The Bacterial Composition of the Gut Microbiota of Mexicans with Overweight and Obesity: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Searches
2.2. Eligibility Criteria and Study Selection
2.3. Data Extraction
2.4. Quality Assessment
3. Results
3.1. Searches and Study Selection
3.2. Quality Assessment
3.3. Data Extraction
| Author | Date | Design | Sample Size | Participant’s Characteristics | Region of Mexico | Dietary Components | Microbiota Assessment Method | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Laura Moreno-Altamirano [36] | 2024 | Cross-sectional study | 91 | Medical students aged 18 years or older (65 women and 26 males) divided according to BMI into normal weight and overweight/obese | Central Mexico (Mexico City) | Specified in the food frequency questionnaire (FFQ) | 16S rRNA gene sequencing | Bacteroides and Prevotella were the predominant genera across different BMI categories. Bacteroides was more prevalent among men with overweight + obesity, while Prevotella was more common in men with normal weight. These trends were not observed in women. |
| 2 | Verónica Riggen-Bueno [40] | 2024 | Cross-sectional, comparative study | 65 | Volunteer male and female participants aged between 18 and 59 years were divided according to BMI into normal weight control and overweight/obese | Western Mexican states (Colima, Jalisco, Michoacán, and Nayarit) | The FFQ was not applied | 16S rRNA gene sequencing | The gut microbiota of the obese group showed a notable increase in Negativicutes, Escherichia/Shigella, Prevotella, and Lachnoclostridium. |
| 3 | Ricardo García-Gamboa [29] | 2024 | Cross-sectional study | 30 | Men and women aged between 20 and 50 years | NS | Specified in the FFQ and in the 24 h dietary recall | 16S rRNA gene sequencing | The Bacillota/Bacteroidota and Bacteroides/Prevotella ratios were positively associated with BMI. In overweight and obese individuals, those with higher levels of Akkermansia muciniphila had more favorable lipid and glucose profiles than those with lower levels of this bacterium. Obese subjects had Allisonella, Subdoligranulum and Dielma, Lachnospira, Romboutsia, and Clostridium. Overweight individuals had Flavonifractor, Eggerthella, and Alloprevotella. Healthy weight individuals had Faecalibacterium, Histophilus, Rikenella, Odoribacter, and Marvinbryantia. |
| 4 | Avilene Rodríguez-Lara [41] | 2022 | Cross-sectional study | 120 | Mexican students (females and males) aged between 18 and 25 years were divided according to BMI into normal weight and overweight/obese | Western Mexico (Guadalajara, Jalisco) | Specified in the FFQ | Species-specific qualitative PCR | The phyla Bacillota with mainly Clostridium coccoides-Eubacterium rectale were found to be mostly expressed in overweight and obese individuals compared to the normal weight subjects. |
| 5 | Sofía Morán-Ramos [35] | 2022 | Short-term, quasi-experimental, longitudinal pilot study. Open, self-controlled clinical trial | 6 | Male children aged 12 years. Obesity status was defined according to the BMI score. Intervention of 6 weeks of diet and exercise | Central Mexico (Mexico City) | Dietary information was obtained. Intervention: participants received a hypo energetic dietary plan (60% energy from carbohydrates, 20–25% from fats, and 15–20% from proteins) | 16S rRNA gene sequencing | The most abundant phylum in children before the intervention was Bacteroidota, Bacillota and Proteobacteria, and no significant modifications in this abundance were observed after the intervention. Odoribacter was associated with the reduction in waist circumference after the intervention. |
| 6 | Marco U. Martinez-Martinez [30] | 2022 | Controlled clinical trial. Randomized double-blind | 38 | Male and female children aged between 6 and 10 years. Obesity status was defined according to the BMI percentile for overweight and obesity | Eastern Mexico (Escalerillas, San Luis Potosí) | All children had a similar diet at the full-time school program. | 16S rRNA gene sequencing | In children with overweight or obesity Veillonella, Catenibacterium, Blautia, Alistipes and Holdemanella showed a positive association with the BMI and BMI Z score. A synbiotic with fructans from Agave salmiana stimulated the abundance and diversity of genera Faecalibacterium and Holdemanella, thus, leading to a healthier gut microbiota. |
