejection of trace antibiotics in wastewater during forward osmosis process. Desalination 2015, 371, 134–143. [Google Scholar] [CrossRef]
- Batista, A.P.S.; Pires, F.C.C.; Teixeira, A. Photochemical degradation of sulfadiazine, sulfamerazine and sulfamethazine: Relevance of concentration and heterocyclic aromatic groups to degradation kinetics. J. Photochem. Photobiol. A Chem. 2014, 286, 40–46. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, W.; Li, J.Y.; Yuan, M.Z.; Zhang, J.H.; Xu, F.; Xu, H.T.; Zheng, X.Y.; Wang, L.Q. Ecotoxicological effects of sulfonamides and fluoroquinolones and their removal by a green alga (Chlorella vulgaris) and a cyanobacterium (Chrysosporum ovalisporum). Environ. Pollut. 2020, 263, 114554. [Google Scholar] [CrossRef]
- Pulicharla, R.; Hegde, K.; Brar, S.K.; Surampalli, R.Y. Tetracyclines metal complexation: Significance and fate of mutual existence in the environment. Environ. Pollut. 2017, 221, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Wang, J.; Li, S. Tetracycline antibiotics in agricultural soil: Dissipation kinetics, transformation pathways, and structure-related toxicity. Sci. Total Environ. 2024, 949, 175126. [Google Scholar] [CrossRef] [PubMed]
- Gopal, G.; Alex, S.A.; Chandrasekaran, N.; Mukherjee, A. A review on tetracycline removal from aqueous systems by advanced treatment techniques. RSC Adv. 2020, 10, 27081–27095. [Google Scholar] [CrossRef] [PubMed]
- McFarland, J.W.; Berger, C.M.; Froshauer, S.A.; Hayashi, S.F.; Hecker, S.J.; Jaynes, B.H.; Jefson, M.R.; Kamicker, B.J.; Lipinski, C.A.; Lundy, K.M.; et al. Quantitative structure-activity relationships among macrolide antibacterial agents: In vitro and in vivo potency against Pasteurella multocida. J. Med. Chem. 1997, 40, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Kortesmäki, E.; Östman, J.R.; Meierjohann, A.; Brozinski, J.M.; Eklund, P.; Kronberg, L. Occurrence of Antibiotics in Influent and Effluent from 3 Major Wastewater-Treatment Plants in Finland. Environ. Toxicol. Chem. 2020, 39, 1774–1789. [Google Scholar] [CrossRef] [PubMed]
- Settimo, L.; Bellman, K.; Knegtel, R.M.A. Comparison of the Accuracy of Experimental and Predicted pKa Values of Basic and Acidic Compounds. Pharm. Res. 2014, 31, 1082–1095. [Google Scholar] [CrossRef]
- Ngigi, A.N.; Magu, M.M.; Muendo, B.M. Occurrence of antibiotics residues in hospital wastewater, wastewater treatment plant, and in surface water in Nairobi County, Kenya. Environ. Monit. Assess. 2020, 192, 18. [Google Scholar] [CrossRef]
- Jones, O.A.H.; Voulvoulis, N.; Lester, J.N. Aquatic environmental assessment of the top 25 English prescription pharmaceuticals. Water Res. 2002, 36, 5013–5022. [Google Scholar] [CrossRef]
- Li, G.A.; Wang, Y.L.; Sun, C.Y.; Liu, F. Determination of the microscopic acid dissociation constant of piperacillin and identification of dissociated molecular forms. Front. Chem. 2023, 11, 1177128. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Guironnet, A.; Sanchez-Cid, C.; Vogel, T.M.; Wiest, L.; Vulliet, E. Aminoglycosides analysis optimization using ion pairing liquid chromatography coupled to tandem mass spectrometry and application on wastewater samples. J. Chromatogr. A 2021, 1651, 462133. [Google Scholar] [CrossRef] [PubMed]
- Muhamadejevs, R.; Haldimann, K.; Gysin, M.; Crich, D.; Jaudzems, K.; Hobbie, S.N. Experimental Determination of the pK a Values of Clinically Relevant Aminoglycoside Antibiotics: Toward Establishing pK a-Activity Relationships. ACS Omega 2024, 9, 5876–5887. [Google Scholar] [CrossRef] [PubMed]
- He, J.S.; Fu, X.; Ni, F.; Yang, G.; Deng, S.H.; Chen, J.P.; Shen, F. Quantitative assessment of interactions of hydrophilic organic contaminants with microplastics in natural water environment. Water Res. 2022, 224, 119024. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim, L.H.; Zoh, K.D. Removal characteristics and mechanism of antibiotics using constructed wetlands. Ecol. Eng. 2016, 91, 85–92. [Google Scholar] [CrossRef]
- Yan, B.; Niu, C.H. Modeling and site energy distribution analysis of levofloxacin sorption by biosorbents. Chem. Eng. J. 2017, 307, 631–642. [Google Scholar] [CrossRef]
- Le, T.H.; Truong, T.; Tran, L.T.; Nguyen, D.H.; Pham, T.P.T.; Ng, C. Antibiotic resistance in the aquatic environments: The need for an interdisciplinary approach. Int. J. Environ. Sci. Technol. 2023, 20, 3395–3408. [Google Scholar] [CrossRef]
- Imtiaz, N.; Anwar, Z.; Waiho, K.; Shi, C.; Mu, C.K.; Wang, C.L.; Wu, Q.Y. A review on aquaculture adaptation for fish treatment from antibiotic to vaccine prophylaxis. Aquac. Int. 2024, 32, 2643–2668. [Google Scholar] [CrossRef]
- Wenning, R. The State Of World Fisheries And Aquaculture (Sofia) 2020 Report; Food and Agriculture Organization of the United Nation: Rome, Italy, 2020; Volume 16, pp. 800–801. [Google Scholar]
- Limbu, S.M.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. A global analysis on the systemic effects of antibiotics in cultured fish and their potential human health risk: A review. Rev. Aquac. 2021, 13, 1015–1059. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Limbu, S.M.; Qiao, F.; Du, Z.Y.; Zhang, M.L. Influence of Long-Term Feeding Antibiotics on the Gut Health of Zebrafish. Zebrafish 2018, 15, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.Y.; An, Z.Y.; Moran, M.J.; Liu, F. Recognition of typical antibiotic residues in environmental media related to groundwater in China (2009–2019). J. Hazard. Mater. 2020, 399, 122813. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Feng, F.; Chai, Y.F.; Meng, X.S.; Sui, Q.W.; Chen, M.X.; Wei, Y.S.; Qi, K.M. Screening and quantitation of residual antibiotics in two different swine wastewater treatment systems during warm and cold seasons. Sci. Total Environ. 2019, 660, 1542–1554. [Google Scholar] [CrossRef]
- Gbadegesin, L.A.; Tang, X.Y.; Liu, C.; Cheng, J.H. Transport of Veterinary Antibiotics in Farmland Soil: Effects of Dissolved Organic Matter. Int. J. Environ. Res. Public Health 2022, 19, 1702. [Google Scholar] [CrossRef]
- Qian, M.R.; Wu, H.Z.; Wang, J.M.; Zhang, H.; Zhang, Z.L.; Zhang, Y.Z.; Lin, H.; Ma, J.W. Occurrence of trace elements and antibiotics in manure- based fertilizers from the Zhejiang Province of China. Sci. Total Environ. 2016, 559, 174–181. [Google Scholar] [CrossRef]
- Ramesh, N.; Tripathi, H. Antibiotic usage practice and knowledge on antimicrobial resistance among livestock and poultry farmers of Telangana state, India. Indian J. Anim. Sci. 2022, 92, 166–173. [Google Scholar] [CrossRef]
- Cox, L.A.; Popken, D.A.; Sun, J.; Liao, X.P.; Fang, L.X. Quantifying Human Health Risks from Virginiamycin Use in Food Animals in China. Risk Anal. 2020, 40, 1244–1257. [Google Scholar] [CrossRef]
- Teillant, A.; Brower, C.H.; Laxminarayan, R. Economics of Antibiotic Growth Promoters in Livestock. In Annual Review of Resource Economics; Rausser, G.C., Ed.; Annual Review of Resource Economics: Palo Alto, CA, USA, 2015; Volume 7, pp. 349–374. [Google Scholar]
- Gothwal, R.; Shashidhar, T. Antibiotic Pollution in the Environment: A Review. Clean-Soil Air Water 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Jia, W.-L.; Song, C.; He, L.-Y.; Wang, B.; Gao, F.-Z.; Zhang, M.; Ying, G.-G. Antibiotics in soil and water: Occurrence, fate, and risk. Curr. Opin. Environ. Sci. Health 2023, 32, 100437. [Google Scholar] [CrossRef]
- Jia, S.Y.; Zhang, X.X.; Miao, Y.; Zhao, Y.T.; Ye, L.; Li, B.; Zhang, T. Fate of antibiotic resistance genes and their associations with bacterial community in livestock breeding wastewater and its receiving river water. Water Res. 2017, 124, 259–268. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Ye, C.S.; Li, J.G.; Yu, X. Increased risk of antibiotic resistance in surface water due to global warming. Environ. Res. 2024, 263, 120149. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wu, C.X.; Zhou, D.S.; Hu, L.F.; Mu, K.; Yin, Z. Insights into incompatible plasmids in multidrug-resistant Raoultella superbugs. BMC Microbiol. 2025, 25, 55. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.E.; Ma, X.D.; Zeng, L.S.; Wang, Q.Q.; Li, M.C.; Teng, L.; He, M.Z.; Liu, C.; Zhao, M.S.; Wang, M.Z.; et al. Interphylum dissemination of NDM-5-positive plasmids in hospital wastewater from Fuzhou, China: A single-centre, culture-independent, plasmid transmission study. Lancet Microbe 2024, 5, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.R.; Li, S.; Zhang, W.; Helbling, D.E.; Xu, N.; Sun, W.L.; Ni, J.R. Animal production predominantly contributes to antibiotic profiles in the Yangtze River. Water Res. 2023, 242, 120214. [Google Scholar] [CrossRef]
- Limbu, S.M.; Zhou, L.; Sun, S.X.; Zhang, M.L.; Du, Z.Y. Chronic exposure to low environmental concentrations and legal aquaculture doses of antibiotics cause systemic adverse effects in Nile tilapia and provoke differential human health risk. Environ. Int. 2018, 115, 205–219. [Google Scholar] [CrossRef]
- Ahmed, Z.S.; Hashad, M.E.; Atef, Y.; Badr, H.; Elhariri, M.; Kadry, M. Public health threat of antimicrobial resistance and virulence genes in Escherichia coli from human-chicken transmission in Egypt. Sci. Rep. 2025, 15, 12627. [Google Scholar] [CrossRef]
- Liao, H.P.; Liu, C.; Zhou, S.G.; Liu, C.Q.; Eldridge, D.J.; Ai, C.F.; Wilhelm, S.W.; Singh, B.K.; Liang, X.L.; Radosevich, M.; et al. Prophage-encoded antibiotic resistance genes are enriched in human-impacted environments. Nat. Commun. 2024, 15, 8315. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, W.J.; Schwarz, S.; Wang, C.Z.; Liu, W.Y.; Chen, F.G.; Luan, T.; Liu, S.G. Characterization of a blaIMP-4-carrying plasmid from Enterobacter cloacae of swine origin. J. Antimicrob. Chemother. 2019, 74, 1799–1806. [Google Scholar] [CrossRef]
- Pei, R.T.; Kim, S.C.; Carlson, K.H.; Pruden, A. Effect of River Landscape on the sediment concentrations of antibiotics and corresponding antibiotic resistance genes (ARG). Water Res. 2006, 40, 2427–2435. [Google Scholar] [CrossRef]
- Lian, F.; Yu, W.C.; Zhou, Q.X.; Gu, S.G.; Wang, Z.Y.; Xing, B.S. Size Matters: Nano-Biochar Triggers Decomposition and Transformation Inhibition of Antibiotic Resistance Genes in Aqueous Environments. Environ. Sci. Technol. 2020, 54, 8821–8829. [Google Scholar] [CrossRef]
- Shao, B.B.; Liu, Z.F.; Zeng, G.M.; Liu, Y.; Liang, Q.H.; He, Q.Y.; Wu, T.; Pan, Y.; Huang, J.; Peng, Z.; et al. Synthesis of 2D/2D CoAl-LDHs/Ti3C2Tx Schottky-junction with enhanced interfacial charge transfer and visible-light photocatalytic performance. Appl. Catal. B-Environ. 2021, 286, 119867. [Google Scholar] [CrossRef]
