Staphylococcus aureus in Foodborne Diseases and Alternative Intervention Strategies to Overcome Antibiotic Resistance by Using Natural Antimicrobials
Abstract
1. Introduction
2. Foodborne Diseases and Food Poisoning Pathogens
2.1. Major Bacterial Pathogens Responsible for Food Poisoning and Illness
2.2. Potential Foods Associated with S. aureus Foodborne Disease and Crisis with MRSA
2.3. Incidence, Prevalence, and Food Disease Associated with S. aureus
2.4. Economic Burden of Disease or Infections Associated with S. aureus
3. Occurrence of Antibiotic Resistance in S. aureus
3.1. Common Antibiotics Used Against S. aureus and the Emergence of Resistance
3.2. Antibiotic Residues in Food and Agriculture
4. Alternative Interventions to Control Food Poisoning with S. aureus
4.1. Bacteriophages
4.2. Plant-Derived Antimicrobials
4.3. Antimicrobial Peptides and Nanoparticles
4.4. Light-Based Methods
4.5. Vaccines
4.6. Limitations of Above-Mentioned Interventions to Control Foodborne Diseases
5. Advantages of Pro-Commensal Strategies in Control of Foodborne S. aureus
5.1. Probiotics
5.2. Synbiotics
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heredia, N.; García, S. Animals as Sources of Food-Borne Pathogens: A Review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef]
- World Health Organization Foodborne Diseases Estimates. Available online: https://www.who.int/data/gho/data/themes/who-estimates-of-the-global-burden-of-foodborne-diseases (accessed on 21 June 2025).
- Tack, D.M.; Ray, L.; Griffin, P.M.; Cieslak, P.R.; Dunn, J.; Rissman, T.; Jervis, R.; Lathrop, S.; Muse, A.; Duwell, M.; et al. Preliminary Incidence and Trends of Infections with Pathogens Transmitted Commonly Through Food—Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2016–2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention Food Safety: People with a Higher Risk of Food Poisoning. Available online: https://www.cdc.gov/food-safety/risk-factors/index.html (accessed on 20 June 2025).
- World Health Organization Food Safety. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 21 June 2025).
- Liu, X.; Yao, H.; Zhao, X.; Ge, C. Biofilm Formation and Control of Foodborne Pathogenic Bacteria. Molecules 2023, 28, 2432. [Google Scholar] [CrossRef] [PubMed]
- Krishnaprasad, V.H.; Nayak, V.; Kumar, S. World Health Organisation’s Bacterial Pathogen Priority List (BPPL) 2017 and BPPL 2024 to Combat Global Antimicrobial Resistance Crisis: ‘Challenges and Opportunities’. J. Antimicrob. Chemother. 2025, dkaf167. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Rajagunala, S.; Chakrabort, S.; Verma, A.K.; Kumar, A.; Tiwari, R.; Kapoor, S. Food-Borne Pathogens of Animal Origin-Diagnosis, Prevention, Control and Their Zoonotic Significance: A Review. Pak. J. Biol. Sci. 2013, 16, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention Food Safety: About Food Safety. Available online: https://www.cdc.gov/food-safety/about/index.html (accessed on 20 June 2025).
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on Major Food-Borne Zoonotic Bacterial Pathogens. J. Trop. Med. 2020, 2020, 4674235. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Department of International Trade, TEI of West Macedonia, Kastoria, Greece Foodborne Pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, A.; Lacy, A.; Koyfman, A.; Long, B. High Risk and Low Prevalence Diseases: Botulism. Am. J. Emerg. Med. 2024, 82, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, E.T.; Jenkins, C.; Barker, C.R.; Painset, A.; Didelot, X.; Simbo, A.; Douglas, A.; Godbole, G.; Jorgensen, F.; Gharbia, S.; et al. Genomic Epidemiology of the Clinically Dominant Clonal Complex 1 in the Listeria Monocytogenes Population in the UK. Microb. Genom. 2024, 10, 001155. [Google Scholar] [CrossRef] [PubMed]
- Brumfield, K.D.; Usmani, M.; Chen, K.M.; Gangwar, M.; Jutla, A.S.; Huq, A.; Colwell, R.R. Environmental Parameters Associated with Incidence and Transmission of Pathogenic Vibrio spp. Environ. Microbiol. 2021, 23, 7314–7340. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.G.; Mills, K.B.; Crosby, H.A.; Horswill, A.R. The Staphylococcus Aureus Regulatory Program in a Human Skin-like Environment. MBio 2024, 15, e00453-24. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shen, Y.; Yang, M.; Chi, K.; Guo, N. Hazard of Staphylococcal Enterotoxins in Food and Promising Strategies for Natural Products against Virulence. J. Agric. Food Chem. 2022, 70, 2450–2465. [Google Scholar] [CrossRef] [PubMed]
- Kasela, M.; Ossowski, M.; Dzikoń, E.; Ignatiuk, K.; Wlazło, Ł.; Malm, A. The Epidemiology of Animal-Associated Methicillin-Resistant Staphylococcus Aureus. Antibiotics 2023, 12, 1079. [Google Scholar] [CrossRef] [PubMed]
- González-Machado, C.; Capita, R.; Alonso-Calleja, C. Methicillin-Resistant Staphylococcus Aureus (MRSA) in Dairy Products and Bulk-Tank Milk (BTM). Antibiotics 2024, 13, 588. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.R.; Davis, J.A.; Barrett, J.B. Prevalence and Characterization of Methicillin-Resistant Staphylococcus Aureus Isolates from Retail Meat and Humans in Georgia. J. Clin. Microbiol. 2013, 51, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Le Loir, Y.; Baron, F.; Gautier, M. Staphylococcus Aureus and Food Poisoning. Genet. Mol. Res. 2003, 2, 63–76. [Google Scholar] [PubMed]
- Fetsch, A.; Contzen, M.; Hartelt, K.; Kleiser, A.; Maassen, S.; Rau, J.; Kraushaar, B.; Layer, F.; Strommenger, B. Staphylococcus Aureus Food-Poisoning Outbreak Associated with the Consumption of Ice-Cream. Int. J. Food Microbiol. 2014, 187, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Masters, B.R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-1-4557-4801-3. [Google Scholar]
- Hennekinne, J.-A.; De Buyser, M.-L.; Dragacci, S. Staphylococcus Aureus and Its Food Poisoning Toxins: Characterization and Outbreak Investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Abdul Halim, H.; Thong, K.; Chai, L. Assessment of Food Safety Knowledge, Attitude, Self-Reported Practices, and Microbiological Hand Hygiene of Food Handlers. IJERPH 2017, 14, 55. [Google Scholar] [CrossRef]
- Raineri, E.J.M.; Altulea, D.; van Dijl, J.M. Staphylococcal Trafficking and Infection—From ‘Nose to Gut’ and Back. FEMS Microbiol. Rev. 2022, 46, fuab041. [Google Scholar] [CrossRef] [PubMed]
- Kozajda, A.; Jeżak, K.; Kapsa, A. Airborne Staphylococcus Aureus in Different Environments—A Review. Environ. Sci. Pollut. Res. 2019, 26, 34741–34753. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2016. EFSA J. 2017, 15, e05077. [Google Scholar] [CrossRef]
- Pillsbury, A.; Chiew, M.; Bates, J.; Sheppeard, V. Outbreak of Staphylococcal Food Poisoning in a Commercially Catered Buffet. Commun. Dis. Intell. (2018) 2013, 37, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Baloch, Z.; Jiang, T.; Zhang, C.; Peng, Z.; Li, F.; Fanning, S.; Ma, A.; Xu, J. Enterotoxigenicity and Antimicrobial Resistance of Staphylococcus Aureus Isolated from Retail Food in China. Front. Microbiol. 2017, 8, 2256. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S.; et al. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus Aureus Bloodstream Infections—United States. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Argudín, M.Á.; Mendoza, M.C.; Rodicio, M.R. Food Poisoning and Staphylococcus Aureus Enterotoxins. Toxins 2010, 2, 1751–1773. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Duan, N.; Gu, H.; Hao, L.; Ye, H.; Gong, W.; Wang, Z. A Review of the Methods for Detection of Staphylococcus Aureus Enterotoxins. Toxins 2016, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.D.; Walsh, K.A.; Gould, L.H. Foodborne Disease Outbreaks Caused by Bacillus Cereus, Clostridium Perfringens, and Staphylococcus Aureus—United States, 1998–2008. Clin. Infect. Dis. 2013, 57, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Pinamonti, D.; Manzano, M.; Maifreni, M.; Bianco, S.; Domi, B.; Ferrin, A.; Anba-Mondoloni, J.; Dechamps, J.; Briandet, R.; Vidic, J. Prevalence and Characterization of Staphylococcus Aureus Isolated from Meat and Milk in Northeastern Italy. J. Food Prot. 2025, 88, 100442. [Google Scholar] [CrossRef] [PubMed]
- Thiran, E.; Di Ciccio, P.A.; Graber, H.U.; Zanardi, E.; Ianieri, A.; Hummerjohann, J. Biofilm Formation of Staphylococcus Aureus Dairy Isolates Representing Different Genotypes. J. Dairy Sci. 2018, 101, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Tenhagen, B.-A.; Alt, K.; Pfefferkorn, B.; Wiehle, L.; Käsbohrer, A.; Fetsch, A. Short Communication: Methicillin-Resistant Staphylococcus Aureus in Conventional and Organic Dairy Herds in Germany. J. Dairy Sci. 2018, 101, 3380–3386. [Google Scholar] [CrossRef] [PubMed]
- Feßler, A.T.; Kadlec, K.; Hassel, M.; Hauschild, T.; Eidam, C.; Ehricht, R.; Monecke, S.; Schwarz, S. Characterization of Methicillin-Resistant Staphylococcus Aureus Isolates from Food and Food Products of Poultry Origin in Germany. Appl. Environ. Microbiol. 2011, 77, 7151–7157. [Google Scholar] [CrossRef] [PubMed]
- Thapaliya, D.; Forshey, B.M.; Kadariya, J.; Quick, M.K.; Farina, S.; O’ Brien, A.; Nair, R.; Nworie, A.; Hanson, B.; Kates, A.; et al. Prevalence and Molecular Characterization of Staphylococcus Aureus in Commercially Available Meat over a One-Year Period in Iowa, USA. Food Microbiol. 2017, 65, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, H.S.; Kim, S.; Kim, M.; Kwak, H.S. Prevalence and Characteristics of Antimicrobial-Resistant Staphylococcus Aureus and Methicillin-Resistant Staphylococcus Aureus from Retail Meat in Korea. Food Sci. Anim. Resour. 2020, 40, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; White, A.E.; McQueen, R.B.; Ahn, J.-W.; Gunn-Sandell, L.B.; Scallan Walter, E.J. Economic Burden of Foodborne Illnesses Acquired in the United States. Foodborne Pathog. Dis. 2024, 22, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Cruz, J.; Gutiérrez, I.F.; Brand-López, K.; Sosa-Rodríguez, Y.A.; Vásquez-Hoyos, P.; Gómez-Cortés, L.C.; Romero-Higuera, L.N.; Rojas-Rojas, D.P.; Ortiz-Mendez, C.A.; Camacho-Moreno, G.; et al. Differences Between Methicillin-Susceptible Versus Methicillin-Resistant Staphylococcus Aureus Infections in Pediatrics: Multicenter Cohort Study Conducted in Bogotá, Colombia, 2014–2018. Pediatr. Infect. Dis. J. 2022, 41, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, A.; Yahara, K.; Oka, K.; Kajihara, T.; Ohkura, T.; Hosaka, Y.; Shibayama, K.; Sugai, M.; Yagi, T. Comparison of Disease and Economic Burden between MRSA Infection and MRSA Colonization in a University Hospital: A Retrospective Data Integration Study. Antimicrob. Resist. Infect. Control. 2024, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Lundborg, C.S.; Zhang, M.; Sun, X.; Li, Y.; Hu, X.; Gu, S.; Gu, Y.; Wei, J.; Dong, H. Clinical and Economic Impact of Methicillin-Resistant Staphylococcus Aureus: A Multicentre Study in China. Sci. Rep. 2020, 10, 3900. [Google Scholar] [CrossRef] [PubMed]
