Varying Effects of Straw-Returning Methods on Soil Microbial Diversity and Community Composition in Northeast China
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Site
2.2. Experimental Design and Soil Sampling
2.3. Soil DNA Extraction and PCR Amplification
2.4. Illumina MiSeq Sequencing
2.5. Bioinformatic Analysis
3. Results
3.1. Illumina MiSeq Sequencing and OTU Cluster Analysis
3.2. Analysis of Soil Bacterial and Fungal Community Composition
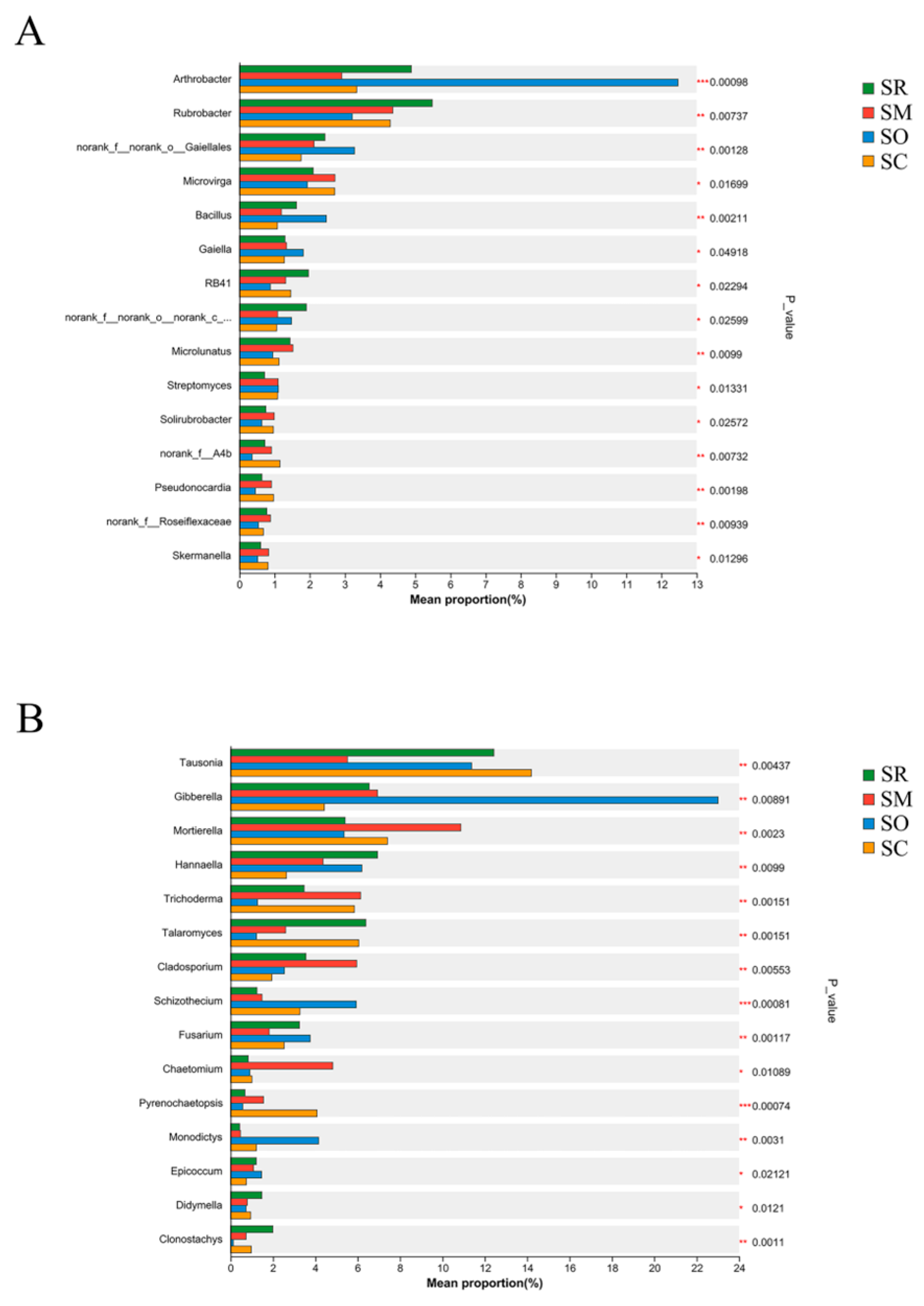
3.3. The Difference of Soil Bacterial and Fungal Community Analysis
3.4. Alpha Diversity Analysis for Soil Bacterial and Fungal Communities
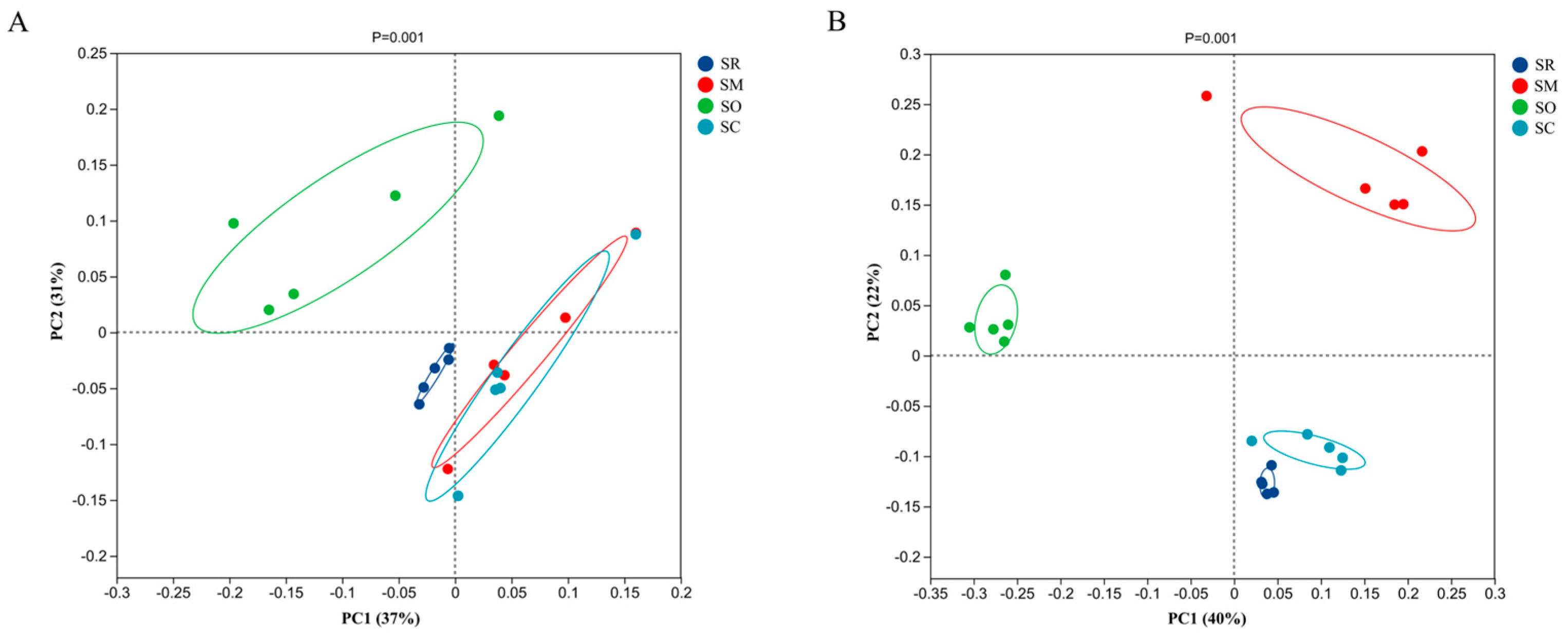
3.5. Beta Diversity Analysis for Soil Bacterial and Fungal Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, J.; Zhu, J.; Huang, Y.G.; Zhang, M.; Zhang, R.C.; Peng, Y.Q. Discussion on the utilization methods of straw. Crop Res. 2019, 33, 597–602. [Google Scholar]
- Li, H.M.; Zhang, M.; Li, X.; Nie, M.; Tian, X.W. Research progress of straw degradation by microorganisms. Shandong Chem. Ind. 2021, 50, 70–74. [Google Scholar]
- Sun, L.N.; Ma, X.Y.; Liu, K.B.; Zheng, X.H.; Zhang, H.L.; Rong, L.G. A Review on research advances on microbial treatment and strengthening techniques of crop straw. J. Shenyang Univ. (Nat. Sci.) 2018, 30, 188–195. [Google Scholar]
- Song, P. Research progress of crop straw development and utilization. Mod. Anim. Husbandry 2018, 2, 26–30. [Google Scholar]
- Huang, L.X.; Wang, Y.J.; Huang, Q.; Wei, L.; Li, X.; Chen, W.S.; Huang, Y.F.; Liu, Z.Z. Effects of two ways of cornstalk returning on soil properties, growth and quality of sweet corn. Guangdong Agr. Sci. 2021, 48, 65–73. [Google Scholar]
- Gou, Z.; Yin, W.; Chai, Q. Straw and residual film management enhances crop yield and weakens CO2 emissions in wheat-maize intercropping system. Sci. Rep. 2021, 11, 14077. [Google Scholar] [CrossRef]
- Chen, L.; Sun, S.; Yao, B.; Peng, Y.; Gao, C.; Qin, T.; Zhou, Y.; Sun, C.; Quan, W. Effects of straw return and straw biochar on soil properties and crop growth: A review. Front. Plant Sci. 2022, 13, 986763. [Google Scholar] [CrossRef]
- Liu, G.Y.; He, A.L.; Du, J.; Yang, Z.P.; Pan, X.Y.; Xu, J.D.; Zheng, N.; Zhang, Y.T. Effects of maize straw returning amount on soil enzyme activity, microbial biomass, and bacterial community in lime concretion black soil. J. Agr. Resour. Environ. 2022, 39, 1033–1040. [Google Scholar]
- Xu, T. Effects of different ways of returning corn stalks to the field on corn growth characteristics, yield and its composition under maize bean rotation. Anhui Agri. Sci. Bull. 2022, 28, 35–37. [Google Scholar]