| 7 | Miguel Vázquez-Moreno [42] | 2020 | Cross-sectional analytical observational study (with case-control statistical analysis) | 330 | Unrelated children with normal weight and obesity aged 6–12 years. Children with a BMI ≥ 5th and <85th percentile were classified as having normal weight and those with a BMI ≥ 95th percentile as having obesity | Central Mexico (Mexico City) Southern Mexico (Oaxaca) | The FFQ was not applied | 16S rRNA amplicon sequencing | Fusicatenibacter, Romboutsia, Ruminococcaceae Ruminiclostridium, Blautia, Clostridium, Anaerostipes and Intestinibacter were associated with obesity. In Mexico City, the most relative abundant bacteria in all the samples were Blautia, Ruminococcaceae UCG-002, and Anaerostipes. Obesity status was positively associated with Fusicatenibacter and Romboutsia, and negatively associated with Ruminococcaceae UCG-002 and Ruminiclostridium. In Oaxaca, the most abundant bacteria were Bacteroides, Alistipes, and Clostridium_sensu_stricto_1. No genera were found to be associated with obesity status. |
| 8 | Tania Aguilar [25] | 2020 | Cross-sectional study | 93 | Normal weight, overweight, and obese school-aged children aged 8.4 ± 1.6 years (girls and boys) | Eastern Mexico (rural communities (Santa Maria Begoña and Santa Cruz) of Queretaro | The FFQ was not applied | Species-specific qualitative PCR | Lower Bacteroidaceae–Porphyromonadaceae–Prevotellaceae and higher abundance of Lactobacillaceae were associated with obesity and metabolic disturbances. |
| 9 | Luigui Gallardo-Becerra [28] | 2020 | Cross-sectional study | 27 | Children (girls and boys) aged 7–10 years: normal weight (NW), obese (O), and obese with metabolic syndrome (OMS) children from a summer-camp of Mexican Health Ministry employees. All children came from households with a middle economic class income and belonged to a similar socio-cultural status. Obesity was defined by body mass index (BMI) based on the guidelines of the Centers for Disease Control and Prevention (CDC). | Central Mexico (Mexico City) | The FFQ was not applied | Sequencing shotgun of the total RNA and a profiling of the 16S rRNA gene using the V4 region | Bacillota were markedly increased in the OMS and O compared to the NW group. Bacteroidota were increased in NW compared to the O and OMS. Novel biomarkers for obesity with MetS consisted of an increased Coriobacteraceae, Collinsela, and Collinsella aerofaciens; Erysipelotrichaceae, Catenibacterium and Catenibacterium spp., and decreased Parabacteroides distasonis, which correlated with clinical and anthropometric parameters associated to obesity and metabolic syndrome. Genus Porphyromonas and an undetermined species within this genus were specifically over-abundant in the obese group. |
| 10 | Sofía Morán-Ramos [34] | 2020 | Cross-sectional study | 926 | School children aged 6–12 years. Obesity status was defined as BMI percentile | Central Mexico (Mexico City) | Specified in the FFQ | 16S rRNA amplicon sequencing | 63% of the children belonged to Bacteroides enterotype, while 37% belonged to Prevotella enterotype. The more abundant taxa were Bacteroides followed by Prevotella and an unclassified Ruminococcaceae genus. Greater abundance of Christensenellaceae family was associated with a lower BMI percentile. |
| 11 | Alejandra Chávez-Carbajal [26] | 2019 | Cross-sectional analytic study | 64 | Volunteer healthy Mexican women (CO), women with obesity (OB), and women with obesity plus metabolic syndrome (OMS) aged from 18 to 59 years | Central Mexico (Mexico City) | Specified in the FFQ | Species-specific qualitative PCR | Bacillota were more abundant in women with OB or OMS than in women of the CO group. There were significant changes in abundances of bacteria belonging to the Ruminococcaceae, Lachnospiraceae, and Erysipelotrichaceae families. The Proteobacteria, Actinobacteria, Tenericutes, Cyanobacteria, and Synergistetes were also not different between the groups. The “Others” category, which included less abundant phyla such as Verrucomicrobia, Spirochaetes, and Fusobacteria, showed a significant difference between groups. Faecalibacterium spp., Roseburia spp., Lachnospira spp., and Coprococcus spp. were significantly more abundant in the OB and OMS groups. The family Erysipelotrichaceae was significantly decreased in the OB and OMS groups. |
| 12 | Otoniel Maya-Lucas [31] | 2019 | Cross-sectional study | 20 | Unrelated children aged between 9 and 11 years selected from a 118 obesity database | Central Mexico (Mexico City) | Specified in the FFQ | Metagenomic shotgun-sequencing of DNA | In obese children, there was an increase in unclassified Methanobrevibacter spp. Normal weight children had a community dominated by Ruminococcus spp. (enterotype 3). Obese children had a community dominated by Prevotella spp. (enterotype 2), Megamonas spp. were overrepresented, and members of the family Oscillospiraceae were depleted. |