- Antonanzas, F.; Lozano, C.; Torres, C. Economic Features of Antibiotic Resistance: The Case of Methicillin-Resistant Staphylococcus Aureus. PharmacoEconomics 2015, 33, 285–325. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.E.; Hyun, D.; Jezek, A.; Samore, M.H. Mortality, Length of Stay, and Healthcare Costs Associated With Multidrug-Resistant Bacterial Infections Among Elderly Hospitalized Patients in the United States. Clin. Infect. Dis. 2022, 74, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.E.; Hatfield, K.M.; Wolford, H.; Samore, M.H.; Scott, R.D.; Reddy, S.C.; Olubajo, B.; Paul, P.; Jernigan, J.A.; Baggs, J. National Estimates of Healthcare Costs Associated with Multidrug-Resistant Bacterial Infections Among Hospitalized Patients in the United States. Clin. Infect. Dis. 2021, 72, S17–S26. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Frees, D.; Ingmer, H. Antibiotic Resistance and the MRSA Problem. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Felgate, H.; Crossman, L.C.; Gray, E.; Clifford, R.; Correia, A.; Dean, R.; Wain, J.; Langridge, G.C. Known Mechanisms Cannot Account for a Third of Reduced Susceptibility in Non-Aureus Staphylococci. Npj Antimicrob. Resist. 2023, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Contarin, R.; Drapeau, A.; François, P.; Madec, J.-Y.; Haenni, M.; Dordet-Frisoni, E. The Interplay between Mobilome and Resistome in Staphylococcus Aureus. MBio 2024, 15, e02428-24. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Ono, D.; Sato, A. Staphylococcal Cassette Chromosome Mec (SCCmec) Analysis of MRSA. In Methicillin-Resistant Staphylococcus Aureus (MRSA) Protocols; Ji, Y., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2020; Volume 2069, pp. 59–78. ISBN 978-1-4939-9848-7. [Google Scholar]
- Bush, K.; Bradford, P.A. Epidemiology of β-Lactamase-Producing Pathogens. Clin. Microbiol. Rev. 2020, 33, e00047-19. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Peng, H.; Rao, X. Molecular Events for Promotion of Vancomycin Resistance in Vancomycin Intermediate Staphylococcus Aureus. Front. Microbiol. 2016, 7, 1601. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Sievert, D.M.; Hageman, J.C.; Boulton, M.L.; Tenover, F.C.; Downes, F.P.; Shah, S.; Rudrik, J.T.; Pupp, G.R.; Brown, W.J.; et al. Infection with Vancomycin-Resistant Staphylococcus Aureus Containing the vanA Resistance Gene. N. Engl. J. Med. 2003, 348, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Walker, M.J.; De Oliveira, D.M.P. Vancomycin Resistance in Enterococcus and Staphylococcus Aureus. Microorganisms 2022, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Stefani, S.; Bongiorno, D.; Mongelli, G.; Campanile, F. Linezolid Resistance in Staphylococci. Pharmaceuticals 2010, 3, 1988–2006. [Google Scholar] [CrossRef] [PubMed]
- Bandín-Vilar, E.; García-Quintanilla, L.; Castro-Balado, A.; Zarra-Ferro, I.; González-Barcia, M.; Campos-Toimil, M.; Mangas-Sanjuan, V.; Mondelo-García, C.; Fernández-Ferreiro, A. A Review of Population Pharmacokinetic Analyses of Linezolid. Clin. Pharmacokinet. 2022, 61, 789–817. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Jiang, Y.; Jiao, L.; Luo, Y.; Wang, X.; Yang, T. Strategies for the Discovery of Oxazolidinone Antibacterial Agents: Development and Future Perspectives. J. Med. Chem. 2023, 66, 13860–13873. [Google Scholar] [CrossRef] [PubMed]
- Tsiodras, S.; Gold, H.S.; Sakoulas, G.; Eliopoulos, G.M.; Wennersten, C.; Venkataraman, L.; Moellering, R.C.; Ferraro, M.J. Linezolid Resistance in a Clinical Isolate of Staphylococcus Aureus. Lancet 2001, 358, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.K.; Sakoulas, G.; Wennersten, C.; Eliopoulos, G.M.; Moellering, R.C., Jr.; Ferraro, M.J.; Gold, H.S. Linezolid Resistance in Staphylococcus Aureus: Characterization and Stability of Resistant Phenotype. J. Infect. Dis. 2002, 186, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.A.; Vallejo, M.; Reyes, J.; Panesso, D.; Moreno, J.; Castañeda, E.; Villegas, M.V.; Murray, B.E.; Quinn, J.P. Clinical and Microbiological Aspects of Linezolid Resistance Mediated by the Cfr Gene Encoding a 23S rRNA Methyltransferase. J. Clin. Microbiol. 2008, 46, 892–896. [Google Scholar] [CrossRef] [PubMed]
- AbdAlhafiz, A.I.; Elleboudy, N.S.; Aboshanab, K.M.; Aboulwafa, M.M.; Hassouna, N.A. Phenotypic and Genotypic Characterization of Linezolid Resistance and the Effect of Antibiotic Combinations on Methicillin-Resistant Staphylococcus Aureus Clinical Isolates. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- LaMarre, J.; Mendes, R.E.; Szal, T.; Schwarz, S.; Jones, R.N.; Mankin, A.S. The Genetic Environment of the Cfr Gene and the Presence of Other Mechanisms Account for the Very High Linezolid Resistance of Staphylococcus Epidermidis Isolate 426-3147L. Antimicrob. Agents Chemother. 2013, 57, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, H.; Zhu, F.; Jiang, S.; Sun, L.; Zhao, F.; Yu, Y.; Chen, Y. Characterization of an ST5-SCCmec II-T311 Methicillin-Resistant Staphylococcus Aureus Strain with a Widespread Cfr-Positive Plasmid. J. Infect. Chemother. 2020, 26, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Schouls, L.M.; Veldman, K.; Brouwer, M.S.M.; Dierikx, C.; Witteveen, S.; Van Santen-Verheuvel, M.; Hendrickx, A.P.A.; Landman, F.; Hengeveld, P.; Wullings, B.; et al. Cfr and fexA Genes in Methicillin-Resistant Staphylococcus Aureus from Humans and Livestock in the Netherlands. Commun. Med. 2022, 2, 135. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Z.; Fu, J.; Cai, J.; Ma, T.; Xie, N.; Fan, R.; Zhai, W.; Feßler, A.T.; Sun, C.; et al. Spreading of Cfr -Carrying Plasmids among Staphylococci from Humans and Animals. Microbiol. Spectr. 2022, 10, e02461-22. [Google Scholar] [CrossRef] [PubMed]
- Salaheen, S.; Chowdhury, N.; Hanning, I.; Biswas, D. Zoonotic Bacterial Pathogens and Mixed Crop-Livestock Farming. Poult. Sci. 2015, 94, 1398–1410. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, H.; Salaheen, S.; Biswas, D. Contamination of Post-Harvest Poultry Products with Multidrug Resistant Staphylococcus Aureus in Maryland-Washington DC Metro Area. Food Control 2016, 65, 132–135. [Google Scholar] [CrossRef]
- Jeżak, K.; Kozajda, A. Occurrence and Spread of Antibiotic-Resistant Bacteria on Animal Farms and in Their Vicinity in Poland and Ukraine—Review. Environ. Sci. Pollut. Res. 2022, 29, 9533–9559. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed]
- Mulchandani, R.; Wang, Y.; Gilbert, M.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food-Producing Animals: 2020 to 2030. PLOS Glob. Public Health 2023, 3, e0001305. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Ahmad, F.; Yaqub, B.; Ramzan, A.; Imran, A.; Afzaal, M.; Mirza, S.A.; Mazhar, I.; Younus, M.; Akram, Q.; et al. Current Trends of Antimicrobials Used in Food Animals and Aquaculture. In Antibiotics and Antimicrobial Resistance Genes in the Environment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 39–69. ISBN 978-0-12-818882-8. [Google Scholar]
- Katz, S.E.; Banerjee, R.; Committee on Infectious Diseases; Council on Environmental Health and Climate Change. Use of Antibiotics in Animal Agriculture: Implications for Pediatrics: Technical Report. Pediatrics 2024, 154, e2024068467. [Google Scholar] [CrossRef] [PubMed]
- Arsène, M.M.J.; Davares, A.K.L.; Viktorovna, P.I.; Andreevna, S.L.; Sarra, S.; Khelifi, I.; Sergueïevna, D.M. The Public Health Issue of Antibiotic Residues in Food and Feed: Causes, Consequences, and Potential Solutions. Vet. World 2022, 15, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, D.; Xie, J.; Zhang, Y.; Wan, Y.; Zhang, Y.; Shi, X. Impacts of Farmland Application of Antibiotic-Contaminated Manures on the Occurrence of Antibiotic Residues and Antibiotic Resistance Genes in Soil: A Meta-Analysis Study. Chemosphere 2022, 300, 134529. [Google Scholar] [CrossRef] [PubMed]
- Lavrukhina, O.I.; Amelin, V.G.; Kish, L.K.; Tretyakov, A.V.; Pen’kov, T.D. Determination of Residual Amounts of Antibiotics in Environmental Samples and Food Products. J. Anal. Chem. 2022, 77, 1349–1385. [Google Scholar] [CrossRef]
- Adegbeye, M.J.; Adetuyi, B.O.; Igirigi, A.I.; Adisa, A.; Palangi, V.; Aiyedun, S.; Alvarado-Ramírez, E.R.; Elghandour, M.M.M.Y.; Molina, O.M.; Oladipo, A.A.; et al. Comprehensive Insights into Antibiotic Residues in Livestock Products: Distribution, Factors, Challenges, Opportunities, and Implications for Food Safety and Public Health. Food Control 2024, 163, 110545. [Google Scholar] [CrossRef]
- Chen, R.-A.; Wu, W.-K.; Panyod, S.; Liu, P.-Y.; Chuang, H.-L.; Chen, Y.-H.; Lyu, Q.; Hsu, H.-C.; Lin, T.-L.; Shen, T.-C.D.; et al. Dietary Exposure to Antibiotic Residues Facilitates Metabolic Disorder by Altering the Gut Microbiota and Bile Acid Composition. Msystems 2022, 7, e00172-22. [Google Scholar] [CrossRef] [PubMed]
- Getahun, M.; Abebe, R.B.; Sendekie, A.K.; Woldeyohanis, A.E.; Kasahun, A.E. Evaluation of Antibiotics Residues in Milk and Meat Using Different Analytical Methods. Int. J. Anal. Chem. 2023, 2023, 4380261. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; El Zowalaty, M.E.; Lundkvist, Å.; Järhult, J.D.; Khan Nayem, M.R.; Tanzin, A.Z.; Badsha, M.R.; Khan, S.A.; Ashour, H.M. Residual Antimicrobial Agents in Food Originating from Animals. Trends Food Sci. Technol. 2021, 111, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Ghimpețeanu, O.M.; Pogurschi, E.N.; Popa, D.C.; Dragomir, N.; Drăgotoiu, T.; Mihai, O.D.; Petcu, C.D. Antibiotic Use in Livestock and Residues in Food—A Public Health Threat: A Review. Foods 2022, 11, 1430. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global Trends in Antimicrobial Use in Food Animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention 2019 Antibiotic Resistance Threats Report. Available online: https://www.cdc.gov/antimicrobial-resistance/data-research/threats/index.html (accessed on 20 June 2025).
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial Resistance in Bacteria: Mechanisms, Evolution, and Persistence. J. Mol. Evol. 2020, 88, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Arnold, B.J.; Huang, I.-T.; Hanage, W.P. Horizontal Gene Transfer and Adaptive Evolution in Bacteria. Nat. Rev. Microbiol. 2022, 20, 206–218. [Google Scholar] [CrossRef] [PubMed]
- McInnes, R.S.; McCallum, G.E.; Lamberte, L.E.; Van Schaik, W. Horizontal Transfer of Antibiotic Resistance Genes in the Human Gut Microbiome. Curr. Opin. Microbiol. 2020, 53, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kot, B.; Wierzchowska, K.; Piechota, M.; Grużewska, A. Antimicrobial Resistance Patterns in Methicillin-Resistant Staphylococcus Aureus from Patients Hospitalized during 2015–2017 in Hospitals in Poland. Med. Princ. Pract. 2020, 29, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Grunenwald, C.M.; Bennett, M.R.; Skaar, E.P. Nonconventional Therapeutics against Staphylococcus Aureus. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Alaoui Mdarhri, H.; Benmessaoud, R.; Yacoubi, H.; Seffar, L.; Guennouni Assimi, H.; Hamam, M.; Boussettine, R.; Filali-Ansari, N.; Lahlou, F.A.; Diawara, I.; et al. Alternatives Therapeutic Approaches to Conventional Antibiotics: Advantages, Limitations and Potential Application in Medicine. Antibiotics 2022, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Kasman, L.M.; Porter, L.D. Bacteriophages. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kwiatek, M.; Parasion, S.; Nakonieczna, A. Therapeutic Bacteriophages as a Rescue Treatment for Drug-resistant Infections—An in Vivo Studies Overview. J. Appl. Microbiol. 2020, 128, 985–1002. [Google Scholar] [CrossRef] [PubMed]
- Chanishvili, N. Phage Therapy—History from Twort and d’Herelle Through Soviet Experience to Current Approaches. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2012; Volume 83, pp. 3–40. ISBN 978-0-12-394438-2. [Google Scholar]
- Gummalla, V.S.; Zhang, Y.; Liao, Y.-T.; Wu, V.C.H. The Role of Temperate Phages in Bacterial Pathogenicity. Microorganisms 2023, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Uchechukwu, C.F.; Shonekan, A. Current Status of Clinical Trials for Phage Therapy. J. Med. Microbiol. 2024, 73, 001895. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Kawano, M.; Sugai, M. Distribution of Antimicrobial Resistance and Virulence Genes within the Prophage-Associated Regions in Nosocomial Pathogens. Msphere 2021, 6, e00452-21. [Google Scholar] [CrossRef] [PubMed]
- USDA-FSIS Safe Suitable Ingredients in the Production of Meat, Poultry, and Egg Products—Revision 59. Available online: https://www.fsis.usda.gov/policy/fsis-directives/7120.1 (accessed on 21 June 2025).