- Li, J.; Ren, L.J.; Li, X.Y.; Bi, R.X.; Jin, X.X.; Yu, N.; Zhang, Y.L.; Zhou, H.T.; Zhang, Y.L. Effects of different straw returning patterns on soil CO2 emission and carbon balance in maize field. Sci. Agric. Sin. 2023, 56, 2738–2750. [Google Scholar]
- Wanmolee, W.; Sornlake, W.; Rattanaphan, N.; Suwannarangsee, S.; Laosiripojana, N.; Champreda, V. Biochemical characterization and synergism of cellulolytic enzyme system from Chaetomium globosum on rice straw saccharification. BMC Biotechnol. 2016, 16, 82. [Google Scholar] [CrossRef]
- Saritha, M.; Arora, A.; Singh, S.; Nain, L. Streptomyces griseorubens mediated delignification of paddy straw for improved enzymatic saccharification yields. Bioresour. Technol. 2013, 135, 12–17. [Google Scholar] [CrossRef]
- Chen, K.J.; Tang, J.C.; Xu, B.H.; Lan, S.L.; Cao, Y. Degradation enhancement of rice straw by co-culture of Phanerochaete chrysosporium and Trichoderma viride. Sci. Rep. 2019, 9, 19708. [Google Scholar] [CrossRef]
- Mei, J.; Shen, X.; Gang, L.; Xu, H.; Wu, F.; Sheng, L. A novel lignin degradation bacteria-Bacillus amyloliquefaciens SL-7 used to degrade straw lignin efficiently. Bioresour. Technol. 2020, 310, 123445. [Google Scholar] [CrossRef]
- Wang, L.; Guan, H.; Hu, J.; Feng, Y.; Li, X.; Yusef, K.K.; Gao, H.; Tian, D. Aspergillus niger enhances organic and inorganic phosphorus release from wheat straw by secretion of degrading enzymes and oxalic acid. J. Agric. Food Chem. 2022, 70, 10738–10746. [Google Scholar] [CrossRef]
- Liu, Z.G.; Lu, H.B.; Zhao, H.C.; Wang, J.Q.; He, J.P.; Wang, Y.Q.; Huang, Z.H.; Zhao, H.X.; Wei, D. Effects of methods for spring maize straw-returning to field on soil microbial biomas C, N, P and enzyme activities in dry farming area. Acta Agr. Boreali-Occident. Sin. 2022, 31, 183–192. [Google Scholar]
- Yang, H.; Zhao, Y.; Ma, J.; Rong, Z.; Chen, J.; Wang, Y.; Zheng, X.; Ye, W. Wheat straw return influences soybean root-associated bacterial and fungal microbiota in a wheat-soybean rotation system. Microorganisms 2022, 10, 667. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Wen, Y.C.; Li, H.Y.; Lin, Z.A.; Zhao, B.Q.; Sun, Z.B.; Yuan, L.; Xu, J.K.; Li, Y.Q. Long-term fertilization alters soil properties and fungal community composition in fluvo-aquic soil of the North China Plain. Sci. Rep. 2020, 10, 7198. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Li, F.; Hitch, T.C.A.; Chen, Y.; Creevey, C.J.; Guan, L.L. Comparative metagenomic and metatranscriptomic analyses reveal the breed effect on the rumen microbiome and its associations with feed efficiency in beef cattle. Microbiome 2019, 7, 6. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, Q.; Cole, J.R.; Rosen, G.L. Using the RDP classifier to predict taxonomic novelty and reduce the search space for finding novel organisms. PLoS ONE 2012, 7, e32491. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Miguel, M.A.; Kim, S.H.; Lee, S.S.; Cho, Y.I. Impact of soil microbes and oxygen availability on bacterial community structure of decomposing poultry carcasses. Animals 2021, 11, 2937. [Google Scholar] [CrossRef]
- Murase, J.; Takenouchi, Y.; Iwasaki, K.; Kimura, M. Microeukaryotic community and oxygen response in rice field soil revealed using a combined rRNA-gene and rRNA-based approach. Microbes Environ. 2014, 29, 74–81. [Google Scholar] [CrossRef]
- Castaño, C.; Lindahl, B.D.; Alday, J.G.; Hagenbo, A.; Martínez de Aragón, J.; Parladé, J.; Pera, J.; Bonet, J.A. Soil microclimate changes affect soil fungal communities in a Mediterranean pine forest. New Phytol. 2018, 220, 1211–1221. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Martin-Closas, L.; Pelacho, A.M.; DeBruyn, J.M. Biodegradable plastic mulch films: Impacts on soil microbial communities and eosystem functions. Front. Microbiol. 2018, 9, 819. [Google Scholar] [CrossRef]
- Bradford, M.A.; Tordoff, G.M.; Eggers, T.; Jones, T.H.; Newington, J.E. Microbiota, fauna, and mesh size interactions in litter decomposition. Oikos 2002, 99, 317–323. [Google Scholar] [CrossRef]
- Zhang, F.T.; Wang, T.S.; Jiang, H.; Zhang, B.; Han, Y.; Yao, S.H. Effects of size and amount and burial depth on early decomposition of maize straw and the links to microbial and nematode communities in the Mollisol of China. Soil Use Manag. 2024, 40, e70003. [Google Scholar] [CrossRef]
- Abdenaceur, R.; Farida, B.T.; Mourad, D.; Rima, H.; Zahia, O.; Fatma, S.H. Effective biofertilizer Trichoderma spp. isolates with enzymatic activity and metabolites enhancing plant growth. Int. Microbiol. 2022, 25, 817–829. [Google Scholar] [CrossRef]
- Ren, X.; Branà, M.T.; Haidukowski, M.; Gallo, A.; Zhang, Q.; Logrieco, A.F.; Li, P.; Zhao, S.; Altomare, C. Potential of Trichoderma spp. for biocontrol of aflatoxin-producing Aspergillus flavus. Toxins 2022, 14, 86. [Google Scholar] [CrossRef]
- TariqJaveed, M.; Farooq, T.; Al-Hazmi, A.S.; Hussain, M.D.; Rehman, A.U. Role of Trichoderma as a biocontrol agent (BCA) of phytoparasitic nematodes and plant growth inducer. J. Invertebr. Pathol. 2021, 183, 107626. [Google Scholar] [CrossRef]