- Duysak, T.; Jeong, J.H.; Kim, K.; Kim, J.S.; Choy, H.E. Analysis of random mutations in Salmonella Gallinarum dihydropteroate synthase conferring sulfonamide resistance. Arch. Microbiol. 2023, 205, 363. [Google Scholar] [CrossRef]
- Shindoh, S.; Kadoya, A.; Kanechi, R.; Watanabe, K.; Suzuki, S. Marine bacteria harbor the sulfonamide resistance gene sul4 without mobile genetic elements. Front. Microbiol. 2023, 14, 1230548. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.C.; Cunha, B.A. Tetracyclines. Med. Clin. N. Am. 1995, 79, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Titilawo, Y.; Obi, L.; Okoh, A. Antimicrobial resistance determinants of Escherichia coli isolates recovered from some rivers in Osun State, South-Western Nigeria: Implications for public health. Sci. Total Environ. 2015, 523, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Nguyen, L.T.; Headrick, S.I.; Murinda, S.E.; Oliver, S.P. Antimicrobial resistance patterns of Shiga toxin-producing Escherichia coli O157:H7 and O157:H7− from different origins. Microb. Drug Resist.-Mech. Epidemiol. Dis. 2007, 13, 44–51. [Google Scholar] [CrossRef]
- Castello, A.; Lo Cascio, G.; Ferraro, C.; Pantano, L.; Costa, A.; Butera, G.; Oliveri, G.; Rizzuto, M.L.; Alduina, R.; Cardamone, C. Food risk associated with vegetable consumption, exposure to antimicrobial-resistant strains and pesticide residues. Ital. J. Food Saf. 2023, 12, 11134. [Google Scholar] [CrossRef]
- Dinos, G.P. The macrolide antibiotic renaissance. Br. J. Pharmacol. 2017, 174, 2967–2983. [Google Scholar] [CrossRef]
- Yi, X.Z.; Wang, M.; Zhou, Z. The potential impact of naturally produced antibiotics, environmental factors, and anthropogenic pressure on the occurrence of erm genes in urban soils. Environ. Pollut. 2019, 245, 282–289. [Google Scholar] [CrossRef]
- Ardanuy, C.; Tubau, F.; Liñares, J.; Domínguez, M.A.; Pallarés, R.; Martín, R.; Spanish Pneumococcal Infection, S. Distribution of subclasses mefA and mefE of the mefA gene among clinical isolates of macrolide-resistant (M-Phenotype) Streptococcus pneumoniae, viridans group Streptococci, and Streptococcus pyogenes. Antimicrob. Agents Chemother. 2005, 49, 827–829. [Google Scholar] [CrossRef]
- Jacobs, L.M.C.; Consol, P.; Chen, Y. Drug Discovery in the Field of β-Lactams: An Academic Perspective. Antibiotics 2024, 13, 59. [Google Scholar] [CrossRef]
- Osinska, A.; Korzeniewska, E.; Harnisz, M.; Felis, E.; Bajkacz, S.; Jachimowicz, P.; Niestepski, S.; Konopka, I. Small-scale wastewater treatment plants as a source of the dissemination of antibiotic resistance genes in the aquatic environment. J. Hazard. Mater. 2020, 381, 121221. [Google Scholar] [CrossRef]
- Wu, W.J.; Feng, Y.; Tang, G.M.; Qiao, F.; McNally, A.; Zong, Z.Y. NDM Metallo-β-Lactamases and Their Bacterial Producers in Health Care Settings. Clin. Microbiol. Rev. 2019, 32, e00115-18. [Google Scholar] [CrossRef] [PubMed]
- McDermott, P.F.; Tyson, G.H.; Kabera, C.; Chen, Y.S.; Li, C.; Folster, J.P.; Ayers, S.L.; Lam, C.; Tate, H.P.; Zhao, S.H. Whole-Genome Sequencing for Detecting Antimicrobial Resistance in Nontyphoidal Salmonella. Antimicrob. Agents Chemother. 2016, 60, 5515–5520. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef] [PubMed]
- Wang-Sheng, Z.; Zu-Huang, M.; Xing-Bei, W. Emergence of five kinds of aminoglycoside-modifying enzyme genes simultaneously in a strain of multidrug-resistant Escherichia coli in China. Clin. Microbiol. Infect. 2012, 18, E11–E12. [Google Scholar] [CrossRef]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef]
- Ruiz, J. Transferable Mechanisms of Quinolone Resistance from 1998 Onward. Clin. Microbiol. Rev. 2019, 32, e00007-19. [Google Scholar] [CrossRef]
- Xiao, Y.Y.; Wang, H.X.; Wang, C.; Gao, H.; Wang, Y.Y.; Xu, J. Trends in and Future Research Direction of Antimicrobial Resistance in Global Aquaculture Systems: A Review. Sustainability 2023, 15, 9012. [Google Scholar] [CrossRef]
- Deng, Y.; Tan, A.; Zhao, F.; Wang, F.; Gong, H.; Lai, Y.; Huang, Z. Global distribution of antimicrobial resistance genes in aquaculture. One Health Adv. 2025, 3, 6. [Google Scholar] [CrossRef]
- Liu, C.; Shan, X.; Zhang, Y.X.; Song, L.T.; Chen, H.Y. Microcosm experiments revealed resistome coalescence of sewage treatment plant effluents in river environment. Environ. Pollut. 2023, 338, 122661. [Google Scholar] [CrossRef]
- Qiao, L.K.; He, L.Y.; Gao, F.Z.; Huang, Z.; Bai, H.; Wang, Y.C.; Shi, Y.J.; Liu, Y.S.; Zhao, J.L.; Ying, G.G. Deciphering key traits and dissemination of antibiotic resistance genes and degradation genes in pharmaceutical wastewater receiving environments. Water Res. 2025, 275, 123241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Zhang, G.Q.; Ju, F. Using Culture-Enriched Phenotypic Metagenomics for Targeted High-Throughput Monitoring of the Clinically Important Fraction of the β-Lactam Resistome. Environ. Sci. Technol. 2022, 56, 11429–11439. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, X.X.; Liu, B.X.; Huang, T.; Liu, R.Y.; Liu, X.C. Metagenome sequencing reveals shifts in phage-associated antibiotic resistance genes from influent to effluent in wastewater treatment plants. Water Res. 2024, 253, 121289. [Google Scholar] [CrossRef] [PubMed]
- Ju, F.; Beck, K.; Yin, X.L.; Maccagnan, A.; McArdell, C.S.; Singer, H.P.; Johnson, D.R.; Zhang, T.; Bürgmann, H. Wastewater treatment plant resistomes are shaped by bacterial composition, genetic exchange, and upregulated expression in the effluent microbiomes. ISME J. 2019, 13, 346–360. [Google Scholar] [CrossRef]
- Roy, S.; Dawson, R.A.; Bradley, J.A.; Hernández, M. Prevalence and dynamics of antimicrobial resistance in pioneer and developing Arctic soils. BMC Microbiol. 2025, 25, 50. [Google Scholar] [CrossRef]
- Zhang, W.G.; Wen, T.; Liu, L.Z.; Li, J.Y.; Gao, Y.; Zhu, D.; He, J.Z.; Zhu, Y.G. Agricultural land-use change and rotation system exert considerable influences on the soil antibiotic resistome in Lake Tai Basin. Sci. Total Environ. 2021, 771, 144848. [Google Scholar] [CrossRef]
- Zhang, D.D.; Li, H.Y.; Yang, Q.F.; Xu, Y. Microbial-mediated conversion of soil organic carbon co-regulates the evolution of antibiotic resistance. J. Hazard. Mater. 2024, 471, 134404. [Google Scholar] [CrossRef]
- Zhao, J.; Ni, T.; Li, J.; Lu, Q.; Fang, Z.Y.; Huang, Q.W.; Zhang, R.F.; Li, R.; Shen, B.; Shen, Q.R. Effects of organic-inorganic compound fertilizer with reduced chemical fertilizer application on crop yields, soil biological activity and bacterial community structure in a rice-wheat cropping system. Appl. Soil Ecol. 2016, 99, 1–12. [Google Scholar] [CrossRef]
- Zheng, D.S.; Yin, G.Y.; Liu, M.; Hou, L.; Yang, Y.; Van Boeckel, T.P.; Zheng, Y.L.; Li, Y. Global biogeography and projection of soil antibiotic resistance genes. Sci. Adv. 2022, 8, eabq8015. [Google Scholar] [CrossRef]
- Yang, Y.; Gunina, A.; Cheng, H.; Liu, L.X.; Wang, B.R.; Dou, Y.X.; Wang, Y.Q.; Liang, C.; An, S.S.; Chang, S.X. Unlocking Mechanisms for Soil Organic Matter Accumulation: Carbon Use Efficiency and Microbial Necromass as the Keys. Glob. Change Biol. 2025, 31, e70033. [Google Scholar] [CrossRef] [PubMed]
- von Wintersdorff, C.J.H.; Penders, J.; van Niekerk, J.M.; Mills, N.D.; Majumder, S.; van Alphen, L.B.; Savelkoul, P.H.M.; Wolffs, P.F.G. Dissemination of Antimicrobial Resistance in Microbial Ecosystems through Horizontal Gene Transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, H.Q.; Zhang, L.W.Y.; Mu, W.P.; Zhang, Y.; Chen, T.J.; Wu, J.X.; Tang, H.Y.; Zheng, S.X.; Liu, Y.F.; et al. Generic Diagramming Platform (GDP): A comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2024, 53, D1670–D1676. [Google Scholar] [CrossRef] [PubMed]
- Heuer, H.; Smalla, K. Plasmids foster diversification and adaptation of bacterial populations in soil. FEMS Microbiol. Rev. 2012, 36, 1083–1104. [Google Scholar] [CrossRef]
- Heuer, H.; Abdo, Z.; Smalla, K. Patchy distribution of flexible genetic elements in bacterial populations mediates robustness to environmental uncertainty. FEMS Microbiol. Ecol. 2008, 65, 361–371. [Google Scholar] [CrossRef]
- Dib, J.R.; Wagenknecht, M.; Farías, M.E.; Meinhardt, F. Strategies and approaches in plasmidome studies uncovering plasmid diversity disregarding of linear elements? Front. Microbiol. 2015, 6, 463. [Google Scholar] [CrossRef]
- Thomas, C.M.; Nielsen, K.M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 2005, 3, 711–721. [Google Scholar] [CrossRef]
- Auchtung, J.M.; Aleksanyan, N.; Bulku, A.; Berkmen, M.B. Biology of ICEBs1, an integrative and conjugative element in Bacillus subtilis. Plasmid 2016, 86, 14–25. [Google Scholar] [CrossRef]
- Varani, A.; He, S.S.; Siguier, P.; Ross, K.; Chandler, M. The IS6 family, a clinically important group of insertion sequences including IS26. Mob. DNA 2021, 12, 11. [Google Scholar] [CrossRef]
- Flores-Rios, R.; Moya-Beltrán, A.; Pareja-Barrueto, C.; Arenas-Salinas, M.; Valenzuela, S.; Orellana, O.; Quatrini, R. The Type IV Secretion System of ICEAfe1: Formation of a Conjugative Pilus in Acidithiobacillus ferrooxidans. Front. Microbiol. 2019, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Waksman, G. From conjugation to T4S systems in Gram-negative bacteria: A mechanistic biology perspective. EMBO Rep. 2019, 20, e47012. [Google Scholar] [CrossRef] [PubMed]
- Cascales, E.; Christie, P.J. Agrobacterium VirB10, an ATP energy sensor required for type IV secretion. Proc. Natl. Acad. Sci. USA 2004, 101, 17228–17233. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Yang, K.; Li, L.; Yang, L.Y.; Zhang, S.; Yu, F.F.; Hua, L.L. Change characteristics, bacteria host, and spread risks of bioaerosol ARGs/MGEs from different stages in sewage and sludge treatment process. J. Hazard. Mater. 2024, 469, 134011. [Google Scholar] [CrossRef]
- Wang, S.; Nie, W.H.; Gu, Q.; Wang, X.; Yang, D.P.; Li, H.Y.; Wang, P.H.; Liao, W.X.; Huang, J.; Yuan, Q.; et al. Spread of antibiotic resistance genes in drinking water reservoirs: Insights from a deep metagenomic study using a curated database. Water Res. 2024, 256, 121572. [Google Scholar] [CrossRef]
- Mukwevho, F.N.; Mbanga, J.; Bester, L.A.; Ismail, A.; Essack, S.Y.; Abia, A.L.K. Potential environmental transmission of antibiotic-resistant Escherichia coli and Enterococcus faecium harbouring multiple antibiotic resistance genes and mobile genetic elements in surface waters close to informal settlements: A tale of two cities. Sci. Total Environ. 2025, 976, 179321. [Google Scholar] [CrossRef]