- Xu, Y. Phage and Phage Lysins: New Era of Bio-preservatives and Food Safety Agents. J. Food Sci. 2021, 86, 3349–3373. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, N.M.; Devequi Gomes Nunes, D.; Shiach, J.; Valeria Saraiva Hodel, K.; Dantas Viana Barbosa, J.; Alencar Pereira Rodrigues, L.; Coler, B.S.; Botelho Pereira Soares, M.; Badaró, R. Current Clinical Landscape and Global Potential of Bacteriophage Therapy. Viruses 2023, 15, 1020. [Google Scholar] [CrossRef] [PubMed]
- Bruttin, A.; Brüssow, H. Human Volunteers Receiving Escherichia Coli Phage T4 Orally: A Safety Test of Phage Therapy. Antimicrob. Agents Chemother. 2005, 49, 2874–2878. [Google Scholar] [CrossRef] [PubMed]
- Panagiotopoulos, A.-P.; Sagona, A.P.; Tsakri, D.; Ferous, S.; Anastassopoulou, C.; Tsakris, A. Virological and Pharmaceutical Properties of Clinically Relevant Phages. Antibiotics 2025, 14, 487. [Google Scholar] [CrossRef] [PubMed]
- Beamud, B.; García-González, N.; Gómez-Ortega, M.; González-Candelas, F.; Domingo-Calap, P.; Sanjuan, R. Genetic Determinants of Host Tropism in Klebsiella Phages. Cell Rep. 2023, 42, 112048. [Google Scholar] [CrossRef] [PubMed]
- Boeckaerts, D.; Stock, M.; Ferriol-González, C.; Oteo-Iglesias, J.; Sanjuán, R.; Domingo-Calap, P.; De Baets, B.; Briers, Y. Prediction of Klebsiella Phage-Host Specificity at the Strain Level. Nat. Commun. 2024, 15, 4355. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, A.; Ebner, P.D.; Syed, Q.A.; Ubaid Ur Rahman, H. Employing List-Shield Bacteriophage as a Bio-Control Intervention for Listeria Monocytogenes from Raw Beef Surface and Maintain Meat Quality during Refrigeration Storage. LWT 2020, 132, 109784. [Google Scholar] [CrossRef]
- Huang, Z.; Yuan, X.; Zhu, Z.; Feng, Y.; Li, N.; Yu, S.; Li, C.; Chen, B.; Wu, S.; Gu, Q.; et al. Isolation and Characterization of Bacillus Cereus Bacteriophage DZ1 and Its Application in Foods. Food Chem. 2024, 431, 137128. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Li, C.; Luo, D.; Zhang, J.; Ding, Y.; Chen, M.; Yang, X.; Lei, T.; Wu, S.; Ye, Q.; et al. Novel Phage vB_CtuP_B1 for Controlling Cronobacter Malonaticus and Cronobacter Turicensis in Ready-to-Eat Lettuce and Powered Infant Formula. Food Res. Int. 2021, 143, 110255. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, J.; Islam, M.S.; Yan, T.; Zhou, Y.; Liang, L.; Connerton, I.F.; Deng, K.; Li, J. Application of a Novel Phage vB_SalS-LPSTLL for the Biological Control of Salmonella in Foods. Food Res. Int. 2021, 147, 110492. [Google Scholar] [CrossRef] [PubMed]
- Shetru, M.N.; Karched, M.; Agsar, D. Locally Isolated Broad Host-Range Bacteriophage Kills Methicillin-Resistant Staphylococcus Aureus in an in Vivo Skin Excisional Wound Model in Mice. Microb. Pathog. 2021, 152, 104744. [Google Scholar] [CrossRef] [PubMed]
- Prazak, J.; Valente, L.G.; Iten, M.; Federer, L.; Grandgirard, D.; Soto, S.; Resch, G.; Leib, S.L.; Jakob, S.M.; Haenggi, M.; et al. Benefits of Aerosolized Phages for the Treatment of Pneumonia Due to Methicillin-Resistant Staphylococcus Aureus: An Experimental Study in Rats. J. Infect. Dis. 2022, 225, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Y.; Wang, J.; Zhao, Y.; Zhong, Q.; Li, G.; Fu, Z.; Lu, S. Phage Endolysin LysP108 Showed Promising Antibacterial Potential Against Methicillin-Resistant Staphylococcus Aureus. Front. Cell. Infect. Microbiol. 2021, 11, 668430. [Google Scholar] [CrossRef] [PubMed]
- Belete, M.A.; Tadesse, S.; Tilahun, M.; Alemayehu, E.; Saravanan, M. Phage Endolysins as New Therapeutic Options for Multidrug Resistant Staphylococcus Aureus: An Emerging Antibiotic-Free Way to Combat Drug Resistant Infections. Front. Cell. Infect. Microbiol. 2024, 14, 1397935. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wei, X.; Wang, Z.; Huang, X.; Li, M.; Hu, Z.; Zhang, K.; Hu, Q.; Peng, H.; Shang, W.; et al. LysSYL: A Broad-Spectrum Phage Endolysin Targeting Staphylococcus Species and Eradicating S. Aureus Biofilms. Microb. Cell. Fact. 2024, 23, 89. [Google Scholar] [CrossRef] [PubMed]
- Ngassam-Tchamba, C.; Duprez, J.N.; Fergestad, M.; De Visscher, A.; L’Abee-Lund, T.; De Vliegher, S.; Wasteson, Y.; Touzain, F.; Blanchard, Y.; Lavigne, R.; et al. In Vitro and in Vivo Assessment of Phage Therapy against Staphylococcus Aureus Causing Bovine Mastitis. J. Glob. Antimicrob. Resist. 2020, 22, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Zhou, W.; Wu, Y.; Li, Y.; Zhu, G.; Zhang, Z.; Gu, X.; Wang, C.; Yang, Z. Effective Treatment of a Broad-Host-Range Lytic Phage SapYZU15 in Eliminating Staphylococcus Aureus from Subcutaneous Infection. Microbiol. Res. 2023, 276, 127484. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.; Kim, J.; Ryu, S.; Bai, J. Characterization of KMSP1, a Newly Isolated Virulent Bacteriophage Infecting Staphylococcus Aureus, and Its Application to Dairy Products. Int. J. Food Microbiol. 2023, 390, 110119. [Google Scholar] [CrossRef] [PubMed]
- Elhamouly, N.A.; Hewedy, O.A.; Zaitoon, A.; Miraples, A.; Elshorbagy, O.T.; Hussien, S.; El-Tahan, A.; Peng, D. The Hidden Power of Secondary Metabolites in Plant-Fungi Interactions and Sustainable Phytoremediation. Front. Plant Sci. 2022, 13, 1044896. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Mukhia, S.; Kumar, R.; Kumar, R. Essential Oil Composition and Antimicrobial Potential of Aromatic Plants Grown in the Mid-Hill Conditions of the Western Himalayas. Sci. Rep. 2023, 13, 4878. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Sulaiman, G.M.; Khan, R.A.; Al-Saffar, A.Z.; Mohsin, M.H.; Albukhaty, S.; Ismail, A. Essential Oils Pharmacological Activity: Chemical Markers, Biogenesis, Plant Sources, and Commercial Products. Process Biochem. 2024, 144, 112–132. [Google Scholar] [CrossRef]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential Oils and Their Application in Food Safety. Front. Sustain. Food Syst. 2021, 5, 653420. [Google Scholar] [CrossRef]
- Muthaiyan, A.; Martin, E.M.; Natesan, S.; Crandall, P.G.; Wilkinson, B.J.; Ricke, S.C. Antimicrobial Effect and Mode of Action of Terpeneless Cold-Pressed Valencia Orange Essential Oil on Methicillin-Resistant Staphylococcus Aureus: Antistaphylococcal Effect of Orange Essential Oil. J. Appl. Microbiol. 2012, 112, 1020–1033. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Phillips, C.A. The Effect of Lemon, Orange and Bergamot Essential Oils and Their Components on the Survival of Campylobacter Jejuni, Escherichia Coli O157, Listeria Monocytogenes, Bacillus Cereus and Staphylococcus Aureus in Vitro and in Food Systems. J. Appl. Microbiol. 2006, 101, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Federman, C.; Ma, C.; Biswas, D. Major Components of Orange Oil Inhibit Staphylococcus Aureus Growth and Biofilm Formation, and Alter Its Virulence Factors. J. Med. Microbiol. 2016, 65, 688–695. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nannapaneni, R.; Chalova, V.I.; Story, R.; Wiggins, K.C.; Crandall, P.G.; Ricke, S.C.; Johnson, M.G. Ciprofloxacin-Sensitive and Ciprofloxacin-Resistant Campylobacter Jejuni are Equally Susceptible to Natural Orange Oil-Based Antimicrobials. J. Environ. Sci. Health Part B 2009, 44, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, C.M.S.; De Alencar, S.M.; De Sousa, R.L.M.; Moreno, A.M.; Da Gloria, E.M. Antimicrobial Activity of Several Essential Oils on Pathogenic and Beneficial Bacteria. Ind. Crops Prod. 2017, 97, 128–136. [Google Scholar] [CrossRef]
- Das, S.; Vishakha, K.; Banerjee, S.; Mondal, S.; Ganguli, A. Sodium Alginate-Based Edible Coating Containing Nanoemulsion of Citrus Sinensis Essential Oil Eradicates Planktonic and Sessile Cells of Food-Borne Pathogens and Increased Quality Attributes of Tomatoes. Int. J. Biol. Macromol. 2020, 162, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Özogul, Y.; Özogul, F.; Kulawik, P. The Antimicrobial Effect of Grapefruit Peel Essential Oil and Its Nanoemulsion on Fish Spoilage Bacteria and Food-Borne Pathogens. LWT 2021, 136, 110362. [Google Scholar] [CrossRef]
- Liu, Y.; McKeever, L.C.; Malik, N.S.A. Assessment of the Antimicrobial Activity of Olive Leaf Extract Against Foodborne Bacterial Pathogens. Front. Microbiol. 2017, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Sudjana, A.N.; D’Orazio, C.; Ryan, V.; Rasool, N.; Ng, J.; Islam, N.; Riley, T.V.; Hammer, K.A. Antimicrobial Activity of Commercial Olea Europaea (Olive) Leaf Extract. Int. J. Antimicrob. Agents 2009, 33, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Ramata-Stunda, A.; Petriņa, Z.; Valkovska, V.; Borodušķis, M.; Gibnere, L.; Gurkovska, E.; Nikolajeva, V. Synergistic Effect of Polyphenol-Rich Complex of Plant and Green Propolis Extracts with Antibiotics against Respiratory Infections Causing Bacteria. Antibiotics 2022, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Al-Rimawi, F.; Sbeih, M.; Amayreh, M.; Rahhal, B.; Mudalal, S. Evaluation of the Antibacterial and Antifungal Properties of Oleuropein, Olea Europea Leaf Extract, and Thymus Vulgaris Oil. BMC Complement. Med. Ther. 2024, 24, 297. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Parmar, P.; Rao, P.; Goswami, D.; Saraf, M. Twin Peaks: Presenting the Antagonistic Molecular Interplay of Curcumin with LasR and LuxR Quorum Sensing Pathways. Curr. Microbiol. 2020, 77, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.-H.; Joung, D.-K.; Kim, Y.-S.; Kang, O.-H.; Kim, S.-B.; Seo, Y.-S.; Kim, Y.-C.; Lee, D.-S.; Shin, D.-W.; Kweon, K.-T.; et al. Synergistic Antibacterial Effect of Curcumin against Methicillin-Resistant Staphylococcus Aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal Activity of Curcumin I Is Associated with Damaging of Bacterial Membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef] [PubMed]