| 13 | Khemlal Nirmalkar [38] | 2018 | Cross-sectional study | 172 | Children aged 6–11 years and adolescents aged 12–18 years. Obesity was defined according to BMI percentile based on Centers for Disease Control and Prevention (CDC) reference data. | Central Mexico (Toluca, State of Mexico) | Diet intake was divided into seven food groups as follows: (1) Starchy staples, (2) legumes, (3) dairy, (4) meat, (5) vitamin A-rich fruits and vegetables, (6) other fruits and vegetables or fruit juices, and (7) foods made with oil, fat, or butter. | 16S rRNA amplicon sequencing | Obese children and adolescents showed an increase in the abundance of members of the family Coriobacteriaceae (p-Actinobacteria). The genus Lactobacillus and family Coriobacteriaceae were enriched in children, and genera Collinsella and Prevotella were enriched in obese adolescents. |
| 14 | Berenice Lopez-Contreras [23] | 2018 | Cross-sectional study | 138 | Normal weight and obese children aged 6–12 years from a summer camp for children of Mexican Health Ministry employees. Obesity was defined according to BMI percentile based on Centers for Disease Control and Prevention (CDC) reference data. | Central Mexico (Mexico City) | Specified in the FFQ | 16S rRNA amplicon sequencing | Normal weight and obese children had no significant differences in phyla abundances or Bacillota/Bacteroidota ratios. In obese children, Bacteroides eggerthii abundance was significantly high and correlated positively with body fat percentage. In normal weight children, Bacteroides plebeius and unclassified Christensenellaceae abundances were significantly higher. |
| 15 | Eder Orlando Méndez-Salazar [33] | 2018 | Cross-sectional study | 36 | Mexican schooled children aged 9–11 years belonging to low-income families | Central Mexico (Chimalhuacán, State of Mexico) | Specified in an unannounced 24 h dietary recalls. | 16S rRNA gene sequencing | Undernourished children had significantly higher levels of bacteria in the Bacillota phylum and in the Lachnospiraceae family than obese children, while the Proteobacteria phylum was overrepresented in the obese group. |
| 16 | Yaneth C Orbe-Orihuela [39] | 2018 | Cross-sectional study | 890 | Normal weight, overweight, and obese children residents aged from 6 to 14 years | Central Mexico (Mexico City) | Specified in the FFQ | Species-specific qualitative PCR | High relative abundance of Bacillota with high Bacillota/Bacteroidota ratio was found in obese children |
| 17 | Lino Mayorga Reyes [32] | 2016 | Cross-sectional study | 9 | Young adults women and men aged from 18 to 39 years from the Universidad Autónoma Metropolitana classified as lean, overweight, and obese | Central Mexico (Mexico City) | Specified in the FFQ | Species-specific qualitative PCR | There were significant differences in the gut microbiota between the overweight and lean groups. There were no significant differences in the abundance of the Bacillota and Bacteroidota within each group. Striking significant differences were observed in the abundance of Faecalibacterium prausnitzii between lean and obese groups and between overweight and obese groups. There were significant differences in the abundance of Clostridium leptum between lean and obese groups and between overweight and obese groups. For Bifidobacterium longum, significant differences were found between lean and overweight groups and between lean and obese groups. Members of the Bacillota phylum and B. longum were more abundant in the lean group. |
| 18 | Barbara Ixchel Estrada-Velasco [27] | 2014 | Cross-sectional study | 1042 | Unrelated children aged 6–14 years | Mexico City (North, South, East and West) | Dietary patterns: Mexican snacks and fast food, fresh fruits and vegetables, fish and foods with saturated fat, tortillas and corn-based foods, bread, cereals, potatoes, rice, pasta, red meat, sausages | Species-specific qualitative PCR | High relative abundance of Bacillota and a low relative abundance of Bacteroidota were associated to obesity |
| 19 | S. Murugesan [37] | 2015 | Cross-sectional study | 190 | Unrelated children aged 9–11 years (normal, overweight, and obese). Obesity was defined according to BMI percentile. | Central Mexico (Ecatepec, State of Mexico) | Specified in a 7-day recall | Ion torrent semiconductor sequencing of 16S rDNA | Overweight and obese children presented an increased abundance of Faecalibacterium spp., Lachnospiraceae, and Roseburia spp. |
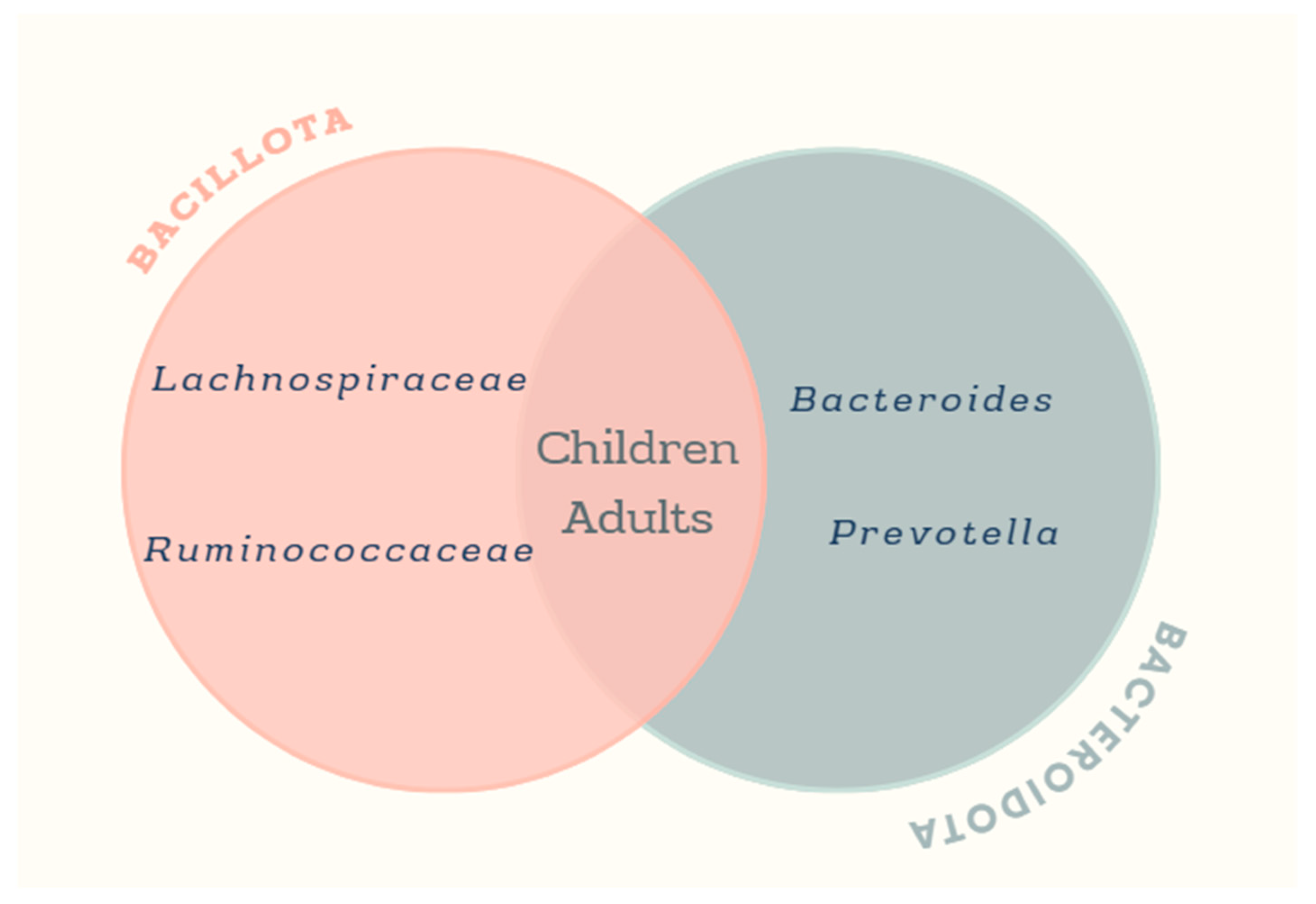
3.4. Studies of the Gut Microbiota of Obese and Overweight Mexican Children
3.5. Studies of the Gut Microbiota in Adult Mexican Population with Obesity
3.6. The Role of the Traditional Mexican Diet in Obese Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obesity. Available online: https://www.who.int/health-topics/obesity (accessed on 10 June 2024).
- Asadi, A.; Shadab Mehr, N.; Mohamadi, M.H.; Shokri, F.; Heidary, M.; Sadeghifard, N.; Khoshnood, S. Obesity and gut-microbiota-brain axis: A narrative review. J. Clin. Lab. Anal. 2022, 36, e24420. [Google Scholar] [CrossRef] [PubMed]
- Encuesta Nacional de Salud y Nutricion 2018. Cuernavaca. Available online: https://ensanut.insp.mx/ (accessed on 12 December 2019).