- Li, X.; Cai, S.J.; Xu, M.Y. Nanoscale zero-valent iron alleviated horizontal transfer of antibiotic resistance genes in soil: The important role of extracellular polymeric substances. J. Hazard. Mater. 2024, 480, 135902. [Google Scholar] [CrossRef]
- Bairoliya, S.; Xiang, J.K.Z.; Cao, B. Extracellular DNA in Environmental Samples: Occurrence, Extraction, Quantification, and Impact on Microbial Biodiversity Assessment. Appl. Environ. Microbiol. 2022, 88, e01845-21. [Google Scholar] [CrossRef]
- Mao, D.Q.; Luo, Y.; Mathieu, J.; Wang, Q.; Feng, L.; Mu, Q.H.; Feng, C.Y.; Alvarez, P.J.J. Persistence of Extracellular DNA in River Sediment Facilitates Antibiotic Resistance Gene Propagation. Environ. Sci. Technol. 2014, 48, 71–78. [Google Scholar] [CrossRef]
- Abe, K.; Nomura, N.; Suzuki, S. Biofilms: Hot spots of horizontal gene transfer (HGT) in aquatic environments, with a focus on a new HGT mechanism. FEMS Microbiol. Ecol. 2020, 96, fiaa031. [Google Scholar] [CrossRef]
- Mantilla-Calderon, D.; Plewa, M.J.; Michoud, G.; Fodelianakis, S.; Daffonchio, D.; Hong, P.Y. Water Disinfection Byproducts Increase Natural Transformation Rates of Environmental DNA in Acinetobacter baylyi ADP1. Environ. Sci. Technol. 2019, 53, 6520–6528. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.L.; Li, L.Y.; Zhang, Y.F.; Ren, D.Y.; Feng, Y.Y.; Li, X.Y.; Wu, D.; Xie, B.; Ma, J.G. Triclosan facilitates the dissemination of antibiotic resistance genes during anaerobic digestion: Focusing on horizontal transfer and microbial response. Bioresour. Technol. 2024, 413, 131522. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Chowdhary, P.; Ahmad, A.; Giri, B.S.; Chaturvedi, P. Hydrothermal liquefaction of rice husk and cow dung in Mixed-Bed-Rotating Pyrolyzer and application of biochar for dye removal. Bioresour. Technol. 2020, 309, 123294. [Google Scholar] [CrossRef] [PubMed]
- Hoang, A.T.; Goldfarb, J.L.; Foley, A.M.; Lichtfouse, E.; Kumar, M.; Xiao, L.L.; Ahmed, S.F.; Said, Z.; Luque, R.; Bui, V.; et al. Production of biochar from crop residues and its application for anaerobic digestion. Bioresour. Technol. 2022, 363, 127970. [Google Scholar] [CrossRef]
- Kumar, A.; Saini, K.; Bhaskar, T. Hydochar and biochar: Production, physicochemical properties and techno- economic analysis. Bioresour. Technol. 2020, 310, 123442. [Google Scholar] [CrossRef]
- Naghipour, D.; Amouei, A.; Ghasemi, K.T.; Taghavi, K. Removal of cefixime from aqueous solutions by the biosorbent prepared from pine cones: Kinetic and isotherm studies. Desalination Water Treat. 2020, 201, 219–227. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.Q.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Liu, L.; Li, C.; Lai, R.T.; Li, H.X.; Lai, L.S.; Liu, X.N. Perturbation and strengthening effects of DOM on the biochar adsorption pathway. Ecotoxicol. Environ. Saf. 2022, 245, 114113. [Google Scholar] [CrossRef]
- Amen, R.; Bashir, H.; Bibi, I.; Shaheen, S.M.; Niazi, N.K.; Shahid, M.; Hussain, M.M.; Antoniadis, V.; Shakoor, M.B.; Al-Solaimani, S.G.; et al. A critical review on arsenic removal from water using biochar-based sorbents: The significance of modification and redox reactions. Chem. Eng. J. 2020, 396, 125195. [Google Scholar] [CrossRef]
- Wang, R.Z.; Huang, D.L.; Liu, Y.G.; Zhang, C.; Lai, C.; Wang, X.; Zeng, G.M.; Zhang, Q.; Gong, X.M.; Xu, P. Synergistic removal of copper and tetracycline from aqueous solution by steam-activated bamboo-derived biochar. J. Hazard. Mater. 2020, 384, 121470. [Google Scholar] [CrossRef]
- Xu, X.Y.; Weng, Y.C.; Zhuang, J.L.; Pei, H.F.; Wu, B.D.; Wu, W.; Yang, J.J.; Wang, B.; Huang, T.Y. Enhanced adsorption capacity of antibiotics by calamus-biochar with phosphoric acid modification: Performance assessment and mechanism analysis. J. Taiwan Inst. Chem. Eng. 2024, 161, 105541. [Google Scholar] [CrossRef]
- Cheng, D.L.; Ngo, H.H.; Guo, W.S.; Chang, S.W.; Nguyen, D.D.; Zhang, X.B.; Varjani, S.; Liu, Y. Feasibility study on a new pomelo peel derived biochar for tetracycline antibiotics removal in swine wastewater. Sci. Total Environ. 2020, 720, 137662. [Google Scholar] [CrossRef]
- Arif, M.; Liu, G.J.; Rehman, M.Z.U.; Yousaf, B.; Ahmed, R.; Miana, M.M.; Ashrafa, A.; Munirf, M.A.M.; Rashida, M.S.; Naeem, A. Carbon dioxide activated biochar-clay mineral composite efficiently removes ciprofloxacin from contaminated water—Reveals an incubation study. J. Clean. Prod. 2022, 332, 130079. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Xiao, J.; Che, H.X.; Xiong, D.Y.; Zhou, Y.L.; Li, B.Y.; Liu, Y.; Wei, G.T. Novel magnetic N-doped biochar derived from sugarcane bagasse and red mud for effective adsorption of tetracycline hydrochloride. J. Environ. Chem. Eng. 2024, 12, 113041. [Google Scholar] [CrossRef]
- Luo, J.W.; Li, X.; Ge, C.J.; Müller, K.; Yu, H.M.; Deng, H.; Shaheen, S.M.; Tsang, D.C.W.; Bolan, N.S.; Rinklebe, J.; et al. Preparation of ammonium-modified cassava waste-derived biochar and its evaluation for synergistic adsorption of ternary antibiotics from aqueous solution. J. Environ. Manag. 2021, 298, 133530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.B.; Zhang, Y.C.; Ngo, H.H.; Guo, W.S.; Wen, H.T.; Zhang, D.; Li, C.C.; Qi, L. Characterization and sulfonamide antibiotics adsorption capacity of spent coffee grounds based biochar and hydrochar. Sci. Total Environ. 2020, 716, 137015. [Google Scholar] [CrossRef]
- Ngigi, A.N.; Ok, Y.S.; Thiele-Bruhn, S. Biochar affects the dissipation of antibiotics and abundance of antibiotic resistance genes in pig manure. Bioresour. Technol. 2020, 315, 123782. [Google Scholar] [CrossRef]
- Calderón-Franco, D.; Apoorva, S.; Medema, G.; van Loosdrecht, M.C.M.; Weissbrodt, D.G. Upgrading residues from wastewater and drinking water treatment plants as low-cost adsorbents to remove extracellular DNA and microorganisms carrying antibiotic resistance genes from treated effluents. Sci. Total Environ. 2021, 778, 146364. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Vithanage, M.; Ahmad, M.; Seo, D.C.; Cho, J.S.; Lee, S.E.; Lee, S.S.; Ok, Y.S. Enhanced sulfamethazine removal by steam-activated invasive plant-derived biochar. J. Hazard. Mater. 2015, 290, 43–50. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhu, X.X.; He, H.L.; Fang, Y.X.; Dong, H.P.; Lü, J.H.; Li, J.F.; Li, Y.M. Adsorption of two antibiotics on biochar prepared in air-containing atmosphere: Influence of biochar porosity and molecular size of antibiotics. J. Mol. Liq. 2019, 274, 353–361. [Google Scholar] [CrossRef]
- Zhu, X.X.; Li, C.Y.; Li, J.F.; Xie, B.; Lü, J.H.; Li, Y.M. Thermal treatment of biochar in the air/nitrogen atmosphere for developed mesoporosity and enhanced adsorption to tetracycline. Bioresour. Technol. 2018, 263, 475–482. [Google Scholar] [CrossRef]
- Xiao, Y.; Lyu, H.H.; Tang, J.C.; Wang, K.; Sun, H.W. Effects of ball milling on the photochemistry of biochar: Enrofloxacin degradation and possible mechanisms. Chem. Eng. J. 2020, 384, 123311. [Google Scholar] [CrossRef]
- Huang, J.S.; Zimmerman, A.R.; Chen, H.; Gao, B. Ball milled biochar effectively removes sulfamethoxazole and sulfapyridine antibiotics from water and wastewater. Environ. Pollut. 2020, 258, 113809. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, D.M.; Cheng, H.R.; Cheng, J.H.; Du, K.S.; Hu, Y.Y.; Chen, Y.C. High mesoporosity phosphorus-containing biochar fabricated from Camellia oleifera shells: Impressive tetracycline adsorption performance and promotion of pyrophosphate-like surface functional groups (C-O-P bond). Bioresour. Technol. 2021, 329, 124922. [Google Scholar] [CrossRef]
- Jiang, B.N.; Lin, Y.Q.; Mbog, J.C. Biochar derived from swine manure digestate and applied on the removals of heavy metals and antibiotics. Bioresour. Technol. 2018, 270, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.Z.; Zhang, X.Y.; Wang, L.P.; Gao, B.; Luo, J.P.; Fang, R.; Zou, W.X.; Meng, N. Sorption of tetracycline on H2O2-modified biochar derived from rape stalk. Environ. Pollut. Bioavailab. 2019, 31, 198–207. [Google Scholar] [CrossRef]
- Liang, H.G.; Zhu, C.X.; Ji, S.; Kannan, P.; Chen, F. Magnetic Fe2O3/biochar composite prepared in a molten salt medium for antibiotic removal in water. Biochar 2022, 4, 3. [Google Scholar] [CrossRef]
- Minaei, S.; Benis, K.Z.; McPhedran, K.N.; Soltan, J. Evaluation of a ZnCl2-modified biochar derived from activated sludge biomass for adsorption of sulfamethoxazole. Chem. Eng. Res. Des. 2023, 190, 407–420. [Google Scholar] [CrossRef]
- Fu, Y.H.; Wang, F.; Sheng, H.J.; Hu, F.; Wang, Z.Q.; Xu, M.; Bian, Y.R.; Jiang, X.; Tiedje, J.M. Removal of extracellular antibiotic resistance genes using magnetic biochar/quaternary phosphonium salt in aquatic environments: A mechanistic study. J. Hazard. Mater. 2021, 411, 125048. [Google Scholar] [CrossRef]
- Yu, X.P.; Bai, M.; Li, X.J.; Yang, P.P.; Wang, Q.Z.; Wang, Z.N.; Weng, L.P.; Ye, H.K. Tetracycline removal by immobilized indigenous bacterial consortium using biochar and biomass: Removal performance and mechanisms. Bioresour. Technol. 2024, 413, 131463. [Google Scholar] [CrossRef]
- Xia, M.M.; Niu, Q.Y.; Qu, X.Y.; Zhang, C.X.; Qu, X.L.; Li, H.R.; Yang, C.P. Simultaneous adsorption and biodegradation of oxytetracycline in wastewater by Mycolicibacterium sp. immobilized on magnetic biochar. Environ. Pollut. 2023, 339, 122728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.N.; Wang, J.H. Removal of chlortetracycline from water by immobilized Bacillus subtilis on honeysuckle residue-derived biochar. Water Air Soil Pollut. 2021, 232, 236. [Google Scholar] [CrossRef]
- Zhang, S.N.; Wang, J.H. Removal of chlortetracycline from water by Bacillus cereus immobilized on Chinese medicine residues biochar. Environ. Technol. Innov. 2021, 240, 101930. [Google Scholar] [CrossRef]
- Fu, H.; Li, X.B.; Wang, J.; Lin, P.F.; Chen, C.; Zhang, X.J.; Suffet, I.H. Activated carbon adsorption of quinolone antibiotics in water: Performance, mechanism, and modeling. J. Environ. Sci. 2017, 56, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.S.; Bandoch, G.F.G.; Asefa, T.; Visentainer, J.V.; Almeida, V.C. Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies. Chem. Eng. J. 2015, 260, 291–299. [Google Scholar] [CrossRef]
- Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Souza, L.S.; Martins, A.C.; Silva, T.L.; Santos, O.O.; Visentainer, J.V.; Almeida, V.C. NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: Kinetic, isotherm and thermodynamic studies. Chem. Eng. J. 2016, 288, 778–788. [Google Scholar] [CrossRef]
- Sharifpour, N.; Moghaddam, F.M.; Mardani, G.; Malakootian, M. Evaluation of the activated carbon coated with multiwalled carbon nanotubes in removal of ciprofloxacin from aqueous solutions. Applied Water Sci. 2020, 10, 140. [Google Scholar] [CrossRef]
- Ncibi, M.C.; Sillanpää, M. Optimized removal of antibiotic drugs from aqueous solutions using single, double and multi-walled carbon nanotubes. J. Hazard. Mater. 2015, 298, 102–110. [Google Scholar] [CrossRef]
- Chen, H.; Gao, B.; Li, H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. Journal of Hazard. Mater. 2015, 282, 201–207. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, Y.; Lin, P.R.; Li, J.L.; Dong, H.; Yu, H.B.; Qi, L.S.; Ren, L.H. Effects of graphite, graphene, and graphene oxide on the anaerobic co-digestion of sewage sludge and food waste: Attention to methane production and the fate of antibiotic resistance genes. Bioresour. Technol. 2021, 339, 125585. [Google Scholar] [CrossRef]
- Modi, A.; Bellare, J. Zeolitic imidazolate framework-67/carboxylated graphene oxide nanosheets incorporated polyethersulfone hollow fiber membranes for removal of toxic heavy metals from contaminated water. Sep. Purif. Technol. 2020, 249, 117160. [Google Scholar] [CrossRef]
- Geng, X.X.; Lv, S.Y.; Yang, J.; Cui, S.H.; Zhao, Z.H. Carboxyl-functionalized biochar derived from walnut shells with enhanced aqueous adsorption of sulfonamide antibiotics. J. Environ. Manag. 2021, 280, 111749. [Google Scholar] [CrossRef]
- Zhao, J.W.; Gao, F.; Sun, Y.; Fang, W.Y.; Li, X.H.; Dai, Y.J. New use for biochar derived from bovine manure for tetracycline removal. J. Environ. Chem. Eng. 2021, 9, 105585. [Google Scholar] [CrossRef]
- Haider, M.I.S.; Liu, G.J.; Yousaf, B.; Arif, M.; Aziz, K.; Ashraf, A.; Safeer, R.; Ijaz, S.; Pikon, K. Synergistic interactions and reaction mechanisms of biochar surface functionalities in antibiotics removal from industrial wastewater. Environ. Pollut. 2024, 356, 124365. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Shang, H.R.; Cao, Y.N.; Yang, C.H.; Feng, Y.J.; Yu, Y.L. High performance removal of sulfamethoxazole using large specific area of biochar derived from corncob xylose residue. Biochar 2022, 4, 11. [Google Scholar] [CrossRef]
- Qin, P.Z.; Huang, D.W.; Tang, R.; Gan, F.Q.; Guan, Y.; Lv, X.X. Enhanced adsorption of sulfonamide antibiotics in water by modified biochar derived from bagasse. Open Chem. 2019, 17, 1309–1316. [Google Scholar] [CrossRef]
- Mei, Y.C.; Zhuang, S.T.; Wang, J.L. Biochar: A potential and green adsorbent for antibiotics removal from aqueous solution. Rev. Environ. Sci. Bio Technol. 2024, 23, 1065–1103. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Sun, P.Z.; Wei, K.J.; Huang, X.; Zhang, X.Y. Enhanced H2O2 activation and sulfamethoxazole degradation by Fe-impregnated biochar. Chem. Eng. J. 2020, 385, 123921. [Google Scholar] [CrossRef]
- Li, R.; Wang, B.; Niu, A.P.; Cheng, N.; Chen, M.; Zhang, X.Y.; Yu, Z.B.; Wang, S.S. Application of biochar immobilized microorganisms for pollutants removal from wastewater: A review. Sci. Total Environ. 2022, 837, 155563. [Google Scholar] [CrossRef]
- Gong, Y.Z.; Niu, Q.Y.; Liu, Y.G.; Dong, J.; Xia, M.M. Development of multifarious carrier materials and impact conditions of immobilised microbial technology for environmental remediation: A review. Environ. Pollut. 2022, 314, 120232. [Google Scholar] [CrossRef]
- Schommer, V.A.; Nazari, M.T.; Melara, F.; Braun, J.C.A.; Rempel, A.; dos Santos, L.F.; Ferrari, V.; Colla, L.M.; Dettmer, A.; Piccin, J.S. Techniques and mechanisms of bacteria immobilization on biochar for further environmental and agricultural applications. Microbiol. Res. 2024, 278, 127534. [Google Scholar] [CrossRef]
- Yang, F.; Jian, H.X.; Wang, C.P.; Wang, Y.; Li, E.H.; Sun, H.W. Effects of biochar on biodegradation of sulfamethoxazole and chloramphenicol by Pseudomonas stutzeri and Shewanella putrefaciens: Microbial growth, fatty acids, and the expression quantity of genes. J. Hazard. Mater. 2021, 406, 124311. [Google Scholar] [CrossRef]
- Wang, J.; Sui, B.; Shen, Y.J.; Meng, H.B.; Zhao, L.X.; Zhou, H.B.; Li, R.; Ding, J.T.; Cheng, H.S.; Zhang, X. Effects of different biochars on antibiotic resistance genes during swine manure thermophilic composting. Int. J. Agric. Biol. Eng. 2018, 11, 166–171. [Google Scholar] [CrossRef]
- Huang, G.Q.; He, X.Q.; Xiong, J.P.; Yang, Z.L.; Han, L.J. Exploring the impact of biochar on antibiotics and antibiotics resistance genes in pig manure aerobic composting through untargeted metabolomics and metagenomics. Bioresour. Technol. 2022, 352, 127118. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Wu, C.R.; Zhou, C.S.; Dong, L.L.; Liu, B.F.; Xing, D.; Yang, S.S.; Fan, J.N.; Feng, L.P.; Cao, G.L.; et al. Fate and removal of antibiotic resistance genes in heavy metals and dye co-contaminated wastewater treatment system amended with β-cyclodextrin functionalized biochar. Sci. Total Environ. 2020, 723, 137991. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Wang, F.; Wang, Z.Q.; Mei, Z.; Jiang, X.; Schäffer, A.; Virta, M.; Tiedje, J.M. Application of magnetic biochar/quaternary phosphonium salt to combat the antibiotic resistome in livestock wastewater. Sci. Total Environ. 2022, 811, 151386. [Google Scholar] [CrossRef]
- Guan, Z.M.; Lv, J.F.; Bai, P.; Guo, X.H. Boron removal from aqueous solutions by adsorption—A review. Desalination 2016, 383, 29–37. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Gómez-Serrano, V.; Alvarez, P.M.; Alvim-Ferraz, M.C.M.; Dias, J.M. Activated carbon modifications to enhance its water treatment applications. An overview. J. Hazard. Mater. 2011, 187, 1–23. [Google Scholar] [CrossRef]
- Moussavi, G.; Alahabadi, A.; Yaghmaeian, K.; Eskandari, M. Preparation, characterization and adsorption potential of the NH4Cl-induced activated carbon for the removal of amoxicillin antibiotic from water. Chem. Eng. J. 2013, 217, 119–128. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.H.; Dong, W.P.; Zhang, L.L.; Kong, Q.; Wang, W.L. Efficient Adsorption of Sulfamethazine onto Modified Activated Carbon: A Plausible Adsorption Mechanism. Sci. Rep. 2017, 7, 12437. [Google Scholar] [CrossRef]
- Kokuloku, L.T.K., Jr.; Miensah, E.D.; Gu, A.T.; Chen, K.W.; Wang, P.; Gong, C.H.; Jiao, Y.; Chen, K.; Yang, Y. A comparative adsorption study of activated carbon and Fe-modified activated carbon for trinitrotoluene removal. J. Taiwan Inst. Chem. Eng. 2024, 161, 105519. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.M.; Li, X.; Li, W.Y.; Mao, S.S.; He, S.Y.; Wu, X.; Tang, C.Q.; Yu, J.; Pan, L.Q.; et al. High efficiency removal of ibuprofen in water using activated carbon derived from Radix Angelica Dahurica residue. Environ. Prog. Sustain. Energy 2024, 43, e14318. [Google Scholar] [CrossRef]
- Biswal, B.K.; Balasubramanian, R. Adsorptive removal of sulfonamides, tetracyclines and quinolones from wastewater and water using carbon-based materials: Recent developments and future directions. J. Clean. Prod. 2022, 349, 131421. [Google Scholar] [CrossRef]
- Zhang, X.B.; Guo, W.S.; Ngo, H.H.; Wen, H.T.; Li, N.; Wu, W. Performance evaluation of powdered activated carbon for removing 28 types of antibiotics from water. J. Environ. Manag. 2016, 172, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.J.; Kim, S.G.; Kim, S.H. Removal of antibiotics by coagulation and granular activated carbon filtration. J. Hazard. Mater. 2008, 151, 38–43. [Google Scholar] [CrossRef]
- Zhang, T.Q.; Lv, K.Y.; Lu, Q.X.; Wang, L.L.; Liu, X.W. Removal of antibiotic-resistant genes during drinking water treatment: A review. J. Environ. Sci. 2021, 104, 415–429. [Google Scholar] [CrossRef]
- Farkas, A.; Butiuc-Keul, A.; Ciatarâs, D.; Neamtu, C.; Craciunas, C.; Podar, D.; Dragan-Bularda, M. Microbiological contamination and resistance genes in biofilms occurring during the drinking water treatment process. Sci. Total Environ. 2013, 443, 932–938. [Google Scholar] [CrossRef]
- Hu, Y.R.; Zhang, T.Y.; Jiang, L.; Luo, Y.; Yao, S.J.; Zhang, D.; Lin, K.F.; Cui, C.Z. Occurrence and reduction of antibiotic resistance genes in conventional and advanced drinking water treatment processes. Sci. Total Environ. 2019, 669, 777–784. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Ren, Z.F.; Chou, T.W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef]
- Zhang, D.; Pan, B.; Zhang, H.; Ning, P.; Xing, B.S. Contribution of Different Sulfamethoxazole Species to Their Overall Adsorption on Functionalized Carbon Nanotubes. Environ. Sci. Technol. 2010, 44, 3806–3811. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Thavorn-amornsri, T.; Pereira, M.F.R.; Serp, P.; Figueiredo, J.L. Comparison between activated carbon, carbon xerogel and carbon nanotubes for the adsorption of the antibiotic ciprofloxacin. Catal. Today 2012, 186, 29–34. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Xia, Y.Z.; Sun, L.; Eyley, S.; Daelemans, B.; Thielemans, W.; Seibel, J.; De Feyter, S. Grafting Ink for Direct Writing: Solvation Activated Covalent Functionalization of Graphene. Adv. Sci. 2022, 9, 2105017. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Li, Y.; Han, S.; Ma, J. Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere 2016, 153, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ma, S.; Si, Y.; Dong, L.F.; Wang, X.L.; Yao, J.; Chen, H.L.; Yi, Z.J.; Yao, W.C.; Xing, B.S. Interaction mechanisms of antibiotic sulfamethoxazole with various graphene-based materials and multiwall carbon nanotubes and the effect of humic acid in water. Carbon 2017, 114, 671–678. [Google Scholar] [CrossRef]
- Tang, Y.L.; Guo, H.G.; Xiao, L.; Yu, S.L.; Gao, N.Y.; Wang, Y.L. Synthesis of reduced graphene oxide/magnetite composites and investigation of their adsorption performance of fluoroquinolone antibiotics. Colloids Surf. A Physicochem. Eng. Asp. 2013, 424, 74–80. [Google Scholar] [CrossRef]
- Saroyan, H.S.; Bele, S.; Giannakoudakis, D.A.; Samanidou, V.F.; Bandosz, T.J.; Deliyanni, E.A. Degradation of endocrine disruptor, bisphenol-A, on an mixed oxidation state manganese oxide/modified graphite oxide composite: A role of carbonaceous phase. J. Colloid Interface Sci. 2019, 539, 516–524. [Google Scholar] [CrossRef]