- Batista De Andrade Neto, J.; Pessoa De Farias Cabral, V.; Brito Nogueira, L.F.; Rocha Da Silva, C.; Gurgel Do Amaral Valente Sá, L.; Ramos Da Silva, A.; Barbosa Da Silva, W.M.; Silva, J.; Marinho, E.S.; Cavalcanti, B.C.; et al. Anti-MRSA Activity of Curcumin in Planktonic Cells and Biofilms and Determination of Possible Action Mechanisms. Microb. Pathog. 2021, 155, 104892. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, F.; Khan, A.U.; Misba, L.; Akhtar, K.; Ali, A. Antimicrobial and Antibiofilm Photodynamic Therapy against Vancomycin Resistant Staphylococcus Aureus (VRSA) Induced Infection In Vitro and In Vivo. Eur. J. Pharm. Biopharm. 2021, 160, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Zhang, B.; Zhao, F. Antibiofilm Effect of Curcumin Against Staphylococcus Aureus Surface Wound Biofilm–Associated Infection: In Vitro and In Silico. Appl. Biochem. Biotechnol. 2023, 195, 5329–5337. [Google Scholar] [CrossRef] [PubMed]
- Khaleghian, M.; Sahrayi, H.; Hafezi, Y.; Mirshafeeyan, M.; Moghaddam, Z.S.; Farasati Far, B.; Noorbazargan, H.; Mirzaie, A.; Ren, Q. In Silico Design and Mechanistic Study of Niosome-Encapsulated Curcumin against Multidrug-Resistant Staphylococcus Aureus Biofilms. Front. Microbiol. 2023, 14, 1277533. [Google Scholar] [CrossRef] [PubMed]
- Guinoiseau, E.; Luciani, A.; Rossi, P.G.; Quilichini, Y.; Ternengo, S.; Bradesi, P.; Berti, L. Cellular Effects Induced by Inula Graveolens and Santolina Corsica Essential Oils on Staphylococcus Aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kamal, A.; Singh, S.; Padalia, R.C.; Tandon, S.; Chauhan, A.; Saikia, D.; Verma, R.S. Chemical Composition, Antimicrobial Activity, Kinetics and Mechanism of Action of Himalayan-Thyme (Thymus Linearis Benth.). J. Essent. Oil Res. 2020, 32, 59–68. [Google Scholar] [CrossRef]
- He, D.; Wu, X.; Wu, K.; Chai, X.; Liang, Y.; Zhang, X.; Cha, Q.; Xie, W. Synergistic Activity of Clove Essential Oil and Thyme Essential Oil and Their Interaction against Malassezia Furfur, Escherichia Coli, Staphylococcus Aureus. LWT 2024, 204, 116431. [Google Scholar] [CrossRef]
- Liu, T.; Kang, J.; Liu, L. Thymol as a Critical Component of Thymus Vulgaris L. Essential Oil Combats Pseudomonas Aeruginosa by Intercalating DNA and Inactivating Biofilm. LWT 2021, 136, 110354. [Google Scholar] [CrossRef]
- Palaniappan, K.; Holley, R.A. Use of Natural Antimicrobials to Increase Antibiotic Susceptibility of Drug Resistant Bacteria. Int. J. Food Microbiol. 2010, 140, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Ghavam, M.; Bacchetta, G.; Castangia, I.; Manca, M.L. Evaluation of the Composition and Antimicrobial Activities of Essential Oils from Four Species of Lamiaceae Martinov Native to Iran. Sci. Rep. 2022, 12, 17044. [Google Scholar] [CrossRef] [PubMed]
- Noui Mehidi, I.; Ait Ouazzou, A.; Tachoua, W.; Hosni, K. Investigating the Antimicrobial Properties of Essential Oil Constituents and Their Mode of Action. Molecules 2024, 29, 4119. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.-M.; Bălăceanu-Gurău, B.; Holban, A.M.; Ilie, C.-I.; Sima, R.M.; Gurău, C.-D.; Dițu, L.-M. Promising Antimicrobial Activities of Essential Oils and Probiotic Strains on Chronic Wound Bacteria. Biomedicines 2025, 13, 962. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.S.; Thilagaraj, W.R.; Gangadharan, P.; Leela, K.V. Comparative Study of Antibacterial Activity between Selected International and Indian Essential Oils against Selected Pathogenic Bacteria. J. Pure Appl. Microbiol. 2024, 18, 401–409. [Google Scholar] [CrossRef]
- Behbahani, B.A.; Shahidi, F.; Yazdi, F.T.; Mortazavi, S.A.; Mohebbi, M. Antioxidant Activity and Antimicrobial Effect of Tarragon (Artemisia Dracunculus) Extract and Chemical Composition of Its Essential Oil . Food Meas. 2017, 11, 847–863. [Google Scholar] [CrossRef]
- Merghni, A.; Belmamoun, A.R.; Urcan, A.C.; Bobiş, O.; Lassoued, M.A. 1,8-Cineol (Eucalyptol) Disrupts Membrane Integrity and Induces Oxidative Stress in Methicillin-Resistant Staphylococcus Aureus. Antioxidants 2023, 12, 1388. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhang, T.; Yuan, Y.; Lin, S.; Xu, J.; Ye, H. Effects of Cinnamaldehyde on Escherichia Coli and Staphylococcus Aureus Membrane. Food Control 2015, 47, 196–202. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Chen, H.; Song, Z.; Guo, H.; Yuan, Y.; Yue, T. Antibacterial Activity of Essential Oils against Stenotrophomonas Maltophilia and the Effect of Citral on Cell Membrane. LWT 2020, 117, 108667. [Google Scholar] [CrossRef]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus Essential Oils (CEOs) and Their Applications in Food: An Overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Nannapaneni, R.; Chalova, V.; Crandall, P.; Ricke, S.; Johnson, M.; Obryan, C. Campylobacter and Arcobacter Species Sensitivity to Commercial Orange Oil Fractions. Int. J. Food Microbiol. 2009, 129, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, Q.; Liu, Y.; Zhou, X.; Wang, X. Isolation and Biological Activities of Decanal, Linalool, Valencene, and Octanal from Sweet Orange Oil. J. Food Sci. 2012, 77, C1156–C1161. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, T.; Wang, L.; Liu, L.; Li, X.; Wu, X. Antibacterial Effects and Mechanism of Mandarin (Citrus Reticulata L.) Essential Oil against Staphylococcus Aureus. Molecules 2020, 25, 4956. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.N.; Elnasser, O.A.; Farghali, S.A.; Ibrahim, O.A.; Ali, H.R.; Barakat, O.S. Formulation and Evaluation of Therapeutic Antimicrobial Citrus and Manuka Honey Creams with Aloe Vera, Mint Essential Oil, and Indian Costus. Sci. Rep. 2025, 15, 7477. [Google Scholar] [CrossRef] [PubMed]
- Elghali, F.; Ibrahim, I.; Guesmi, M.; Frikha, F.; Mnif, S. Unveiling the Impact of Selected Essential Oils on MRSA Strain ATCC 33591: Antibacterial Efficiency, Biofilm Disruption, and Staphyloxanthin Inhibition. Braz. J. Microbiol. 2024, 55, 2057–2069. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.P.; Ferreira, I.C.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic Compounds and Antimicrobial Activity of Olive (Olea Europaea L. Cv. Cobrançosa) Leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Zorofchian Moghadamtousi, S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef] [PubMed]
- Praditya, D.; Kirchhoff, L.; Brüning, J.; Rachmawati, H.; Steinmann, J.; Steinmann, E. Anti-Infective Properties of the Golden Spice Curcumin. Front. Microbiol. 2019, 10, 912. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Anasane, N.; Santos, C.A.D. Curcumin and Curcumin-Loaded Nanoparticles: Antipathogenic and Antiparasitic Activities. Expert Rev. Anti-Infect. Ther. 2020, 18, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Schraufstätter, E.; Bernt, H. Antibacterial Action of Curcumin and Related Compounds. Nature 1949, 164, 456–457. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, H.; Misba, L.; Ahmad, S.; Khan, A.U. Curcumin Induced Photodynamic Therapy Mediated Suppression of Quorum Sensing Pathway of Pseudomonas Aeruginosa: An Approach to Inhibit Biofilm in Vitro. Photodiagn. Photodyn. Ther. 2020, 30, 101645. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Subhan, N.; Jazayeri, J.A.; John, G.; Vanniasinkam, T.; Obied, H.K. Plant Phenols as Antibiotic Boosters: In Vitro Interaction of Olive Leaf Phenols with Ampicillin. Phytother. Res. 2016, 30, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Diarra, M.S.; Block, G.; Rempel, H.; Oomah, B.D.; Harrison, J.; McCallum, J.; Boulanger, S.; Brouillette, É.; Gattuso, M.; Malouin, F. In Vitro and in Vivo Antibacterial Activities of Cranberry Press Cake Extracts Alone or in Combination with β-Lactams against Staphylococcus Aureus. BMC Complement. Altern. Med. 2013, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Punz, B.; Christ, C.; Waldl, A.; Li, S.; Liu, Y.; Johnson, L.; Auer, V.; Cardozo, O.; Farias, P.M.A.; Andrade, A.C.D.S.; et al. Nano-Scaled Advanced Materials for Antimicrobial Applications—Mechanistic Insight, Functional Performance Measures, and Potential towards Sustainability and Circularity. Environ. Sci. Nano 2025, 12, 1710–1739. [Google Scholar] [CrossRef]
- Gao, J.; Yao, T.; Zhao, L.; Xu, H. Application of Metal-Based Nanomaterials in Antibacterial Food Packaging. Trends Food Sci. Technol. 2025, 161, 105060. [Google Scholar] [CrossRef]
- Rosli, N.A.; Teow, Y.H.; Mahmoudi, E. Current Approaches for the Exploration of Antimicrobial Activities of Nanoparticles. Sci. Technol. Adv. Mater. 2021, 22, 885–907. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, B.S. Food Nanotechnology—An Overview. Nanotechnol. Sci. Appl. 2010, 3, 1–15. [Google Scholar] [PubMed]
- Mardones, J.; Gómez, M.L.; Díaz, C.; Galleguillos, C.; Covarrubias, C. In Vitro Antibacterial Properties of Copper Nanoparticles as Endodontic Medicament against Enterococcus Faecalis. J. Dent. Oral. Disord. 2018, 4, 1107. [Google Scholar]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential Antibacterial Mechanism of Silver Nanoparticles and the Optimization of Orthopedic Implants by Advanced Modification Technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed]
- Rossner, P.; Cervena, T.; Echalar, B.; Palacka, K.; Milcova, A.; Novakova, Z.; Sima, M.; Simova, Z.; Vankova, J.; Holan, V. Metal Nanoparticles with Antimicrobial Properties: The Toxicity Response in Mouse Mesenchymal Stem Cells. Toxics 2023, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.J.; Manshian, B.; Montenegro, J.M.; Amin, F.; Meermann, B.; Thiron, T.; Cornelissen, M.; Vanhaecke, F.; Doak, S.; Parak, W.J.; et al. Cytotoxic Effects of Gold Nanoparticles: A Multiparametric Study. ACS Nano 2012, 6, 5767–5783. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Vijver, M.G.; Richardson, M.K.; Ahmad, F.; Peijnenburg, W.J.G.M. Particle-Specific Toxic Effects of Differently Shaped Zinc Oxide Nanoparticles to Zebrafish Embryos (Danio Rerio). Environ. Toxicol. Chem. 2014, 33, 2859–2868. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.K.; Chakraborty, S.; Manna, S.; Mandal, S.M. Antimicrobial Nanoparticles: Current Landscape and Future Challenges. RSC Pharm. 2024, 1, 388–402. [Google Scholar] [CrossRef]
- Zhou, K.; Li, C.; Chen, D.; Pan, Y.; Tao, Y.; Qu, W.; Liu, Z.; Wang, X.; Xie, S. A Review on Nanosystems as an Effective Approach against Infections of Staphylococcus Aureus. IJN 2018, 13, 7333–7347. [Google Scholar] [CrossRef] [PubMed]