- Barquera, S.; Rivera, J.A. Obesity in Mexico: Rapid epidemiological transition and food industry interference in health policies. Lancet Diabetes Endocrinol. 2020, 8, 746–747. [Google Scholar] [CrossRef] [PubMed]
- Shamah-Levy, T.; Cuevas-Nasu, L.; Romero-Martínez, M.; Gómez-Humaran, I.M.; Ávila-Arcos, M.A.; Rivera, J.A. Nutrition Status of Children, Teenagers, and Adults From National Health and Nutrition Surveys in Mexico From 2006 to 2020. Front. Nutr. 2021, 8, 777246. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Armougom, F.; Million, M.; Raoult, D. The relationship between gut microbiota and weight gain in humans. Future Microbiol. 2012, 7, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Kassis, A.; Major, G.; Chou, C.J. Is the gut microbiota a new factor contributing to obesity and its metabolic disorders? J. Obes. 2012, 2012, 879151. [Google Scholar] [CrossRef] [PubMed]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A.; et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Holleman, F.; Zoetendal, E.G.; de Vos, W.M.; Hoekstra, J.B.; Nieuwdorp, M. The environment within: How gut microbiota may influence metabolism and body composition. Diabetologia 2010, 53, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Melancholic microbes: A link between gut microbiota and depression? Neurogastroenterol Motil. 2013, 25, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Dave, M.; Higgins, P.D.; Middha, S.; Rioux, K.P. The human gut microbiome: Current knowledge, challenges, and future directions. Transl. Res. 2012, 160, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Bikel, S.; Valdez-Lara, A.; Cornejo-Granados, F.; Rico, K.; Canizales-Quinteros, S.; Soberón, X.; Del Pozo-Yauner, L.; Ochoa-Leyva, A. Combining metagenomics, metatranscriptomics and viromics to explore novel microbial interactions: Towards a systems-level understanding of human microbiome. Comput. Struct. Biotechnol. J. 2015, 13, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Deschasaux, M.; Bouter, K.E.; Prodan, A.; Levin, E.; Groen, A.K.; Herrema, H.; Tremaroli, V.; Bakker, G.J.; Attaye, I.; Pinto-Sietsma, S.J.; et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med. 2018, 24, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Walters, W.A.; Xu, Z.; Knight, R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014, 588, 4223–4233. [Google Scholar] [CrossRef] [PubMed]
- Dziarski, R.; Park, S.Y.; Kashyap, D.R.; Dowd, S.E.; Gupta, D. Pglyrp-Regulated Gut Microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii Enhance and Alistipes finegoldii Attenuates Colitis in Mice. PLoS ONE 2016, 11, e0146162. [Google Scholar] [CrossRef] [PubMed]
- Kant, A.K. Dietary patterns and health outcomes. J. Am. Diet. Assoc. 2004, 104, 615–635. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- González-Bonilla, A.; Meneses, M.E.; Pérez-Herrera, A.; Armengol-Álvarez, D.; Martínez-Carrera, D. Dietary Supplementation with Oyster Culinary-Medicinal Mushroom, Pleurotus ostreatus (Agaricomycetes), Reduces Visceral Fat and Hyperlipidemia in Inhabitants of a Rural Community in Mexico. Int. J. Med. Mushrooms 2022, 24, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Kawachi, I. Diets and Health: How Food Decisions Are Shaped by Biology, Economics, Geography, and Social Interactions. Big Data 2015, 3, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Granados, F.; Calderón de la Barca, A.M.; Torres, N.; Martínez-Romero, E.; Torres, J.; López-Vidal, Y.; Soberón, X.; Partida-Martínez, L.P.; Pinto-Cardoso, S.; Alcaraz, L.D.; et al. Microbiome-MX 2018: Microbiota and microbiome opportunities in Mexico, a megadiverse country. Res. Microbiol. 2019, 170, 235–241. [Google Scholar] [CrossRef] [PubMed]
- López-Contreras, B.E.; Morán-Ramos, S.; Villarruel-Vázquez, R.; Macías-Kauffer, L.; Villamil-Ramírez, H.; León-Mimila, P.; Vega-Badillo, J.; Sánchez-Muñoz, F.; Llanos-Moreno, L.E.; Canizalez-Román, A.; et al. Composition of gut microbiota in obese and normal-weight Mexican school-age children and its association with metabolic traits. Pediatr. Obes. 2018, 13, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Parums, D.V. Review articles, systematic reviews, meta-analysis, and the updated preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 guidelines. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e934475. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, T.; Nava, G.M.; Olvera-Ramírez, A.M.; Ronquillo, D.; Camacho, M.; Zavala, G.A.; Caamaño, M.C.; Acevedo-Whitehouse, K.; Rosado, J.L.; García, O.P. Gut Bacterial Families Are Associated with Body Composition and Metabolic Risk Markers in School-Aged Children in Rural Mexico. Child Obes. 2020, 16, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Carbajal, A.; Nirmalkar, K.; Pérez-Lizaur, A.; Hernández-Quiroz, F.; Ramírez-Del-Alto, S.; García-Mena, J.; Hernández-Guerrero, C. Gut Microbiota and Predicted Metabolic Pathways in a Sample of Mexican Women Affected by Obesity and Obesity Plus Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 438. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Velasco, B.I.; Cruz, M.; Garcia-Mena, J.; Valladares Salgado, A.; Peralta Romero, J.; Guna Serrano Mde, L.; Madrid-Marina, V.; Orbe Orihuela, C.; López Islas, C.; Burguete-García, A.I. Childhood obesity is associated to the interaction between firmicutes and high energy food consumption. Nutr. Hosp. 2014, 31, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Becerra, L.; Cornejo-Granados, F.; García-López, R.; Valdez-Lara, A.; Bikel, S.; Canizales-Quinteros, S.; López-Contreras, B.E.; Mendoza-Vargas, A.; Nielsen, H.; Ochoa-Leyva, A. Metatranscriptomic analysis to define the Secrebiome, and 16S rRNA profiling of the gut microbiome in obesity and metabolic syndrome of Mexican children. Microb. Cell Fact. 2020, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- García-Gamboa, R.; Díaz-Torres, O.; Senés-Guerrero, C.; Gradilla-Hernández, M.S.; Moya, A.; Pérez-Brocal, V.; Garcia-Gonzalez, A.; González-Avila, M. Associations between bacterial and fungal communities in the human gut microbiota and their implications for nutritional status and body weight. Sci. Rep. 2024, 14, 5703. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, M.U.; Vazquez-Maldonado, D.; Ratering, S.; Godinez-Hernandez, C.; Ortiz-Basurto, R.I.; Soria-Guerra, R.E.; Schneider, B.; Juárez-Flores, B.I.; Portales-Perez, D.P.; Schnell, S. Fructans from Agave enhance probiotic yoghurt by modulating gut microbiota on children with overweight or obesity. Food Biosci. 2022, 46, 101516. [Google Scholar] [CrossRef]