- Swain, H.; Adak, T.; Mukherjee, A.K.; Sarangi, S.; Samal, P.; Khandual, A.; Jena, R.; Bhattacharyya, P.; Naik, S.K.; Mehetre, S.T.; et al. Seed biopriming with Trichoderma strains isolated from tree bark improves plant growth, antioxidative defense system in rice and enhance straw degradationcapacity. Front. Microbiol. 2021, 12, 633881. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, J.; Gao, W.; Yang, Q.; Chen, L.; Yang, L.; Sun, Q.; Zhang, H. Effects of rice straw structure on chaetoglobosin A production by Chaetomium globosum CGMCC 6882. Int. J. Biol. Macromol. 2020, 150, 1223–1228. [Google Scholar] [CrossRef]
- Han, C.; Yang, R.R.; Sun, Y.X.; Liu, M.Y.; Zhou, L.F.; Li, D.C. Identification and characterization of a novel hyperthermostable bifunctional cellobiohydrolase- xylanase enzyme for synergistic effect with commercial cellulase on pretreated wheat straw degradation. Front. Bioeng. Biotechnol. 2020, 8, 296. [Google Scholar] [CrossRef]
- Singh, R.K.; Tiwari, M.K.; Kim, D.; Kang, Y.C.; Ramachandran, P.; Lee, J.K. Molecular cloning and characterization of a GH11 endoxylanase from Chaetomium globosum, and its use in enzymatic pretreatment of biomass. Appl. Microbiol. Biotechnol. 2013, 97, 7205–7214. [Google Scholar] [CrossRef]
- Tian, Y.; Fu, X.; Zhang, G.; Zhang, R.; Kang, Z.; Gao, K.; Mendgen, K. Mechanisms in growth-promoting of cucumber by the endophytic fungus Chaetomium globosum strain ND35. J. Fungi 2022, 8, 180. [Google Scholar] [CrossRef]
- Zhao, S.S.; Zhang, Y.Y.; Yan, W.; Cao, L.L.; Xiao, Y.; Ye, Y.H. Chaetomium globosum CDW7, a potential biological control strain and its antifungal metabolites. FEMS Microbiol. Lett. 2017, 364, fnw287. [Google Scholar] [CrossRef]
- Gao, W.L.; Fang, J.L.; Zhu, C.Y.; Xu, W.F.; Lyu, Z.Y.; Chan, X.A.; Zhao, Q.W.; Li, Y.Q. Identification and characterization of a new regulator, TagR, for environmental stress resistance based on the DNA methylome of Streptomyces roseosporus. Microbiol. Spectr. 2023, 11, e0038023. [Google Scholar] [CrossRef]
- Le, K.D.; Yu, N.H.; Park, A.R.; Park, D.J.; Kim, C.J.; Kim, J.C. Streptomyces sp. AN090126 as a biocontrol agent against bacterial and fungal plant diseases. Microorganisms 2022, 10, 791. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef]
- Xu, J.; Yang, Q. Isolation and characterization of rice straw degrading Streptomyces griseorubens C-5. Biodegradation 2010, 21, 107–116. [Google Scholar] [CrossRef]