- Maya, S.; Indulekha, S.; Sukhithasri, V.; Smitha, K.T.; Nair, S.V.; Jayakumar, R.; Biswas, R. Efficacy of Tetracycline Encapsulated O-Carboxymethyl Chitosan Nanoparticles against Intracellular Infections of Staphylococcus Aureus. Int. J. Biol. Macromol. 2012, 51, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.H.; Zhou, W.M.; He, Y.Z.; Wang, Y.; Lv, B.; Wang, X.M. Effects of Lipopeptide Carboxymethyl Chitosan Nanoparticles on Staphylococcus Aureus Biofilm. J. Biol. Regul. Homeost. Agents 2017, 31, 737–743. [Google Scholar] [PubMed]
- Divya, K.; Vijayan, S.; George, T.K.; Jisha, M.S. Antimicrobial Properties of Chitosan Nanoparticles: Mode of Action and Factors Affecting Activity. Fibers Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and Antibacterial Activity of Chitosan Nanoparticles. Carbohydr. Res. 2004, 339, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, C.M.B.; Lopes, N.A.; Brandelli, A. Lipid-Based Nanostructures for the Delivery of Natural Antimicrobials. Molecules 2021, 26, 3587. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhou, H.; Lin, L. The Specific Antibacterial Effect of the Salvia Oil Nanoliposomes against Staphylococcus Aureus Biofilms on Milk Container. Food Control 2016, 61, 92–98. [Google Scholar] [CrossRef]
- Mokarizadeh, M.; Kafil, H.; Ghanbarzadeh, S.; Alizadeh, A.; Hamishehkar, H. Improvement of Citral Antimicrobial Activity by Incorporation into Nanostructured Lipid Carriers: A Potential Application in Food Stuffs as a Natural Preservative. Res. Pharma Sci. 2017, 12, 409. [Google Scholar] [CrossRef]
- Shu, G.; Xu, D.; Zhang, W.; Zhao, X.; Li, H.; Xu, F.; Yin, L.; Peng, X.; Fu, H.; Chang, L.-J.; et al. Preparation of Shikonin Liposome and Evaluation of Its in Vitro Antibacterial and in Vivo Infected Wound Healing Activity. Phytomedicine 2022, 99, 154035. [Google Scholar] [CrossRef] [PubMed]
- Parachin, N.S.; Mulder, K.C.; Viana, A.A.B.; Dias, S.C.; Franco, O.L. Expression Systems for Heterologous Production of Antimicrobial Peptides. Peptides 2012, 38, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The Antimicrobial Peptides and Their Potential Clinical Applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- Liu, Y.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W. Antimicrobial Peptides and Their Application in Food Packaging. Trends Food Sci. Technol. 2021, 112, 471–483. [Google Scholar] [CrossRef]
- Bisht, V.; Das, B.; Hussain, A.; Kumar, V.; Navani, N.K. Understanding of Probiotic Origin Antimicrobial Peptides: A Sustainable Approach Ensuring Food Safety. Npj Sci. Food 2024, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Anumudu, C.; Hart, A.; Miri, T.; Onyeaka, H. Recent Advances in the Application of the Antimicrobial Peptide Nisin in the Inactivation of Spore-Forming Bacteria in Foods. Molecules 2021, 26, 5552. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, A.; López, R.L.; Pulido, R.P.; Burgos, M.J.G. Natural Antimicrobials for Food Biopreservation. In Food Biopreservation; Briefs in Food, Health, and Nutrition; Springer: New York, NY, USA, 2014; pp. 3–14. ISBN 978-1-4939-2028-0. [Google Scholar]
- Maitra, M.; Chakraborty, A.; Ray Chowdhury, B.; Das Sharma, A.; Misra, S.R.; Dutta, C.; Mandal, T.P.; Bhunia, S. Application of Lantibiotics in Canned Food Preservation. In Lantibiotics as Alternative Therapeutics; Elsevier: Amsterdam, The Netherlands, 2023; pp. 147–167. ISBN 978-0-323-99141-4. [Google Scholar]
- Zheng, S.; Tu, Y.; Li, B.; Qu, G.; Li, A.; Peng, X.; Li, S.; Shao, C. Antimicrobial Peptide Biological Activity, Delivery Systems and Clinical Translation Status and Challenges. J. Transl. Med. 2025, 23, 292. [Google Scholar] [CrossRef] [PubMed]
- Onyeaka, H.; Passaretti, P.; Miri, T.; Al-Sharify, Z.T. The Safety of Nanomaterials in Food Production and Packaging. Curr. Res. Food Sci. 2022, 5, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Zohri, M.; Shafiee Alavidjeh, M.; Mirdamadi, S.S.; Behmadi, H.; Hossaini Nasr, S.M.; Eshghi Gonbaki, S.; Shafiee Ardestani, M.; Jabbari Arabzadeh, A. Nisin-Loaded Chitosan/Alginate Nanoparticles: A Hopeful Hybrid Biopreservative. J. Food Saf. 2013, 33, 40–49. [Google Scholar] [CrossRef]
- Ramôa, A.M.; Campos, F.; Moreira, L.; Teixeira, C.; Leiro, V.; Gomes, P.; Das Neves, J.; Martins, M.C.L.; Monteiro, C. Antimicrobial Peptide-Grafted PLGA-PEG Nanoparticles to Fight Bacterial Wound Infections. Biomater. Sci. 2023, 11, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Yemmireddy, V.; Adhikari, A.; Moreira, J. Effect of Ultraviolet Light Treatment on Microbiological Safety and Quality of Fresh Produce: An Overview. Front. Nutr. 2022, 9, 871243. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Hung, Y. Inactivation of E. Coli O157:H7 on Blueberries by Electrolyzed Water, Ultraviolet Light, and Ozone. J. Food Sci. 2012, 77, M206–M211. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Bohrerova, Z.; Lee, J. Inactivation of Internalized Salmonella Typhimurium in Lettuce and Green Onion Using Ultraviolet C Irradiation and Chemical Sanitizers. J. Appl. Microbiol. 2013, 114, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Hadi, J.; Wu, S.; Brightwell, G. Antimicrobial Blue Light versus Pathogenic Bacteria: Mechanism, Application in the Food Industry, Hurdle Technologies and Potential Resistance. Foods 2020, 9, 1895. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos, C.; Sellera, F.P.; De Freitas, L.M.; Gargano, R.G.; Telles, E.O.; Freitas, R.O.; Baptista, M.S.; Ribeiro, M.S.; Lincopan, N.; Pogliani, F.C.; et al. Inactivation of Milk-Borne Pathogens by Blue Light Exposure. J. Dairy Sci. 2020, 103, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Gunther, N.W.; Phillips, J.G.; Sommers, C. The Effects of 405-Nm Visible Light on the Survival of Campylobacter on Chicken Skin and Stainless Steel. Foodborne Pathog. Dis. 2016, 13, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Glueck, M.; Schamberger, B.; Eckl, P.; Plaetzer, K. New Horizons in Microbiological Food Safety: Photodynamic Decontamination Based on a Curcumin Derivative. Photochem. Photobiol. Sci. 2017, 16, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Buchovec, I.; Lukseviciute, V.; Marsalka, A.; Reklaitis, I.; Luksiene, Z. Effective Photosensitization-Based Inactivation of Gram (−) Food Pathogens and Molds Using the Chlorophyllin—Chitosan Complex: Towards Photoactive Edible Coatings to Preserve Strawberries. Photochem. Photobiol. Sci. 2016, 15, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Xu, X.; Jeong, S.-M.; Kim, S.-R.; Kim, H.-H.; Kang, W.-S.; Ryu, S.-H.; Lee, G.-H.; Ahn, D.-H. Effect of Various LED Light Wavelengths on the Growth of Food-Borne Bacteria. J. Life Sci. 2021, 31, 905–912. [Google Scholar] [CrossRef]
- Chen, H.; Moraru, C.I. Exposure to 222 Nm Far UV-C Effectively Inactivates Planktonic Foodborne Pathogens and Inhibits Biofilm Formation. Innov. Food Sci. Emerg. Technol. 2023, 87, 103411. [Google Scholar] [CrossRef]
- Tortik, N.; Spaeth, A.; Plaetzer, K. Photodynamic Decontamination of Foodstuff from Staphylococcus Aureus Based on Novel Formulations of Curcumin. Photochem. Photobiol. Sci. 2014, 13, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.Q.; Blanco, K.C.; Garcia, É.B.; Perez, S.M.L.; Chianfrone, D.J.; Morais, V.S.; Bagnato, V.S. Effects of Ultraviolet Light and Curcumin-Mediated Photodynamic Inactivation on Microbiological Food Safety: A Study in Meat and Fruit. Photodiagnosis Photodyn. Ther. 2020, 30, 101678. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhao, Y.; Matthews, K.; Gao, J.; Hao, J.; Wang, S.; Han, J.; Jia, Y. Antibacterial Activity against Staphylococcus Aureus of Curcumin-Loaded Chitosan Spray Coupled with Photodynamic Treatment. LWT 2020, 134, 110073. [Google Scholar] [CrossRef]
- Callaway, T.R.; Anderson, R.C.; Edrington, T.S.; Genovese, K.J.; Poole, T.L.; Harvey, R.B.; Nisbet, D.J. Probiotics, Vaccines and Other Interventions for Pathogen Control in Animals. In Improving the Safety of Fresh Meat; Elsevier: Amsterdam, The Netherlands, 2005; pp. 192–213. ISBN 978-1-85573-955-0. [Google Scholar]
- Osterloh, A. Vaccination against Bacterial Infections: Challenges, Progress, and New Approaches with a Focus on Intracellular Bacteria. Vaccines 2022, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S. Critical Analysis of Compositions and Protective Efficacies of Oral Killed Cholera Vaccines. Clin. Vaccine Immunol. 2014, 21, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramaniam, A.; Adhikari, R.P.; Kort, T.; Liao, G.C.; Conley, S.; Abaandou, L.; Kailasan, S.; Onodera, Y.; Krishnan, S.; Djagbare, D.M.; et al. TBA225, a Fusion Toxoid Vaccine for Protection and Broad Neutralization of Staphylococcal Superantigens. Sci. Rep. 2019, 9, 3279. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Parekh, S.; Pujari, P.; Shewale, S.; Desai, S.; Kawade, A.; Lalwani, S.; Ravi, M.D.; Kamath, V.; Mahopatra, J.; et al. A Phase III Randomized-Controlled Study of Safety and Immunogenicity of DTwP-HepB-IPV-Hib Vaccine (HEXASIIL®) in Infants. Npj Vaccines 2024, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Matthews, K.R.; Dhewa, T.; Puniya, A.K. Antimicrobial Resistance in the Food Chain: Trends, Mechanisms, Pathways, and Possible Regulation Strategies. Foods 2022, 11, 2966. [Google Scholar] [CrossRef] [PubMed]
- Finco, O.; Rappuoli, R. Designing Vaccines for the Twenty-First Century Society. Front. Immunol. 2014, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.; Soldaini, E.; McLoughlin, R.M.; Rittenhouse, S.; Bagnoli, F.; Phogat, S. Staphylococcus Aureus Vaccine Research and Development: The Past, Present and Future, Including Novel Therapeutic Strategies. Front. Immunol. 2021, 12, 705360. [Google Scholar] [CrossRef] [PubMed]
- Fattom, A.I.; Horwith, G.; Fuller, S.; Propst, M.; Naso, R. Development of StaphVAXTM, a Polysaccharide Conjugate Vaccine against S. Aureus Infection: From the Lab Bench to Phase III Clinical Trials. Vaccine 2004, 22, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.G.; Allen, K.B.; Moreira, E.D.; Moustafa, M.; Isgro, F.; Boucher, H.W.; Corey, G.R.; Carmeli, Y.; Betts, R.; Hartzel, J.S.; et al. Effect of an Investigational Vaccine for Preventing Staphylococcus Aureus Infections After Cardiothoracic Surgery: A Randomized Trial. JAMA 2013, 309, 1368. [Google Scholar] [CrossRef] [PubMed]