- Yu, W.C.; Zhan, S.H.; Shen, Z.Q.; Zhou, Q.X.; Yang, D. Efficient removal mechanism for antibiotic resistance genes from aquatic environments by graphene oxide nanosheet. Chem. Eng. J. 2017, 313, 836–846. [Google Scholar] [CrossRef]
- Lee, K.M.; Castro, E.; Ratcliffe, J.; Lerner, C.; Caglayan, M. Nick sealing of polbeta mismatch insertion products by LIG1 and LIG3alpha during 8-oxoG bypass leads to mutagenic or error-free base excision repair. J. Biol. Chem. 2025, 301, 108540. [Google Scholar] [CrossRef]
- Zou, W.; Li, X.K.; Lai, Z.Y.; Zhang, X.L.; Hu, X.G.; Zhou, Q.X. Graphene Oxide Inhibits Antibiotic Uptake and Antibiotic Resistance Gene Propagation. ACS Appl. Mater. Interfaces 2016, 8, 33165–33174. [Google Scholar] [CrossRef]
- Quang, H.H.P.; Dinh, N.T.; Nguyen, P.K.T.; Nguyen, V.H. Exploring algae-based biochar strategies for adsorptive removal of antibiotics: A green leap towards environmental sustainability. Biomass Convers. Biorefin. 2025, online published, 1–40. [Google Scholar] [CrossRef]
- Zhang, X.T.; Hou, J.J.; Zhang, S.D.; Cai, T.; Liu, S.J.; Hu, W.J.; Zhang, Q.Z. Standardization and micromechanistic study of tetracycline adsorption by biochar. Biochar 2024, 6, 12. [Google Scholar] [CrossRef]
- Shen, Q.B.; Wang, Z.Y.; Yu, Q.; Cheng, Y.; Liu, Z.D.; Zhang, T.P.; Zhou, S.Q. Removal of tetracycline from an aqueous solution using manganese dioxide modified biochar derived from Chinese herbal medicine residues. Environ. Res. 2020, 183, 109195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Wang, Z.Q.; Liang, Y.H.; Wu, T.X.; Chen, Y.L.; Yan, J.R.; Zhu, Y.Y.; Ding, D.H. Adsorptive decontamination of antibiotics from livestock wastewater by using alkaline-modified biochar. Environ. Res. 2023, 226, 115676. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, Y.X.; Wang, H.T.; Lu, W.J.; Zhou, Z.Y.; Zhang, Y.C.; Ren, L.L. Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour. Technol. 2014, 164, 47–54. [Google Scholar] [CrossRef]
- Chun, Y.; Sheng, G.Y.; Chiou, C.T.; Xing, B.S. Compositions and sorptive properties of crop residue-derived chars. Environ. Sci. Technol. 2004, 38, 4649–4655. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, L.L.; Zheng, X.Y.; Xiao, D.; Yang, Y.; Zhang, Z.K.; Ai, B.L.; Sheng, Z.W. Adsorption of Sulfonamides in Aqueous Solution on Reusable Coconut-Shell Biochar Modified by Alkaline Activation and Magnetization. Front. Chem. 2022, 9, 814647. [Google Scholar] [CrossRef]
- Zhuang, S.T.; Mei, Y.C.; Wang, J.L. Adsorption performance and mechanisms of Co2+ onto carboxyl-functionalized carbon nanotubes. J. Clean. Prod. 2023, 430, 139709. [Google Scholar] [CrossRef]
- Ma, Y.T.; Wang, R.; Gao, C.P.; Han, R.P. Carbon nanotube-loaded copper-nickel ferrite activated persulfate system for adsorption and degradation of oxytetracycline hydrochloride. J. Colloid Interface Sci. 2023, 640, 761–774. [Google Scholar] [CrossRef]
- Liu, M.Y.; Gao, C.P.; Han, R.P.; Qu, L.B. Assessment of β-cyclodextrin-immobilizing hydrolyzed polyacrylonitrile membrane for enhanced remediation of bisphenol A and tetracyclines: Adsorption and antibacterial studies. J. Clean. Prod. 2023, 387, 135839. [Google Scholar] [CrossRef]
- Wu, C.Y.; Fu, L.Y.; Li, H.Q.; Liu, X.; Wan, C.L. Using biochar to strengthen the removal of antibiotic resistance genes: Performance and mechanism. Sci. Total Environ. 2022, 816, 151554. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; He, Y.Z.; He, Y.Z.; Liu, X.C.; Xu, B.; Yu, J.F.; Dai, C.H.; Huang, A.Q.; Pang, Y.; Luo, L. Analyses of tetracycline adsorption on alkali-acid modified magnetic biochar: Site energy distribution consideration. Sci. Total Environ. 2019, 650, 2260–2266. [Google Scholar] [CrossRef] [PubMed]
- Ninwiwek, N.; Hongsawat, P.; Punyapalakul, P.; Prarat, P. Removal of the antibiotic sulfamethoxazole from environmental water by mesoporous silica-magnetic graphene oxide nanocomposite technology: Adsorption characteristics, coadsorption and uptake mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123716. [Google Scholar] [CrossRef]
- Fang, J.; Jin, L.; Meng, Q.K.; Wang, D.J.; Lin, D.H. Interactions of extracellular DNA with aromatized biochar and protection against degradation by DNase I. J. Environ. Sci. 2021, 101, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Z.; Ali, S.; Rizwan, M.; Zaheer, I.E.; Malik, A.; Riaz, M.A.; Shahid, M.R.; Rehman, M.Z.U.; Al-Wabel, M.I. A critical review of mechanisms involved in the adsorption of organic and inorganic contaminants through biochar. Arab. J. Geosci. 2018, 11, 448. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravindakumar, C.T.; Shah, N.S.; Boczkaj, G. Advanced oxidation processes (AOPs) based wastewater treatment—Unexpected nitration side reactions-a serious environmental issue: A review. Chem. Eng. J. 2022, 430, 133002. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.A.; Zheng, H.S.; Li, H.B.; Zheng, Y.J.; Nan, J.; Ma, J.; Nagarajan, D.; Chang, J.S. Antibiotics degradation by advanced oxidation process (AOPs): Recent advances in ecotoxicity and antibiotic-resistance genes induction of degradation products. Chemosphere 2023, 311, 136977. [Google Scholar] [CrossRef]
- Mohammadi, S.; Moussavi, G.; Yaghmaeian, K.; Giannakis, S. Development of a percarbonate-enhanced Vacuum UV process for simultaneous fluoroquinolone antibiotics removal and fecal bacteria inactivation under a continuous flow mode of operation. Chem. Eng. J. 2022, 431, 134064. [Google Scholar] [CrossRef]
- Masood, Z.; Ikhlaq, A.; Farooq, U.; Qi, F.; Javed, F.; Aziz, H.A. Removal of anti-biotics from veterinary pharmaceutical wastewater using combined Electroflocculation and Fe-Zn loaded zeolite 5A based catalytic ozonation process. J. Water Process Eng. 2022, 49, 103039. [Google Scholar] [CrossRef]
- Chen, G.Y.; Yu, Y.; Liang, L.; Duan, X.G.; Li, R.; Lu, X.K.; Yan, B.B.; Li, N.; Wang, S.B. Remediation of antibiotic wastewater by coupled photocatalytic and persulfate oxidation system: A critical review. J. Hazard. Mater. 2021, 408, 124461. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.L. Multivalent metal catalysts in Fenton/Fenton-like oxidation system: A critical review. Chem. Eng. J. 2023, 466, 143147. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Pishbin, E. Ozone-based advanced oxidation processes in water treatment: Recent advances, challenges, and perspective. Environ. Sci. Pollut. Res. Int. 2025, 32, 3531–3570. [Google Scholar] [CrossRef] [PubMed]
- Marson, E.O.; Paniagua, C.E.S.; Costa-Serge, N.M.; Sousa, R.M.F.; Silva, G.D.; Becker, R.W.; Sirtori, C.; Starling, M.; Carvalho, S.R.; Trovó, A.G. Chemical and toxicological evaluation along with unprecedented transformation products during photolysis and heterogeneous photocatalysis of chloramphenicol in different aqueous matrices. Environ. Sci. Pollut. Res. 2021, 28, 23582–23594. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.H.; Zhou, L.; Wang, G.Q.; Feng, Y.H.; Wang, Z.Y.; Yang, X. Aqueous photodegradation of antibiotic florfenicol: Kinetics and degradation pathway studies. Environ. Sci. Pollut. Res. 2016, 23, 6982–6989. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, C.; Niu, L.H.; Cai, W. Occurrence of endocrine disrupting compounds in aqueous environment and their bacterial degradation: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1–59. [Google Scholar] [CrossRef]
- Sun, S.P.; Guo, H.Q.; Ke, Q.; Sun, J.H.; Shi, S.H.; Zhang, M.L.; Zhou, Q. Degradation of Antibiotic Ciprofloxacin Hydrochloride by Photo-Fenton Oxidation Process. Environ. Eng. Sci. 2009, 26, 753–759. [Google Scholar] [CrossRef]
- Fiorentino, A.; Soriano-Molina, P.; Abeledo-Lameiro, M.J.; de la Obra, I.; Proto, A.; Polo-Lopez, M.I.; Perez, J.A.S.; Rizzo, L. Neutral (Fe3+-NTA) and acidic (Fe2+) pH solar photo-Fenton Vs chlorination: Effective urban wastewater disinfection does not mean control of antibiotic resistance. J. Environ. Chem. Eng. 2022, 10, 108777. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; de Araújo, M.J.G.; Costa, E.; Santos, J.E.L.; dos Santos, E.V.; Martínez-Huitle, C.A.; Pergher, S.B.C. Design of highly efficient porous carbon foam cathode for electro-Fenton degradation of antimicrobial sulfanilamide. Appl. Catal. B-Environ. 2021, 283, 119652. [Google Scholar] [CrossRef]
- Li, L.; Gao, J.; Yuan, Y.M.; Zhang, S.; Liang, M.; Liu, Y. Study on FeS2/g-C3N4 as a photo-Fenton heterojunction catalyst for tetracycline degradation with H2O2 under visible light irradiation. J. Taiwan Inst. Chem. Eng. 2021, 126, 134–144. [Google Scholar] [CrossRef]
- Snowberger, S.; Adejumo, H.; He, K.; Mangalgiri, K.P.; Hopanna, M.; Soares, A.D.; Blaney, L. Direct Photolysis of Fluoroquinolone Antibiotics at 253.7 nm: Specific Reaction Kinetics and Formation of Equally Potent Fluoroquinolone Antibiotics. Environ. Sci. Technol. 2016, 50, 9533–9542. [Google Scholar] [CrossRef]
- Dai, Y.J.; Liu, M.; Li, J.J.; Yang, S.S.; Sun, Y.; Sun, Q.Y.; Wang, W.S.; Lu, L.; Zhang, K.X.; Xu, J.Y.; et al. A review on pollution situation and treatment methods of tetracycline in groundwater. Sep. Sci. Technol. 2020, 55, 1005–1021. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.X.; Luo, L.; Gao, H.B.; Zheng, F. Photodegradation of Moxifloxacin Hydrochloride Solutions under Visible Light Irradiation: Identification of Products and the Effect of pH on their Formation. AAPS Pharmscitech 2018, 19, 1182–1190. [Google Scholar] [CrossRef]
- Hubicka, U.; Zmudzki, P.; Talik, P.; Zuromska-Witek, B.; Krzek, J. Photodegradation assessment of ciprofloxacin, moxifloxacin, norfloxacin and ofloxacin in the presence of excipients from tablets by UPLC-MS/MS and DSC. Chem. Cent. J. 2013, 7, 133. [Google Scholar] [CrossRef]
- Zheng, S.M.; Wang, Y.D.; Chen, C.H.; Zhou, X.J.; Liu, Y.; Yang, J.M.; Geng, Q.J.; Chen, G.; Ding, Y.Z.; Yang, F.X. Current Progress in Natural Degradation and Enhanced Removal Techniques of Antibiotics in the Environment: A Review. Int. J. Environ. Res. Public Health 2022, 19, 10919. [Google Scholar] [CrossRef]
- Wenk, J.; Nguyen, M.T.; Nelson, K.L. Natural Photosensitizers in Constructed Unit Process Wetlands: Photochemical Characterization and Inactivation of Pathogen Indicator Organisms. Environ. Sci. Technol. 2019, 53, 7724–7735. [Google Scholar] [CrossRef]