- Feng, H.; Sun, Y.; Zhi, Y.; Mao, L.; Luo, Y.; Wei, X.; Zhou, P. Lignocellulose degradation by the isolate of Streptomyces griseorubens JSD-1. Funct. Integr. Genom. 2015, 15, 163–173. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, Y.; Chu, S.; Zhi, Y.; Wang, B.; Zhou, P. Biological pretreatment of rice straw with Streptomyces griseorubens JSD-1 and its optimized production of cellulase and xylanase for improved enzymatic saccharification efficiency. Prep. Biochem. Biotechnol. 2016, 46, 575–585. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological control of plant pathogens: A global perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Khan, S.; Srivastava, S.; Karnwal, A.; Malik, T. Streptomyces as a promising biological control agents for plant pathogens. Front. Microbiol. 2023, 14, 1285543. [Google Scholar] [CrossRef]
- Marra, R.; Lombardi, N.; d’Errico, G.; Troisi, J.; Scala, G.; Vinale, F.; Woo, S.L.; Bonanomi, G.; Lorito, M. Application of Trichoderma strains and metabolites enhances soybean productivity and nutrient content. J. Agric. Food Chem. 2019, 67, 1814–1822. [Google Scholar] [CrossRef]
- Mitrović, I.; Čanak, P.; Tančić Živanov, S.; Farkaš, H.; Vasiljević, M.; Ćujić, S.; Zorić, M.; Mitrović, B. Trichoderma harzianum in biocontrol of maize fungal diseases and relevant mycotoxins: From the laboratory to the field. J. Fungi 2025, 11, 416. [Google Scholar] [CrossRef]
- Ali, I.; Khan, A.; Ali, A.; Ullah, Z.; Dai, D.Q.; Khan, N.; Khan, A.; Al-Tawaha, A.R.; Sher, H. Iron and zinc micronutrients and soil inoculation of Trichoderma harzianum enhance wheat grain quality and yield. Front. Plant Sci. 2022, 13, 960948. [Google Scholar] [CrossRef]
- Pandey, N.; Vaishnav, R.; Rajavat, A.S.; Singh, A.N.; Kumar, S.; Tripathi, R.M.; Kumar, M.; Shrivastava, N. Exploring the potential of Bacillus for crop productivity and sustainable solution for combating rice false smut disease. Front. Microbiol. 2024, 15, 1405090. [Google Scholar] [CrossRef]
- Zahra, S.T.; Tariq, M.; Abdullah, M.; Azeem, F.; Ashraf, M.A. Dominance of Bacillus species in the wheat (Triticum aestivum L.) rhizosphere and their plant growth promoting potential under salt stress conditions. PeerJ 2023, 11, e14621. [Google Scholar] [CrossRef]
- Tahir, M.; Ahmad, I.; Shahid, M.; Shah, G.M.; Farooq, A.B.U.; Akram, M.; Tabassum, S.A.; Naeem, M.A.; Khalid, U.; Ahmad, S.; et al. Regulation of antioxidant production, ion uptake and productivity in potato (Solanum tuberosum L.) plant inoculated with growth promoting salt tolerant Bacillus strains. Ecotoxicol. Environ. Saf. 2019, 178, 33–42. [Google Scholar] [CrossRef]