- Maya-Lucas, O.; Murugesan, S.; Nirmalkar, K.; Alcaraz, L.D.; Hoyo-Vadillo, C.; Pizano-Zárate, M.L.; García-Mena, J. The gut microbiome of Mexican children affected by obesity. Anaerobe 2019, 55, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Mayorga Reyes, L.; González Vázquez, R.; Cruz Arroyo, S.M.; Melendez Avalos, A.; Reyes Castillo, P.A.; Chavaro Pérez, D.A.; Ramos Terrones, I.; Ramos Ibáñez, N.; Rodríguez Magallanes, M.M.; Langella, P.; et al. Correlation between diet and gut bacteria in a population of young adults. Int. J. Food Sci. Nutr. 2016, 67, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Salazar, E.O.; Ortiz-López, M.G.; Granados-Silvestre, M.; Palacios-González, B.; Menjivar, M. Altered Gut Microbiota and Compositional Changes in Firmicutes and Proteobacteria in Mexican Undernourished and Obese Children. Front. Microbiol. 2018, 9, 2494. [Google Scholar] [CrossRef] [PubMed]
- Moran-Ramos, S.; Lopez-Contreras, B.E.; Villarruel-Vazquez, R.; Ocampo-Medina, E.; Macias-Kauffer, L.; Martinez-Medina, J.N.; Villamil-Ramirez, H.; León-Mimila, P.; Del Rio-Navarro, B.E.; Ibarra-Gonzalez, I.; et al. Environmental and intrinsic factors shaping gut microbiota composition and diversity and its relation to metabolic health in children and early adolescents: A population-based study. Gut Microbes 2020, 11, 900–917. [Google Scholar] [CrossRef] [PubMed]
- Morán-Ramos, S.; Siliceo-Bernardi, M.T.; Villalpando-Carrión, S.; Canizales-Quinteros, S.; Frigolet, M.E.; Gutiérrez-Aguilar, R. Gut microbiota composition after a dietary and physical activity intervention: A pilot study in Mexican children with obesity. Bol. Med. Hosp. Infant. Mex. 2022, 79, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Altamirano, L.; Robles-Rivera, K.; Castelán-Sánchez, H.G.; Vaca-Paniagua, F.; Iñarritu Pérez, M.D.C.; Hernández-Valencia, S.E.; Cruz-Casarrubias, C.; García-García, J.J.; Ruíz de la Cruz, M.; Martínez-Gregorio, H.; et al. Gut Microbiota: Association with Fiber Intake, Ultra-Processed Food Consumption, Sex, Body Mass Index, and Socioeconomic Status in Medical Students. Nutrients 2024, 16, 4241. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, S.; Ulloa-Martínez, M.; Martínez-Rojano, H.; Galván-Rodríguez, F.M.; Miranda-Brito, C.; Romano, M.C.; Piña-Escobedo, A.; Pizano-Zárate, M.L.; Hoyo-Vadillo, C.; García-Mena, J. Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Nirmalkar, K.; Murugesan, S.; Pizano-Zárate, M.L.; Villalobos-Flores, L.E.; García-González, C.; Morales-Hernández, R.M.; Nuñez-Hernández, J.A.; Hernández-Quiroz, F.; Romero-Figueroa, M.D.S.; Hernández-Guerrero, C.; et al. Gut Microbiota and Endothelial Dysfunction Markers in Obese Mexican Children and Adolescents. Nutrients 2018, 10, 2009. [Google Scholar] [CrossRef] [PubMed]
- Orbe-Orihuela, Y.C.; Lagunas-Martínez, A.; Bahena-Román, M.; Madrid-Marina, V.; Torres-Poveda, K.; Flores-Alfaro, E.; Méndez-Padrón, A.; Díaz-Benítez, C.E.; Peralta-Zaragoza, O.; Antúnez-Ortiz, D.; et al. High relative abundance of firmicutes and increased TNF-α levels correlate with obesity in children. Salud Publica Mex. 2018, 60, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Riggen-Bueno, V.; Del Toro-Arreola, S.; Baltazar-Díaz, T.A.; Vega-Magaña, A.N.; Peña-Rodríguez, M.; Castaño-Jiménez, P.A.; Sánchez-Orozco, L.V.; Vera-Cruz, J.M.; Bueno-Topete, M.R. Intestinal Dysbiosis in Subjects with Obesity from Western Mexico and Its Association with a Proinflammatory Profile and Disturbances of Folate (B9) and Carbohydrate Metabolism. Metabolites 2024, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lara, A.; Plaza-Díaz, J.; López-Uriarte, P.; Vázquez-Aguilar, A.; Reyes-Castillo, Z.; Álvarez-Mercado, A.I. Fiber Consumption Mediates Differences in Several Gut Microbes in a Subpopulation of Young Mexican Adults. Nutrients 2022, 14, 1214. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Moreno, M.; Perez-Herrera, A.; Locia-Morales, D.; Dizzel, S.; Meyre, D.; Stearns, J.C.; Cruz, M. Association of gut microbiome with fasting triglycerides, fasting insulin and obesity status in Mexican children. Pediatr. Obes. 2021, 16, e12748. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G. Part two: The impact of agricultural Development on child nutrition in Tabasco, Mexico. Med. Anthropol. 1980, 4, 21–54. [Google Scholar] [CrossRef]
- Guibrunet, L.; Ortega-Avila, A.G.; Arnés, E.; Mora Ardila, F. Socioeconomic, demographic and geographic determinants of food consumption in Mexico. PLoS ONE 2023, 18, e0288235. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Aceves-Martins, M.; Llauradó, E.; Tarro, L.; Solà, R.; Giralt, M. Obesity-promoting factors in Mexican children and adolescents: Challenges and opportunities. Glob. Health Action 2016, 9, 29625. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Nasu, L.; Muñoz-Espinosa, A.; Shamah-Levy, T.; García-Feregrino, R.; Gómez-Acosta, L.M.; Ávila-Arcos, M.A.; Rivera-Dommarco, J.A. Estado de nutrición de niñas y niños menores de cinco años en México. Ensanut 2022. Salud Publica Mex. 2023, 65, s211–s217. [Google Scholar] [CrossRef] [PubMed]
- Shamah-Levy, T.; Gaona-Pineda, E.B.; Cuevas-Nasu, L.; Morales-Ruan, C.; Valenzuela-Bravo, D.G.; Méndez-Gómez Humaran, I.; Ávila-Arcos, M.A. Prevalencias de sobrepeso y obesidad en población escolar y adolescente de México. Ensanut Continua 2020–2022. Salud Publica Mex. 2023, 65, s218–s224. [Google Scholar] [CrossRef] [PubMed]
- Abay, K.A.; Ibrahim, H.; Breisinger, C. Food policies and obesity in low-and middle-income countries. World Dev. 2022, 151, 105775. [Google Scholar] [CrossRef]
- Angulo Moreno, M.E.; Barrios González, E.; Benítez Brito, N.; Cansino Campuzano, Á.; Díaz Fernández, P.; Duarte Curbelo, Á.D.P.; Fernández Betancor, N.; García Mérida, M.J.; Guillén Díaz, O.; de la Huerga Moreno, S. Abordaje a la Obesidad Infantil y Juvenil en Canarias. Prevención Cardiovascular Desde la Infancia; Gobierno de Canarias: Las Palmas, Spain, 2023; ISBN 978-84-16878-32-1.