- Feng, L.K.; Wu, H.M.; Zhang, J.; Brix, H. Simultaneous elimination of antibiotics resistance genes and dissolved organic matter in treatment wetlands: Characteristics and associated relationship. Chem. Eng. J. 2021, 415, 128966. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, D.; Bai, B.; Ma, Z.Y.; Zong, S.C. Insight into antibiotic removal by advanced oxidation processes (AOPs): Performance, mechanism, degradation pathways, and ecotoxicity assessment. Chem. Eng. J. 2024, 500, 157134. [Google Scholar] [CrossRef]
- Li, A.Y.; Weng, J.Q.; Yan, X.M.; Li, H.; Shi, H.B.; Wu, X.D. Electrochemical oxidation of acid orange 74 using Ru, IrO2, PbO2, and boron doped diamond anodes: Direct and indirect oxidation. J. Electroanal. Chem. 2021, 898, 115622. [Google Scholar] [CrossRef]
- Oturan, N.; Wu, J.; Zhang, H.; Sharma, V.K.; Oturan, M.A. Electrocatalytic destruction of the antibiotic tetracycline in aqueous medium by electrochemical advanced oxidation processes: Effect of electrode materials. Appl. Catal. B-Environ. 2013, 140, 92–97. [Google Scholar] [CrossRef]
- Dai, Q.Z.; Zhou, J.Z.; Weng, M.L.; Luo, X.B.; Feng, D.L.; Chen, J.M. Electrochemical oxidation metronidazole with Co modified PbO2 electrode: Degradation and mechanism. Sep. Purif. Technol. 2016, 166, 109–116. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Oturan, M.A. Electrochemical advanced oxidation processes for wastewater treatment: Advances in formation and detection of reactive species and mechanisms. Curr. Opin. Electrochem. 2021, 27, 100678. [Google Scholar] [CrossRef]
- Bracamontes-Ruelas, A.R.; Ordaz-Díaz, L.A.; Bailón-Salas, A.M.; Ríos-Saucedo, J.C.; Reyes-Vidal, Y.; Reynoso-Cuevas, L. Emerging Pollutants in Wastewater, Advanced Oxidation Processes as an Alternative Treatment and Perspectives. Processes 2022, 10, 1041. [Google Scholar] [CrossRef]
- Wang, S.; Yang, S.; Quispe, E.; Yang, H.; Sanfiorenzo, C.; Rogers, S.W.; Wang, K.; Yang, Y.; Hoffmann, M.R. Removal of Antibiotic Resistant Bacteria and Genes by UV-Assisted Electrochemical Oxidation on Degenerative TiO2 Nanotube Arrays. ACS EST Eng. 2021, 1, 612–622. [Google Scholar] [CrossRef]
- Wang, B.B.; Shi, H.H.; Habteselassie, M.Y.; Deng, X.Y.; Teng, Y.; Wang, Y.Y.; Huang, Q.G. Simultaneous removal of multidrug-resistant Salmonella enterica serotype typhimurium, antibiotics and antibiotic resistance genes from water by electrooxidation on a Magneli phase Ti4O7 anode. Chem. Eng. J. 2021, 407, 127134. [Google Scholar] [CrossRef]
- Barhoumi, N.; Oturan, N.; Olvera-Vargas, H.; Brillas, E.; Gadri, A.; Ammar, S.; Oturan, M.A. Pyrite as a sustainable catalyst in electro-Fenton process for improving oxidation of sulfamethazine. Kinetics, mechanism and toxicity assessment. Water Res. 2016, 94, 52–61. [Google Scholar] [CrossRef]
- Oturan, M.A.; Oturan, N.; Edelahi, M.C.; Podvorica, F.I.; El Kacemi, K. Oxidative degradation of herbicide diuron in aqueous medium by Fenton’s reaction based advanced oxidation processes. Chem. Eng. J. 2011, 171, 127–135. [Google Scholar] [CrossRef]
- Elmolla, E.; Chaudhuri, M. Optimization of Fenton process for treatment of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution. J. Hazard. Mater. 2009, 170, 666–672. [Google Scholar] [CrossRef]
- Bokhari, T.H.; Ahmad, N.; Jilani, M.I.; Saeed, M.; Usman, M.; Ul Haq, A.; Rehman, R.; Iqbal, M.; Nazir, A.; Javed, T. UV/H2O2, UV/H2O2/SnO2 and Fe/H2O2 based advanced oxidation processes for the degradation of disperse violet 63 in aqueous medium. Mater. Res. Express 2020, 7, 015531. [Google Scholar] [CrossRef]
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced oxidation processes in the removal of organic substances from produced water: Potential, configurations, and research needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Zhang, M.H.; Dong, H.; Zhao, L.; Wang, D.X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.Q.; Zhi, D.; Tang, H.M.; Jiang, L.; Luo, S.; Zhou, Y.Y. Effect of Fe2+, Mn2+ catalysts on the performance of electro-Fenton degradation of antibiotic ciprofloxacin, and expanding the utilizing of acid mine drainage. Sci. Total Environ. 2020, 720, 137560. [Google Scholar] [CrossRef] [PubMed]
- Ioannou-Ttofa, L.; Raj, S.; Prakash, H.; Fatta-Kassinos, D. Solar photo-Fenton oxidation for the removal of ampicillin, total cultivable and resistant E-coli and ecotoxicity from secondary-treated wastewater effluents. Chem. Eng. J. 2019, 355, 91–102. [Google Scholar] [CrossRef]
- Manoharan, R.K.; Ishaque, F.; Ahn, Y.H. Fate of antibiotic resistant genes in wastewater environments and treatment strategies-A review. Chemosphere 2022, 298, 134671. [Google Scholar] [CrossRef]
- Fiorentino, A.; Ferro, G.; Alferez, M.C.; Polo-López, M.I.; Fernández-Ibañez, P.; Rizzo, L. Inactivation and regrowth of multidrug resistant bacteria in urban wastewater after disinfection by solar-driven and chlorination processes. J. Photochem. Photobiol. B Biol. 2015, 148, 43–50. [Google Scholar] [CrossRef]
- Karaolia, P.; Michael, I.; García-Fernández, I.; Agüera, A.; Malato, S.; Fernández-Ibáñez, P.; Fatta-Kassinos, D. Reduction of clarithromycin and sulfamethoxazole-resistant Enterococcus by pilot-scale solar-driven Fenton oxidation. Sci. Total Environ. 2014, 468, 19–27. [Google Scholar] [CrossRef]
- Michael, S.G.; Michael-Kordatou, I.; Beretsou, V.G.; Jäger, T.; Michael, C.; Schwartz, T.; Fatta-Kassinos, D. Solar photo-Fenton oxidation followed by adsorption on activated carbon for the minimisation of antibiotic resistance determinants and toxicity present in urban wastewater. Appl. Catal. B Environ. 2019, 244, 871–880. [Google Scholar] [CrossRef]
- Vijayaraghavan, P.; Lourthuraj, A.A.; Arasu, M.V.; AbdullahAl-Dhabi, N.; Ravindran, B.; WoongChang, S. Effective removal of pharmaceutical impurities and nutrients using biocatalyst from the municipal wastewater with moving bed packed reactor. Environ. Res. 2021, 200, 111777. [Google Scholar] [CrossRef]
- Wang, S.Z.; Wang, J.L. Comparative study on sulfamethoxazole degradation by Fenton and Fe(II)-activated persulfate process. RSC Adv. 2017, 7, 48670–48677. [Google Scholar] [CrossRef]
- Herzog, B.; Lemmer, H.; Horn, H.; Müller, E. Characterization of pure cultures isolated from sulfamethoxazole-acclimated activated sludge with respect to taxonomic identification and sulfamethoxazole biodegradation potential. BMC Microbiol. 2013, 13, 276. [Google Scholar] [CrossRef]
- Larcher, S.; Yargeau, V. Biodegradation of sulfamethoxazole by individual and mixed bacteria. Appl. Microbiol. Biotechnol. 2011, 91, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Q.M.; Cheng, J.S.; Yuan, Y.J. Improving the bioremoval of sulfamethoxazole and alleviating cytotoxicity of its biotransformation by laccase producing system under coculture of Pycnoporus sanguineus and Alcaligenes faecalis. Bioresour. Technol. 2016, 220, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Akrout, I.; Staita, K.; Zouari-Mechichi, H.; Ghariani, B.; Khmaissa, M.; Navarro, D.; Doan, A.; Albert, Q.; Faulds, C.; Sciara, G.; et al. Valorizing fungal diversity for the degradation of fluoroquinolones. Heliyon 2024, 10, e30611. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Ji, D.G.; Wang, C. Interaction between earthworms and arbuscular mycorrhizal fungi on the degradation of oxytetracycline in soils. Soil Biol. Biochem. 2015, 90, 283–292. [Google Scholar] [CrossRef]
- Cao, J.; Wang, C.; Dou, Z.X.; Liu, M.L.; Ji, D.G. Hyphospheric impacts of earthworms and arbuscular mycorrhizal fungus on soil bacterial community to promote oxytetracycline degradation. J. Hazard. Mater. 2018, 341, 346–354. [Google Scholar] [CrossRef]
- Zumstein, M.T.; Helbling, D.E. Biotransformation of antibiotics: Exploring the activity of extracellular and intracellular enzymes derived from wastewater microbial communities. Water Res. 2019, 155, 115–123. [Google Scholar] [CrossRef]
- Terzic, S.; Udikovic-Kolic, N.; Jurina, T.; Krizman-Matasic, I.; Senta, I.; Mihaljevic, I.; Loncar, J.; Smital, T.; Ahel, M. Biotransformation of macrolide antibiotics using enriched activated sludge culture: Kinetics, transformation routes and ecotoxicological evaluation. J. Hazard. Mater. 2018, 349, 143–152. [Google Scholar] [CrossRef]
- Shi, Y.K.; Lin, H.; Ma, J.W.; Zhu, R.R.; Sun, W.C.; Lin, X.Y.; Zhang, J.; Zheng, H.B.; Zhang, X. Degradation of tetracycline antibiotics by Arthrobacter nicotianae OTC-16. J. Hazard. Mater. 2021, 403, 123996. [Google Scholar] [CrossRef]
- Yin, Z.F.; Xia, D.; Shen, M.; Zhu, D.W.; Cai, H.J.; Wu, M.; Zhu, Q.R.; Kang, Y.J. Tetracycline degradation by Klebsiella sp. strain TR5: Proposed degradation pathway and possible genes involved. Chemosphere 2020, 253, 126729. [Google Scholar] [CrossRef]
- Tan, Z.W.; Chen, J.C.; Liu, Y.L.; Chen, L.; Xu, Y.Q.; Zou, Y.X.; Li, Y.T.; Gong, B.N. The survival and removal mechanism of Sphingobacterium changzhouense TC931 under tetracycline stress and its’ ecological safety after application. Bioresour. Technol. 2021, 333, 125067. [Google Scholar] [CrossRef]
- Huang, X.C.; Zhang, X.Y.; Feng, F.X.; Xu, X.P. Biodegradation of tetracycline by the yeast strain Trichosporon mycotoxinivorans XPY-10. Prep. Biochem. Biotechnol. 2016, 46, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.M.; Brugnari, T.; Haminiuk, C.W.I.; Maciel, G.M. Production and immobilization of laccases from monoculture and co-culture of Trametes villosa and Pycnoporus sanguineus for sustainable biodegradation of ciprofloxacin. Process Biochem. 2024, 141, 132–143. [Google Scholar] [CrossRef]
- Tian, Q.P.; Dou, X.; Huang, L.; Wang, L.; Meng, D.; Zhai, L.X.; Shen, Y.; You, C.P.; Guan, Z.B.; Liao, X.R. Characterization of a robust cold-adapted and thermostable laccase from Pycnoporus sp. SYBC-L10 with a strong ability for the degradation of tetracycline and oxytetracycline by laccase-mediated oxidation. J. Hazard. Mater. 2020, 382, 121084. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, C.A.V.; Maciel, G.M.; Rodrigues, E.A.; Silva, L.L.; Oliveira, R.F.; Brugnari, T.; Peralta, R.M.; de Souza, C.G.M. Simultaneous Removal of the Antimicrobial Activity and Toxicity of Sulfamethoxazole and Trimethoprim by White Rot Fungi. Water Air Soil Pollut. 2017, 228, 341. [Google Scholar] [CrossRef]
- Guo, X.L.; Zhu, Z.W.; Li, H.L. Biodegradation of sulfamethoxazole by Phanerochaete chrysosporium. J. Mol. Liq. 2014, 198, 169–172. [Google Scholar] [CrossRef]
- Xie, P.; Chen, C.; Zhang, C.F.; Su, G.Y.; Ren, N.Q.; Ho, S.H. Revealing the role of adsorption in ciprofloxacin and sulfadiazine elimination routes in microalgae. Water Res. 2020, 172, 115475. [Google Scholar] [CrossRef]