- Pauli, N.T.; Kim, H.K.; Falugi, F.; Huang, M.; Dulac, J.; Henry Dunand, C.; Zheng, N.-Y.; Kaur, K.; Andrews, S.F.; Huang, Y.; et al. Staphylococcus Aureus Infection Induces Protein A–Mediated Immune Evasion in Humans. J. Exp. Med. 2014, 211, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Moscoso, M.; García, P.; Cabral, M.P.; Rumbo, C.; Bou, G. A D-Alanine Auxotrophic Live Vaccine Is Effective against Lethal Infection Caused by Staphylococcus Aureus. Virulence 2018, 9, 604–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mukherjee, I.; Venkatasubramaniam, A.; Dikeman, D.; Orlando, N.; Zhang, J.; Ortines, R.; Mednikov, M.; Sherchand, S.P.; Kanipakala, T.; et al. Dry and Liquid Formulations of IBT-V02, a Novel Multi-Component Toxoid Vaccine, Are Effective against Staphylococcus Aureus Isolates from Low-to-Middle Income Countries. Front. Immunol. 2024, 15, 1373367. [Google Scholar] [CrossRef] [PubMed]
- Poolman, J.T.; Torres, V.J.; Missiakas, D.; Welten, S.P.M.; Fernandez, J.; DuMont, A.L.; O’Keeffe, A.; Konstantinov, S.R.; Morrow, B.; Burghout, P.; et al. A SpA+LukAB Vaccine Targeting Staphylococcus Aureus Evasion Factors Restricts Infection in Two Minipig Infection Models. Npj Vaccines 2025, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Filler, S.G.; Schmidt, C.S.; Ibrahim, A.S.; Edwards, J.E.; Hennessey, J.P. Applying Convergent Immunity to Innovative Vaccines Targeting Staphylococcus Aureus. Front. Immunol. 2014, 5, 463. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Yang, F.; Feng, Q.; Zhang, J.; Gu, J.; Jing, H.; Cai, C.; Xu, L.; Yang, X.; Xia, X.; et al. Rapid and Broad Immune Efficacy of a Recombinant Five-Antigen Vaccine against Staphylococcus Aureus Infection in Animal Models. Vaccines 2020, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Fajardo-Lubian, A.; Venturini, C. Use of Bacteriophages to Target Intracellular Pathogens. Clin. Infect. Dis. 2023, 77, S423–S432. [Google Scholar] [CrossRef] [PubMed]
- Bichet, M.C.; Chin, W.H.; Richards, W.; Lin, Y.-W.; Avellaneda-Franco, L.; Hernandez, C.A.; Oddo, A.; Chernyavskiy, O.; Hilsenstein, V.; Neild, A.; et al. Bacteriophage Uptake by Mammalian Cell Layers Represents a Potential Sink That May Impact Phage Therapy. IScience 2021, 24, 102287. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, E.; Bonnin, R.A.; Rocha, E.P.C. Phage-Plasmids Spread Antibiotic Resistance Genes through Infection and Lysogenic Conversion. MBio 2022, 13, e01851-22. [Google Scholar] [CrossRef] [PubMed]
- Meaden, S.; Koskella, B. Exploring the Risks of Phage Application in the Environment. Front. Microbiol. 2013, 4, 358. [Google Scholar] [CrossRef] [PubMed]
- Pilati, G.V.T.; Cadamuro, R.D.; Filho, V.B.; Dahmer, M.; Elois, M.A.; Savi, B.P.; Salles, G.B.C.; Muniz, E.C.; Fongaro, G. Bacteriophage-Associated Antimicrobial Resistance Genes in Avian Pathogenic Escherichia Coli Isolated from Brazilian Poultry. Viruses 2023, 15, 1485. [Google Scholar] [CrossRef] [PubMed]
- Sawa, T.; Moriyama, K.; Kinoshita, M. Current Status of Bacteriophage Therapy for Severe Bacterial Infections. J. Intensive Care 2024, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, P.E.; Baig, S.; Stegger, M.; Ingmer, H.; Garmyn, A.; Butaye, P. Spontaneous Phage Resistance in Avian Pathogenic Escherichia Coli. Front. Microbiol. 2021, 12, 782757. [Google Scholar] [CrossRef] [PubMed]
- Depardieu, F.; Didier, J.-P.; Bernheim, A.; Sherlock, A.; Molina, H.; Duclos, B.; Bikard, D. A Eukaryotic-like Serine/Threonine Kinase Protects Staphylococci against Phages. Cell Host Microbe 2016, 20, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, H.; Ju, Z.; Huang, J.; Lin, C.; Wu, J.; Wu, Y.; Sun, S.; Wang, H.; Hao, G.; et al. Increased Mutations in Lipopolysaccharide Biosynthetic Genes Cause Time-Dependent Development of Phage Resistance in Salmonella. Antimicrob. Agents Chemother. 2024, 68, e00594-23. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, B.J.; Barth, Z.K.; McKitterick, A.C.; Seed, K.D. A Highly Specific Phage Defense System Is a Conserved Feature of the Vibrio Cholerae Mobilome. PLoS Genet. 2017, 13, e1006838. [Google Scholar] [CrossRef] [PubMed]
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage Therapy: From Biological Mechanisms to Future Directions. Cell 2023, 186, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Berida, T.I.; Adekunle, Y.A.; Dada-Adegbola, H.; Kdimy, A.; Roy, S.; Sarker, S.D. Plant Antibacterials: The Challenges and Opportunities. Heliyon 2024, 10, e31145. [Google Scholar] [CrossRef] [PubMed]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Kengni, F.; Fodouop, S.P.C.; Tala, D.S.; Djimeli, M.N.; Fokunang, C.; Gatsing, D. Antityphoid Properties and Toxicity Evaluation of Harungana Madagascariensis Lam (Hypericaceae) Aqueous Leaf Extract. J. Ethnopharmacol. 2016, 179, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Pabón, W.; Schlievert, P.M. Models Matter: The Search for an Effective Staphylococcus Aureus Vaccine. Nat. Rev. Microbiol. 2014, 12, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and Virulence of Staphylococcus Aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Gwynne, P.J.; Gallagher, M.P. Light as a Broad-Spectrum Antimicrobial. Front. Microbiol. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Syamaladevi, R.M.; Killinger, K.; Sablani, S.S. Ultraviolet-C Light Inactivation of Escherichia Coli O157:H7 and Listeria Monocytogenes on Organic Fruit Surfaces. Int. J. Food Microbiol. 2015, 210, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Syamaladevi, R.M.; Adhikari, A.; Lupien, S.L.; Dugan, F.; Bhunia, K.; Dhingra, A.; Sablani, S.S. Ultraviolet-C Light Inactivation of Penicillium Expansum on Fruit Surfaces. Food Control 2015, 50, 297–303. [Google Scholar] [CrossRef]
- Mendez-Pfeiffer, P.; Ballesteros Monrreal, M.G.; Mendez-Encinas, M.A.; Valencia, D.; Ortiz, B.; González-Davis, O.; Cadena-Nava, R.D. Nanoparticles in Antibacterial Therapy: A Systematic Review of Enhanced Efficacy against Intracellular Bacteria. ACS Omega 2025, 10, 17070–17086. [Google Scholar] [CrossRef] [PubMed]
- Park, M.V.D.Z.; Neigh, A.M.; Vermeulen, J.P.; De La Fonteyne, L.J.J.; Verharen, H.W.; Briedé, J.J.; Van Loveren, H.; De Jong, W.H. The Effect of Particle Size on the Cytotoxicity, Inflammation, Developmental Toxicity and Genotoxicity of Silver Nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A Systematic Review on Silver Nanoparticles-Induced Cytotoxicity: Physicochemical Properties and Perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Attarilar, S.; Yang, J.; Ebrahimi, M.; Wang, Q.; Liu, J.; Tang, Y.; Yang, J. The Toxicity Phenomenon and the Related Occurrence in Metal and Metal Oxide Nanoparticles: A Brief Review from the Biomedical Perspective. Front. Bioeng. Biotechnol. 2020, 8, 822. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.; Aqib, A.I.; Ali, M.M.; Chang, Y.F. Non-Metallic Nanoparticles Eliminating Bacteria. In Nanomaterials in the Battle Against Pathogens and Disease; Pal, K., Zaheer, T., Eds.; CRC Press: Boca Raton, FL, USA, 2022; p. 26. [Google Scholar]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. IJN 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Trush, E.A.; Poluektova, E.A.; Beniashvilli, A.G.; Shifrin, O.S.; Poluektov, Y.M.; Ivashkin, V.T. The Evolution of Human Probiotics: Challenges and Prospects. Probiotics Antimicrob. Prot. 2020, 12, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Maftei, N.-M.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, Y.-Q.; Liu, H.-Y.; Lai, T.; Ma, J.-L.; Wang, J.-F.; Zhu, Y.-H. Evaluation of Lactobacillus Rhamnosus GG Using an Escherichia Coli K88 Model of Piglet Diarrhoea: Effects on Diarrhoea Incidence, Faecal Microflora and Immune Responses. Vet. Microbiol. 2010, 141, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Monachese, M.; Cunningham-Rundles, S.; Diaz, M.; Guerrant, R.; Hummelen, R.; Kemperman, R.; Kerac, M.; Kort, R.; Merenstein, D.J.; Panigrahi, P.; et al. Probiotics and Prebiotics to Combat Enteric Infections and HIV in the Developing World: A Consensus Report. Gut Microbes 2011, 2, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.; Vilchez-Padial, L.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef] [PubMed]
- Calo-Mata, P.; Arlindo, S.; Boehme, K.; De Miguel, T.; Pascoal, A.; Barros-Velazquez, J. Current Applications and Future Trends of Lactic Acid Bacteria and Their Bacteriocins for the Biopreservation of Aquatic Food Products. Food Bioprocess. Technol. 2008, 1, 43–63. [Google Scholar] [CrossRef]
- Varma, P.; Dinesh, K.R.; Menon, K.K.; Biswas, R. Lactobacillus Fermentum Isolated from Human Colonic Mucosal Biopsy Inhibits the Growth and Adhesion of Enteric and Foodborne Pathogens. J. Food Sci. 2010, 75, M546–M551. [Google Scholar] [CrossRef] [PubMed]
- Acharjee, M.; Hasan, F.; Islam, T.; Nur, I.T.; Begum, N.; Mazumder, C.; Lubna, M.A.; Zerin, N.; Shahriar, A.; Mahmud, M.R. In-Vitro Antibacterial Activity of Commercially Available Probiotics on Food-Borne Pathogens along with Their Synergistic Effects with Synthetic Drugs. Metab. Open 2022, 14, 100187. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Ren, F.; Qin, H.; Bukhari, I.; Yang, J.; Gao, D.; Ouwehand, A.C.; Lehtinen, M.J.; Zheng, P.; Mi, Y. Lactobacillus Acidophilus NCFM and Lactiplantibacillus Plantarum Lp-115 Inhibit Helicobacter Pylori Colonization and Gastric Inflammation in a Murine Model. Front. Cell. Infect. Microbiol. 2023, 13, 1196084. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, S.; Zhang, T.; Teng, X.; Ling, X.; Li, B.; Xiao, G.; Huang, S. A Bifidobacterium Animalis Subsp. Lactis Strain That Can Suppress Helicobacter Pylori: Isolation, in Vitro and in Vivo Validation. Lett. Appl. Microbiol. 2024, 77, ovae005. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xia, Y.; Sun, X.; Dou, W.; Chen, R.; Huang, P.; Yu, L.; Tian, F.; Zhao, J.; Chen, W.; et al. Bifidobacterium Breve Modulates Lactic Acid to Curtail Escherichia Coli Expansion and Alleviate Inflammatory Bowel Disease. Food Biosci. 2024, 61, 104626. [Google Scholar] [CrossRef]