- García-Mena, J.; Corona-Cervantes, K.; Cuervo-Zanatta, D.; Benitez-Guerrero, T.; Vélez-Ixta, J.M.; Zavala-Torres, N.G.; Villalobos-Flores, L.E.; Hernández-Quiroz, F.; Perez-Cruz, C.; Murugesan, S.; et al. Gut microbiota in a population highly affected by obesity and type 2 diabetes and susceptibility to COVID-19. World J. Gastroenterol. 2021, 27, 7065–7079. [Google Scholar] [CrossRef] [PubMed]
- Garza-Velasco, R.; Garza-Manero, S.P.; Perea-Mejía, L.M. Gut microbiota: A fundamental ally of the human organism. Educ. Química 2021, 32, 10–19. [Google Scholar] [CrossRef]
- Munoz, R.; Rosselló-Móra, R.; Amann, R. Revised phylogeny of Bacteroidetes and proposal of sixteen new taxa and two new combinations including Rhodothermaeota phyl. nov. Syst. Appl. Microbiol. 2016, 39, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Ávila, E.E.; Ramírez-Silva, I.; Torres-Sánchez, L.E.; Díaz-Benítez, C.E.; Orbe-Orihuela, Y.C.; Lagunas-Martínez, A.; Galván-Portillo, M.; Flores, M.; Cruz, M.; Burguete-García, A.I. High Relative Abundance of Lactobacillus reuteri and Fructose Intake are Associated with Adiposity and Cardiometabolic Risk Factors in Children from Mexico City. Nutrients 2019, 11, 1207. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Barreto, G.; Burrello, C.; Diaz-Basabe, A.; Suutarinen, M.; Kainulainen, V.; Bowers, J.R.; Lemmer, D.; Engelthaler, D.M.; Eklund, K.K.; et al. Novel Odoribacter splanchnicus Strain and Its Outer Membrane Vesicles Exert Immunoregulatory Effects in vitro. Front. Microbiol. 2020, 11, 575455. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Attilio, R.R. Actualización en el manejo del colesterol HDL bajo. Rev. Médica Clínica Las. Condes 2012, 23, 689–692. [Google Scholar] [CrossRef]
- Hadi, H.A.; Carr, C.S.; Al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- González Olmo, B.M.; Butler, M.J.; Barrientos, R.M. Evolution of the Human Diet and Its Impact on Gut Microbiota, Immune Responses, and Brain Health. Nutrients 2021, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Carrizales-Sánchez, A.K.; García-Cayuela, T.; Hernández-Brenes, C.; Senés-Guerrero, C. Gut microbiota associations with metabolic syndrome and relevance of its study in pediatric subjects. Gut Microbes 2021, 13, 1960135. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Qin, Q.; Chen, J.; Yan, S.; Li, T.; Gao, X.; Yang, Y.; Li, A.; Ding, S. Gut Microbiome Alterations in Patients With Visceral Obesity Based on Quantitative Computed Tomography. Front. Cell Infect. Microbiol. 2021, 11, 823262. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Fang, X.; Zhou, Y.; Dou, L.; Dou, T. Machine learning-based investigation of the relationship between gut microbiome and obesity status. Microbes Infect. 2022, 24, 104892. [Google Scholar] [CrossRef] [PubMed]
- Loftfield, E.; Herzig, K.H.; Caporaso, J.G.; Derkach, A.; Wan, Y.; Byrd, D.A.; Vogtmann, E.; Männikkö, M.; Karhunen, V.; Knight, R.; et al. Association of Body Mass Index with Fecal Microbial Diversity and Metabolites in the Northern Finland Birth Cohort. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Mena-Vázquez, N.; Ruiz-Limón, P.; Moreno-Indias, I.; Manrique-Arija, S.; Lisbona-Montañez, J.M.; Rioja, J.; Mucientes, A.; Martin-Núñez, G.M.; Cano-García, L.; Tinahones, F.J.; et al. Adiposity is associated with expansion of the genus Dialister in rheumatoid arthritis patients. Biomed. Pharmacother. 2023, 160, 114388. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Fang, X.; Chen, Y.; Ding, M.; Gong, M. Gut flora influences the hypothalamic-gonadal axis to regulate the pathogenesis of obesity-associated precocious puberty. Sci. Rep. 2024, 14, 28844. [Google Scholar] [CrossRef] [PubMed]
- Salah, M.; Azab, M.; Ramadan, A.; Hanora, A. New Insights on Obesity and Diabetes from Gut Microbiome Alterations in Egyptian Adults. Omics 2019, 23, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Li, D.; He, Y.; Li, Y.; Yang, Z.; Zhao, X.; Liu, Y.; Wang, Y.; Sun, J.; Feng, X.; et al. Discrepant gut microbiota markers for the classification of obesity-related metabolic abnormalities. Sci. Rep. 2019, 9, 13424. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Pan, C.H.; Wei, C.C.; Huang, H.Y. Lactobacillus plantarum PS128 Improves Physiological Adaptation and Performance in Triathletes through Gut Microbiota Modulation. Nutrients 2020, 12, 2315. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.H.; Chu, C.W.; Khoo, J.J.C.; Cheong, M.; Soon, G.H.; Ho, E.X.P.; Law, N.M.; De Sessions, P.F.; Fock, K.M.; Ang, T.L.; et al. A pilot study to examine the association between human gut microbiota and the host’s central obesity. JGH Open 2019, 3, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Li, F.J.; Zhang, R.Y.; Li, J.Y.; Liu, Y.N.; Zhang, Z.X.; Du, L.; Li, Y.D.; Liu, X.; Zhang, W.; Cui, G.Y.; et al. Pain, obesity, adenosine salvage disruption, and smoking behavior mediate the effect of gut microbiota on sleep disorders: Results from network Mendelian randomization and 16S rDNA sequencing. Front. Microbiol. 2024, 15, 1413218. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Sierra, A.; Romo-Hualde, A.; Aranaz, P.; Goni, L.; Cuervo, M.; Martínez, J.A.; Milagro, F.I.; Riezu-Boj, J.I. Diet- and sex-related changes of gut microbiota composition and functional profiles after 4 months of weight loss intervention. Eur. J. Nutr. 2021, 60, 3279–3301. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, P.; Ramos-Lopez, O.; Cuevas-Sierra, A.; Martinez, J.A.; Milagro, F.I.; Riezu-Boj, J.I. A predictive regression model of the obesity-related inflammatory status based on gut microbiota composition. Int. J. Obes. 2021, 45, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Ma, J.; Wu, X.; Qiu, L.; Huang, R.; Zhang, H.; Huang, H.; Chen, X. Impact of Visceral Obesity on Structural and Functional Alterations of Gut Microbiota in Polycystic Ovary Syndrome (PCOS): A Pilot Study Using Metagenomic Analysis. Diabetes Metab. Syndr. Obes. 2023, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Burakova, I.; Smirnova, Y.; Gryaznova, M.; Syromyatnikov, M.; Chizhkov, P.; Popov, E.; Popov, V. The Effect of Short-Term Consumption of Lactic Acid Bacteria on the Gut Microbiota in Obese People. Nutrients 2022, 14, 3384. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Fan, Y.; Schlezinger, J.J.; Ehrlich, S.D.; Webster, T.F.; Hyötyläinen, T.; Pedersen, O.; Orešič, M. Exposure to environmental toxicants is associated with gut microbiome dysbiosis, insulin resistance and obesity. Environ. Int. 2024, 186, 108569. [Google Scholar] [CrossRef] [PubMed]
- López-Montoya, P.; Rivera-Paredez, B.; Palacios-González, B.; Morán-Ramos, S.; López-Contreras, B.E.; Canizales-Quinteros, S.; Salmerón, J.; Velázquez-Cruz, R. Dietary Patterns Are Associated with the Gut Microbiome and Metabolic Syndrome in Mexican Postmenopausal Women. Nutrients 2023, 15, 4704. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A Beneficial Gut Organism From the Discoveries in Genus and Species. Front. Cell Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef] [PubMed]
- Masood, B.; Moorthy, M. Causes of obesity: A review. Clin. Med. 2023, 23, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.R.; Lee, Y.R.; Kim, Y.S.; Park, H.Y. Diet-Induced Gut Dysbiosis and Leaky Gut Syndrome. J. Microbiol. Biotechnol. 2024, 34, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Agrobiodiversidad. Available online: https://siagro.conabio.gob.mx/ (accessed on 28 April 2023).
- Dieta de la Milpa: Modelo de Alimentación Mesoamericana Biocompatible. Available online: https://www.gob.mx/cms/uploads/attachment/file/98453/La_Dieta_de_la_Milpa.pdf (accessed on 28 February 2019).
- Gaona-Pineda, E.B.; Martínez-Tapia, B.; Arango-Angarita, A.; Valenzuela-Bravo, D.; Gómez-Acosta, L.M.; Shamah-Levy, T.; Rodríguez-Ramírez, S. Food groups consumption and sociodemographic characteristics in Mexican population. Salud Publica Mex. 2018, 60, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Gaona-Pineda, E.B.; Rodríguez-Ramírez, S.; Medina-Zacarías, M.C.; Valenzuela-Bravo, D.G.; Martinez-Tapia, B.; Arango-Angarita, A. Consumidores de grupos de alimentos en población mexicana. Ensanut Continua 2020–2022. Salud Publica Mex. 2023, 65, s248–s258. [Google Scholar] [CrossRef] [PubMed]
- Aburto, T.C.; Batis, C.; Pedroza-Tobías, A.; Pedraza, L.S.; Ramírez-Silva, I.; Rivera, J.A. Dietary intake of the Mexican population: Comparing food group contribution to recommendations, 2012–2016. Salud Publica Mex. 2022, 64, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Corona-Cervantes, K.; Parra-Carriedo, A.; Hernández-Quiroz, F.; Martínez-Castro, N.; Vélez-Ixta, J.M.; Guajardo-López, D.; García-Mena, J.; Hernández-Guerrero, C. Physical and Dietary Intervention with Opuntia ficus-indica (Nopal) in Women with Obesity Improves Health Condition through Gut Microbiota Adjustment. Nutrients 2022, 14, 1008. [Google Scholar] [CrossRef] [PubMed]
- Murga-Garrido, S.M.; Orbe-Orihuela, Y.C.; Díaz-Benítez, C.E.; Castañeda-Márquez, A.C.; Cornejo-Granados, F.; Ochoa-Leyva, A.; Sanchez-Flores, A.; Cruz, M.; Burguete-García, A.I.; Lagunas-Martínez, A. Alterations of the Gut Microbiome Associated to Methane Metabolism in Mexican Children with Obesity. Children 2022, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Aguayo-Patrón, S.V.; Calderón de la Barca, A.M. Old Fashioned vs. Ultra-Processed-Based Current Diets: Possible Implication in the Increased Susceptibility to Type 1 Diabetes and Celiac Disease in Childhood. Foods 2017, 6, 100. [Google Scholar] [CrossRef] [PubMed]
- Arreola-Ornelas, H.; Merino-Juárez, G.A.; Contreras-Loya, D.; Méndez-Carniado, O.; Morales-Juárez, L.; Bernal-Serrano, D.; Arizmendi-Barrera, K.A.; Vargas-Martínez, C.; Razo, C.; Knaul, F.M.; et al. Burden of overweight and obesity in Mexico from 1990 to 2021. Gac. Med. Mex. 2023, 159, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Valerino-Perea, S.; Lara-Castor, L.; Armstrong, M.E.G.; Papadaki, A. Definition of the Traditional Mexican Diet and Its Role in Health: A Systematic Review. Nutrients 2019, 11, 2803. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human genetics shape the gut microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, H.D.; Morrison, M.; Dekker Nitert, M. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 2018, 9, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef] [PubMed]