- Xiao, G.X.; Chen, J.Q.; Show, P.L.; Yang, Q.L.; Ke, J.; Zhao, Q.; Guo, R.X.; Liu, Y.H. Evaluating the application of antibiotic treatment using algae-algae/activated sludge system. Chemosphere 2021, 282, 130966. [Google Scholar] [CrossRef]
- Garcia-Rodríguez, A.; Matamoros, V.; Fontàs, C.; Salvadó, V. The influence of light exposure, water quality and vegetation on the removal of sulfonamides and tetracyclines: A laboratory-scale study. Chemosphere 2013, 90, 2297–2302. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Fan, H.Y.; Huang, X.; Wei, L.L.; Liu, C.X. Fate of antibiotics from swine wastewater in constructed wetlands with different flow configurations. Int. Biodeterior. Biodegrad. 2019, 140, 119–125. [Google Scholar] [CrossRef]
- Fang, H.S.; Zhang, Q.; Nie, X.P.; Chen, B.W.; Xiao, Y.D.; Zhou, Q.B.; Liao, W.; Liang, X.M. Occurrence and elimination of antibiotic resistance genes in a long-term operation integrated surface flow constructed wetland. Chemosphere 2017, 173, 99–106. [Google Scholar] [CrossRef]
- Chen, J.; Ying, G.G.; Wei, X.D.; Liu, Y.S.; Liu, S.S.; Hu, L.X.; He, L.Y.; Chen, Z.F.; Chen, F.R.; Yang, Y.Q. Removal of antibiotics and antibiotic resistance genes from domestic sewage by constructed wetlands: Effect of flow configuration and plant species. Sci. Total Environ. 2016, 571, 974–982. [Google Scholar] [CrossRef]
- Sabri, N.A.; Schmitt, H.; van der Zaan, B.M.; Gerritsen, H.W.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Performance of full scale constructed wetlands in removing antibiotics and antibiotic resistance genes. Sci. Total Environ. 2021, 786, 147368. [Google Scholar] [CrossRef]
- Bai, S.Y.; Wang, X.; Zhang, Y.; Liu, F.; Shi, L.L.; Ding, Y.L.; Wang, M.; Lyu, T. Constructed Wetlands as Nature-Based Solutions for the Removal of Antibiotics: Performance, Microbial Response, and Emergence of Antimicrobial Resistance (AMR). Sustainability 2022, 14, 14989. [Google Scholar] [CrossRef]
- Hena, S.; Gutierrez, L.; Croué, J.P. Removal of metronidazole from aqueous media by C. vulgaris. J. Hazard. Mater. 2020, 384, 121400. [Google Scholar] [CrossRef] [PubMed]
- Angulo, E.; Bula, L.; Mercado, I.; Montaño, A.; Cubillán, N. Bioremediation of Cephalexin with non-living Chlorella sp., biomass after lipid extraction. Bioresour. Technol. 2018, 257, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Song, C.F.; Wei, Y.L.; Qiu, Y.T.; Qi, Y.; Li, Y.; Kitamura, Y. Biodegradability and mechanism of florfenicol via Chlorella sp UTEX1602 and L38: Experimental study. Bioresour. Technol. 2019, 272, 529–534. [Google Scholar] [CrossRef]
- Chen, Q.H.; Zhang, L.; Han, Y.H.; Fang, J.Y.; Wang, H.Y. Degradation and metabolic pathways of sulfamethazine and enrofloxacin in Chlorella vulgaris and Scenedesmus obliquus treatment systems. Environ. Sci. Pollut. Res. 2020, 27, 28198–28208. [Google Scholar] [CrossRef]
- Li, H.T.; Pan, Y.; Wang, Z.Z.; Chen, S.; Guo, R.X.; Chen, J.Q. An algal process treatment combined with the Fenton reaction for high concentrations of amoxicillin and cefradine. RSC Adv. 2015, 5, 100775–100782. [Google Scholar] [CrossRef]
- Xiong, J.Q.; Kurade, M.B.; Kim, J.R.; Roh, H.S.; Jeon, B.H. Ciprofloxacin toxicity and its co-metabolic removal by a freshwater microalga Chlamydomonas mexicana. J. Hazard. Mater. 2017, 323, 212–219. [Google Scholar] [CrossRef]
- Yang, M.Z.; Liang, S.; Hu, Z.; Xie, H.J.; Zhuang, L.L.; Zhang, J. Antibiotic Removal Based on Constructed Wetland: Mechanism, Performance, and Regulation. Curr. Pollut. Rep. 2024, 11, 3. [Google Scholar] [CrossRef]
- Lei, Y.; Rijnaarts, H.; Langenhoff, A. Mesocosm constructed wetlands to remove micropollutants from wastewater treatment plant effluent: Effect of matrices and pre-treatments. Chemosphere 2022, 305, 135306. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yan, C.; Zhang, L.; Zhang, G.; Fang, H. Remediation of sulfonamide antibiotic-containing wastewater by constructed wetlands: Importance and action mechanism of plants. J. Environ. Manag. 2025, 383, 125520. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.M.; Liu, X.H.; Lv, Y.; Guo, X.C.; Lu, S.Y. Response of Cyperus involucratus to sulfamethoxazole and ofloxacin-contaminated environments: Growth physiology, transportation, and microbial community. Ecotoxicol. Environ. Saf. 2020, 206, 111332. [Google Scholar] [CrossRef]
- Wang, S.Q.; Cui, Y.B.; Li, A.M.; Wang, D.; Zhang, W.J.; Chen, Z.B. Seasonal dynamics of bacterial communities associated with antibiotic removal and sludge stabilization in three different sludge treatment wetlands. J. Environ. Manag. 2019, 240, 231–237. [Google Scholar] [CrossRef]
- Man, Y.; Wang, J.X.; Tam, N.F.Y.; Wan, X.; Huang, W.D.; Zheng, Y.; Tang, J.P.; Tao, R.; Yang, Y. Responses of rhizosphere and bulk substrate microbiome to wastewater-borne sulfonamides in constructed wetlands with different plant species. Sci. Total Environ. 2020, 706, 135955. [Google Scholar] [CrossRef]
- Zheng, Y.C.; Liu, Y.; Qu, M.W.; Hao, M.Q.; Yang, D.; Yang, Q.; Wang, X.C.; Dzakpasu, M. Fate of an antibiotic and its effects on nitrogen transformation functional bacteria in integrated vertical flow constructed wetlands. Chem. Eng. J. 2021, 417, 129272. [Google Scholar] [CrossRef]
- Liu, X.H.; Guo, X.C.; Liu, Y.; Lu, S.Y.; Xi, B.D.; Zhang, J.; Wang, Z.; Bi, B. A review on removing antibiotics and antibiotic resistance genes from wastewater by constructed wetlands: Performance and microbial response. Environ. Pollut. 2019, 254, 112996. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, J.L.; Liu, C.X.; Liu, L.; Liu, Y.H.; Fan, H.Y.; Zhang, T.F. Performance and bacterial community dynamics of vertical flow constructed wetlands during the treatment of antibiotics-enriched swine wastewater. Chem. Eng. J. 2017, 316, 727–735. [Google Scholar] [CrossRef]
- Shan, A.Q.; Wang, W.J.; Kang, K.J.; Hou, D.D.; Luo, J.P.; Wang, G.; Pan, M.H.; Feng, Y.; He, Z.L.; Yang, X.E. The Removal of Antibiotics in Relation to a Microbial Community in an Integrated Constructed Wetland for Tail Water Decontamination. Wetlands 2020, 40, 993–1004. [Google Scholar] [CrossRef]
- Alexandrino, D.A.M.; Mucha, A.P.; Almeida, C.M.R.; Gao, W.; Jia, Z.J.; Carvalho, M.F. Biodegradation of the veterinary antibiotics enrofloxacin and ceftiofur and associated microbial community dynamics. Sci. Total Environ. 2017, 581, 359–368. [Google Scholar] [CrossRef]
- Chen, P.P.; Yu, X.F.; Zhang, J.Y.; Wang, Y.Q. New and traditional methods for antibiotic resistance genes removal: Constructed wetland technology and photocatalysis technology. Front. Microbiol. 2023, 13, 1110793. [Google Scholar] [CrossRef]
- Chen, J.; Wei, X.D.; Liu, Y.S.; Ying, G.G.; Liu, S.S.; He, L.Y.; Su, H.C.; Hu, L.X.; Chen, F.R.; Yang, Y.Q. Removal of antibiotics and antibiotic resistance genes from domestic sewage by constructed wetlands: Optimization of wetland substrates and hydraulic loading. Sci. Total Environ. 2016, 565, 240–248. [Google Scholar] [CrossRef]
- Savin, M.; Alexander, J.; Bierbaum, G.; Hammerl, J.A.; Hembach, N.; Schwartz, T.; Schmithausen, R.M.; Sib, E.; Voigt, A.; Kreyenschmidt, J. Antibiotic-resistant bacteria, antibiotic resistance genes, and antibiotic residues in wastewater from a poultry slaughterhouse after conventional and advanced treatments. Sci. Rep. 2021, 11, 16622. [Google Scholar] [CrossRef] [PubMed]
- Vacca, G.; Wand, H.; Nikolausz, M.; Kuschk, P.; Kästner, M. Effect of plants and filter materials on bacteria removal in pilot-scale constructed wetlands. Water Res. 2005, 39, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Avila, C.; García-Galán, M.J.; Borrego, C.M.; Rodríguez-Mozaz, S.; García, J.; Barceló, D. New insights on the combined removal of antibiotics and ARGs in urban wastewater through the use of two configurations of vertical subsurface flow constructed wetlands. Sci. Total Environ. 2021, 755, 142554. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhao, Y.; Wang, C.; Zhang, H.P.; Chen, Q.R.; Zhang, X.; Zhang, L.P.; Wu, J.M.; Wu, Z.B.; Zhou, Q.H. Removal performance of antibiotics and antibiotic resistance genes in swine wastewater by integrated vertical-flow constructed wetlands with zeolite substrate. Sci. Total Environ. 2020, 721, 137765. [Google Scholar] [CrossRef]
- Chen, J.; Deng, W.J.; Liu, Y.S.; Hu, L.X.; He, L.Y.; Zhao, J.L.; Wang, T.T.; Ying, G.G. Fate and removal of antibiotics and antibiotic resistance genes in hybrid constructed wetlands. Environ. Pollut. 2019, 249, 894–903. [Google Scholar] [CrossRef]
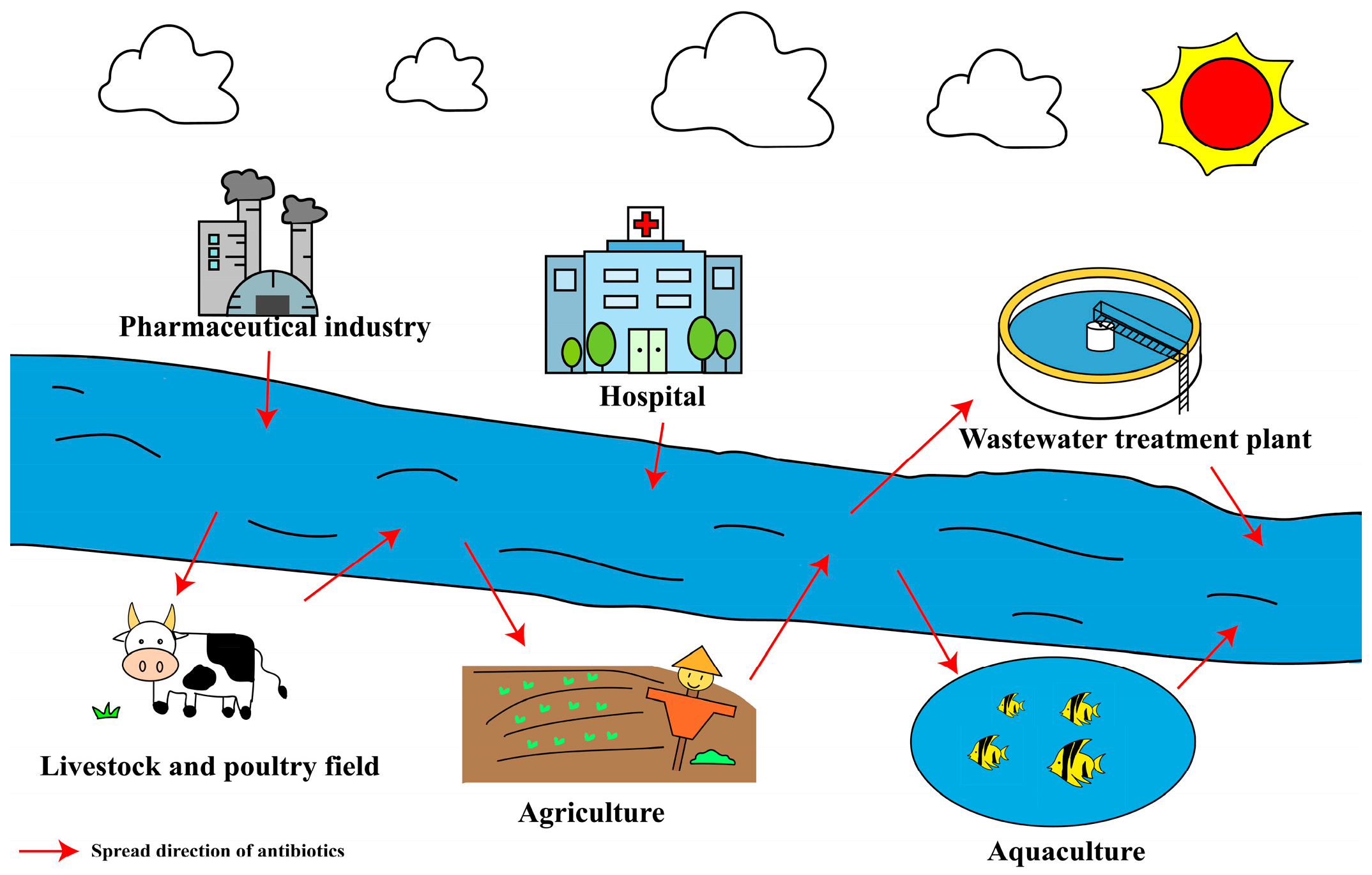

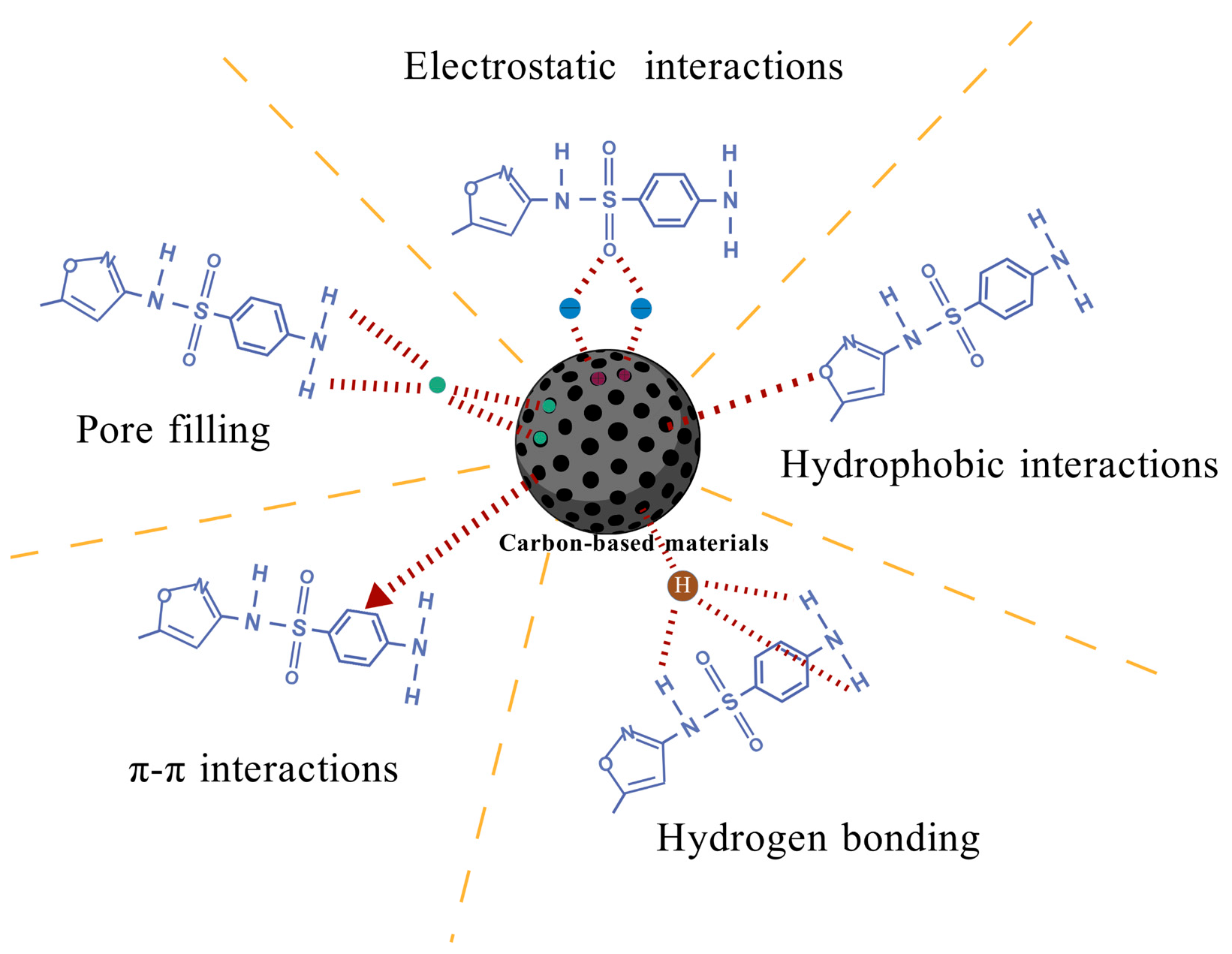
| Types | Compound | CAS | Molecular Formula | Molecular Weight | pKa Values | Log Kow | References |
|---|---|---|---|---|---|---|---|
| Sulfonamides (SAs) | Sulfamethoxazole (SMX) | 723-46-6 | C10H11N3O3S | 253.3 | 1.6; 5.7 | 0.89 | [29,30] |
| Sulfamerazine (SMZ) | 127-79-7 | C11H12N4O2S | 264.3 | 2.24; 6.92 | 1.41 | [31] | |
| Sulfadiazine (SDZ) | 68-35-9 | C10H10N4O2S | 250.3 | 6.36 | −0.0314 | [32] | |
| Tetracyclines (TCs) | Oxytetracycline (OTC) | 79-57-2 | C22H24N2O9 | 460.4 | 3.53; 7.25; 9.58 | −0.9 | [33] |
| Chlortetracycline (CTC) | 57-62-5 | C22H23CIN2O8 | 478.9 | 7.435 | −0.62 | [34] | |
| Tetracycline (TC) | 60-54-8 | C22H24N2O8 | 444.4 | 3.3; 7.68; 9.69 | −1.3 | [35] | |
| Macrolides (MLs) | Erythromycin (ERY) | 114-07-8 | C37H67NO13 | 733.9 | 3.06 | 8.9 | [36,37] |
| Azithromycin (AZM) | 83905-01-5 | C38H72N2O12 | 749.0 | 8.74 | 4.02 | [36] | |
| Clarithromycin (CLR) | 81103-11-9 | C38H69NO13 | 748.0 | 8.99 | 3.16 | [38] | |
| β-lactams | Penicillin G (PEN) | 61-33-6 | C16H18N2O4S | 334.4 | 2.74 | 1.83 | [39] |
| Amoxicillin (AMX) | 26787-78-0 | C16H19N3O5S | 365.4 | 3.37; 8.96 | 0.87 | [40,41] | |
| Aminoglycosides (AGs) | Kanamycin (KAN) | 59-01-8 | C18H36N4O11 | 484.5 | 7.2 | <−3 | [42,43] |
| Streptomycin (SM) | 57-92-1 | C21H39N7O12 | 581.6 | -- | −7.53 | [43] | |
| Tobramycin (TO) | 32986-56-4 | C18H37N5O9 | 467.5 | 6.98 | -- | [44] | |
| Quinolones (QNs) | Ciprofloxacin (CIP) | 85721-33-1 | C17H18FN3O3 | 331.3 | 6.09; 8.74 | 0.28 | [45] |
| Enrofloxacin (ENR) | 93106-60-6 | C19H22FN3O3 | 359.4 | 2.7−3.9 | -- | [46] | |
| Ofloxacin (OFX) | 82419-36-1 | C18H20FN3O4 | 361.4 | 5.97; 9.28 | −0.39 | [45] | |
| Levofloxacin (LEV) | 100986-85-4 | C18H20FN3O4 | 361.4 | 6.02; 8.05 | −0.39 | [47] |
| Types | Mechanisms | ARGs | References |
|---|---|---|---|