- Closs, G.; Bhandari, M.; Helmy, Y.A.; Kathayat, D.; Lokesh, D.; Jung, K.; Suazo, I.D.; Srivastava, V.; Deblais, L.; Rajashekara, G. The Probiotic Lacticaseibacillus Rhamnosus GG Supplementation Reduces Salmonella Load and Modulates Growth, Intestinal Morphology, Gut Microbiota, and Immune Responses in Chickens. Infect. Immun. 2025, 93, e00420-24. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.Z.; Yap, C.; Lytvyn, L.; Lo, C.K.-F.; Beardsley, J.; Mertz, D.; Johnston, B.C. Probiotics for the Prevention of Clostridium Difficile-Associated Diarrhea in Adults and Children. Cochrane Database Syst. Rev. 2017, 12, CD006095. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gibson, G.R.; Walton, G.E. An In Vitro Approach to Study Effects of Prebiotics and Probiotics on the Faecal Microbiota and Selected Immune Parameters Relevant to the Elderly. PLoS ONE 2016, 11, e0162604. [Google Scholar] [CrossRef] [PubMed]
- Gou, H.-Z.; Zhang, Y.-L.; Ren, L.-F.; Li, Z.-J.; Zhang, L. How Do Intestinal Probiotics Restore the Intestinal Barrier? Front. Microbiol. 2022, 13, 929346. [Google Scholar] [CrossRef] [PubMed]
- Raja, S. Evaluation of Potential Antioxidant Probiotics in in Vitro Models of the Gut Epithelium. Masters’s Thesis, Harvard Medical School, Boston, MA, USA, 2022. [Google Scholar]
- Fang, H.-W.; Fang, S.-B.; Chiang Chiau, J.-S.; Yeung, C.-Y.; Chan, W.-T.; Jiang, C.-B.; Cheng, M.-L.; Lee, H.-C. Inhibitory Effects of Lactobacillus Casei Subsp. Rhamnosus on Salmonella Lipopolysaccharide-Induced Inflammation and Epithelial Barrier Dysfunction in a Co-Culture Model Using Caco-2/Peripheral Blood Mononuclear Cells. J. Med. Microbiol. 2010, 59, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Davoodabadi, A.; Soltan Dallal, M.M.; Lashani, E.; Tajabadi Ebrahimi, M. Antimicrobial Activity of Lactobacillus spp. Isolated from Fecal Flora of Healthy Breast-Fed Infants Against Diarrheagenic Escherichia Coli. Jundishapur J. Microbiol. 2015, 8, e27852. [Google Scholar] [CrossRef] [PubMed]
- Piewngam, P.; Khongthong, S.; Roekngam, N.; Theapparat, Y.; Sunpaweravong, S.; Faroongsarng, D.; Otto, M. Probiotic for Pathogen-Specific Staphylococcus Aureus Decolonisation in Thailand: A Phase 2, Double-Blind, Randomised, Placebo-Controlled Trial. Lancet Microbe 2023, 4, e75–e83. [Google Scholar] [CrossRef] [PubMed]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and Inputs from Lactic Acid Bacteria and Their Bacteriocins as Alternatives to Antibiotic Growth Promoters During Food-Animal Production. Front. Microbiol. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhou, G.; Yang, X.-M.; Chen, G.-J.; Chen, H.-B.; Liao, Z.-L.; Zhong, Q.-P.; Wang, L.; Fang, X.; Wang, J. Transcriptomic Analysis Revealed Antimicrobial Mechanisms of Lactobacillus Rhamnosus SCB0119 against Escherichia Coli and Staphylococcus Aureus. Int. J. Mol. Sci. 2022, 23, 15159. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Gong, S.; Shu, J.; Zhu, J.; Liu, H.; Chen, P. Effects of Mixed Lactic Acid Bacteria on Intestinal Microbiota of Mice Infected with Staphylococcus Aureus. BMC Microbiol. 2018, 18, 109. [Google Scholar] [CrossRef] [PubMed]
- Warrack, S.; Panjikar, P.; Duster, M.; Safdar, N. Tolerability of a Probiotic in Subjects with a History of Methicillin-Resistant Staphylococcus Aureus Colonisation. Benef. Microbes 2014, 5, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Eggers, S.; Barker, A.K.; Valentine, S.; Hess, T.; Duster, M.; Safdar, N. Effect of Lactobacillus Rhamnosus HN001 on Carriage of Staphylococcus Aureus: Results of the Impact of Probiotics for Reducing Infections in Veterans (IMPROVE) Study. BMC Infect. Dis. 2018, 18, 129. [Google Scholar] [CrossRef] [PubMed]
- Gan, B.S.; Kim, J.; Reid, G.; Cadieux, P.; Howard, J.C. Lactobacillus Fermentum RC-14 Inhibits Staphylococcus Aureus Infection of Surgical Implants in Rats. J. Infect. Dis. 2002, 185, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Edalati, E.; Saneei, B.; Alizadeh, M.; Hosseini, S.S.; Zahedi Bialvaei, A.; Taheri, K. Isolation of Probiotic Bacteria from Raw Camel’s Milk and Their Antagonistic Effects on Two Bacteria Causing Food Poisoning. New Microbes New Infect. 2019, 27, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Lakhtin, M.; Alyoshkin, V.; Lakhtin, V.; Afanasyev, S.; Pozhalostina, L.; Pospelova, V. Probiotic Lactobacillus and Bifidobacterial Lectins Against Candida Albicans and Staphylococcus Aureus Clinical Strains: New Class of the Pathogen Biofilm Destructors. Probiotics Antimicrob. Prot. 2010, 2, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Voravuthikunchai, S.P.; Bilasoi, S.; Supamala, O. Antagonistic Activity against Pathogenic Bacteria by Human Vaginal Lactobacilli. Anaerobe 2006, 12, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Millette, M.; Luquet, F.M.; Lacroix, M. In Vitro Growth Control of Selected Pathogens by Lactobacillus Acidophilus- and Lactobacillus Casei-Fermented Milk. Lett. Appl. Microbiol. 2007, 44, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Karska-Wysocki, B.; Bazo, M.; Smoragiewicz, W. Antibacterial Activity of Lactobacillus Acidophilus and Lactobacillus Casei against Methicillin-Resistant Staphylococcus Aureus (MRSA). Microbiol. Res. 2010, 165, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Parsaeimehr, M.; Azizkhani, M.; Jebelli Javan, A. The Inhibitory Effects of 2 Commercial Probiotic Strains on the Growth of Staphylococcus Aureus and Gene Expression of Enterotoxin A. Int. J. Enteric Pathog. 2017, 5, 70–75. [Google Scholar] [CrossRef]
- Saidi, N.; Owlia, P.; Marashi, S.M.A.; Saderi, H. Inhibitory Effect of Probiotic Yeast Saccharomyces Cerevisiae on Biofilm Formation and Expression of α-Hemolysin and Enterotoxin A Genes of Staphylococcus Aureus. Iran. J. Microbiol. 2019, 11, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Pang, B.; Li, N.; Jin, H.; Li, J.; Wu, W.; Ai, C.; Jiang, C.; Shi, J. Therapeutic Effect of Lactobacillus Rhamnosus SHA113 on Intestinal Infection by Multi-Drug-Resistant Staphylococcus Aureus and Its Underlying Mechanisms. Food Funct. 2020, 11, 6226–6239. [Google Scholar] [CrossRef] [PubMed]
- Frickmann, H.; Klenk, C.; Warnke, P.; Redanz, S.; Podbielski, A. Influence of Probiotic Culture Supernatants on in Vitro Biofilm Formation of Staphylococci. Eur. J. Microbiol. Immunol. 2018, 8, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.; Globa, L.; Barbaree, J.; Vodyanoy, V.; Sorokulova, I. Antagonistic Activity of Bacillus Bacteria against Food-Borne Pathogens. J. Prob. Health 2013, 1, 110. [Google Scholar] [CrossRef]
- El-Kholy, A.M.; El-Shinawy, S.H.; Meshref, A.M.S.; Korny, A.M. Screening of Antagonistic Activity of Probiotic Bacteria against Some Food-Borne Pathogens. Appl. Environ. Microbiol. 2014, 2, 53–60. [Google Scholar] [CrossRef]
- Denkova, R.; Denkova, Z.; Yanakieva, V.; Blazheva, D. Antimicrobial Activity of Probiotic Lactobacilli, Bifidobacteria and Propionic Acid Bacteria, Isolated from Various Sources. Microb. Pathog. Strateg. Combat. Them Sci. Technol. Educ. 2013, 2, 857–864. [Google Scholar]
- Yang, J.; Wang, J.; Yang, K.; Liu, M.; Qi, Y.; Zhang, T.; Fan, M.; Wei, X. Antibacterial Activity of Selenium-Enriched Lactic Acid Bacteria against Common Food-Borne Pathogens in Vitro. J. Dairy Sci. 2018, 101, 1930–1942. [Google Scholar] [CrossRef] [PubMed]
- Tejero-Sariñena, S.; Barlow, J.; Costabile, A.; Gibson, G.R.; Rowland, I. In Vitro Evaluation of the Antimicrobial Activity of a Range of Probiotics against Pathogens: Evidence for the Effects of Organic Acids. Anaerobe 2012, 18, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Evivie, S.E.; Ogwu, M.C.; Abdelazez, A.; Bian, X.; Liu, F.; Li, B.; Huo, G. Correction: Suppressive Effects of Streptococcus Thermophilus KLDS 3.1003 on Some Foodborne Pathogens Revealed through in Vitro, in Vivo and Genomic Insights. Food Funct. 2021, 12, 3280. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gulhane, R.D.; Singh, A.; Goel, M.; Udelal, P.P.; Sangwan, V.; Sihag, M.K.; Goel, G.; Panwar, H.; Puniya, A.K. Exploring the Antimicrobial Potential of Lactobacilli Against Early-Stage and Mature Biofilms of Staphylococcus Aureus and Pseudomonas Aeruginosa. Front. Chem. 2025, 13, 1425666. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, G.; Rosato, R.; Cicchinelli, M.; Iavarone, F.; Urbani, A.; Sanguinetti, M.; Delogu, G.; De Maio, F. The Activity of Cell-Free Supernatant of Lactobacillus Crispatus M247: A Promising Treatment against Vaginal Infections. Front. Cell. Infect. Microbiol. 2025, 15, 1586442. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, H.; Smoragiewicz, W. Role of Probiotics in the Prevention and Treatment of Meticillin-Resistant Staphylococcus Aureus Infections. Int. J. Antimicrob. Agents 2013, 42, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Sana, S.; Datta, S.; Biswas, D.; Sengupta, D. Assessment of Synergistic Antibacterial Activity of Combined Biosurfactants Revealed by Bacterial Cell Envelop Damage. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2018, 1860, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yan, X.; Gu, S.; Yang, Y.; Zhao, L.; He, X.; Chen, H.; Ge, J.; Liu, D. Biosurfactants of Lactobacillus Helveticus for Biodiversity Inhibit the Biofilm Formation of Staphylococcus Aureus and Cell Invasion. Future Microbiol. 2019, 14, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Gudiña, E.J.; Rocha, V.; Teixeira, J.A.; Rodrigues, L.R. Antimicrobial and Antiadhesive Properties of a Biosurfactant Isolated from Lactobacillus Paracasei Ssp. Paracasei A20. Lett. Appl. Microbiol. 2010, 50, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Gu, S.; Cui, X.; Shi, Y.; Wen, S.; Chen, H.; Ge, J. Antimicrobial, Anti-Adhesive and Anti-Biofilm Potential of Biosurfactants Isolated from Pediococcus Acidilactici and Lactobacillus Plantarum against Staphylococcus Aureus CMCC26003. Microb. Pathog. 2019, 127, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Dore, M.P.; Bibbò, S.; Fresi, G.; Bassotti, G.; Pes, G.M. Side Effects Associated with Probiotic Use in Adult Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2019, 11, 2913. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Snydman, D.R. Risk and Safety of Probiotics. Clin. Infect. Dis. 2015, 60, S129–S134. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Strain-Specificity and Disease-Specificity of Probiotic Efficacy: A Systematic Review and Meta-Analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut Microbiota Metabolism of Dietary Fiber Influences Allergic Airway Disease and Hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A. The Use of Probiotics, Prebiotics, and Synbiotics as an Alternative to Antibiotics. In Alternatives to Antibiotics; Saha, T., Deb Adhikari, M., Tiwary, B.K., Eds.; Springer Nature: Singapore, 2022; pp. 449–465. ISBN 978-981-19-1853-7. [Google Scholar]