- Silva-Boghossian, C.M.; Cesário, P.C.; Leão, A.T.T.; Colombo, A.P.V. Subgingival microbial profile of obese women with periodontal disease. J. Periodontol. 2018, 89, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest Pathophysiol. 2015, 6, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Lin, T.L.; Tsai, Y.L.; Wu, T.R.; Lai, W.F.; Lu, C.C.; Lai, H.C. Next generation probiotics in disease amelioration. J. Food Drug Anal. 2019, 27, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Falalyeyeva, T.; Kyriachenko, Y.; Tseyslyer, Y.; Kovalchuk, O.; Hadiliia, O.; Eslami, M.; Yousefi, B.; Abenavoli, L.; Fagoonee, S.; et al. Akkermansia muciniphila as a novel powerful bacterial player in the treatment of metabolic disorders. Minerva Endocrinol. 2022, 47, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, S.; Hu, X.; Chen, F.; Li, D. A Review of Healthy Dietary Choices for Cardiovascular Disease: From Individual Nutrients and Foods to Dietary Patterns. Nutrients 2023, 15, 4898. [Google Scholar] [CrossRef] [PubMed]
- Valerino-Perea, S.; Armstrong, M.E.G.; Papadaki, A. Adherence to a traditional Mexican diet and non-communicable disease-related outcomes: Secondary data analysis of the cross-sectional Mexican National Health and Nutrition Survey. Br. J. Nutr. 2023, 129, 1266–1279. [Google Scholar] [CrossRef] [PubMed]
- Zavala, G.A.; Ainscough, T.S.; Jimenez-Moreno, A.C. Barriers to a healthy diet and physical activity in Mexican adults: Results from the Mexican Health and Nutrition Survey. Nutr. Bull. 2022, 47, 298–306. [Google Scholar] [CrossRef] [PubMed]

| Central Mexico | Eastern | Southern | References | |||||
|---|---|---|---|---|---|---|---|---|
| State of Mexico | ||||||||
| Bacteria | Mexico City | Chimalhuacán | Ecatepec | Toluca | Querétaro (Rural Areas) | San Luis Potosí | Oaxaca | |
| Proteobacteria | Present | Present | [33,35] | |||||
| Lachnospira and Roseburia | Present | Present | Present | [33,37] | ||||
| Lactobacillus | Present | Present | Present | [25,38] | ||||
| Faecalibacterium | Present | [37] | ||||||
| Bacteroides | Present | Present | Present | [25,34,42] | ||||
| Porphyromonas | Present | Present | [25,28] | |||||
| Family Ruminococcaceae | Present | [42] | ||||||
| Prevotella | Present | Present | Present | [25,31,38] | ||||
| Collinsella | Present | Present | [28,38] | |||||
| Alistipes | Present | Present | [30,42] | |||||
| Family Coriobacteriaceae | Present | Present | [28,38] | |||||
| Catenibacterium | Present | Present | [28,30] | |||||
| Bacteria | Central Mexico (Mexico City) | Western Mexico (Colima, Jalisco, Michoacán, and Nayarit) | Reference |
|---|---|---|---|
| Bacteroides | Present | [36] | |
| Prevotella | Present | Present | [36,40] |
| Negativicutes | Present | [40] | |
| Escherichia/Shigella | Present | [40] | |
| Lachnoclostridium | Present | [40] | |
| Allisonella | Present | [29] | |
| Lachnospira | Present | Present | [26,28] |
| Romboutsia | Present | [29] | |
| Subdoligranorum | Present | [29] | |
| Clostridium | Present (and C. leptum) | Present (and C. coccoides) | [29,32] |
| Dilema | Present | [29] | |
| Eubacterium rectale | Present | [41] | |
| Faecalibacterium | Present (and F. praunitzii) | [26,32] | |
| Roseburia | Present | [26] | |
| Coprococcus | Present | [26] | |
| Erysipelotrichaceae | Present | [26] | |
| Bifidobacterium longum | Present | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samudio-Cruz, M.A.; Luna-Angulo, A.; Cabrera-Ruiz, E.; Landa-Solis, C.; Rangel-López, E.; Carrillo-Mora, P.; Ríos-Martínez, J.; Toledo-Pérez, R.; Paniagua-Pérez, R.; Martínez-Canseco, C.J.; et al. The Bacterial Composition of the Gut Microbiota of Mexicans with Overweight and Obesity: A Systematic Review. Microorganisms 2025, 13, 1727. https://doi.org/10.3390/microorganisms13081727
Samudio-Cruz MA, Luna-Angulo A, Cabrera-Ruiz E, Landa-Solis C, Rangel-López E, Carrillo-Mora P, Ríos-Martínez J, Toledo-Pérez R, Paniagua-Pérez R, Martínez-Canseco CJ, et al. The Bacterial Composition of the Gut Microbiota of Mexicans with Overweight and Obesity: A Systematic Review. Microorganisms. 2025; 13(8):1727. https://doi.org/10.3390/microorganisms13081727
Chicago/Turabian StyleSamudio-Cruz, María Alejandra, Alexandra Luna-Angulo, Elizabeth Cabrera-Ruiz, Carlos Landa-Solis, Edgar Rangel-López, Paul Carrillo-Mora, Juan Ríos-Martínez, Rafael Toledo-Pérez, Rogelio Paniagua-Pérez, Carlos Jorge Martínez-Canseco, and et al. 2025. "The Bacterial Composition of the Gut Microbiota of Mexicans with Overweight and Obesity: A Systematic Review" Microorganisms 13, no. 8: 1727. https://doi.org/10.3390/microorganisms13081727
APA StyleSamudio-Cruz, M. A., Luna-Angulo, A., Cabrera-Ruiz, E., Landa-Solis, C., Rangel-López, E., Carrillo-Mora, P., Ríos-Martínez, J., Toledo-Pérez, R., Paniagua-Pérez, R., Martínez-Canseco, C. J., Lino-González, A. L., Saldaña Solano, A. J., & Sánchez-Chapul, L. (2025). The Bacterial Composition of the Gut Microbiota of Mexicans with Overweight and Obesity: A Systematic Review. Microorganisms, 13(8), 1727. https://doi.org/10.3390/microorganisms13081727