| Sulfonamides (SAs) | Similar in structure to para-aminobenzoic acid (PABA), competitively binding to dihydropteroic acid (DHP), preventing the synthesis of tetrahydrofolate (the conversion of DHP), and hindering the synthesis of nucleic acids | sul1, sul2, sul3, and sul4 | [75,76] |
| Tetracyclines (TCs) | Specifically binding to the 30S subunit of bacterial ribosomes, preventing the binding of aminoacyl tRNA to mRNA complexes, blocking peptide chain extension, and interfering with protein synthesis required for bacterial growth | tetA, tetB, tetC, tetD, tetE, tetG, tetK, tetM, and tetW | [77,78,79,80] |
| Macrolides (MLs) | Binding to the peptide donor site (P site) of the 50S subunit of bacterial ribosomes, inhibiting the translocation or transfer of peptidyl tRNA, hindering peptide chain extension, and interfering with protein synthesis | ermA, ermB, ermC, ermD, ermF, ermG, ermT, ermY, mefA, and mefE | [81,82,83] |
| β-lactams | Binding to penicillin-binding proteins (PBPs), inhibiting the transpeptidases in peptidoglycan synthesis, leading to structural defects in the cell wall, and causing bacterial expansion and lysis | blaTEM, blaNDM, blaOXA, blaCTX-M, and blaSHV | [84,85,86,87] |
| Aminoglycosides (AGs) | Combining bacterial ribosome 30S and the A-site on 16S to further interfere with protein synthesis | aac(3)-I, aac(6′)-I, and aph(3′)-I | [88,89] |
| Quinolones (QNs) | Inhibiting DNA helicase and topoisomerase IV, disrupting bacterial DNA replication, and interfering with normal DNA metabolism | qnrA, qnrB, qnrC, qnrD, qnrE, qnrS, and qnrVC | [90,91] |
| Adsorbents | Raw Materials | Antibiotics/ ARGs | Initial Concentration | Condition | Efficiency/ Uptake | References | |
|---|---|---|---|---|---|---|---|
| Biochar | Calamus | ERY | 50 mg/L | pH = 8.5; t = 1 h; T = 25 °C | 325 mg/g | [133] | |
| Pomelo peel | TC | 10 mg/L | pH = 8.5; t = 48 h; T = 25 °C | 476.19 mg/g | [134] | ||
| Rice husk | CIP | 25 mg/L | pH = 6.0; t = 12 h; T = 25 °C | 50.32 mg/g | [135] | ||
| Pomelo peel | CTC | 10 mg/L | pH = 8.5; t = 48 h; T = 25 °C | 555.56 mg/g | [134] | ||
| Sugarcane bagasse | TC | 20 mg/L | pH = 8.5; t = 2 h; T = 25 °C | 85.5 mg/g | [136] | ||
| Cassava waste | OTC | 1 mg/L | pH = 7.0; t = 24 h; T = 25 °C | 2.43 mg/g | [137] | ||
| Coffee grounds | SMX | 0.5 mg/L | pH = 6.8; t = 24 h; T = 25 °C | 0.13 mg/g | [138] | ||
| Pinecone biochar | sul1, tetW | 10 mg/L | t = 30 d; T = 20 °C | 13–21% | [139] | ||
| Sewage-sludge biochar | eDNA | 100 mg/L | pH < 5.0; t = 5 h; T = 25 °C | 1 mg/g | [140] | ||
| Physical modification | Steam activation | Burcucumber plants; H2O | SMZ | 50 mg/L | pH = 3.0; t = 72 h; T = 25 °C | 37.7 mg/g | [141] |
| Bamboo; H2O | TC | 100 mmol/L | pH = 5.0; t = 50 h; T = 25 °C | 95.75% | [132] | ||
| Heat treatment | Pinewood sawdust; 800 °C | TC | 48 mg/L | pH = 5.0; t = 48 h; T = 25 °C | 18.8-fold | [142] | |
| Softwood sawdust; 700 °C | TC | 25 mg/L | pH = 6.8; t = 48 h; T = 25 °C | 5.5–9.2-fold | [143] | ||
| Ball milling | Poplar woodchips; 300 °C | ENR | 20 mg/L | pH = 6.8; t = 3 h; T = 25 °C | 93.4 mg/g | [144] | |
| Hickory chips; 450 °C | SMX | 10 mg/L | pH = 6.0; t = 12 h; T = 25 °C; r = 250 r | 83.3% | [145] | ||
| Chemical modification | Acid modification | Camellia oleifera shells; H3PO4 | TC | 25 mg/L | pH = 6.0; t = 4 h; T = 25 °C | 451.5 mg/g | [146] |
| Swine manure; HCl | SMZ | 9 mg/L | pH = 3.0–9.0; t = 12 h; T = 25 °C | 1.58 mg/g | [147] | ||
| Alkali modification | Pomelo peel derived biochar; KOH | TC | 40 mg/L | pH = 7.0 ± 0.5; t = 48 h; T = 25 °C | 402.86 mg/g | [134] | |
| Pomelo peel derived biochar; KOH | CTC | 40 mg/L | pH = 7.0 ± 0.5; t = 48 h; T = 25 °C | 456.68 mg/g | [134] | ||
| Oxidative modification | Rape stalk; H2O2 | TC | 20 mg/L | pH = 9.0; t = 22 h; T = 25 °C | 42.45 mg/g | [148] | |
| Metal oxide and metal salt modification | Poplar wood chips; Fe2O3 | NOR | 50 mg/L | pH = 6.0; t = 24 h; T = 25 °C | 38.77 mg/g | [149] | |
| Municipal wastewater sludge; ZnCl2 | SMX | 100 mg/L | pH = 3; t = 24 h; T = 25 °C | 50.6 mg/g | [150] | ||
| Magnetic biochar; quaternary phosphonium salt | Calf thymus DNA | 100 μg/mL | pH = 7.0; t = 24 h; T = 25 °C | >92.7% | [151] | ||
| Biological modification | Reed charcoal and wheat bran; Achromobacter and Parapedobacter | TC | 20 mg/L | W:V = 1:10; t = 24 h; T = 30 °C; r = 180 r | 1.37-fold and 11.44-fold | [152] | |
| Straw magnetic biochar; Mycolicibacterium sp. | OTC | 25 mg/L | W:V = 1:200; t = 24 h; T = 30 °C; r = 160 r | 71.8% | [153] | ||
| Honeysuckle residue-derived biochar; Bacillus subtilis | CTC | 50 mg/L | pH = 7.0; t = 72 h; T = 30 °C; r = 180 r | 78.35% | [154] | ||
| Forsythia, erding and chrysanthemum; Bacillus cereus | CTC | 50 mg/L | W:V = 1:10; t = 48 h; T = 30 °C; r = 180 r | 82.34% | [155] | ||
| Activated carbons | Powdered activated carbon | CIP | 2 mg/L | pH = 3.9; t = 48 h; T = 25 °C | 291.96 mg/g | [156] | |
| Macadamia nut shells; NaOH | TC | 600 mg/L | pH = 3.0; t = 3 h; T = 25 °C | 455.33 mg/g | [157] | ||
| Guava seeds; NaOH | AMX | 800 mg/L | pH = 4.0; t = 4 h; T = 25 °C | 570.48 mg/g | [158] | ||
| Carbon nanotubes | Multi-walled carbon nanotubes | CIP | 20 mg/L | pH = 7.0; t = 0.5 h; T = 40 °C | 73% | [159] | |
| Single-walled carbon nanotubes | OTC | 50 mg/L | pH = 6.7–7.0; t = 26 h; T = 25 °C | 375 mg/g | [160] | ||
| CIP | 50 mg/L | pH = 6.7–7.0; t = 26 h; T = 25 °C | 520 mg/g | [160] | |||
| Graphene | Graphene-oxide | CIP | 20 mg/L | pH = 5.0; t = 26 h; T = 25 °C | 379 mg/g | [161] | |
| SMX | 40 mg/L | pH = 5.0; t = 26 h; T = 25 °C | 240 mg/g | [161] | |||
| sul2 | 1.50 × 105 copies/g | t = 18 d; T = 55 °C | 76.12% | [162] | |||
| Reduced graphene oxide | SMX | 5 mg/L | pH = 6.0; t = 3 h; T = 25 °C | 92% | [163] | ||
| Types | Antibiotics/ ARGs | Initial Concentration | Condition | Degradation Efficiency | References | ||
|---|---|---|---|---|---|---|---|
| Photolysis | CAP | 3.0 μmol/L | t = 0.5 h; T = 26 °C; pH = 5.7; dark light | 20% | [227] | ||
| Florfenicol | 20 μmol/L | t = 80 h; T = 25 °C; pH = 7.0; solar irradiation | 24% | [228] | |||
| Electrochemical oxidation | PbO2/Ti/Na2SO4 | LEV | 500 mg/L | Current density = 50 mA·cm−2; Voltage = 9.8 V; t = 2.67 h; pH = 7 | 98.41% | [228] | |
| Stainless steel/stainless steel/Peroxydisulfate | OFX | 5 mg/L | Current density = 25 mA·cm−2; Voltage = 2.6–3.1 V; t = 1.5 h; pH = 4 | 89.6% | [228] | ||
| Fenton/Fenton-like technology | Fenton technology | Fe2+/H2O2 | intI1, sul1, tetX | -- | -- | 2.58–3.79 logs | [229] |
| Photo-Fenton oxidation | UV/H2O2/Fe2+ | CPFX | 15 mg/L | Fe2+ = 0.05 mmol/L; H2O2 = 5.0 mmol/L; pH = 4.0 | 71% | [230] | |
| Fe2+/H2O2 | intI1 | 0.5 | Fe2+ = 0.1 mmol/L; H2O2 = 1.47 mmol/L; pH = 2.8; natural sunlight | 23% | [231] | ||
| Electro-Fenton oxidation | H2O2/Fe2+/Current | SAs | 0.5 mmol/L | Fe2+ = 0.5 mmol/L; H2O2 = 7 mg/L; pH = 3.0 | 92% | [232] | |
| Heterogeneous photo/Electro-Fenton oxidation | UV/H2O2/Fe3S4 | SMX | 5 mg/L | Fe3S4 = 15 mg; H2O2 = 9.79 mmol/L; pH = 5.0 | 93% | [233] | |
| Types | Strain/ Plant | Antibiotics/ ARGs | Initial Concentration | Operating Condition | Degradation Efficiency | References | |
|---|---|---|---|---|---|---|---|
| Bacterium | Arthrobacter nicotianae OTC-16 | OTC | 100 mg/L | t = 8 d; T = 30 °C; r = 180 r | 98.5% | [271] | |
| Klebsiella sp. strain TR5 | TC | 200 mg/L | pH = 7.0; t = 36 h; T = 25 °C; r = 180 r | 90% | [272] | ||
| Sphingobacterium changzhouense TC931 | TC | 10 mg/L | pH = 7.0; t = 36 h; T = 30 °C; r = 150 r | 87.38% | [273] | ||
| Fungi | Trichosporon mycotoxinivorans XPY-10 | TC | 800 mg/L | t = 7 d; T = 30 °C; r = 120 r | 78.28% | [274] | |
| Trametes villosa and Pycnoporus sanguineus | CIP | 2.5 mg/L | t = 24 h; T = 40 °C; r = 120 r; in the dark | 25% | [275] | ||
| Pycnoporus sp. SYBC-L10 | OTC | 500 mg/L | t = 5 min; T = 30 °C; r = 200 r | 100% | [276] | ||
| Pleurotus ostreatus | SMX | 50 mg/L | t = 15 d; T = 25 °C; r = 120 r | 74% | [277] | ||
| Phanerochaete chrysosporium | SMX | 10 mg/L | pH = 4.5; t = 10 d; T = 35 °C; r = 160 r | 74% | [278] | ||
| Algaes | Chlamydomonas sp. Tai-03 | CIP | 10 mg/L | pH = 6.2; t = 9 d; r = 300 r; 2%CO2 | 65.05% | [279] | |
| SDZ | 10 mg/L | pH = 6.2; t = 9 d; r = 300 r; 2%CO2 | 17.05% | [279] | |||
| Chlorella pyrenoidosa | CED | 50 mg/L | t = 24 h; T = 25 ± 1 °C; light/dark cycle = 12 h:12 h; 70 μmol∙photons∙m−2∙s−1 | 41.47 ± 0.62% | [280] | ||
| Spyrogira sp. | STZ | 200 μg/L | t = 20 d; T = 20 °C; light/dark cycle = 12 h:12 h; 15 μmol∙photons∙m−2∙s−1 | 36% | [281] | ||
| Constructed wetlands | SF-CWs | Phragmites australis | OTC | 30 μg/L | t = 15 d | 99 ± 0.27% | [282] |
| CIP | 30 μg/L | t = 15 d | 97 ± 0.26% | [282] | |||
| sul1, tetA, tetC, tetE, and qnrS | 5.68 × 107 copies/g | t = 30 d | 77.8% | [283] | |||
| HSF-CWs | Thalia dealbata Fraser | sul1 | (1.26 ± 0.01) × 105 copies/g | t = 7 d | 70.0 ± 6.82% | [284] | |
| sul2 | (9.17 ± 0.42) × 107 copies/g | t = 7 d | 47.2 ± 13.8% | [284] | |||
| Phragmites australis | SAs | 400 μg/kg | t = 3 d | 95% | [285] | ||
| Thalia dealbata Fraser | floR | (2.16 ± 0.01) × 106 copies/g | t = 7 d | 88.2 ± 1.54% | [284] | ||
| cmlA | (1.30 ± 0.01) × 106 copies/g | t = 7 d | 80.9 ± 2.78% | [284] | |||
| VSF-CWs | Pontederia cordata | TC | 254.53 mg/L | t = 4 d | 91% | [286] | |
| OTC | 228.53 mg/L | t = 4 d | 90% | [286] | |||
| Thalia dealbata Fraser | tetO | (1.20 ± 0.02) × 103 copies/g | t = 7 d | 76.9 ± 8.56% | [284] | ||
| ermB | (7.41 ± 0.05) × 104 copies/g | t = 7 d | 85.2 ± 3.30% | [284] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Shao, X.; Wang, Q. Antibiotics and Antibiotic Resistance Genes in the Environment: Dissemination, Ecological Risks, and Remediation Approaches. Microorganisms 2025, 13, 1763. https://doi.org/10.3390/microorganisms13081763
Wu Z, Shao X, Wang Q. Antibiotics and Antibiotic Resistance Genes in the Environment: Dissemination, Ecological Risks, and Remediation Approaches. Microorganisms. 2025; 13(8):1763. https://doi.org/10.3390/microorganisms13081763
Chicago/Turabian StyleWu, Zhaomeng, Xiaohou Shao, and Qilin Wang. 2025. "Antibiotics and Antibiotic Resistance Genes in the Environment: Dissemination, Ecological Risks, and Remediation Approaches" Microorganisms 13, no. 8: 1763. https://doi.org/10.3390/microorganisms13081763
APA StyleWu, Z., Shao, X., & Wang, Q. (2025). Antibiotics and Antibiotic Resistance Genes in the Environment: Dissemination, Ecological Risks, and Remediation Approaches. Microorganisms, 13(8), 1763. https://doi.org/10.3390/microorganisms13081763