- Li, X.; Hu, S.; Yin, J.; Peng, X.; King, L.; Li, L.; Xu, Z.; Zhou, L.; Peng, Z.; Ze, X.; et al. Effect of Synbiotic Supplementation on Immune Parameters and Gut Microbiota in Healthy Adults: A Double-Blind Randomized Controlled Trial. Gut Microbes 2023, 15, 2247025. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Zhang, Y.; Qiu, Y.; Wu, Z.; Zhong, Z.; Zeng, X.; Zeng, Y.; Xiong, L.; Wen, Y.; Liu, R. Fructooligosaccharide Supplementation Alleviated the Pathological Immune Response and Prevented the Impairment of Intestinal Barrier in DSS-Induced Acute Colitis Mice. Food Funct. 2021, 12, 9844–9854. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, K.M.G.M.M.; Yang, S.J.; Lee, N.-K.; Paik, H.-D. Probiotic Properties of Lactobacillus Brevis KU200019 and Synergistic Activity with Fructooligosaccharides in Antagonistic Activity against Foodborne Pathogens. Food Sci. Anim. Resour. 2020, 40, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, Y.; Li, J.; Ban, L.; Yang, L.; Wang, S.; Zhu, L.; Song, H.; Liu, H. The Adhesion of the Gut Microbiota to Insoluble Dietary Fiber from Soy Hulls Promoted the Proliferation of Probiotics in Vitro. LWT 2022, 153, 112560. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Y.; Huang, J.; Zhang, H.; Lin, Q.; Han, L.; Liu, J.; Wang, J.; Liu, H. Insoluble Dietary Fiber from Soy Hulls Regulates the Gut Microbiota in Vitro and Increases the Abundance of Bifidobacteriales and Lactobacillales. J. Food Sci. Technol. 2020, 57, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Malos, I.G.; Pasarin, D.; Ghizdareanu, A.-I.; Frunzareanu, B. A Promising Approach for the Food Industry: Enhancing Probiotic Viability Through Microencapsulated Synbiotics. Microorganisms 2025, 13, 336. [Google Scholar] [CrossRef] [PubMed]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R.; Liu, W.; Tromp, R.H. Microencapsulation of Probiotics in Multi-Polysaccharide Microcapsules by Electro-Hydrodynamic Atomization and Incorporation into Ice-Cream Formulation. Food Struct. 2020, 25, 100147. [Google Scholar] [CrossRef]
- Srisuk, N.; Nopharatana, M.; Jirasatid, S. Co-Encapsulation of Dictyophora Indusiata to Improve Lactobacillus Acidophilus Survival and Its Effect on Quality of Sweet Fermented Rice (Khoa-Mak) Sap Beverage. J. Food Sci. Technol. 2021, 58, 3598–3610. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Alharbi, M.A.; Alharbi, N.K.; Rafiq, S.; Shahbaz, M.; Murtaza, S.; Raza, N.; Farooq, U.; Ali, M.; Imran, M.; et al. Effect of Inulin on Organic Acids and Microstructure of Synbiotic Cheddar-Type Cheese Made from Buffalo Milk. Molecules 2022, 27, 5137. [Google Scholar] [CrossRef] [PubMed]
- Mileriene, J.; Serniene, L.; Kondrotiene, K.; Santarmaki, V.; Kourkoutas, Y.; Vasiliauskaite, A.; Lauciene, L.; Malakauskas, M. Indigenous Lactococcus Lactis with Probiotic Properties: Evaluation of Wet, Thermally- and Freeze-Dried Raisins as Supports for Cell Immobilization, Viability and Aromatic Profile in Fresh Curd Cheese. Foods 2022, 11, 1311. [Google Scholar] [CrossRef] [PubMed]
- Gomez Quintero, D.F.; Kok, C.R.; Hutkins, R. The Future of Synbiotics: Rational Formulation and Design. Front. Microbiol. 2022, 13, 919725. [Google Scholar] [CrossRef] [PubMed]
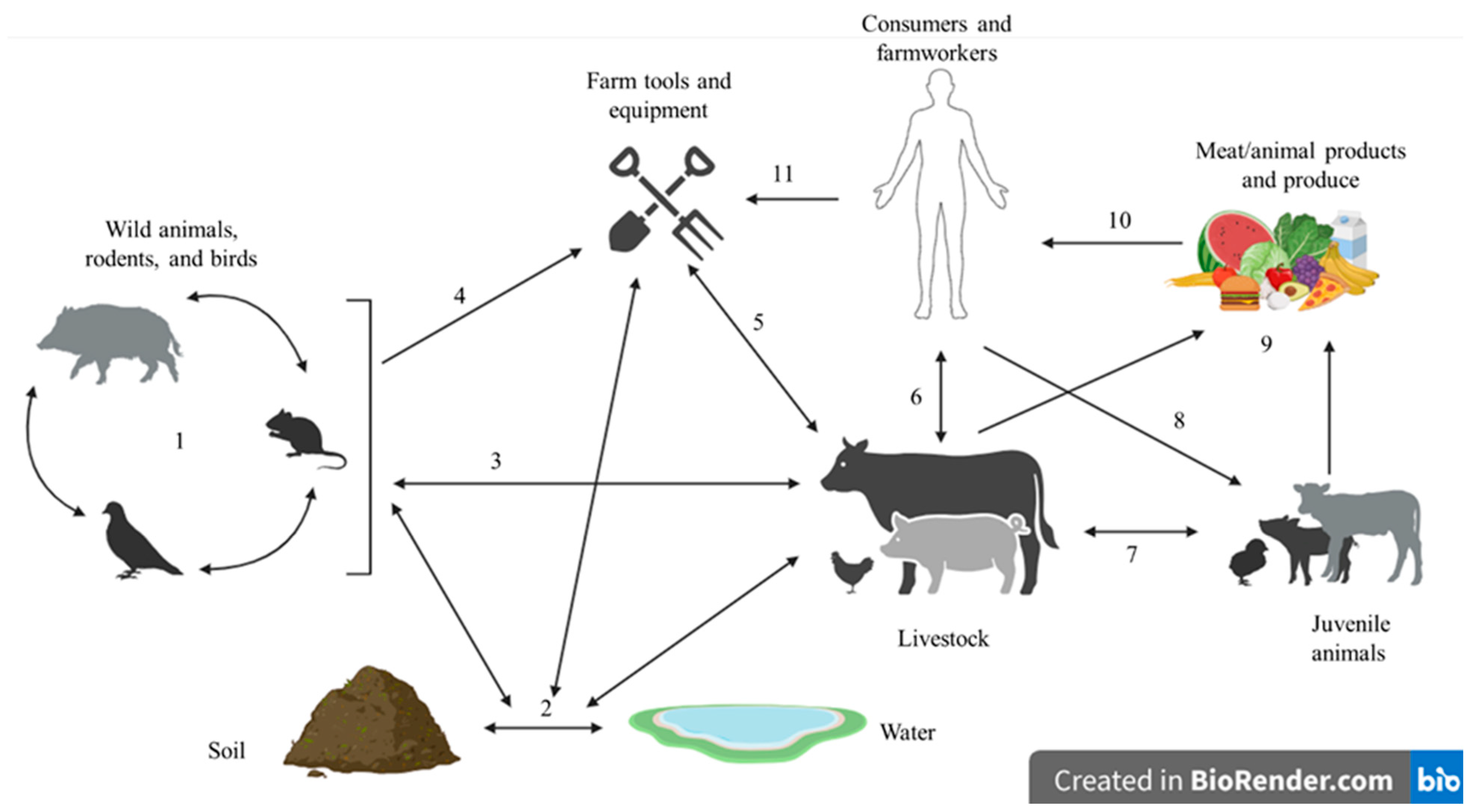
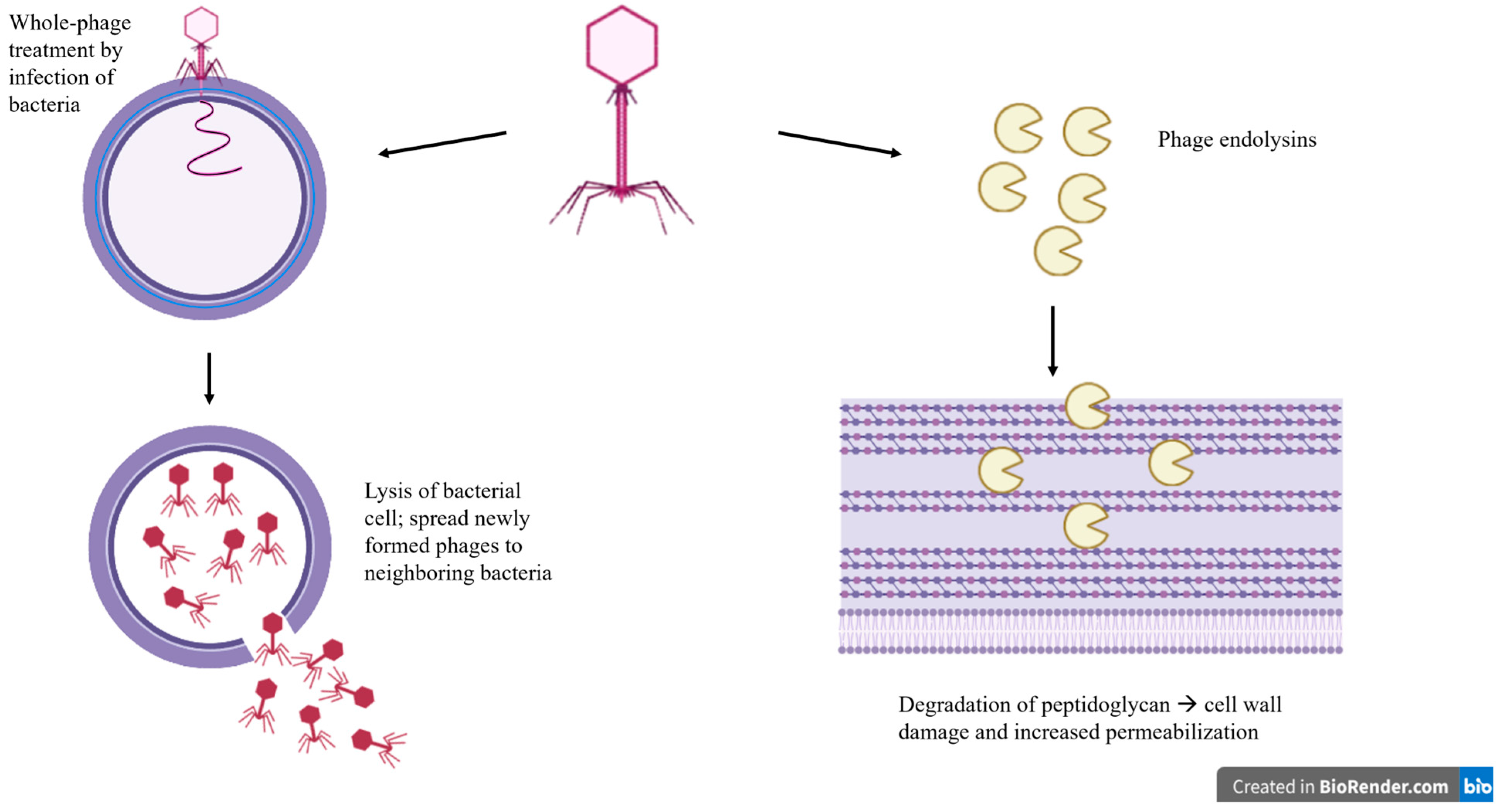
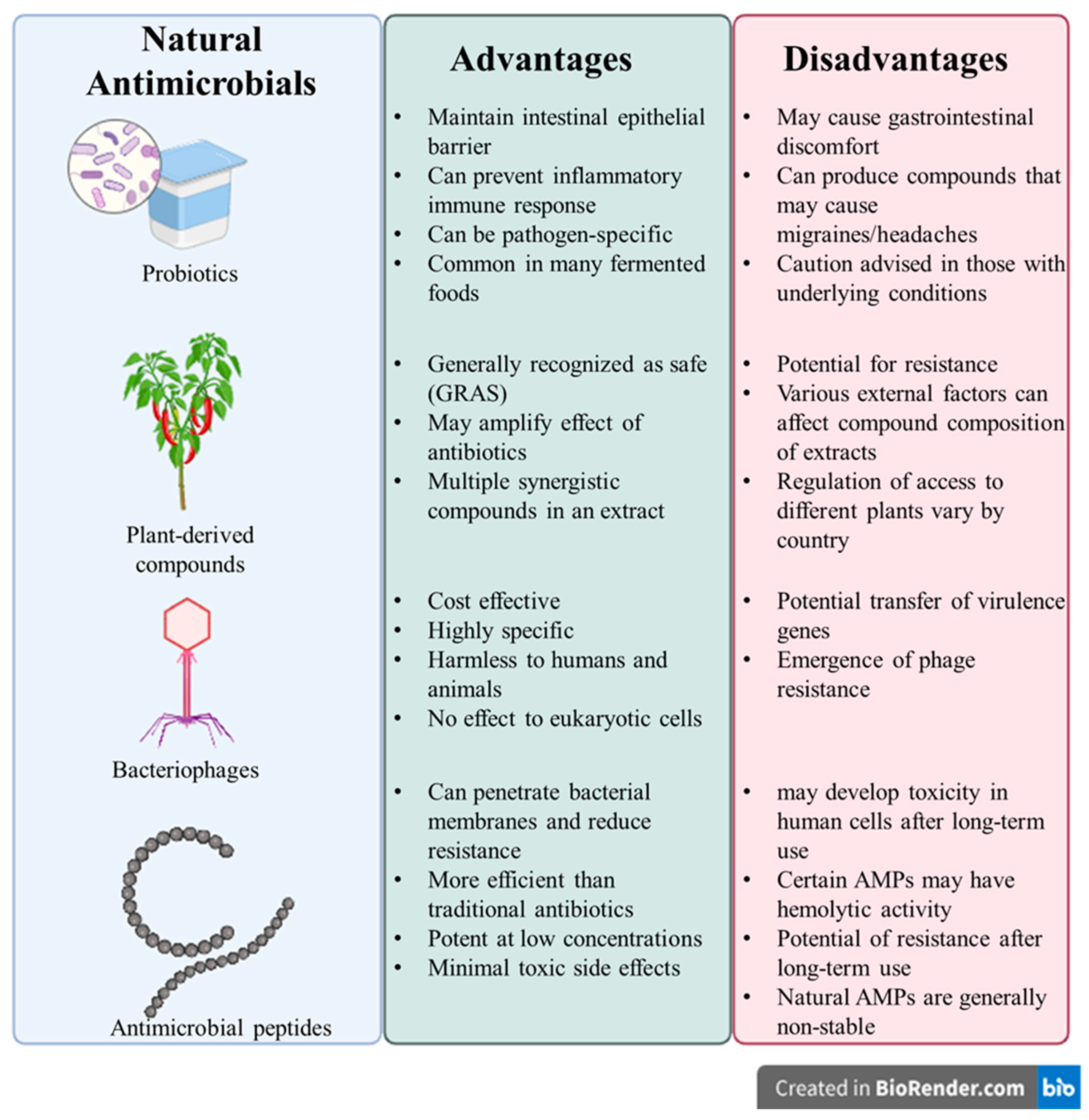
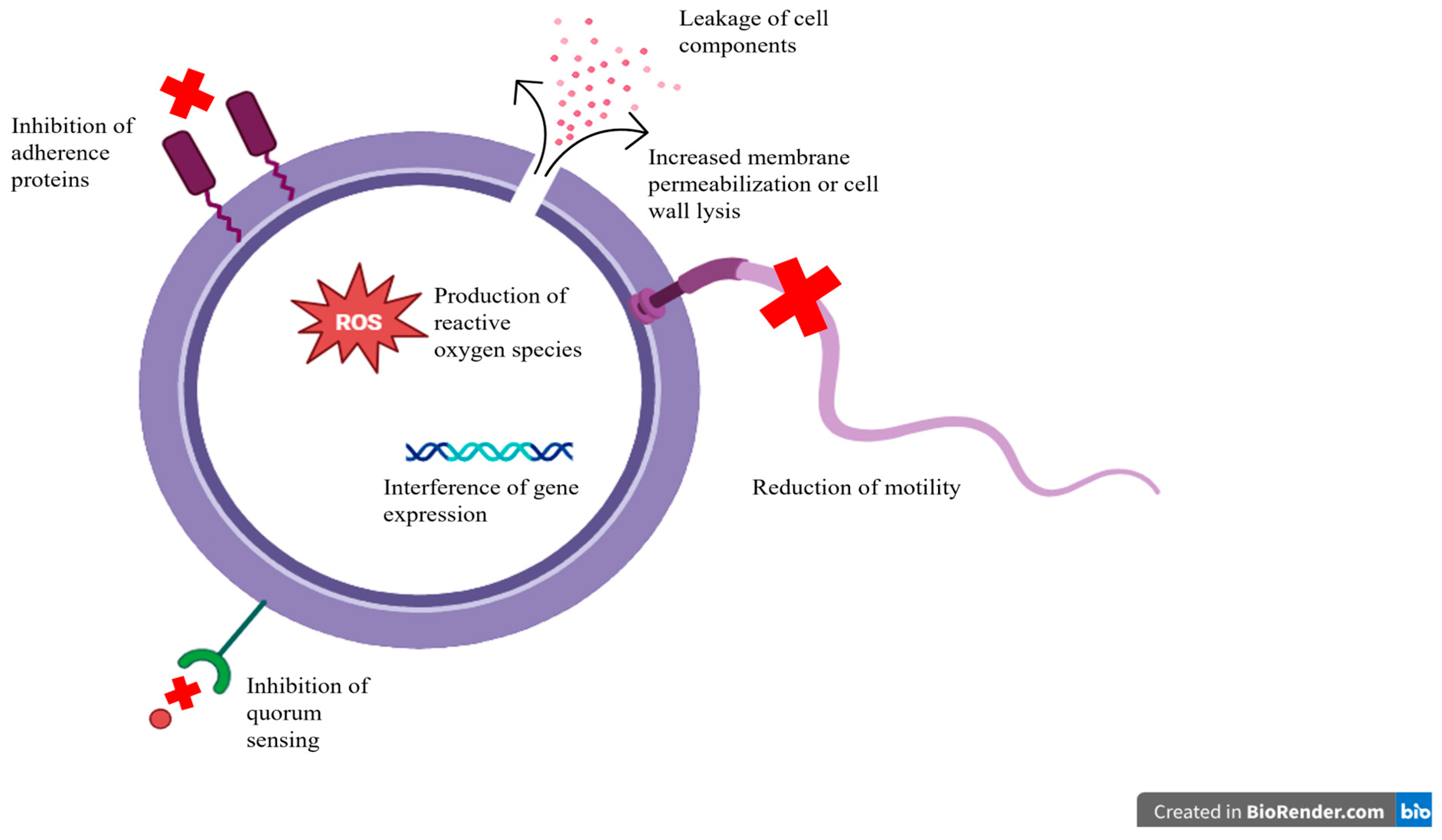
| Natural Antimicrobial Sources | Mechanism of Action | References |
|---|---|---|
| Citrus oil | -downregulation of cell wall synthesis genes -Induction of cell wall damage and lysis | [119,120,121,122,123,124,125] |
| Olive leaf extract | -reduction of flagella/motility -induction of cell wall and cell membrane damage | [126,127,128,129] |
| Curcumin | -inhibition of bacterial quorum sensing -inhibition of biofilm formation | [130,131,132,133,134,135,136] |
| Inula graveolens and Santolina corsica essential oil | -alteration of cell membrane -induction of cytoplasm leakage | [137] |
| Thyme essential oil | -damage of membrane integrity -increased membrane permeability -production of ROS -reduction of motility | [138,139,140,141] |
| Probiotic | S. aureus Strain | Antagonistic Activity | Mechanism | Reference |
|---|---|---|---|---|
| L. fermentum RC-14 | S. aureus ID50 (Oxford strain) | Inhibited S. aureus infection of surgical implants, completed inhibited subcutaneous abscess formation | Inhibition of adhesion to surfaces in vitro | [272] |
| L. plantarum MH734175, Leuconostoc mesenteroides CAU111 | S. aureus subsp. aureus PTCC 1431 | Antagonistic and proteolytic activity of probiotics against S. aureus with a zone of inhibition of 0.5 mm or larger | Bactericidal effect of protease-sensitive bacteriocins | [273] |
| L. acidophilus NK1, Bifidobacterium adolescentis MC 42 | Clinical strains of S. aureus (478, 502, 46 m, 83p) | Destructive properties against S. aureus biofilm | Biofilm degradation, biofilm refinement, segmentation, detachment of fragment and lysis | [274] |
| L. reuteri L 22 | S. aureus ATCC 25923 | The numbers of MRSA 02 were significantly lowered by more than 2-log-fold and no growth after 24 h of incubation | Antibacterial properties attributed by the production of H2O2 and bacteriocin | [275] |
| L. acidophilus CL1285, L. casei | S. aureus ATCC 29213 | The consumption of the CL1285 in mice reduced S. aureus after 18 days by 85% | Production of organic acids and bacteriocin | [276] |
| L. acidophilus CL1285 and L. casei LBC80R | S. aureus ATCC 43300 and MRSA clinical isolate | The inhibition of MRSA growth observed with the inhibition zones ranging from 1.4 to 2.9 cm | [277] | |
| L. acidophilus (LA5), L. casei 01 | S. aureus ATCC 29213 | The reduction of S. aureus up to 3-log/CFU in a co-culture method and suppression of SEA, SEC and SEE production up to 10.31-fold in co-incubation | Production of bacteriocin and hydrogen peroxide, competition for nutrients, and acidification | [278] |
| Saccharomyces cerevisiae S3 | S. aureus ATCC 29213, S. aureus ATCC 33591 | Supernatant and lysate extract reduced biofilm formation up to 69% and 80%, respectively. The hemolytic activity was reduced up to 93% by supernatant extract. The reduction of sea gene expression by 12-fold | Mannoproteins extracted S. cerevisiae cell wall believed to reduce biofilm growth | [279] |
| L. rhamnosus SHA113 | S. aureus ZBQ006, S. aureus 29213 | Up to 79% inhibition of S. aureus growth and reduced biofilm formation | Production of organic acids, hydrogen peroxide and biosurfactants. Inhibition of the expression of TNF-alpha and IL-6 Expression of up regulatory tight junction proteins ZO-1 and occluding. inflammatory factors | [280] |
| Streptococcus salivarius K12 | S. aureus ATCC 25923 | Inhibited the formation and maturation of fresh S. aureus biofilm by more than 60% | Acidification of the growth media | [281] |
| B. subtilis BSB3, 16k | S. aureus 10292, S. aureus 10378, S. aureus 12600, S. aureus 10203, MRSA 13, 34, 26, 2, 5 | The inhibition of S. aureus observed with zones of inhibition measured up to 20 mm | The production of biosurfactants | [282] |
| L. acidophilus La-5 and Bifidobacterium longum ATCC 15707 | S. aureus | Inhibited the growth with inhibition zone of >17 mm based on agar spot and well diffusion assay | Production of organic acids (lactic acid, acetic acid), hydrogen peroxide and bacteriocins | [283] |
| L. acidophilus AC, Bifidobacterium bifidum 4 | S. aureus ATCC 25903 | Inhibited S. aureus concentration up to 106 CFU/cm3 during joint cultivation at 60 and 72 h of incubation | Competition for nutrients, production of short-chain acids, and inhibitory substances of protein and non-protein nature | [284] |
| S. thermophilus (XN-S) | S. aureus ATCC 29213 | Cell-free culture supernatant from selenium (Se)-enriched S. thermophilus exerted stronger antibacterial activity than those from the non-Se strains | Cell structure damage | [285] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, A.; Mijar, S.; Harvey, C.; Biswas, D. Staphylococcus aureus in Foodborne Diseases and Alternative Intervention Strategies to Overcome Antibiotic Resistance by Using Natural Antimicrobials. Microorganisms 2025, 13, 1732. https://doi.org/10.3390/microorganisms13081732
Phan A, Mijar S, Harvey C, Biswas D. Staphylococcus aureus in Foodborne Diseases and Alternative Intervention Strategies to Overcome Antibiotic Resistance by Using Natural Antimicrobials. Microorganisms. 2025; 13(8):1732. https://doi.org/10.3390/microorganisms13081732
Chicago/Turabian StylePhan, Anna, Sanjaya Mijar, Catherine Harvey, and Debabrata Biswas. 2025. "Staphylococcus aureus in Foodborne Diseases and Alternative Intervention Strategies to Overcome Antibiotic Resistance by Using Natural Antimicrobials" Microorganisms 13, no. 8: 1732. https://doi.org/10.3390/microorganisms13081732
APA StylePhan, A., Mijar, S., Harvey, C., & Biswas, D. (2025). Staphylococcus aureus in Foodborne Diseases and Alternative Intervention Strategies to Overcome Antibiotic Resistance by Using Natural Antimicrobials. Microorganisms, 13(8), 1732. https://doi.org/10.3390/microorganisms13081732