- de Oliveira-Paiva, C.A.; Bini, D.; de Sousa, S.M.; Ribeiro, V.P.; Dos Santos, F.C.; de Paula Lana, U.G.; de Souza, F.F.; Gomes, E.A.; Marriel, I.E. Inoculation with Bacillus megaterium CNPMS B119 and Bacillus subtilis CNPMS B2084 improve P-acquisition and maize yield in Brazil. Front. Microbiol. 2024, 15, 1426166. [Google Scholar] [CrossRef]
- Villafañe, D.L.; Maldonado, R.A.; Bianchi, J.S.; Kurth, D.; Gramajo, H.; Chiesa, M.A.; Rodríguez, E. Streptomyces N2A, an endophytic actinobacteria that promotes soybean growth and increases yield and seed quality under field conditions. Plant Sci. 2024, 343, 112073. [Google Scholar] [CrossRef]
- Gao, Y.; Ning, Q.; Yang, Y.; Liu, Y.; Niu, S.; Hu, X.; Pan, H.; Bu, Z.; Chen, N.; Guo, J.; et al. Endophytic Streptomyces hygroscopicus OsiSh-2-mediated balancing between growth and disease resistance in host rice. mBio 2021, 12, e0156621. [Google Scholar] [CrossRef]
- Bhuiyan, A.U.; Chowdhury, M.Z.H.; Mim, M.F.; Siddique, S.S.; Haque, M.A.; Rahman, M.S.; Islam, S.M.N. Seed priming with Metarhizium anisopliae (MetA1) improves physiology, growth and yield of wheat. Heliyon 2024, 10, e36600. [Google Scholar] [CrossRef]
- Chowdhury, M.Z.H.; Mostofa, M.G.; Mim, M.F.; Haque, M.A.; Karim, M.A.; Sultana, R.; Rohman, M.M.; Bhuiyan, A.U.; Rupok, M.R.B.; Islam, S.M.N. The fungal endophyte Metarhizium anisopliae (MetA1) coordinates salt tolerance mechanisms of rice to enhance growth and yield. Plant Physiol. Biochem. 2024, 207, 108328. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, H.; Wang, J. Synthesis and application of a compound microbial inoculant for effective soil remediation. Environ. Sci. Pollut. Res Int. 2023, 30, 120915–120929. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, Y.; Sun, Z. Varying Effects of Straw-Returning Methods on Soil Microbial Diversity and Community Composition in Northeast China. Microorganisms 2025, 13, 1749. https://doi.org/10.3390/microorganisms13081749
Zhang Y, Wang Y, Sun Z. Varying Effects of Straw-Returning Methods on Soil Microbial Diversity and Community Composition in Northeast China. Microorganisms. 2025; 13(8):1749. https://doi.org/10.3390/microorganisms13081749
Chicago/Turabian StyleZhang, Yitao, Yuxian Wang, and Zhanbin Sun. 2025. "Varying Effects of Straw-Returning Methods on Soil Microbial Diversity and Community Composition in Northeast China" Microorganisms 13, no. 8: 1749. https://doi.org/10.3390/microorganisms13081749
APA StyleZhang, Y., Wang, Y., & Sun, Z. (2025). Varying Effects of Straw-Returning Methods on Soil Microbial Diversity and Community Composition in Northeast China. Microorganisms, 13(8), 1749. https://doi.org/10.3390/microorganisms13081749





